Treatment of Status Epilepticus after Traumatic Brain Injury Using an Antiseizure Drug Combined with a Tissue Recovery Enhancer Revealed by Systems Biology
Abstract
1. Introduction
2. Results
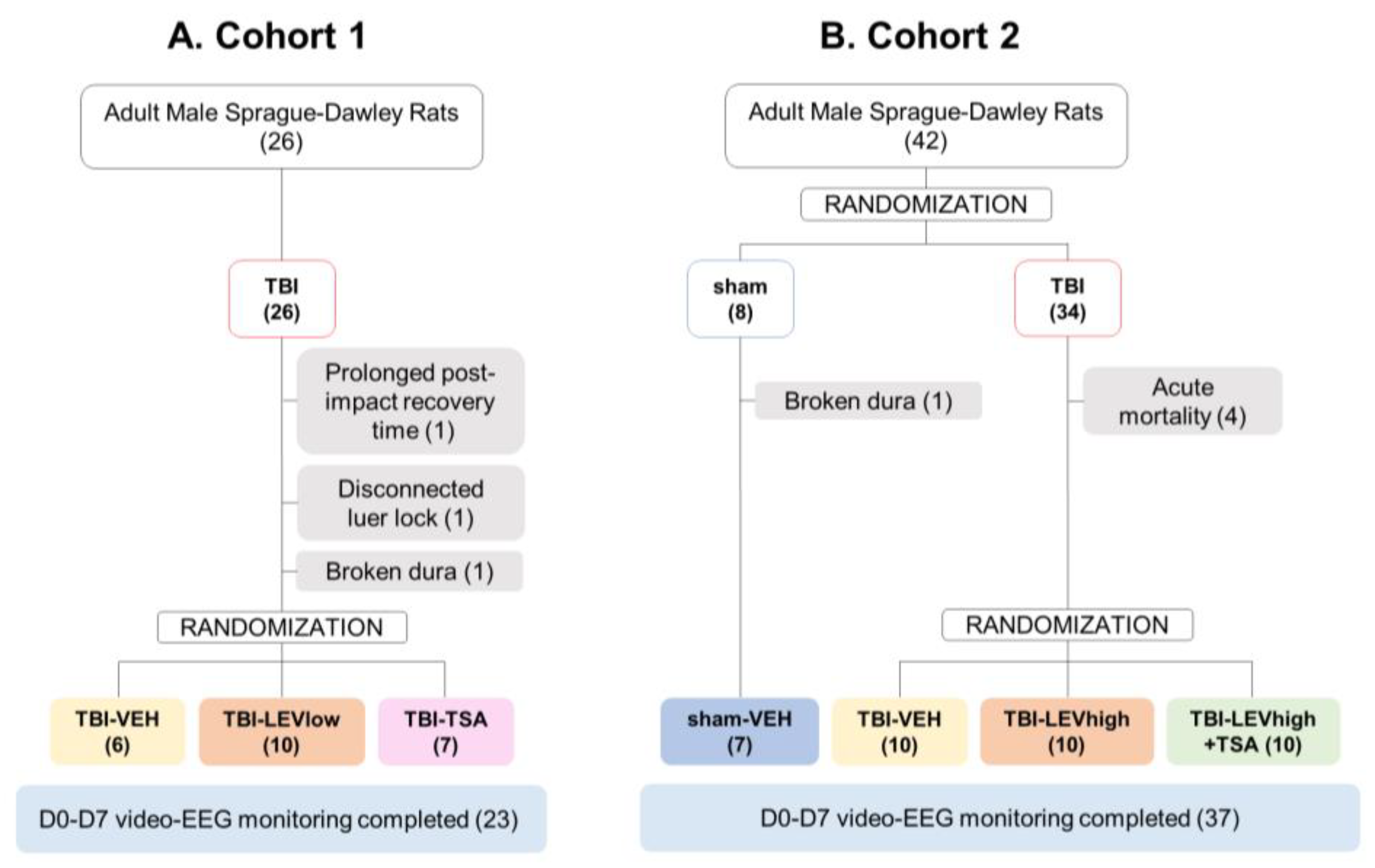
2.1. Mortality and Exclusions
2.2. Compound Selection for In Vitro Validation
2.3. Effect of Compounds on Neuronal Viability, Oxidative Stress, and Neuroinflammation In Vitro
2.3.1. Neuronal Viability
2.3.2. Nitrite Levels
2.3.3. TNFα Levels
2.4. Compound Selection for In Vivo Validation
2.5. Effect of TSA on In Vivo Outcome Measures
2.5.1. Preliminary Data from Cohort 1
2.5.2. Acute Seizures
Percentage of Rats with Acute Seizures during the First 72 h
Latency to the First Acute Seizure
Number of Acute Electrographic Seizures during the First 72 h
Seizure Duration
Cumulative Seizure Duration
Behavioral Severity of Electrographic Seizures
3. Discussion
3.1. LEV Showed a Remarkable Antiseizure Effect on Early Seizures after Severe TBI
3.2. Systems Biology Analysis Revealed Trichostatin A as the Most Promising TBI-Related Pathology-Modifying Compound
3.3. Trichostatin A Monotherapy Showed a Mild Neuroprotective Effect In Vivo But Had a Meager Treatment Effect In Vivo
3.4. Hurdles in Translation—Why Were Favorable In Vitro Neuroprotective Effects of TBI Not Found In Vivo?
4. Materials and Methods
4.1. Generation of TBI Gene Expression Signature in the Perilesional Cortex (TBI-Sig)
4.2. Selection of Drugs for In Vitro Validation
4.3. In Vitro Validation of Compound Effects on Neuronal Viability, Oxidative Stress, and Neuroinflammation
4.3.1. In Vitro Assessment of Neuroprotective, Antioxidant, and Anti-Inflammatory Effects of Compounds
Preparation of Mouse Primary Cortical Neurons and BV-2 Co-Culture
In Vitro Assessment of Treatments Effects
4.3.2. In Vitro Outcome Measures
Neuronal Viability
Nitrite Assay
TNFα ELISA from Cell Culture Medium
4.4. Selection of Compounds for In Vivo Validation
4.5. In Vivo Validation: Effect on Acute Seizures, Cortical Lesion Area, and Plasma Phosphorylated Neurofilament Heavy Chain (pNF-H) Levels
4.5.1. Animals
4.5.2. Induction of Lateral Fluid-Percussion TBI
4.5.3. Electrode Implantation
4.5.4. Preparation and Implantation of Subcutaneous Alzet Minipumps
4.5.5. Post-Impact Monitoring and Care
4.5.6. Video-Electroencephalography (Video-EEG) Monitoring
4.5.7. Video-EEG Analysis
4.5.8. Randomization of Animals into Treatment Groups
4.5.9. Drug Preparation
4.5.10. Drug Administration
Cohort 1
Cohort 2
4.5.11. Blood Sampling and Plasma Preparation
4.5.12. Quantification of Plasma pNF-H
4.5.13. Histology
4.5.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position Statement: Definition of Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Selassie, A.W.; Zaloshnja, E.; Langlois, J.A.; Miller, T.; Jones, P.; Steiner, C. Incidence of Long-Term Disability Following Traumatic Brain Injury Hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008, 23, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Corrigan John, D.; Selassie, A.W.; Orman, J.A.L. The Epidemiology of Traumatic Brain Injury. J. Head Trauma Rehabil. 2010, 25, 72–80. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic Brain Injury: Integrated Approaches to Improve Prevention, Clinical Care, and Research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- Horneman, G.; Emanuelson, I. Cognitive Outcome in Children and Young Adults Who Sustained Severe and Moderate Traumatic Brain Injury 10 Years Earlier. Brain Inj. 2009, 23, 907–914. [Google Scholar] [CrossRef]
- Walker, K.R.; Tesco, G. Molecular Mechanisms of Cognitive Dysfunction Following Traumatic Brain Injury. Front. Aging Neurosci. 2013, 5, 29. [Google Scholar] [CrossRef]
- Frey, L.C. Epidemiology of Posttraumatic Epilepsy: A Critical Review. Epilepsia 2003, 44, 11–17. [Google Scholar] [CrossRef]
