Yeast Heterologous Expression Systems for the Study of Plant Membrane Proteins
Abstract
:1. Introduction
2. Baker’s Yeast Saccharomyces cerevisiae for Functional Study of Plant Membrane Proteins
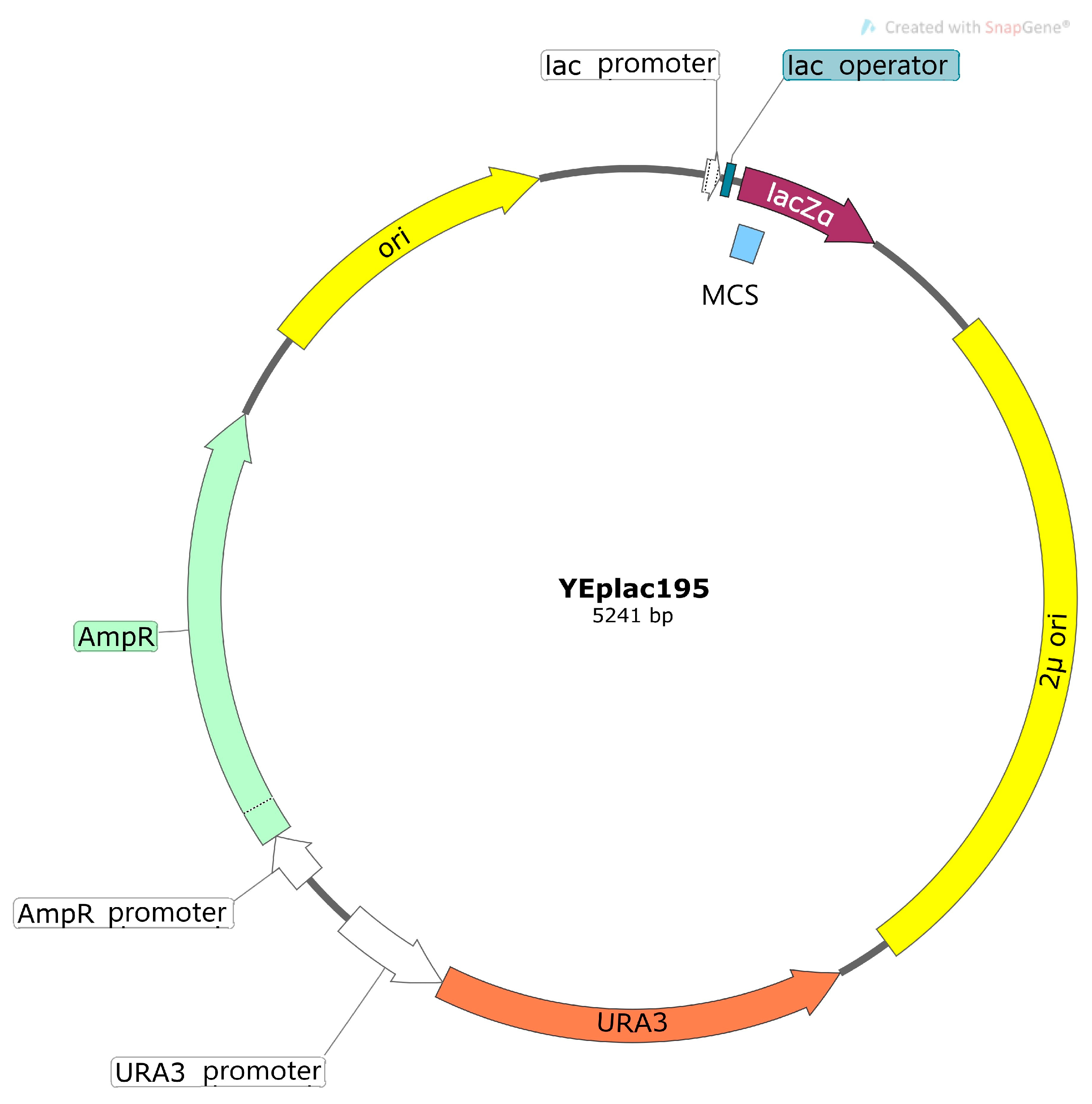
2.1. Vector Systems Used for Heterologous Expression in Cells of S. cerevisiae
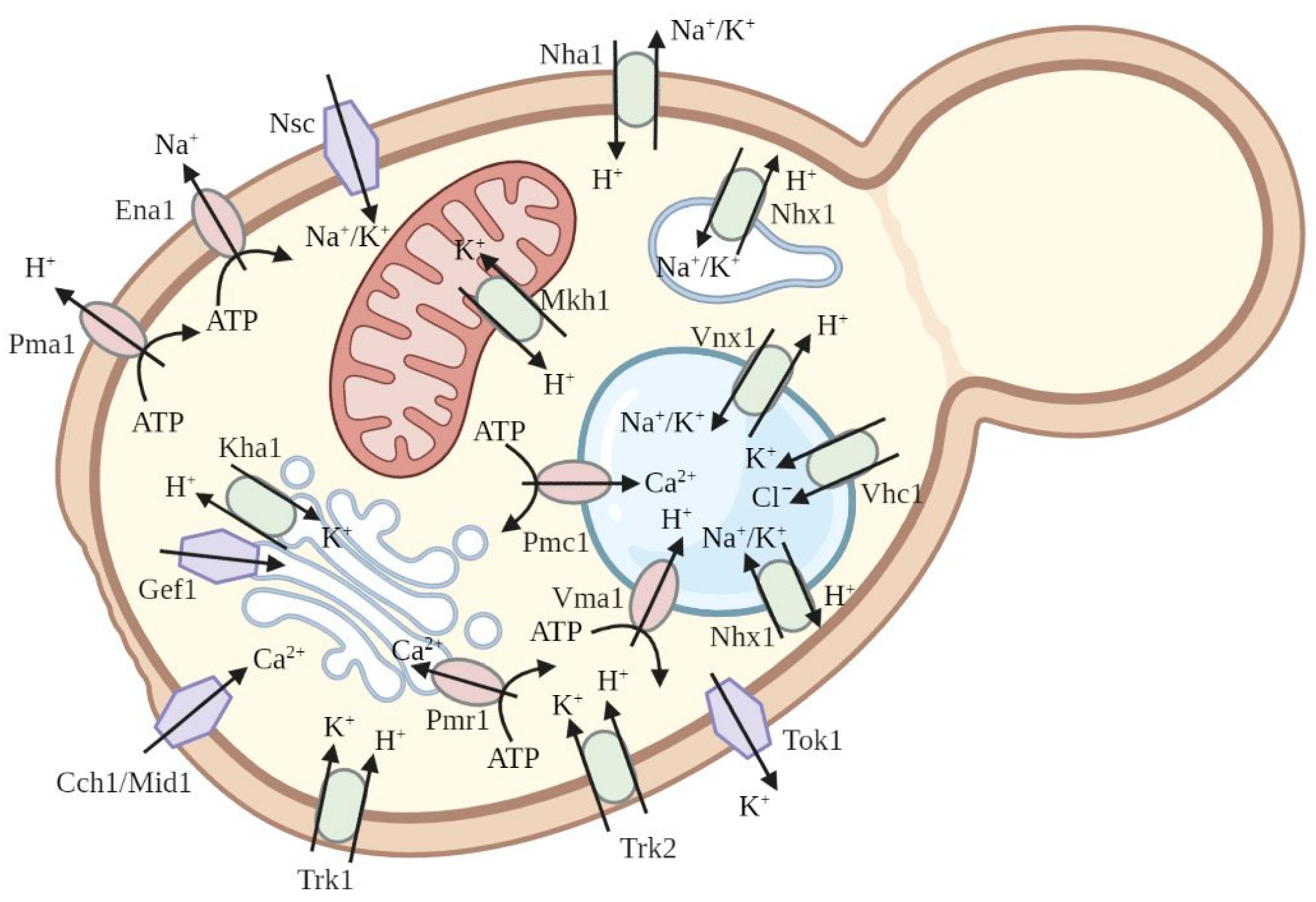
2.2. Functional Complementation of Yeast Saccharomyces cerevisiae as a Method to Study Plant Potassium and Sodium Channels and Transporters
2.3. Functional Complementation of S. cerevisiae Mutants to Study Other Plant Ion Channels
2.4. Investigation of Plant Ion Pumps Using Mutants of Saccharomyces cerevisiae
2.5. Systems of Heterologous Expression and Research on Function of Mutant Protein Forms
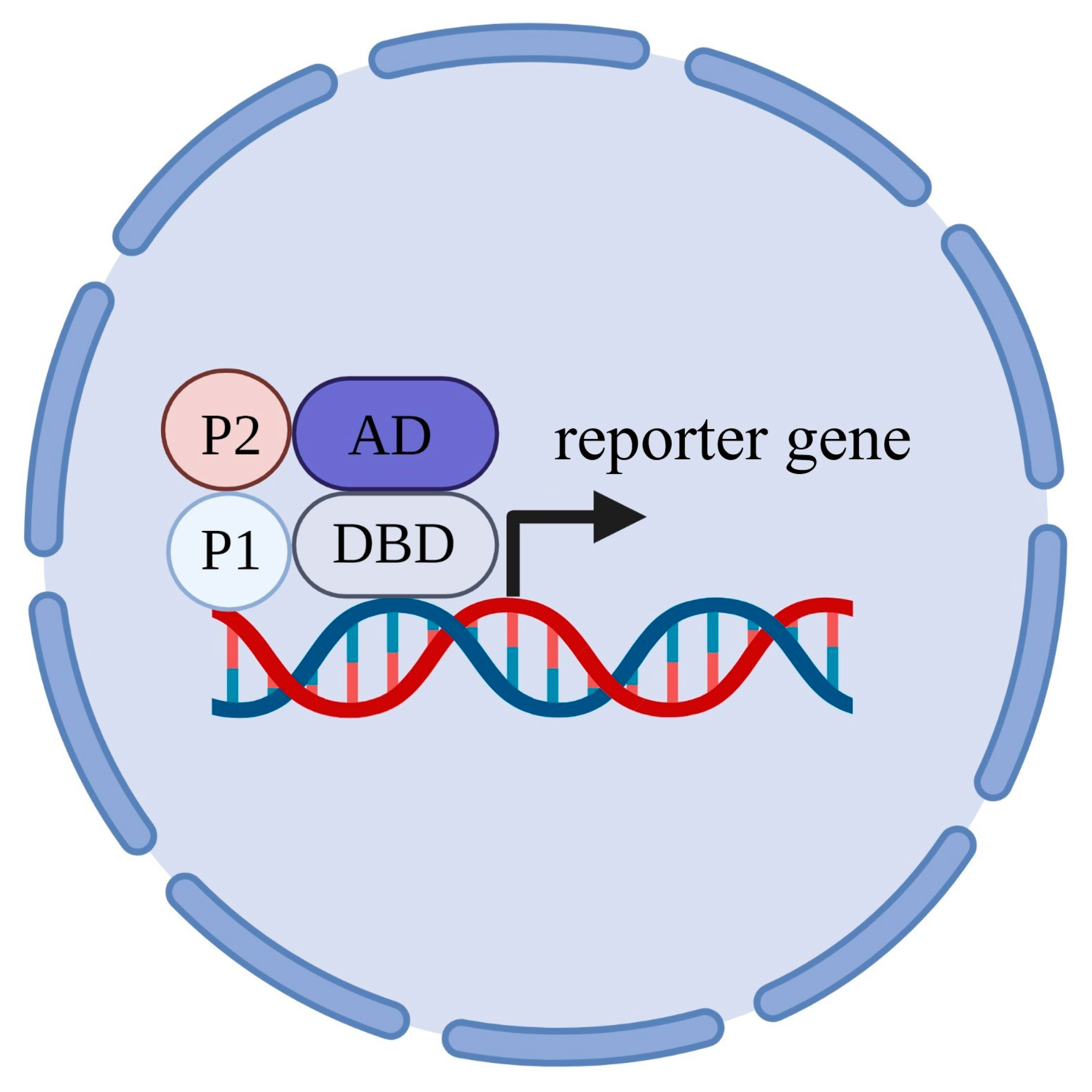
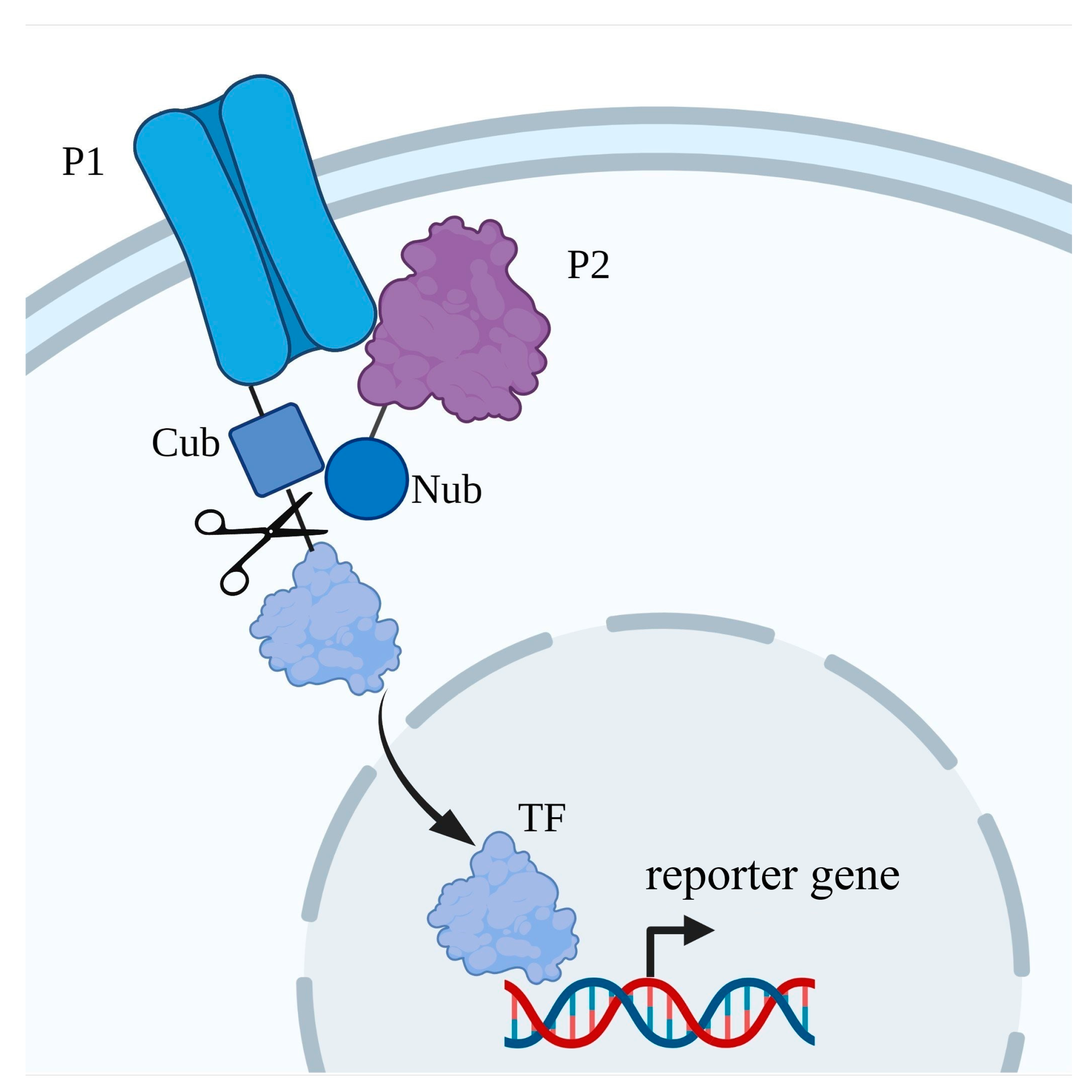
2.6. S. cerevisiae Serves as a Toolkit to Study Protein–Protein Interactions
2.7. Protein–Protein Interaction with Ion Transporters of Plants That Were Discovered in S. cerevisiae
3. Methylotrophic Yeast Pichia pastoris for Heterologous Expression of Proteins
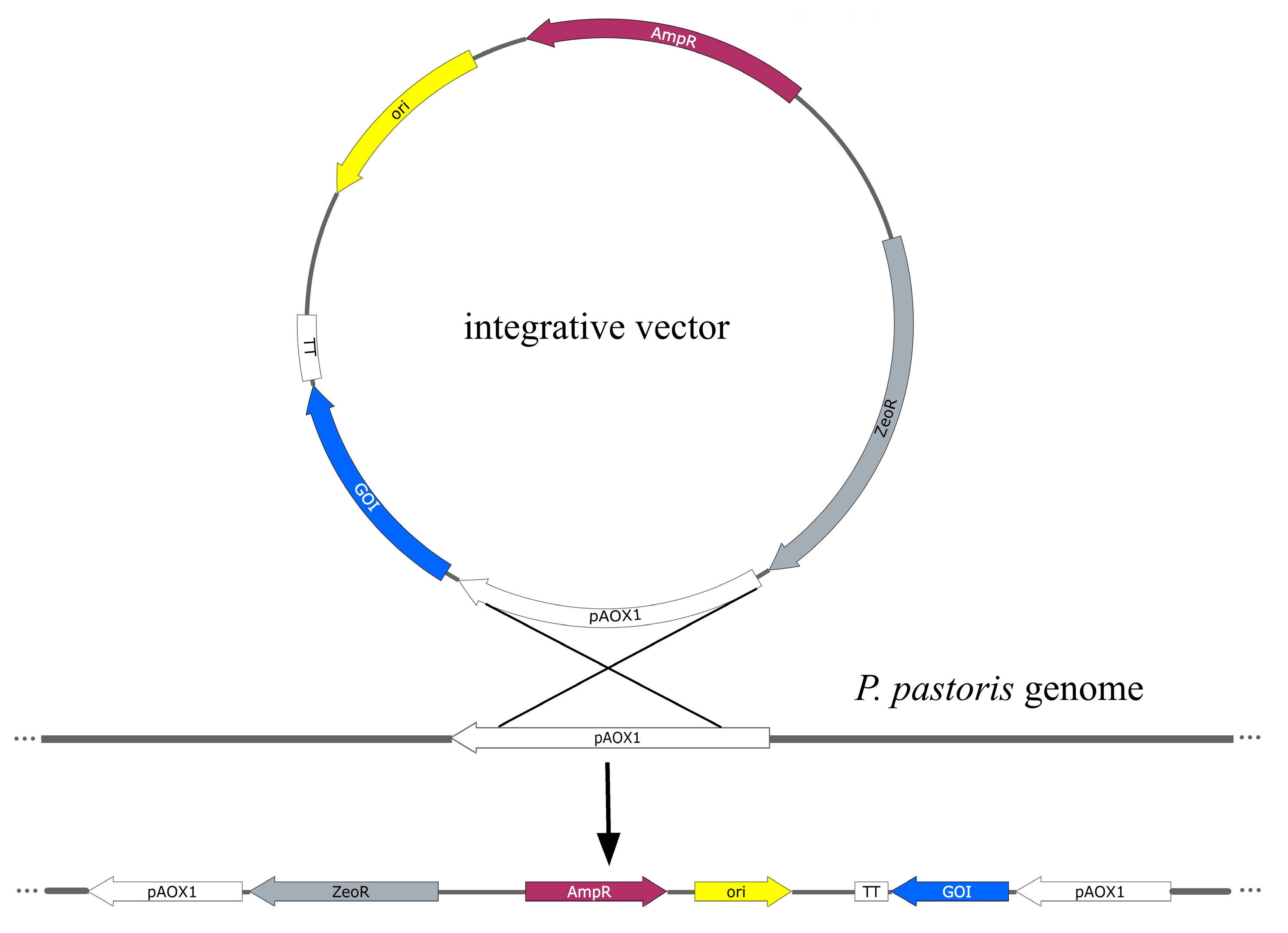
3.1. Vector Systems for Heterologous Expression in Cells of P. pastoris
3.2. The Factors That Influence Expression of Recombinant Proteins in P. pastoris
3.3. Approaches for Purification of Recombinant Proteins
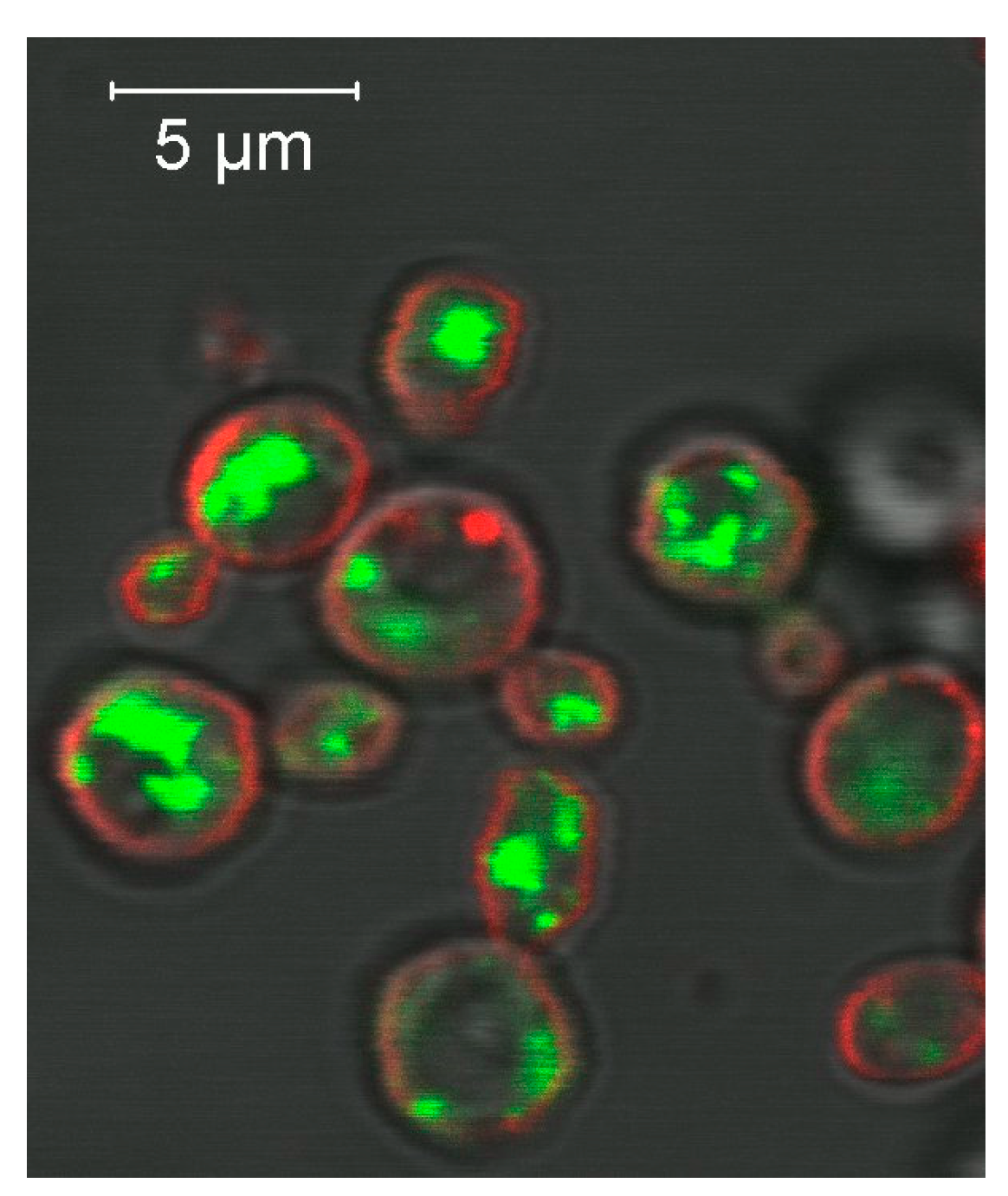
3.4. Heterologous Expression of Plant Proteins in Pichia pastoris
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, S.; Bader, M.L.; Drew, D.; de Gier, J.-W. Rationalizing membrane protein overexpression. Trends Biotech. 2006, 24, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, S.; Klepsch, M.M.; Schlegel, S.; Appel, A.; Draheim, R.; Tarry, M.; Högbom, M.; van Wijk, K.J.; Slotboom, D.J.; Persson, J.O.; et al. Tuning Escherichia coli for membrane protein overexpression. Proc. Natl. Acad. Sci. USA 2008, 105, 14371–14386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubellini, F.; Verdon, G.; Karpowich, N.K.; Luff, J.D.; Boёl, G.; Gauthier, N.; Handelman, S.K.; Ades, S.E.; Hunt, J.F. Physiological Response to Membrane Protein Overexpression in E. coli. Mol. Cell. Proteom. 2011, 10, M111.007930. [Google Scholar] [CrossRef] [Green Version]
- Kunji, E.R.S.; Chan, K.W.; Slotboom, D.J.; Floyd, S.; O’Connor, R.; Monné, M. Eukaryotic membrane protein overproduction in Lactococcus lactis. Curr. Opin. Biotechnol. 2005, 16, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Uozumi, N.; Nakamura, T.; Schroeder, J.I.; Muto, S.S. Determination of transmembrane topology of an inward-rectifying potassium channel from Arabidopsis thaliana based on functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 1998, 95, 9773–9778. [Google Scholar] [CrossRef] [Green Version]
- Hamamoto, S.; Marui, J.; Matsuoka, K.; Higashi, K.; Igarashi, K.; Nakagawa, T.; Kuroda, T.; Mori, Y.; Murata, Y.; Nakanishi, Y.; et al. Characterization of a tobacco TPK-type K+ channel as a novel tonoplast K+ channel using yeast tonoplasts. J. Biol. Chem. 2008, 283, 1911–1920. [Google Scholar] [CrossRef] [Green Version]
- Hafercamp, I.; Linka, N. Functional expression and characterization of membrane transport proteins. Plant Biol. 2012, 14, 675–690. [Google Scholar] [CrossRef]
- Maeda, S.; Schertler, G.F.X. Production of GPCR and GPCR complexes for structure determination. Curr. Opin. Struct. Biol. 2013, 23, 381–392. [Google Scholar] [CrossRef]
- Byrne, B. Pichia pastoris as an expression host for membrane protein structural biology. Curr. Opin. Struct. Biol. 2015, 32, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böer, E.; Steinborn, G.; Kunze, G.; Gellissen, G. Yeast expression platforms. Appl. Microbiol. Biotechno. 2007, 77, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Schwacke, R.; Flügge, U.-I.; Kunze, R. Plant membrane proteome databases. Plant Physiol. Biochem. 2004, 42, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Gellissen, G.; Hollenberg, C.P. Application of yeasts in gene expression studies: A comparison of Saccharomyces cerevisiae, Hansenula polymorpha and Kluyveromyces lactis—A review. Gene 1997, 190, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Muchhal, U.S.; Pardo, J.M.; and Raghothama, K.G. Phosphate transporters from the higher plant Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 10519–10523. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Cunningham, K.W.; Harper, J.F.; Sze, H. ECA1 complements yeast mutant defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1997, 94, 8579–8584. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.L.; Newstead, S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 2014, 507, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, D.; Hammes, U.; Thor, K.; Suter-Grotemeyer, M.; Flückiger, R.; Slusarenko, A.J.; Ward, J.M.; Rentsch, D. AtPTR1, a plasma membrane peptide transporter expressed during seed germination and in vascular tissue of Arabidopsis. Plant J. 2004, 40, 488–499. [Google Scholar] [CrossRef]
- Howarth, J.R.; Fourcroy, P.; Davidian, J.-C.; Smith, F.W.; Hawkesford, M.J. Cloning of two contrasting high-affinty sulfate transporters from tomato induced by low sulfate and infection by the vascular pathogen Verticillium dahliae. Planta 2003, 218, 58–64. [Google Scholar] [CrossRef]
- Benito, B.; and Rodriguez-Navarro, A. Molecular cloning and characterization of a sodium-pump ATPase of the moss Physcomitrella patens. Plant J. 2003, 36, 382–389. [Google Scholar] [CrossRef]
- Ichikawa, T.; Nakazawa, M.; Kawashima, M.; Iizumi, H.; Kuroda, H.; Kondou, Y.; Tsuhara, Y.; Suzuki, K.; Ishikawa, A.; Seki, M.; et al. The FOX hunting system: An alnernative gain-of-function gene hunting technique. Plant J. 2006, 45, 974–985. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Zheng, J.-X.; Mo, H.; Xia, K.-F.; Jian, S.-G. Functional Identification of Salt-Stress-Related Genes Using the FOX Hunting System from Ipomoea pes-caprae. Int. J. Mol. Sci. 2018, 19, 3446. [Google Scholar] [CrossRef] [Green Version]