- Salazar, A.M.; Grafman, J. Post-traumatic epilepsy. Clinical clues to pathogenesis and paths to prevention. In Handbook of Clinical Neurology; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 128, pp. 525–538. [Google Scholar]
- Abdul-Muneer, P.M.; Chandra, N.; Haorah, J. Interactions of Oxidative Stress and Neurovascular Inflammation in the Pathogenesis of Traumatic Brain Injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef]
- Werner, C.; Engelhard, K. Pathophysiology of Traumatic Brain Injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef]
- Jarrahi, A.; Braun, M.; Ahluwalia, M.; Gupta, R.V.; Wilson, M.; Munie, S.; Ahluwalia, P.; Vender, J.R.; Vale, F.L.; Dhandapani, K.M.; et al. Revisiting Traumatic Brain Injury: From Molecular Mechanisms to Therapeutic Interventions. Biomedicines 2020, 8, 389. [Google Scholar] [CrossRef]
- Webster, K.M.; Sun, M.; Crack, P.; O’Brien, T.J.; Shultz, S.R.; Semple, B.D. Inflammation in Epileptogenesis after Traumatic Brain Injury. J. Neuroinflammation 2017, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R.; Weisz, H.A.; Willey, H.E.; Torres, K.E.O.; Falduto, M.T.; Sinha, M.; Spratt, H.; Bolding, I.J.; Johnson, K.M.; Parsley, M.A.; et al. Traumatic Brain Injury Induces Long-Lasting Changes in Immune and Regenerative Signaling. PLoS ONE 2019, 14, e0214741. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.M.; Nuwer, M.R.; Nenov, V.; Ronne-Engstrom, E.; Hovda, D.A.; Bergsneider, M.; Kelly, D.F.; Martin, N.A.; Becker, D.P. Increased Incidence and Impact of Nonconvulsive and Convulsive Seizures after Traumatic Brain Injury as Detected by Continuous Electroencephalographic Monitoring. J. Neurosurg. 1999, 91, 750–760. [Google Scholar] [CrossRef]
- Vespa, P.M.; Miller, C.; McArthur, D.; Eliseo, M.; Etchepare, M.; Hirt, D.; Glenn, T.C.; Martin, N.; Hovda, D. Nonconvulsive Electrographic Seizures after Traumatic Brain Injury Result in a Delayed, Prolonged Increase in Intracranial Pressure and Metabolic Crisis. Crit. Care Med. 2007, 35, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.M.; McArthur, D.L.; Xu, Y.; Eliseo, M.; Etchepare, M.; Dinov, I.; Alger, J.; Glenn, T.P.; Hovda, D. Nonconvulsive Seizures after Traumatic Brain Injury Are Associated with Hippocampal Atrophy. Neurology 2010, 75, 792–798. [Google Scholar] [CrossRef]
- Zimmermann, L.L.; Diaz-Arrastia, R.; Vespa, P.M. Seizures and the Role of Anticonvulsants After Traumatic Brain Injury. Neurosurg. Clin. N. Am. 2016, 27, 499–508. [Google Scholar] [CrossRef]
- Tubi, M.A.; Lutkenhoff, E.; Blanco, M.B.; McArthur, D.; Villablanca, P.; Ellingson, B.; Diaz-Arrastia, R.; Van Ness, P.; Real, C.; Shrestha, V.; et al. Early Seizures and Temporal Lobe Trauma Predict Post-Traumatic Epilepsy: A Longitudinal Study. Neurobiol. Dis. 2019, 123, 115–121. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, J.; Yuan, Q.; Wu, X.; Wu, X.; Hu, J. Early Post-Traumatic Seizures Are Associated with Valproic Acid Plasma Concentrations and UGT1A6/CYP2C9 Genetic Polymorphisms in Patients with Severe Traumatic Brain Injury. Scand. J. Trauma. Resusc. Emerg. Med. 2017, 25, 85. [Google Scholar] [CrossRef]
- Andrade, P.; Banuelos-Cabrera, I.; Lapinlampi, N.; Paananen, T.; Ciszek, R.; Ndode-Ekane, X.E.; Pitkänen, A. Acute Non-Convulsive Status Epilepticus after Experimental Traumatic Brain Injury in Rats. J. Neurotrauma 2019, 36, 1890–1907. [Google Scholar] [CrossRef]
- Schouten, J.W. Neuroprotection in Traumatic Brain Injury: A Complex Struggle against the Biology of Nature. Curr. Opin. Crit. Care 2007, 13, 134–142. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.-R. Traumatic Brain Injury. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.; Hicks, R. Combination Therapies for Traumatic Brain Injury: Prospective Considerations. J. Neurotrauma 2009, 26, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Gerring, Z.F.; Gamazon, E.R.; White, A.; Derks, E.M. Integrative Network-Based Analysis Reveals Gene Networks and Novel Drug Repositioning Candidates for Alzheimer Disease. Neurol. Genet. 2021, 7, e622. [Google Scholar] [CrossRef]