- Gnügge, R.; Rudolf, F. Saccharomyces cerevisiae shuttle vectors. Yeast 2017, 34, 205–221. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, J. The genomic landscape of position effects on protein expression level and noise in yeast. Cell Syst. 2016, 2, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amen, T.; Kaganovich, D. Integrative modules for efficient genome engineering in yeast. Microb. Cell 2017, 4, 182–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnon, J.T. Genomic considerations for the modification of Saccharomyces cerevisiae for biofuel and metabolite biosynthesis. Microorganisms 2020, 8, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ton, V.-K.; Rao, R. Functional expression of heterologous proteins in yeast: Insights into Ca2+ signaling and Ca2+-transporting ATPases. Am. J. Physiol. Cell Physiol. 2004, 287, C580–C589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast expression systems: Overview and recent advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Serrano, R.; Rodriguez-Navarro, A. Ion homeostasis during salt stress in plants. Curr. Opin. Cell Biol. 2001, 13, 399–404. [Google Scholar] [CrossRef]
- Locascio, A.; Andres-Colas, N.; Mulet, J.M.; Yenush, L. Saccharomyces cerevisiae as a tool to investigate plant potassium and sodium transporter. Int. J. Mol. Sci. 2019, 20, 2133. [Google Scholar] [CrossRef] [Green Version]
- Gaber, R.F.; Styles, C.A.; Fink, G.R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 1988, 8, 2848–2859. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Buckley, A.M.; Gaber, R.F. TRK2 is required for low affinity K+ transport in Saccharomyces cerevisiae. Genet. 1990, 125, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ariño, J.; Ramos, J.; Sychrova, H. Monovalent cation transporters at the plasma membrane in yeasts. Yeast 2018, 36, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.; Mason, B.; Slayman, C. The yeast Pma1 proton pump: A model for understanding the biogenesis of plasma membrane proteins. J. Biol. Chem. 2001, 276, 29613–29616. [Google Scholar] [CrossRef] [Green Version]
- Ariño, J.; Ramos, J.; Sychrova, H. Alkali metal cation transport and homeostasis in yeasts. Microbio. Mol. Biol. Rev. 2010, 74, 95–120. [Google Scholar] [CrossRef] [Green Version]
- Haro, R.; Garciadeblas, B.; Rodríguez-Navarro, A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991, 291, 189–191. [Google Scholar] [CrossRef] [Green Version]
- Benito, B.; Garciadeblas, B.; Rodriguez-Navarro, A. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology 2002, 148, 933–941. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, A.; Ariño, J. Function and regulation of the Saccharomyces cerevisiae ENA sodium ATPase system. Eukaryot. Cell 2007, 6, 2175–2183. [Google Scholar] [CrossRef] [Green Version]
- Bañuelos, M.A.; Sychrova, H.; Bleykasten-Grosshans, C.; Souciet, J.L.; Potier, S. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology 1998, 144 Pt 1, 2749–2758. [Google Scholar] [CrossRef] [Green Version]
- Ohgaki, R.; Nakamura, N.; Mitsui, K.; Kanazawa, H. Characterization of the ion transport activity of the budding yeast Na+/H+ antiporter, Nha1p, using isolated secretory vesicles. Biochim. Biophys. Acta 2005, 1712, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Volkov, V. Quantitative description of ion transport via plasma membrane of yeast and small cells. Front. Plant Sci. 2015, 6, 425. [Google Scholar] [CrossRef] [Green Version]
- Almagro, A.; Prista, C.; Benito, B.; Loureiro-Dias, M.C.; Ramos, J. Cloning and expression of two genes coding for sodium pumps in the salt-tolerant yeast Debaryomyces hansenii. J. Bacteriol. 2001, 183, 3251–3255. [Google Scholar] [CrossRef] [Green Version]
- Cagnac, O.; Leterrier, M.; Yeager, M.; Blumwald, E. Identification and characterization of Vnx1p, a novel type of vacuolar monovalent cation/H+ antiporter of Saccharomyces cerevisiae. J. Biol. Chem. 2007, 282, 24284–24293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrezselyova, S.; Kinclova-Zimmermannova, O.; Sychrova, H. Vhc1, a novel transporter belonging to the family of electroneutral cation-Cl− cotransporters, participates in the regulation of cation content and morphology of Saccharomyces cerevisiae vacuoles. Biochim. Biophys. Acta 2013, 1828, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Přibylova, L.; Papouškova, K.; Zauřel, M.; Souciet, J.-L.; Sychrova, H. Exploration of yeast alkali metal cation/H+ antiporters: Sequence and structure comparison. Folia Microbiol. 2006, 51, 413–424. [Google Scholar] [CrossRef]
- Anderson, J.A.; Huprikar, S.S.; Kochian, L.V.; Lucas, W.J.; Gaber, R.F. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1992, 89, 3736–3740. [Google Scholar] [CrossRef] [Green Version]
- Sentenac, H.; Bonneaud, N.; Minet, M.; Lacroute, F.; Salmon, J.M.; Gaymard, F.; Grignon, C. Cloning and expression in yeast of a plant potassium ion transport system. Science 1992, 256, 663–665. [Google Scholar] [CrossRef]
- Lebaudy, A.; Very, A.-A.; Sentenac, H. K+-channel activity in plants: Genes, regulation and functions. FEBS Lett. 2007, 581, 2357–2366. [Google Scholar] [CrossRef]
- Obata, T.; Kitamoto, H.K.; Nakamura, A.; Fukuda, A.; Tanaka, Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiol. 2007, 144, 1978–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachtman, D.P.; Schroeder, J.I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 1994, 370, 655–658. [Google Scholar] [CrossRef]
- Platten, J.D.; Cotsaftis, O.; Berthomieu, P.; Bohnert, H.; Davenport, R.J.; Fairbairn, D.J.; Horie, T.; Leigh, R.A.; Lin, H.-X.; Luan, S.; et al. Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci. 2006, 11, 372–374. [Google Scholar] [CrossRef]