- Mirza, N.; Sills, G.J.; Pirmohamed, M.; Marson, A.G. Identifying New Antiepileptic Drugs through Genomics- Based Drug Repurposing. Hum. Mol. Genet. 2017, 26, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Stathias, V.; Jermakowicz, A.M.; Maloof, M.E.; Forlin, M.; Walters, W.; Suter, R.K.; Durante, M.A.; Williams, S.L.; Harbour, J.W.; Volmar, C.-H.; et al. Drug and Disease Signature Integration Identifies Synergistic Combinations in Glioblastoma. Nat. Commun. 2018, 9, 5315. [Google Scholar] [CrossRef]
- Lipponen, A.; Paananen, J.; Puhakka, N.; Pitkänen, A. Analysis of Post-Traumatic Brain Injury Gene Expression Signature Reveals Tubulins, Nfe2l2, Nfkb, Cd44 and S100a4 as Treatment Targets. Sci. Rep. 2016, 6, 31570. [Google Scholar] [CrossRef]
- Lipponen, A.; El-Osta, A.; Kaspi, A.; Ziemann, M.; Khurana, I.; KN, H.; Navarro-Ferrandis, V.; Puhakka, N.; Paananen, J.; Pitkänen, A. Transcription Factors Tp73, Cebpd, Pax6, and Spi1 Rather than DNA Methylation Regulate Chronic Transcriptomics Changes after Experimental Traumatic Brain Injury. Acta Neuropathol. Commun. 2018, 6, 17. [Google Scholar] [CrossRef]
- Lipponen, A.; Natunen, T.; Hujo, M.; Ciszek, R.; Hämäläinen, E.; Tohka, J.; Hiltunen, M.; Paananen, J.; Poulsen, D.; Kansanen, E.; et al. In Vitro and In Vivo Pipeline for Validation of Disease-Modifying Effects of Systems Biology-Derived Network Treatments for Traumatic Brain Injury—Lessons Learned. Int. J. Mol. Sci. 2019, 20, 5395. [Google Scholar] [CrossRef]
- Perks, A.; Cheema, S.; Mohanraj, R. Anaesthesia and Epilepsy. Br. J. Anaesth. 2012, 108, 562–571. [Google Scholar] [CrossRef]
- Grover, E.H.; Nazzal, Y.; Hirsch, L.J. Treatment of Convulsive Status Epilepticus. Curr. Treat. Options Neurol. 2016, 18, 11. [Google Scholar] [CrossRef]
- Ndode-Ekane, X.E.; Kharatishvili, I.; Pitkänen, A. Unfolded Maps for Quantitative Analysis of Cortical Lesion Location and Extent after Traumatic Brain Injury. J. Neurotrauma 2017, 34, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Immonen, R.J.; Kharatishvili, I.; Niskanen, J.P.; Gröhn, H.; Pitkänen, A.; Gröhn, O.H.J. Distinct MRI Pattern in Lesional and Perilesional Area after Traumatic Brain Injury in Rat–11 Months Follow-Up. Exp. Neurol. 2009, 215, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of Seizure Activity by Electrical Stimulation: II. Motor Seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Ciszek, R.; Paananen, T.; Ndode-Ekane, X.E. Seizure semiology in rats with post-traumatic epilepsy. In Proceedings of the American Epilepsy Society, Nashville, TN, USA, 2–6 December 2022. [Google Scholar]
- Gultekin, R.; Huang, S.; Clavisi, O.; Pattuwage, L.; König, T.C.; Gruen, R. Pharmacological Interventions in Traumatic Brain Injury: Can We Rely on Systematic Reviews for Evidence? Injury 2016, 47, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Vella, M.A.; Crandall, M.; Patel, M.B.; Surgery, A.C.; Sciences, S.; Care, S.C.; Surgery, E.G.; Building, M.A. Acute Management of TBI. Surg. Clin. North Am. 2017, 97, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Vedantam, A.; Gopinath, S.P.; Robertson, C.S. Acute management of traumatic brain injury. In Rehabilitation After Traumatic Brain Injury; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–11. ISBN 9780323544566. [Google Scholar]
- Aiguabella, M.; Falip, M.; Villanueva, V.; de la Peña, P.; Molins, A.; Garcia-Morales, I.; Saiz, R.A.; Pardo, J.; Tortosa, D.; Sansa, G.; et al. Efficacy of Intravenous Levetiracetam as an Add-on Treatment in Status Epilepticus: A Multicentric Observational Study. Seizure 2011, 20, 60–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hedges, A.; Findlay, M.C.; Davis, G.E.; Wolfe, B.M.; Hawryluk, G.W.J.; Menacho, S.T.; Ansari, S. Levetiracetam Dosing for Seizure Prophylaxis in Neurocritical Care Patients. Brain Inj. 2023, 37, 1167–1172. [Google Scholar] [CrossRef]