- Almeida, P.; Katsching, D.; de Boer, A.H. HKT transporters–state of the art. Int. J. Mol. Sci. 2013, 14, 20359–20385. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Luo, W.; Lin, W.; Ma, L.; Kabir, M.H. Model of cation transportation mediated by high-affinity potassium transporters (HKTs) in higher plants. Biol. Proced. Online 2015, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Hamamoto, S.; Horie, T.; Hauser, F.; Deinlein, U.; Schroeder, J.I.; Uozumi, N. HKT transporters mediate salt stress resistance in plants: From structure and function to the field. Curr. Opin. Biotechnol. 2015, 32, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, F.; Horie, T. A conserved primary salt tolerance mechanism mediated by HKT transporters: A mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Physiol. 2010, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Berthomieu, P.; Conejero, G.; Nublat, A.; Brackenbury, W.J.; Lambert, C.; Savio, C.; Uozumi, N.; Oiki, S.; Yamada, K.; Cellier, F.; et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003, 22, 2004–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunarpi; Horie, T.; Motoda, J.; Kubo, M.; Yang, H.; Yoda, K.; Horie, R.; Chan, W.-Y.; Leung, H.-Y.; Hattori, K.; et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005, 44, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.J.; Muñoz-Mayor, A.; Jha, D.; Essah, P.A.; Rus, A.; Tester, M. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 2007, 30, 497–507. [Google Scholar] [CrossRef]
- Gassmann, W.; Rubio, F.; Schroeder, J.I. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996, 10, 869–882. [Google Scholar] [CrossRef]
- Rubio, F.; Gassmann, W.; Schroeder, J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 1995, 270, 1660–1663. [Google Scholar] [CrossRef]
- Uozumi, N.; Kim, E.J.; Rubio, F.; Yamaguchi, T.; Muto, S.; Tsuboi, A.; Bakker, E.P.; Nakamura, T.; Schroeder, J.I. The Arabidopsis HKT1 gene homolog mediates inward Na+ current in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol. 2000, 122, 1249–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haxim, Y.; Wang, L.; Pan, Z.; Fan, X.; Ma, J. A novel high-affinity potassium transporter SeHKT1;2 from halophyte Salicornia europaea shows strong selectivity for Na+ rather than K+. Front. Plant Sci. 2023, 14, 1104070. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Kumar, R.; Schroeder, J.I.; Marsh, E.L. Molecular and functional characterization of a novel low-affinity cation transporter (LCT1) in higher plants. Proc. Natl. Acad. Sci. USA 1997, 94, 11079–11084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iida, K.; Teng, J.; Cho, T.; Yoshikawa-Kimura, S.; Iida, H. Post-translational processing and membrane translocation of the yeast regulatory Mid1 subunit of the Cch1/VGCC/NALCN cation channel family. Membr. Biol. 2017, 292, 20570–20582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, S.; Antosiewicz, D.M.; Ward, J.M.; Schroeder, J.I. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc. Natl. Acad. Sci. USA 1998, 95, 12043–12048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Rao, R.; Sherman, A.; Grisafi, P.; Alper, S.; Fink, G. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl. Acad. Sci. USA 1999, 96, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Darley, C.P.; van Wuytswinkel, O.C.; van der Woude, K.; Mager, W.H.; de Boer, A.H. Arabidopsis thaliana and Saccharomyces cerevisiae NHX1 genes encode amiloride sensitive electroneutral Na+/H+ exchangers. Biochem. J. 2000, 351 Pt 1, 241–249. [Google Scholar] [CrossRef]
- Aharon, G.S.; Apse, M.P.; Duan, S.; Hua, X.; Blumwald, E. Characterization of a family of vacuolar Na+/H+ antiporters in Arabidopsis thaliana. Plant Soil. 2003, 253, 245–256. [Google Scholar] [CrossRef]
- Wu, C.; Gao, X.; Kong, X.; Zhao, Y.; Zhang, H. Molecular cloning and functional analysis of a Na+/H+ antiporter gene ThNHX1 from a halophytic plant Thellungiella halophila. Plant Mol. Biol. Rep. 2009, 27, 1–12. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Hong, S.; Xia, Z.; Cui, D.; Guo, J.; Xu, H.; Jiang, X. Functional characterization of a wheat NHX antiporter gene TaNHX2 that encodes a K+/H+ exchanger. PLoS ONE 2013, 8, e78098. [Google Scholar] [CrossRef]
- Ali, R.; Zielinski, R.E.; Berkowitz, G.A. Expression of plant cyclic nucleotide-gated cation channels in yeast. J. Exp. Bot. 2006, 57, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, P.; Moeder, W.; Yoshioka, K. Plant cyclic nucleotide-gated channels: New insights on their functions and regulation. Plant Physiol. 2020, 184, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Sherman, T.; Fromm, H. Cyclic nucleotide-gated channels in plants. FEBS Lett. 2007, 581, 2237–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarratt-Barnham, E.; Wang, L.; Ning, Y.; Davis, J.M. The complex story of plant cyclic nucleotide-gated channels. Int. J. Mol. Sci. 2021, 22, 874. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.M.; Sanders, D.; et al. Phylogenetic relationship within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, C.; Merkler, T.; Neuhaus, G. Characterization of a novel gene family of putative cyclic nucleotide- and calmoduline-regulated ion channels in Arabidopsis thaliana. Plant J. 1999, 18, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Leng, O.; Mercier, R.W.; Yao, W.; Berkowitz, G.A. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999, 121, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kang, Y.; Ma, C.; Miao, R.; Wu, C.; Long, Y.; Ge, T.; Wu, Z.; Hou, X.; Zhang, J.; et al. CNGC2 is a Ca2+ influx channel that prevents accumulation of apoplatic Ca2+ in the leaf. Plant Physiol. 2017, 173, 1342–1354. [Google Scholar] [CrossRef] [Green Version]
- Gobert, A.; Park, G.; Amtmann, A.; Sanders, D.; Maathuis, F.J. Arabidopsis thaliana cyclic nucleotide-gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J. Exp. Bot. 2006, 57, 791–800. [Google Scholar] [CrossRef]