- Atwood, R.; Walker, P.; Walper, D.; Elster, E.; Bradley, M. Use of Levetiracetam for Post-Traumatic Seizure Prophylaxis in Combat-Related Traumatic Brain Injury. Mil. Med. 2023, 00, 1–5. [Google Scholar] [CrossRef]
- Yan, H.-D.; Ji-qun, C.; Ishihara, K.; Nagayama, T.; Serikawa, T.; Sasa, M. Separation of Antiepileptogenic and Antiseizure Effects of Levetiracetam in the Spontaneously Epileptic Rat (SER). Epilepsia 2005, 46, 1170–1177. [Google Scholar] [CrossRef]
- Itoh, K.; Inamine, M.; Oshima, W.; Kotani, M.; Chiba, Y.; Ueno, M.; Ishihara, Y. Prevention of Status Epilepticus-Induced Brain Edema and Neuronal Cell Loss by Repeated Treatment with High-Dose Levetiracetam. Brain Res. 2015, 1608, 225–234. [Google Scholar] [CrossRef]
- Contreras-García, I.J.; Cárdenas-Rodríguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macías, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals 2022, 15, 475. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.; Shear, D.A.; Bramlett, H.M.; Dixon, C.E.; Mondello, S.; Schmid, K.E.; Poloyac, S.M.; Dietrich, W.D.; Hayes, R.L.; Wang, K.K.W.; et al. Levetiracetam Treatment in Traumatic Brain Injury: Operation Brain Trauma Therapy. J. Neurotrauma 2016, 33, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Saletti, P.G.; Mowrey, W.B.; Liu, W.; Li, Q.; McCullough, J.; Aniceto, R.; Lin, I.; Eklund, M.; Casillas-Espinosa, P.M.; Ali, I.; et al. Early Preclinical Plasma Protein Biomarkers of Brain Trauma Are Influenced by Early Seizures and Levetiracetam. Epilepsia Open 2023, 8, 586–608. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Afroz, S.; Valsamis, H.A.; Michelson, H.B.; Goodman, J.H.; Ling, D.S.F. Early Intervention with Levetiracetam Prevents the Development of Cortical Hyperexcitability and Spontaneous Epileptiform Activity in Two Models of Neurotrauma in Rats. Exp. Neurol. 2021, 337, 113571. [Google Scholar] [CrossRef] [PubMed]
- Doheny, H.C.; Whittington, M.A.; Jefferys, J.G.R.; Patsalos, P.N. A Comparison of the Efficacy of Carbamazepine and the Novel Anti-Epileptic Drug Levetiracetam in the Tetanus Toxin Model of Focal Complex Partial Epilepsy. Br. J. Pharmacol. 2002, 135, 1425–1434. [Google Scholar] [CrossRef]
- Coles, L.D.; Saletti, P.G.; Lisgaras, C.P.; Casillas-Espinosa, P.M.; Liu, W.; Li, Q.; Jones, N.C.; Shultz, S.; Ali, I.; Brady, R.; et al. Levetiracetam Pharmacokinetics and Brain Uptake in a Lateral Fluid Percussion Injury Rat Model. J. Pharmacol. Exp. Ther. 2023, 386, 259–265. [Google Scholar] [CrossRef]
- Doheny, H.; Ratnaraj, N.; Whittington, M.; Jefferys, J.G.; Patsalos, P. Blood and Cerebrospinal Fluid Pharmacokinetics of the Novel Anticonvulsant Levetiracetam (Ucb L059) in the Rat. Epilepsy Res. 1999, 34, 161–168. [Google Scholar] [CrossRef]
- Trinka, E.; Leitinger, M. Which EEG Patterns in Coma Are Nonconvulsive Status Epilepticus? Epilepsy Behav. 2015, 49, 203–222. [Google Scholar] [CrossRef]
- Kane, N.; Acharya, J.; Beniczky, S.; Caboclo, L.; Finnigan, S.; Kaplan, P.W.; Shibasaki, H.; Pressler, R.; van Putten, M.J.A.M. A Revised Glossary of Terms Most Commonly Used by Clinical Electroencephalographers and Updated Proposal for the Report Format of the EEG Findings. Revision 2017. Clin. Neurophysiol. Pract. 2017, 2, 170–185. [Google Scholar] [CrossRef]
- Pitkänen, A. Therapeutic Approaches to Epileptogenesis-Hope on the Horizon. Epilepsia 2010, 51, 2–17. [Google Scholar] [CrossRef]
- Gresa-Arribas, N.; Viéitez, C.; Dentesano, G.; Serratosa, J.; Saura, J.; Solà, C. Modelling Neuroinflammation In Vitro: A Tool to Test the Potential Neuroprotective Effect of Anti-Inflammatory Agents. PLoS One 2012, 7, e45227. [Google Scholar] [CrossRef] [PubMed]