- Mercier, R.W.; Rabinowitz, N.M.; Ali, R.; Gaxiola, R.A.; Berkowitz, G.A. Yeast hygromycin sensitivity as a functional assay of cyclic nucleotide gated cation channels. Plant Physiol. Biochem. 2004, 42, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Davenport, R.J.; and Tester, M. Nonselective cation channels in plants. Annu. Rev. Plant Biol. 2002, 53, 67–107. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Tester, M. Sodium Fluxes through Nonselective Cation Channels in the Plasma Membrane of Protoplasts from Arabidopsis Roots. Plant Physiol. 2002, 128, 379–387. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81. [Google Scholar] [CrossRef]
- Jin, Y.; Jing, W.; Zhang, Q.; Zhang, W. Cyclic nucleotide gated 10 negatively regulates salt tolerance by mediating Na+ transport in Arabidopsis. J. Plant Res. 2015, 128, 211–220. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl− transport contributing to salt tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Pusch, M. CLC chloride channels and transporters: Structure, function, physiology, and disease. Physiol. Rev. 2018, 98, 1493–1590. [Google Scholar] [CrossRef]
- De Angeli, A.; Monachello, D.; Ephritikhine, G.; Frachisse, J.-M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. CLC-mediated anion transport in plant cells. Phil. Trans. R. Soc. B 2009, 364, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Zifarelli, G.; Pusch, M. CLC transport proteins in plants. FEBS Lett. 2010, 584, 2122–2127. [Google Scholar] [CrossRef] [Green Version]
- Barbier-Brygoo, H.; De Angeli, A.; Filleur, S.; Frachisse, J.-M.; Gambale, F.; Thomine, S.; Wege, S. Anion channels/transporters in plants: From molecular bases to regulatory networks. Annu. Rev. Plant Biol. 2011, 62, 25–51. [Google Scholar] [CrossRef]
- Lv, Q.D.; Tang, R.; Liu, H.; Gao, X.S.; Li, Y.Z.; Zheng, H.Q.; Zhang, H.X. Cloning and molecular analyses of the Arabidopsis thaliana chloride channel gene family. Plant Sci. 2009, 176, 650–661. [Google Scholar] [CrossRef]
- Gaxiola, R.A.; Yuan, D.S.; Klausner, R.D.; Fink, G.R. The yeast CLC chloride channel functions in cation homeostasis. Proc. Natl. Acad. Sci. USA 1998, 95, 4046–4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmagne, A.; Vinauger-Douard, M.; Monachello, D.; de Longevialle, A.F.; Charon, C.; Allot, M.; Rappaport, F.; Wollman, F.-A.; Barbier-Brygoo, H.; Ephritikhine, G. Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. J. Exp. Bot. 2007, 58, 3385–3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, A.; Fukuda, A.; Sakai, S.; Tanaka, Y. Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.). Plant Cell Physiol. 2006, 47, 32–42. [Google Scholar] [CrossRef]
- Wei, P.; Wang, L.; Liu, A.; Yu, B.; Lam, H.M. GmCLC1 confers enhanced salt tolerance through regulating chloride accumulation in soybean. Front. Plant Sci. 2016, 7, 1082. [Google Scholar] [CrossRef] [Green Version]
- Wei, P.; Che, B.; Shen, L.; Cui, Y.; Wu, S.; Cheng, C.; Liu, F.; Li, M.W.; Yu, B.; Lam, H.M. Identification and functional characterization of the chloride channel gene, GsCLC-c2 from wild soybean. BMC Plant Biol. 2019, 19, 121. [Google Scholar] [CrossRef]
- Nedelyaeva, O.I.; Shuvalov, A.V.; Mayorova, O.V.; Yurchenko, A.A.; Popova, L.G.; Balnokin, Y.V.; Karpichev, I.V. Cloning and functional analysis of SaCLCc1, a gene belonging to the chloride channel family (CLC), from the halophyte Suaeda altissima (L.) Pall. Dokl. Biochem. Biophys. 2018, 481, 186–189. [Google Scholar] [CrossRef]
- Nedelyaeva, O.; Shuvalov, A.; Karpichev, I.; Beliaev, D.; Myasoedov, N.; Khalilova, L.; Khramov, D.; Popova, L.; Balnokin, Y. Molecular cloning and characterization of SaCLCa1, a novel protein of the chloride channel (CLC) family from the halophyte Suaeda altissima (L.) Pall. J. Plant Physiol. 2019, 240, 152995. [Google Scholar] [CrossRef]
- Nedelyaeva, O.I.; Popova, L.G.; Volkov, V.S.; Balnokin, Y.V. Molecular cloning and characterization of SaCLCd, SaCLCf, and SaCLCg, novel proteins of the chloride channel family (CLC) from the halophyte Suaeda altissima (L.) Pall. Plants 2022, 11, 409. [Google Scholar] [CrossRef]
- Nedelyaeva, O.I.; Popova, L.G.; Khramov, D.E.; Volkov, V.S.; Balnokin, Y.V. Chloride channel family in the euhalophyte Suaeda altissima (L.) Pall: Cloning of novel members SaCLCa2 and SaCLCc2, general characterization of the family. Int. J. Mol. Sci. 2023, 24, 941. [Google Scholar] [CrossRef]
- De Angeli, A.; Monachello, D.; Ephritikhin, G.; Frachisse, J.M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 2006, 442, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Jossier, M.; Kroniewicz, L.; Dalmas, F.; Le Thiec, D.; Ephritikhine, G.; Thomine, S.; Barbier-Brygoo, H.; Vavasseur, A.; Filleur, S.; Leonhardt, N. The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J. 2010, 64, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Von der Fecht-Bartenbach, J.; Bogner, M.; Krebs, M.; Stierhof, Y.D.; Schumacher, K.; Ludewig, U. Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J. 2007, 50, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.T.; Agorio, A.; Jossier, M.; Depré, S.; Thomine, S.; Filleur, S. Characterization of the chloride channel-like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2015, 57, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Villalba, J.M.; Palmgren, M.G.; Berberian, G.E.; Ferguson, C.; Serrano, R. Functional expression of plant plasma membrane H+-ATPase in yeast endoplasmic reticulum. J. Biol. Chem. 1992, 267, 12341–12359. [Google Scholar] [CrossRef]
- Serrano, R. Structure and function of plasma membrane H+-ATPase. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 61–94. [Google Scholar] [CrossRef]
- Serrano, R.; Kielland-Brandt, M.C.; Fink, G.R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na++K+)-, K+- and Ca2+-ATPases. Nature 1986, 319, 689–693. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Christensen, G. Functional comparison between plant plasma membrane H+-ATPase isoforms expressed in yeast. J. Biol. Chem. 1994, 269, 3027–3033. [Google Scholar] [CrossRef]
- Cunningham, K.W.; Fink, G.R. Ca2+ transport in Saccharomyces cerevisiae. J. Exp. Biol. 1994, 196, 157–166. [Google Scholar] [CrossRef]