- Natunen, T.A.; Gynther, M.; Rostalski, H.; Jaako, K.; Jalkanen, A.J. Extracellular Prolyl Oligopeptidase Derived from Activated Microglia Is a Potential Neuroprotection Target. Basic Clin. Pharmacol. Toxicol. 2019, 124, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Martiskainen, H.; Paldanius, K.M.A.; Natunen, T.; Takalo, M.; Marttinen, M.; Leskelä, S.; Huber, N.; Mäkinen, P.; Bertling, E.; Dhungana, H.; et al. DHCR24 Exerts Neuroprotection upon Inflammation-Induced Neuronal Death. J. Neuroinflammation 2017, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Deboer, C.; Meulman, P.A.; Wnuk, R.J.; Peterson, D. Geldanamycin, a New Antibiotic. J. Antibiot. 1970, 23, 442–447. [Google Scholar] [CrossRef]
- Supko, J.G.; Hickman, R.L.; Grever, M.R.; Malspeis, L. Preclinical Pharmacologic Evaluation of Geldanamycin as an Antitumor Agent. Cancer Chemother. Pharmacol. 1995, 36, 305–315. [Google Scholar] [CrossRef]
- Xiao, N.; Callaway, C.W.; Lipinski, C.A.; Hicks, S.D.; DeFranco, D.B. Geldanamycin Provides Posttreatment Protection Against Glutamate-Induced Oxidative Toxicity in a Mouse Hippocampal Cell Line. J. Neurochem. 1999, 72, 95–101. [Google Scholar] [CrossRef]
- Atkinson, R.M.; Ditman, K.S. Tranylcypromine: A Review. Clin. Pharmacol. Ther. 1965, 6, 631–655. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Tang, H.; Wu, H.; Li, L.; Wu, R.; Zhao, J.; Wu, Y.; Liu, Z.; Chen, J. Tranylcypromine Causes Neurotoxicity and Represses BHC110/LSD1 in Human-Induced Pluripotent Stem Cell-Derived Cerebral Organoids Model. Front. Neurol. 2017, 8, 626. [Google Scholar] [CrossRef]
- Caraci, F.; Pappalardo, G.; Basile, L.; Giuffrida, A.; Copani, A.; Tosto, R.; Sinopoli, A.; Giuffrida, M.L.; Pirrone, E.; Drago, F.; et al. Neuroprotective Effects of the Monoamine Oxidase Inhibitor Tranylcypromine and Its Amide Derivatives against Aβ(1–42)-Induced Toxicity. Eur. J. Pharmacol. 2015, 764, 256–263. [Google Scholar] [CrossRef]
- Pubchem Website. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acetylleucyl-leucyl-norleucinal (accessed on 24 May 2018).
- Haas, M.; Page, S.; Page, M.; Neumann, F.J.; Marx, N.; Adam, M.; Ziegler-Heitbrock, H.W.L.; Neumeier, D.; Brand, K. Effect of Proteasome Inhibitors on Monocytic IκB-α and -β Depletion, NF-ΚB Activation, and Cytokine Production. J. Leukoc. Biol. 1998, 63, 395–404. [Google Scholar] [CrossRef]
- Li, N.; Shang, L.; Wang, S.-C.; Liao, L.-S.; Chen, D.; Huang, J.-F.; Xiong, K. The Toxic Effect of ALLN on Primary Rat Retinal Neurons. Neurotox. Res. 2016, 30, 392–406. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, F.; Alamo, C.; Cuenca, E.; Shen, W.; Clervoy, P.; Rubio, G. History of the Discovery and Clinical Introduction of Chlorpromazine. Ann. Clin. Psychiatry 2005, 17, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.W. A History of Antipsychotic Drug Development. Compr. Psychiatry 1999, 40, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Ruskin, B.H. Determination of Serum Chlorpromazine Metabolites in Psychotic Patients. J. Nerv. Ment. Dis. 1964, 139, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Labuzek, K.; Kowalski, J.; Gabryel, B.; Herman, Z.S. Chlorpromazine and Loxapine Reduce Interleukin-1β and Interleukin-2 Release by Rat Mixed Glial and Microglial Cell Cultures. Eur. Neuropsychopharmacol. 2005, 15, 23–30. [Google Scholar] [CrossRef]
- Bastianetto, S.; Danik, M.; Mennicken, F.; Williams, S.; Quirion, R. Prototypical Antipsychotic Drugs Protect Hippocampal Neuronal Cultures against Cell Death Induced by Growth Medium Deprivation. BMC Neurosci. 2006, 7, 28. [Google Scholar] [CrossRef]
- Langley, B.; Gensert, J.; Beal, M.; Ratan, R. Remodeling Chromatin and Stress Resistance in the Central Nervous System: Histone Deacetylase Inhibitors as Novel and Broadly Effective Neuroprotective Agents. Curr. Drug Target-CNS Neurol. Disord. 2005, 4, 41–50. [Google Scholar] [CrossRef]