- Cunningham, K.W.; Fink, G.R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+-ATPases. J. Cell Biol. 1994, 124, 351–363. [Google Scholar] [CrossRef]
- Geisler, M.; Axelsen, K.B.; Harper, J.F.; Palmgren, M.G. Molecular aspects of higher plant P-type Ca2+-ATPases. Biochim. Biophys. Acta 2000, 1465, 52–78. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.F.; Hong, B.; Hwang, I.; Guo, H.Q.; Stoddard, R.; Huang, J.F.; Palmgren, M.G.; Sze, H. A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J. Biol. Chem. 1998, 273, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Geisler, M.; Frangne, N.; Gomès, E.; Martinoia, E.; and Palmgren, M.G. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000, 124, 1814–1827. [Google Scholar] [CrossRef] [Green Version]
- Qudeimat, E.; Faltusz, A.M.C.; Wheeler, G.; Lang, D.; Holtorf, H.; Brownlee, C.; Reski, R.; Frank, W. A PIIB-type Ca2+-ATPase is essential for stress adaptation in Physcomitrella patens. Proc. Natl. Acad. Sci. USA 2008, 105, 19555–19560. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yang, H.; Peer, W.A.; Richter, G.; Blakeslee, J.; Bandyopadhyay, A.; Titapiwantakun, B.; Undurraga, S.; Khodakovskaya, M.; Richards, E.L.; et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 2005, 310, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Perez-Castiñeira, J.R.; Hernandez, A.; Drake, R.; Serrano, A. A plant proton-pumping inorganic pyrophosphatase functionally complements the vacuolar ATPase transport activity and confers bafilomycin resistance in yeast. Biochem. J. 2011, 437, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Uozumi, N.; Gassmann, W.; Cao, Y.; Schroeder, J. Identification of strong modifications in cation selectivity in an Arabidopsis inward rectifying potassium channel by mutant selection in yeast. J. Biol. Chem. 1995, 270, 24276–24281. [Google Scholar] [CrossRef] [Green Version]
- Aleman, F.; Nieves-Cordones, M.; Martinez, V.; Rubio, F. Root K+ acquisition in plants: The Arabidopsis thaliana model. Plant Cell Physiol. 2011, 52, 1603–1612. [Google Scholar] [CrossRef]
- Pyo, Y.J.; Gierth, M.; Schroeder, J.I.; Cho, M.H. High affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 2010, 153, 863–875. [Google Scholar] [CrossRef] [Green Version]
- Rubio, F.; Aleman, F.; Nieves-Cordones, M.; Martinez, V. Studies on Arabidopsis athak5, atakt1 double mutants disclose the range of concentrations at which AtHAK5, AtAKT1 and unknown systems mediate K+ uptake. Physiol. Plant 2010, 139, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Aleman, F.; Martinez, V.; Rubio, F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol. Plant 2010, 3, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Aleman, F.; Caballero, F.; Rodenas, R.; Rivero, R.; Martinez, V.; Rubio, F. The F130S point mutation in the Arabidopsis high-affinity K+ transporter AtHAK5 increases K+ over Na+ and Cs+ selectivity and confers Na+ and Cs+ tolerance to yeast under heterologous expression. Front. Plant Sci. 2014, 5, 430. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Wallmeroth, N.; Berendzen, K.W.; Grefen, C. Techniques for the analysis of protein-protein interactions in vivo. Plant Physiol. 2016, 171, 727–758. [Google Scholar] [CrossRef] [Green Version]
- Loper, J.; Mukhtar, M.S. Mapping protein-protein interaction using high-throughput yeast 2-hybrid. Methods Mol. Biol. 2017, 1610, 217–230. [Google Scholar] [CrossRef]
- Ferro, E.; Trabalzini, L. The yeast two-hybrid and related methods as powerful tools to study plant cell signalling. Plant Mol. Biol. 2013, 83, 287–301. [Google Scholar] [CrossRef]
- Fields, S.; Song, O. A novel genetic system to detect protein-protein interactions. Nature 1989, 340, 245–256. [Google Scholar] [CrossRef]
- Moosavi, B.; Mousavi, B.; Yang, W.-C.; Yang, G.-F. Yeast-based assays for detecting protein-protein/drug interactions and their inhibitors. Eur. J. Cell Biol. 2017, 96, 529–541. [Google Scholar] [CrossRef]
- Hirst, M.; Ho, C.; Sabourin, L.; Rudnicki, M.; Penn, L.; Sadowski, I. A two-hybrid system for transactivator bait proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 8726–8731. [Google Scholar] [CrossRef] [Green Version]
- Dube, D.H.; Li, B.; Greenblatt, E.J.; Nimer, S.; Raymond, A.K.; Kohler, J.J. A two-hybrid assay to study protein interactions within the secretory pathway. PLoS ONE 2010, 5, e15648. [Google Scholar] [CrossRef] [Green Version]
- Stagljar, I.; Korostensky, C.; Johnsson, N.; Heesen, S. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 5187–5192. [Google Scholar] [CrossRef] [Green Version]
- Kittanakom, S.; Chuk, M.; Wong, V.; Snyder, J.; Edmonds, D.; Lydakis, A.; Zhang, Z.; Auerbach, D.; Stagljar, I. Analysis of membrane protein complexes using the split-ubiquitin membrane yeast two-hybrid system. Methods Mol. Biol. 2009, 548, 247–271. [Google Scholar] [CrossRef]
- Mockli, N.; Deplazes, A.; Hassa, P.O.; Zhang, Z.; Peter, M.; Hottiger, M.O.; Stagljar, I.; Auerbach, D. Yeast split-ubiquitin-based cytosolic screening system to detect interactions between transcriptionally active proteins. Biotechniques 2007, 42, 725–730. [Google Scholar] [CrossRef]
- Bruckner, A.; Polge, C.; Lentze, N.; Auerbach, D.; Schlattner, U. Yeast two-hybrid, a powerful tool for systems biology. Int. J. Mol. Sci. 2009, 10, 2763. [Google Scholar] [CrossRef] [Green Version]
- Hedrich, R. Ion channels in plants. Physiol. Rev. 2012, 92, 1777–1811. [Google Scholar] [CrossRef]
- Zhu, J.K.; Liu, J.; Xiong, L. Genetic analysis of salt tolerance in arabidopsis. Evidence for a critical role of potassium nutrition. Plant Cell 1998, 10, 1181–1191. [Google Scholar] [CrossRef] [Green Version]
- Gong, D.; Guo, Y.; Schumaker, K.S.; Zhu, J.-K. The SOS3 Family of Calcium Sensors and SOS2 Family of Protein Kinases in Arabidopsis. Plant Physiol. 2004, 134, 919–926. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- Quintero, F.J.; Ohta, M.; Shi, H.; Zhu, J.-K.; Pardo, J.M. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. USA 2002, 99, 9061–9066. [Google Scholar] [CrossRef] [Green Version]
- Lebaudy, A.; Vavasseur, A.; Hosy, E.; Dreyer, I.; Leonhardt, N.; Thibaud, J.B.; Véry, A.A.; Simonneau, T.; Sentenac, H. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl. Acad. Sci. USA 2008, 105, 5271–5276. [Google Scholar] [CrossRef] [Green Version]
- Kashtoh, H.; Baek, K.-H. Structural and functional insights into the role of guard cell ion channels in abiotic stress-induced stomatal closure. Plants 2021, 10, 2774. [Google Scholar] [CrossRef]
- Sato, A.; Sato, Y.; Fukao, Y.; Fujiwara, M.; Umezawa, T.; Shinozaki, K.; Hibi, T.; Taniguchi, M.; Miyake, H.; Goto, D.B.; et al. Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 2009, 424, 439–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.C.; Lan, W.; Kim, B.; Li, L.; Cheong, Y.H.; Pandey, G.K.; Lu, G.; Buchanan, B.B.; Luan, S. A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc. Natl. Acad. Sci. USA 2007, 104, 15959–15964. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef] [Green Version]
- Boscari, A.; Clément, M.; Volkov, V.; Golldack, D.; Hybiak, J.; Miller, A.J.; Amtmann, A.; Fricke, W. Potassium channels in barley: Cloning, functional characterization and expression analyses in relation to leaf growth and development. Plant Cell Environ. 2009, 32, 1761–1777. [Google Scholar] [CrossRef]
- Grefen, C.; Blatt, M.R. Do Calcineurin B-Like Proteins Interact Independently of the Serine Threonine Kinase CIPK23 with the K+ Channel AKT1? Lessons Learned from a Ménage à Trois. Plant Physiol. 2012, 159, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Cereghino, G.P.L.; Cereghino, J.L.; Ilgen, C.; Cregg, J.M. Production of recombinant proteins in fermenter cultures of the yeast Pichia pastoris. Curr. Opin. Biotechnol. 2002, 13, 329–332. [Google Scholar] [CrossRef]
- Looser, V.; Bruhlmann, B.; Bumbak, F.; Stenger, C.; Costa, M.; Camattari, A.; Fotiadis, D.; Kovar, K. Cultivation strategies to enhance productivity of Pichia pastoris: A review. Biotechnol. Adv. 2015, 33 Pt 2, 1177–1193. [Google Scholar] [CrossRef] [Green Version]
- Hino, T.; Arakawa, T.; Iwanari, H.; Yurugi-Kobayashi, T.; Ikeda-Suno, C.; Nakada-Nakura, Y.; Kusano-Arai, O.; Weyand, S.; Shimamura, T.; Nomura, N.; et al. G-protein-coupled receptor inactivation by an allosteric inverse-agonist antibody. Nature 2012, 482, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Long, S.B.; Campbell, E.B.; Mackinnon, R. Crystal structure of a mammalian voltage-dependent shaker family K+ channel. Science 2005, 309, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Nyblom, M.; Frick, A.; Wang, Y.; Ekvall, M.; Hallgren, K.; Hedfalk, K.; Neutze, R.; Tajkhorshid, E.; Tornroth-Horsefield, S. Structural and functional analysis of SoPIP2;1 mutants adds insight into plant aquaporin gating. J. Mol. Biol. 2009, 387, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Szewczyk, P.; Grimard, V.; Lee, C.-W.; Martinez, L.; Doshi, R.; Caya, A.; Villaluz, M.; Pardon, E.; Cregger, C.; et al. Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain. Proc. Natl. Acad. Sci. USA 2013, 110, 13386–13391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, N.; Cherouati, N.; Prual, C.; Steffan, T.; Zeder-Lutz, G.; Magnin, T.; Pattus, F.; Michel, H.; Wagner, R.; Reinhart, C. Enhancing functional production of G protein-coupled receptors in Pichia pastoris to level required for structural studies via a single expression screen. Protein Sci. 2006, 15, 1115–1126. [Google Scholar] [CrossRef] [Green Version]
- Abad, S.; Kitz, K.; Hörmann, A.; Schreiner, U.; Hartner, F.S.; Glieder, A. Real-time PCR-based determination of gene copy numbers in Pichia pastoris. Biotechnol. J. 2010, 5, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Zahrl, R.J.; Pena, D.A.; Mattanovich, D.; Gasser, B. Systems biotechnology for protein production in Pichia pastoris. FEMS Yeast Res. 2017, 17, fox068. [Google Scholar] [CrossRef]
- Inan, M.; Meagher, M.M. Non-repressing carbon sources for alcohol oxidase (AOX1) promoter of Pichia pastoris. J. Biosci. Bioeng. 2001, 92, 585–589. [Google Scholar] [CrossRef]
- Waterham, H.R.; Digan, M.E.; Koutz, P.J.; Lair, S.V.; Cregg, J.M. Isolation of the Pichia pastoris glyceraldehyde-3-phosphate dehydrogenase gene and regulation and use of its promoter. Gene 1997, 186, 37–44. [Google Scholar] [CrossRef]
- Takagi, S.; Tsutsumi, N.; Terui, Y.; Kong, X.Y. Method for Methanol Independent Induction from Methanol Inducible Promoters in Pichia 2012. U.S. Patent 8,236,528 b2, 7 August 2012. [Google Scholar]
- Wang, X.; Wang, Q.; Wang, J.; Bai, P.; Shi, L.; Shen, W.; Zhou, M.; Zhou, X.; Zhang, Y.; Cai, M. Mit1 transcription factor mediates methanol signaling and regulates alcohol oxidase 1 promoter in Pichia pastoris. J. Biol. Chem. 2016, 291, 6245–6261. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, X.; Shi, L.; Qi, F.; Zhang, P.; Zhang, Y.; Zhou, X.; Song, Z.; Cai, M. Methanol-independent protein expression by AOX1 promoter with trans-acting elements engineering and glucose-glycerol-shift induction in Pichia pastoris. Sci. Rep. 2017, 7, 41850. [Google Scholar] [CrossRef] [Green Version]
- Rinnofner, C.; Felber, M.; Pichler, H. Strains and molecular tools for recombinant protein production in Pichia pastoris. Methods Mol. Biol. 2022, 2513, 79–112. [Google Scholar] [CrossRef]
- Kellogg, M.K.; Miller, S.C.; Tikhonova, E.B.; Karamyshev, A.L. SRPassing co-translational targeting: The role of the signal recognition particle in protein targeting and mRNA protection. Int. J. Mol. Sci. 2021, 22, 6284. [Google Scholar] [CrossRef] [PubMed]
- Wickner, W.; Schekman, R. Protein translocation across biological membranes. Science 2005, 310, 1452–1456. [Google Scholar] [CrossRef] [Green Version]
- Deuerling, E.; Bukan, B. Chaperone-assisted folding of newly synthesized proteins in the cytosol. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, P.J.; Hegde, R.S. An intramembrane chaperone complex facilitates membrane protein biogenesis. Nature 2020, 584, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Qi, L. Quality control in the endoplasmic reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 2018, 43, 593–605. [Google Scholar] [CrossRef]