- Yoshida, M.; Kijima, M.; Akita, M.; Beppu, T. Potent and Specific Inhibition of Mammalian Histone Deacetylase Both in Vivo and in Vitro by Trichostatin A. J. Biol. Chem. 1990, 265, 17174–17179. [Google Scholar] [CrossRef]
- Agudelo, M.; Gandhi, N.; Saiyed, Z.; Pichili, V.; Thangavel, S.; Khatavkar, P.; Yndart-Arias, A.; Nair, M. NIH Public Access. Alcohol. Clin. Exp. Res. 2012, 35, 1550–1556. [Google Scholar]
- Ryu, H.; Lee, J.; Olofsson, B.A.; Mwidau, A.; Deodoglu, A.; Escudero, M.; Flemington, E.; Azizkhan-Clifford, J.; Ferrante, R.J.; Ratan, R.R. Histone Deacetylase Inhibitors Prevent Oxidative Neuronal Death Independent of Expanded Polyglutamine Repeats via an Sp1-Dependent Pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 4281–4286. [Google Scholar] [CrossRef]
- Citraro, R.; Leo, A.; Santoro, M.; D’agostino, G.; Constanti, A.; Russo, E. Role of Histone Deacetylases (HDACs) in Epilepsy and Epileptogenesis. Curr. Pharm. Des. 2017, 23, 5546–5562. [Google Scholar] [CrossRef] [PubMed]
- Broide, R.S.; Redwine, J.M.; Aftahi, N.; Young, W.; Bloom, F.E.; Winrow, C.J. Distribution of Histone Deacetylases 1–11 in the Rat Brain. J. Mol. Neurosci. 2007, 31, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; West, E.J.; Van, K.C.; Gurkoff, G.G.; Zhou, J.; Zhang, X.-M.; Kozikowski, A.P.; Lyeth, B.G. HDAC Inhibitor Increases Histone H3 Acetylation and Reduces Microglia Inflammatory Response Following Traumatic Brain Injury in Rats. Brain Res. 2008, 1226, 181–191. [Google Scholar] [CrossRef]
- Gao, W.-M.; Chadha, M.S.; Kline, A.E.; Clark, R.S.B.; Kochanek, P.M.; Dixon, C.E.; Jenkins, L.W. Immunohistochemical Analysis of Histone H3 Acetylation and Methylation—Evidence for Altered Epigenetic Signaling Following Traumatic Brain Injury in Immature Rats. Brain Res. 2006, 1070, 31–34. [Google Scholar] [CrossRef]
- Sagarkar, S.; Balasubramanian, N.; Mishra, S.; Choudhary, A.G.; Kokare, D.M.; Sakharkar, A.J. Repeated Mild Traumatic Brain Injury Causes Persistent Changes in Histone Deacetylase Function in Hippocampus: Implications in Learning and Memory Deficits in Rats. Brain Res. 2019, 1711, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Banerjee, J.; Dubey, V.; Tripathi, M.; Chandra, P.S.; Sharma, M.C.; Lalwani, S.; Siraj, F.; Doddamani, R.; Dixit, A.B. Role of Altered Expression, Activity and Sub-Cellular Distribution of Various Histone Deacetylases (HDACs) in Mesial Temporal Lobe Epilepsy with Hippocampal Sclerosis. Cell. Mol. Neurobiol. 2022, 42, 1049–1064. [Google Scholar] [CrossRef]
- Sanderson, L.; Taylor, G.W.; Aboagye, E.O.; Alao, J.P.; Latigo, J.R.; Coombes, R.C.; Vigushin, D.M. Plasma Pharmacokinetics and Metabolism of the Histone Deacetylase Inhibitor Trichostatin a after Intraperitoneal Administration to Mice. Drug Metab. Dispos. 2004, 32, 1132–1138. [Google Scholar] [CrossRef]
- Hyeon, J.K.; Rowe, M.; Ren, M.; Hong, J.S.; Chen, P.S.; Chuang, D.M. Histone Deacetylase Inhibitors Exhibit Anti-Inflammatory and Neuroprotective Effects in a Rat Permanent Ischemic Model of Stroke: Multiple Mechanisms of Action. J. Pharmacol. Exp. Ther. 2007, 321, 892–901. [Google Scholar] [CrossRef]
- Moreno-Yruela, C.; Fass, D.M.; Cheng, C.; Herz, J.; Olsen, C.A.; Haggarty, S.J. Kinetic Tuning of HDAC Inhibitors Affords Potent Inducers of Progranulin Expression. ACS Chem. Neurosci. 2019, 10, 3769–3777. [Google Scholar] [CrossRef]
- Ma, X.-H.; Gao, Q.; Jia, Z.; Zhang, Z.-W. Neuroprotective Capabilities of TSA against Cerebral Ischemia/Reperfusion Injury via PI3K/Akt Signaling Pathway in Rats. Int. J. Neurosci. 2015, 125, 140–146. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Ali, S.; Pace, P.E.; Mirsaidi, N.; Ito, K.; Adcock, I.; Coombes, R.C. Trichostatin A Is a Histone Deacetylase Inhibitor with Potent Antitumor Activity against Breast Cancer in Vivo. Clin. Cancer Res. 2001, 7, 971–976. [Google Scholar] [PubMed]
- Hoffmann, K.; Czapp, M.; Löscher, W. Increase in Antiepileptic Efficacy during Prolonged Treatment with Valproic Acid: Role of Inhibition of Histone Deacetylases? Epilepsy Res. 2008, 81, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.-H.; Wu, C.-C.; Wu, J.; Lai, H.-Y.; Chen, K.-Y.; Jheng, B.-R.; Chen, M.-C.; Chang, T.-H.; Chen, B.-S. Temporal Genetic Modifications after Controlled Cortical Impact—Understanding Traumatic Brain Injury through a Systematic Network Approach. Int. J. Mol. Sci. 2016, 17, 216. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Kemppainen, S.; Ndode-Ekane, X.E.; Huusko, N.; Huttunen, J.K.; Gröhn, O.; Immonen, R.; Sierra, A.; Bolkvadze, T. Posttraumatic Epilepsy—Disease or Comorbidity? Epilepsy Behav. 2014, 38, 19–24. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Kim, Y.; Li, J.; Huang, S.; Saleem, S.; Li, R.C.; Xu, Y.; Dore, S.; Cao, W. Histone Deacetylase Inhibition Activates Transcription Factor Nrf2 and Protects against Cerebral Ischemic Damage. Free Radic. Biol. Med. 2012, 52, 928–936. [Google Scholar] [CrossRef]
- Rau, T.; Kothiwal, A.; Rova, A.; Rhoderick, J.; Poulsen, D. Phenoxybenzamine Is Neuroprotective in a Rat Model of Severe Traumatic Brain Injury. Int. J. Mol. Sci. 2014, 15, 1402–1417. [Google Scholar] [CrossRef]
- ILINCS Website. Available online: http://www.ilincs.org/ilincs/ (accessed on 4 April 2018).
- Pilarczyk, M.; Fazel-Najafabadi, M.; Kouril, M.; Shamsaei, B.; Vasiliauskas, J.; Niu, W.; Mahi, N.; Zhang, L.; Clark, N.A.; Ren, Y.; et al. Connecting Omics Signatures and Revealing Biological Mechanisms with ILINCS. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Brooke, S.M.; Bliss, T.M.; Franklin, L.R.; Sapolsky, R.M. Quantification of Neuron Survival in Monolayer Cultures Using an Enzyme-Linked Immunosorbent Assay Approach, Rather than by Cell Counting. Neurosci. Lett. 1999, 267, 21–24. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Vink, R.; Noble, L.; Yamakami, I.; Fernyak, S.; Soares, H.; Faden, A.L. Traumatic Brain Injury in the Rat: Characterization of a Lateral Fluid-Percussion Model. Neuroscience 1989, 28, 233–244. [Google Scholar] [CrossRef]
- Kharatishvili, I.; Nissinen, J.P.; McIntosh, T.K.; Pitkänen, A. A Model of Posttraumatic Epilepsy Induced by Lateral Fluid-Percussion Brain Injury in Rats. Neuroscience 2006, 140, 685–697. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 6, ISBN 978-0-12-374121-9. [Google Scholar]
- Nissinen, A.J.; Andrade, P.; Natunen, T.; Shatillo, O.; Sallinen, J.; Ekolle, X. Disease-Modifying Effect of Atipamezole in a Model of Post-Traumatic Epilepsy. Epilepsy Res. 2017, 136, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, J.; Lassiter, T.F.; McDonagh, D.L.; Sheng, H.; Warner, D.S.; Lynch, J.R.; Laskowitz, D.T. Levetiracetam Is Neuroprotective in Murine Models of Closed Head Injury and Subarachnoid Hemorrhage. Neurocrit. Care 2006, 5, 71–78. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, E.A.; Puhakka, N.; Mills, J.D.; Srivastava, P.K.; Johnson, M.R.; Roncon, P.; Das Gupta, S.; Karttunen, J.; Simonato, M.; Lukasiuk, K.; et al. Standardization Procedure for Plasma Biomarker Analysis in Rat Models of Epileptogenesis: Focus on Circulating MicroRNAs. Epilepsia 2017, 58, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Kamnaksh, A.; Puhakka, N.; Ali, I.; Smith, G.; Aniceto, R.; Mccullough, J.; Das, S.; Ndode-ekane, X.E.; Brady, R.; Casillas-espinosa, P.; et al. Harmonization of Pipeline for Preclinical Multicenter Plasma Protein and MiRNA Biomarker Discovery in a Rat Model of Post-Traumatic. Epilepsy Res. 2019, 149, 92–101. [Google Scholar] [CrossRef]
- Huusko, N.; Römer, C.; Ndode-Ekane, X.E.; Lukasiuk, K.; Pitkänen, A. Loss of Hippocampal Interneurons and Epileptogenesis: A Comparison of Two Animal Models of Acquired Epilepsy. Brain Struct. Funct. 2015, 220, 153–191. [Google Scholar] [CrossRef] [PubMed]