- Sun, Z.; Brodsky, J.L. Protein quality control in the secretory pathway. J. Cell Biol. 2019, 218, 3171–3187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travers, K.J.; Patil, C.K.; Wodicka, L.; Lockhart, D.J.; Weissman, J.S.; Walter, P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 2000, 101, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Kimata, Y.; Ishiwata-Kimata, Y.; Ito, T.; Hirata, A.; Suzuki, T.; Oikawa, D.; Takeuchi, M.; Kohno, K. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J. Cell Biol. 2007, 179, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Ruegsegger, U.; Leber, J.H.; Walter, P. Block of HAC1 mRNA translation by long-range base pairing is released by cytoplasmic splicing upon induction of the unfolded protein response. Cell 2001, 107, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Whyteside, G.; Nor, R.M.; Alcocer, M.J.C.; Archer, D.B. Activation of the unfolded protein response in Pichia pastoris requires splicing of a HAC1 mRNA intron and retention of the C-terminal tail of Hac1p. FEBS Lett. 2011, 585, 1037–1041. [Google Scholar] [CrossRef] [Green Version]
- Guerfal, M.; Ryckaert, S.; Jacobs, P.P.; Ameloot, P.; van Craenenbroeck, K.; Derycke, R.; Callewaert, N. The HAC1 gene from Pichia pastoris: Characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins. Microb. Cell Factories 2010, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Swartz, D.J.; Protasevich, I.I.; Brouillette, C.G.; Harrell, P.M.; Hildebrandt, E.; Gasser, B.; Mattanovich, D.; Ward, A.; Chang, G.; et al. A gene optimization strategy that enhances production of fully functional P-glycoprotein in Pichia pastoris. PLoS ONE 2011, 6, 22577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, N. Asparagine-linked glycosylation in the yeast Golgi. Biochim. Biophys. Acta 1999, 1426, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Vervecken, W.; Kaigorodov, V.; Callewaert, N.; Geysens, S.; De Vusser, K.; Contreras, R. In vivo synthesis of mammalian-like, hybrid-type N-glycans in Pichia pastoris. Appl. Environ. Microbiol. 2004, 70, 2639–2646. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.S.; Oldham, M.L.; Zhang, Q.; Chen, J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature 2012, 490, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Davidson, R.C.; Nett, J.H.; Renfer, E.; Li, H.; Stadheim, T.A.; Miller, B.J.; Miele, R.G.; Hamilton, S.R.; Choi, B.K.; Mitchell, T.I.; et al. Functional analysis of the ALG3 gene encoding the Dol-P-Man: Man5GlcNAc2-PP-Dol mannosyltransferase enzyme of P. pastoris. Glycobiology 2004, 14, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Krainer, F.W.; Gmeiner, C.; Neutsch, L.; Windwarder, M.; Pletzenauer, R.; Herwig, C.; Altmann, F.; Glieder, A.; Spadiut, O. Knockout of an endogenous mannosyltransferase increases the homogeneity of glycoproteins produced in Pichia pastoris. Sci. Rep. 2013, 3, 3279. [Google Scholar] [CrossRef] [Green Version]
- Claes, K.; Vandewalle, K.; Laukens, B.; Laeremans, T.; Vosters, O.; Langer, I.; Parmentier, M.; Steyaert, J.; Callewaert, N. Modular integrated secretory system engineering in Pichia pastoris to enhance G-protein coupled receptor expression. ACS Synth. Biol. 2016, 5, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.; Newstead, S.; Sonoda, Y.; Kim, H.; von Heijne, G.; Iwata, S. GFP-based optimization scheme for the overexpression and purification of eukaryotic membrane proteins in Saccharomyces cerevisiae. Nat. Protoc. 2008, 3, 784–798. [Google Scholar] [CrossRef] [Green Version]
- Lichty, J.J.; Malecki, J.L.; Agnew, H.D.; Michelson-Horowitz, D.J.; Tan, S. Comparison of affinity tags for protein purification. Protein Exp. Purif. 2005, 41, 98–105. [Google Scholar] [CrossRef]
- Newstead, S.; Kim, H.; vonHeijne, G.; Iwata, S.; Drew, D. High-throughput fluorescent-based optimization of eukaryotic membrane protein overexpression and purification in Saccharomyces serevisiae. Proc. Natl. Acad. Sci. USA 2007, 104, 13936–13941. [Google Scholar] [CrossRef] [Green Version]
- Nagaraj, V.J.; Galati, V.; Luscher, M.; Boller, T.; Wiemken, A. Cloning and functional characterization of a cDNA encoding barley soluble acid invertase (HvINV1). Plant Sci. 2005, 168, 249–258. [Google Scholar] [CrossRef]
- Glöckner, A.; and Voigt, C.A. Expression, purification and in vitro enzyme activity assay of plant derived GTPase. Bio-protocol 2015, 5, e1651. [Google Scholar] [CrossRef]
- Wang, M.; Zou, Z.; Li, Q.; Xin, H.; Zhu, X.; Chen, X.; Li, X. Heterologous expression of three Camellia sinensis small heat shock protein genes confers temperature stress tolerance in yeast and Arabidopsis thaliana. Plant Cell Rep. 2017, 36, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Cabral, K.M.; Almeida, M.S.; Valente, A.P.; Almeida, F.C.; Kurtenbach, E. Production of the active antifungal Pisum sativum defensin 1 (Psd1) in Pichia pastoris: Overcoming the inefficiency of the STE13 protease. Protein Expr. Purif. 2003, 31, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liang, Y.; Zhang, H.; Wu, G.; Wang, L.; Qian, H.; Qi, X. Production of a recombinant carrot antifreeze protein by Pichia pastoris GS115 and its cryoprotective effects on frozen dough properties and bread quality. LWT Food Sci. Technol. 2018, 96, 543–550. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Diatloff, E.; Forde, B.G.; Roberts, S.K. Expression and transport characterization of the wheat low-affinity cation transporter (LCT1) in the methylotrophic yeast Pichia pastoris. Biochem. Biophys. Res. Comm. 2006, 344, 807–8013. [Google Scholar] [CrossRef]