- Ciszek, R.; Andrade, P.; Tapiala, J.; Pitkänen, A.; Ndode-Ekane, X.E. Web Application for Quantification of Traumatic Brain Injury-Induced Cortical Lesions in Adult Mice. Neuroinformatics 2020, 18, 307–317. [Google Scholar] [CrossRef]








| Compound | Concentration | Neuronal Viability | Nitrite | TNFα | Total Score |
|---|---|---|---|---|---|
| Trichostatin A | 50 nM | 0.75 | 0.75 | 1 | 2.5 |
| 7,8-dihydroxychlorpromazine | 50 µM | 0.45 | 0.30 | 1 | 1.75 |
| Calpain inhibitor | 50 µM | 0.60 | 1 | 1 | 2.60 |
| (+)-Tranylcypromine | 1 µM | 0.15 | 0.15 | N/A | 0.30 |
| Geldanamycin | 10 nM | 0.15 | 0.15 | N/A | 0.30 |
| Treatment Group | All Seizures (K-W 0.005) | Time after TBI (h) | Intragroup Statistics (Friedman’s Two-Way ANOVA) | ||
|---|---|---|---|---|---|
| T1 = 0–24 h (K-W 0.070) | T2 = 25–48 h (K-W 0.009) | T3 = 49–72 h (K-W 0.383) | |||
| TBI-Veh (16) | 727 ± 688 (198) [537, 0–1833] | 438 ± 510 (110) [208, 0–1406] | 169 ± 290 (57) [10, 0–953] | 120 ± 176 (31) [0, 0–475] | ns |
| TBI-TSA (7) | 898 ± 937 (90) [596, 0–2344] (Cohen’s d −0.222) | 372 ± 632 (31) [0, 0–1391] (Cohen’s d 0.120) | 414 ± 330 (49) [596, 0–734] (Cohen’s d −0.813) | 112 ± 175 (10) [0, 0–419] (Cohen’s d 0.053) | ns |
| TBI-LEVlow (10) | 358 ± 715 (60) [41, 0–2111] (Cohen’s d 0.528) | 201 ± 444 (28) [0, 0–1287] (Cohen’s d, 0.487) | 67 ± 146 (18) # [0, 0–462] (Cohen’s d, 0.414) | 95 ± 258 (14) [0, 0–824] (Cohen’s d, 0.123) | ns |
| TBI-LEVhigh (10) | 42 ± 64 (10) **, # [0, 0–186] (Cohen’s d 1.256) C d to LEVlow 0.623 | 10 ± 22 (2) * [0, 0–66] (Cohen’s d 1.060) C d to LEVlow 0.607 | 14 ± 23 (5) # [0, 0–58] (Cohen’s d 0.675) C d to LEVlow 0.511 | 18 ± 49 (3) [0, 0–154] (Cohen’s d 0.719) C d to LEVlow 0.414 | ns |
| TBI-LEVhigh + TSA (10) | 109 ± 282 (21) **, # [0, 0–898] (Cohen’s d 1.083) | 62 ± 137 (16) [0, 0–424] (Cohen’s d 0.912) | 29 ± 92 (3) ## [0, 0–291] (Cohen’s d 0.593) | 18 ± 58 (2) [0, 0–183] (Cohen’s d 0.713) | ns |
| Compound | 32 h | 3 Months | ||
|---|---|---|---|---|
| Concordance Value | Cell Line | Concordance Value | Cell Line | |
| Trichostatin A | 0.331 | Neu | 0.373 | Neu |
| Geldanamycin | 0.281 | Neu-KCL | 0.375 | Neu-KCL |
| Calpain inhibitor | 0.268 | Neu-KCL | 0.367 | Neu-KCL |
| 7,8-dihydroxychlorpromazine | 0.221 | Neu-KCL | 0.226 | Neu-KCL |
| (+)-Tranylcypromine | −0.206 | Neu-KCL | −0.251 | Neu-KCL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajevu, N.; Lipponen, A.; Andrade, P.; Bañuelos, I.; Puhakka, N.; Hämäläinen, E.; Natunen, T.; Hiltunen, M.; Pitkänen, A. Treatment of Status Epilepticus after Traumatic Brain Injury Using an Antiseizure Drug Combined with a Tissue Recovery Enhancer Revealed by Systems Biology. Int. J. Mol. Sci. 2023, 24, 14049. https://doi.org/10.3390/ijms241814049
Kajevu N, Lipponen A, Andrade P, Bañuelos I, Puhakka N, Hämäläinen E, Natunen T, Hiltunen M, Pitkänen A. Treatment of Status Epilepticus after Traumatic Brain Injury Using an Antiseizure Drug Combined with a Tissue Recovery Enhancer Revealed by Systems Biology. International Journal of Molecular Sciences. 2023; 24(18):14049. https://doi.org/10.3390/ijms241814049
Chicago/Turabian StyleKajevu, Natallie, Anssi Lipponen, Pedro Andrade, Ivette Bañuelos, Noora Puhakka, Elina Hämäläinen, Teemu Natunen, Mikko Hiltunen, and Asla Pitkänen. 2023. "Treatment of Status Epilepticus after Traumatic Brain Injury Using an Antiseizure Drug Combined with a Tissue Recovery Enhancer Revealed by Systems Biology" International Journal of Molecular Sciences 24, no. 18: 14049. https://doi.org/10.3390/ijms241814049
APA StyleKajevu, N., Lipponen, A., Andrade, P., Bañuelos, I., Puhakka, N., Hämäläinen, E., Natunen, T., Hiltunen, M., & Pitkänen, A. (2023). Treatment of Status Epilepticus after Traumatic Brain Injury Using an Antiseizure Drug Combined with a Tissue Recovery Enhancer Revealed by Systems Biology. International Journal of Molecular Sciences, 24(18), 14049. https://doi.org/10.3390/ijms241814049