- Karlsson, M.; Fotiadis, D.; Sjövall, S.; Johansson, I.; Hedfalk, K.; Engel, A.; Kjellbom, P. Reconstitution of water channel function of an aquaporin overexpressed and purified from Pichia pastoris. FEBS Lett. 2003, 537, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Plasencia, I.; Survery, S.; Ibragimova, S.; Hansen, J.S.; Kjellbom, P.; Heliz-Nielsen, C.; Johanson, U.; Mouritsen, O.G. Structure and stability of the spinach aquaporin SoPIP2;1 in detergent micelles and lipid membranes. PLoS ONE 2011, 6, e14674. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structure mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, J.; Choi, H.; Burla, B.; Kretzschmar, T.; Lee, Y.; Martinoja, E. Plant ABC transporters. Arab. Book. 2011, 9, e0153. [Google Scholar] [CrossRef] [Green Version]
- Borghi, L.; Kang, J.; Ko, D.; Lee, Y.; Martinoia, E. The role of ABCG-type ABC transporters in phytohormone transport. Biochem. Soc. Trans. 2015, 43, 924–930. [Google Scholar] [CrossRef]
- Verrier, P.J.; Bird, D.; Burla, B.; Dassa, E.; Forestier, C.; Geisler, M.; Klein, M.; Kolukisaoglu, Ü.; Lee, Y.; Martinoia, E.; et al. Plant ABC proteins–a unified nomenclature and updated inventory. Trends Plant Sci. 2008, 13, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Gräfe, K.; Shanmuyarajah, K.; Zobel, T.; Weidtkamp-Peters, S.; Kleinschrodt, D.; Smits, S.H.J.; Schmitt, L. Cloning and expression of selected ABC transporters from the Arabidopsis thaliana ABCG family in Pichia pastoris. PLoS ONE 2019, 14, e0211156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, D.; Esmaili, M.; Overduin, M.; Fliegel, L. Expression and detergent free purification and recostitution of the plant plasma membrane Na+/H+ antiporter SOS1 overexpressed in Pichia pastoris. BBA-Biomembr. 2020, 1862, 183111. [Google Scholar] [CrossRef]

| Protein Expressed | Source Species | Protein Function | Reference, Yeast Species |
|---|---|---|---|
| AtPT1, AtPT2 | Arabidopsis thaliana | Phosphate transporters | [15], S.c. |
| ECA1 | “ | Ca2+-ATPase of ER | [16], S.c. |
| NRT1.1 | “ | Nitrate transporter | [17], S.c. |
| AtPTR1 | “ | Peptide transporter of PM | [18], S.c. |
| LeST1-1; LeST1-2 | Lycopersicon esculentum | Sulphate transporters | [19], S.c. |
| KAT1 | A. thaliana | PM K+ inward rectifying channel (guard cells) | [46,142], S.c. |
| OsKAT1 | Oriza sativa | PM K+ inward rectifying channel (guard cells) | [49], S.c. |
| AKT1 | A. thaliana | PM K+ inward rectifying channel (root cells) | [47], S.c.; |
| TaHKT2;1 (HKT1) | Triticum aestivum | K+/Na+-symport (subfamily II of HKT family) | [59,60], S.c. |
| AtHKT1;1 | A. thaliana | Na+ transport (subfamily I of HKT family) | [61], S.c. |
| SeHKT1;2 | Salicornia europaea | Na+ transport (subfamily I of HKT family) | [62], S.c. |
| LCT1 | Triticum aestivum | Low affinity cation transporter; low affinity Na+ and K+ uptake; high affinity Ca2+ and Cd2+ uptake | [63], S.c. [189], P.p. |
| AtNHX1 | A. thaliana | Tonoplast Na+/H+ antiporter | [67], S.c.; [68], S.c.; |
| ThNHX1 | Thellungiella halophila | Tonoplast Na+/H+ antiporter | [70], S.c. |
| TaNHX2 | Triticum aestivum | K+/H+ antiporter of endomembranes | [71], S.c. |
| AtCNGC1 | A. thaliana | Cyclic nucleotide gated channels, permeable to K+, calmodulin regulated (putative Ca2+ permeability) | [78], S.c. |
| AtCNGC2 | A. thaliana | Cyclic nucleotide-gated channel, permeable to K+ | [78], S.c. |
| AtCNGC3 | A. thaliana | Cyclic nucleotide-gated channel, permeable to Na+, K+ | [80], S.c. |
| AtCNGC10 | “ | Cyclic nucleotide-gated channel, permeable to Na+ | [85], S.c. |
| AHA1 AHA2 AHA3 | H+-ATPase of PM | [106] S.c. [109], S.c. | |
| PpPCA1 | Physcomitrella patens | PIIB-type Ca2+-ATPase | [115], S.c. |
| PpENA1 | Physcomitrella patens | Na+-ATPase | [20], S.c. |
| AVP1 | A. thaliana | Vacuolar H+-Pyrophosphatase | [117], S.c. |
| AtCLCa | “ | NO3−/H+ antiporter (vacuolar membrane) | [92,102], S.c. |
| AtCLCb | “ | NO3−/H+ antiporter (vacuolar membrane) | [92], S.c. |
| AtCLCc | “ | vacuolar Cl− transporter (probably, Cl−/H+ antiporter) | [92,103], S.c. |
| AtCLCd | “ | Cl−/H+ antiporter (trans-Golgi network) | [95], S.c. |
| AtCLCe | “ | Cl− channel of thylakoid membranes | [94], S.c. |
| AtCLCf | “ | Cl− channel of Golgi membranes | [94], S.c. |
| AtCLCg | “ | Cl− channel (vacuolar membrane) | [92], S.c |
| OsCLC-1; OsCLC-2 | O. sativa | Cl− channels (vacuolar putative) | [95], S.c. |
| GmCLC1 | Glycine max | Cl−/H+ antiporter | [96], S.c. |
| GsCLC-c2 | Glycine soja | Anionic (Cl− and NO3−) channel | [97], S.c. |
| SaCLCa1/a2 | Suaeda altissima | NO3−/H+ antiporter | [99,101], S.c. |
| SaCLCc1/c2 | S. altissima | Cl−/H+ antiporter | [98,101], S.c. |
| SaCLCd | S. altissima | Cl−/H+ antiporter | [100], S.c. |
| SaCLCf; SaCLCg | S. altissima | Cl− channels, putative | [100], S.c. |
| AtHAK5 | A. thaliana | High-affinity K+-transporter of PM | [123], S.c. |
| SOS1 | A. thaliana | Na+/H+ antiporter of PM | [139], S.c.; [198], P.p. [190], P.p. |
| SoPIP2;1 (PM28A) | Spinacia oleracea | Aquaporin of PM | [193], P.p.; |
| PDR2; PDR8 | A. thaliana | Potential transporters of phytohormones in PM (ABC family transporter) | [197], P.p. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, L.G.; Khramov, D.E.; Nedelyaeva, O.I.; Volkov, V.S. Yeast Heterologous Expression Systems for the Study of Plant Membrane Proteins. Int. J. Mol. Sci. 2023, 24, 10768. https://doi.org/10.3390/ijms241310768
Popova LG, Khramov DE, Nedelyaeva OI, Volkov VS. Yeast Heterologous Expression Systems for the Study of Plant Membrane Proteins. International Journal of Molecular Sciences. 2023; 24(13):10768. https://doi.org/10.3390/ijms241310768
Chicago/Turabian StylePopova, Larissa G., Dmitrii E. Khramov, Olga I. Nedelyaeva, and Vadim S. Volkov. 2023. "Yeast Heterologous Expression Systems for the Study of Plant Membrane Proteins" International Journal of Molecular Sciences 24, no. 13: 10768. https://doi.org/10.3390/ijms241310768
APA StylePopova, L. G., Khramov, D. E., Nedelyaeva, O. I., & Volkov, V. S. (2023). Yeast Heterologous Expression Systems for the Study of Plant Membrane Proteins. International Journal of Molecular Sciences, 24(13), 10768. https://doi.org/10.3390/ijms241310768






