Unique Features of the m6A Methylome and Its Response to Salt Stress in the Roots of Sugar Beet (Beta vulgaris)
Abstract
1. Introduction
2. Results
2.1. Physiological and Ionic Response to Salt Stress in Seedling Roots
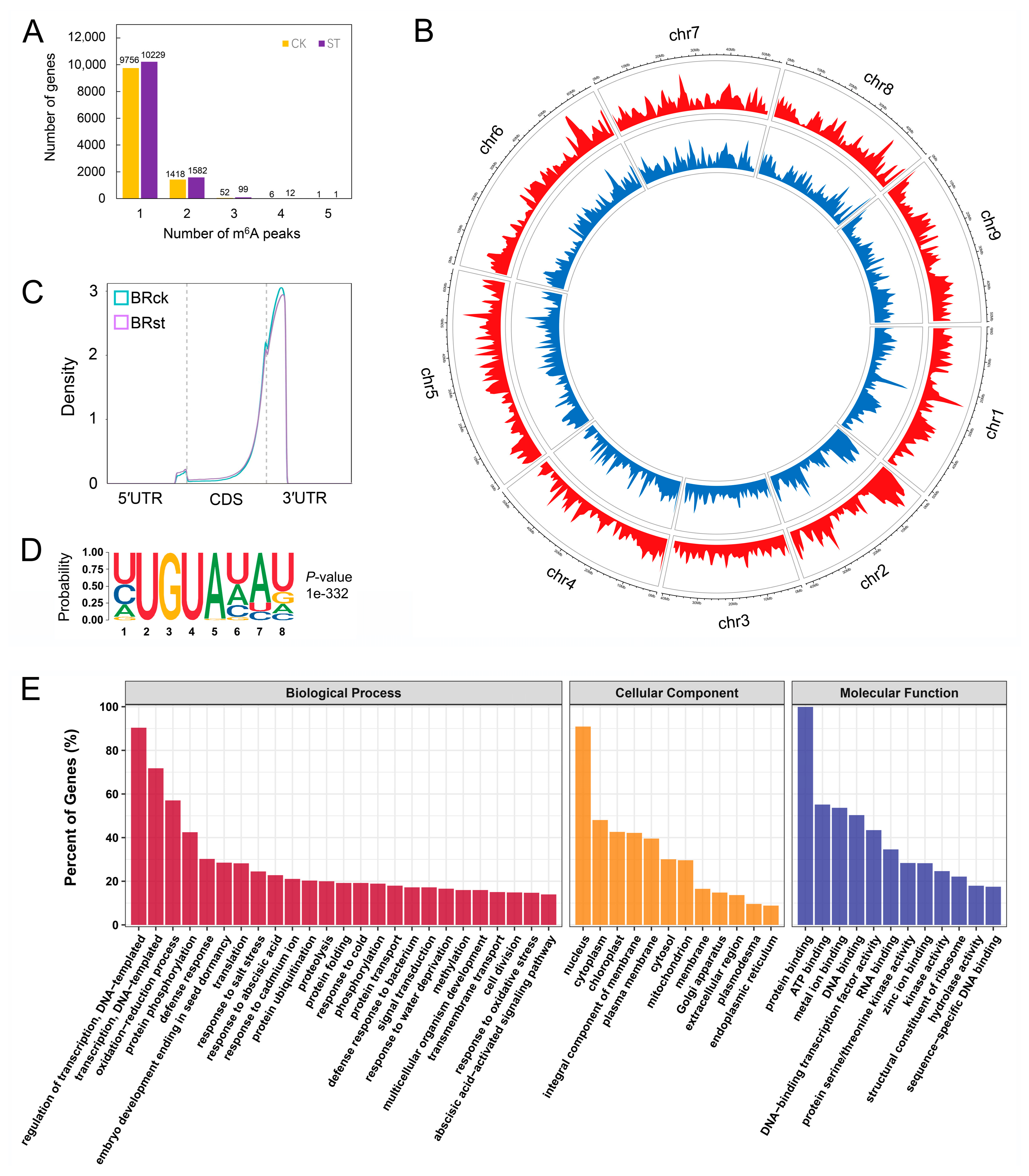
2.2. Generation of m6A Methylation Profiles for Sugar Beet Roots
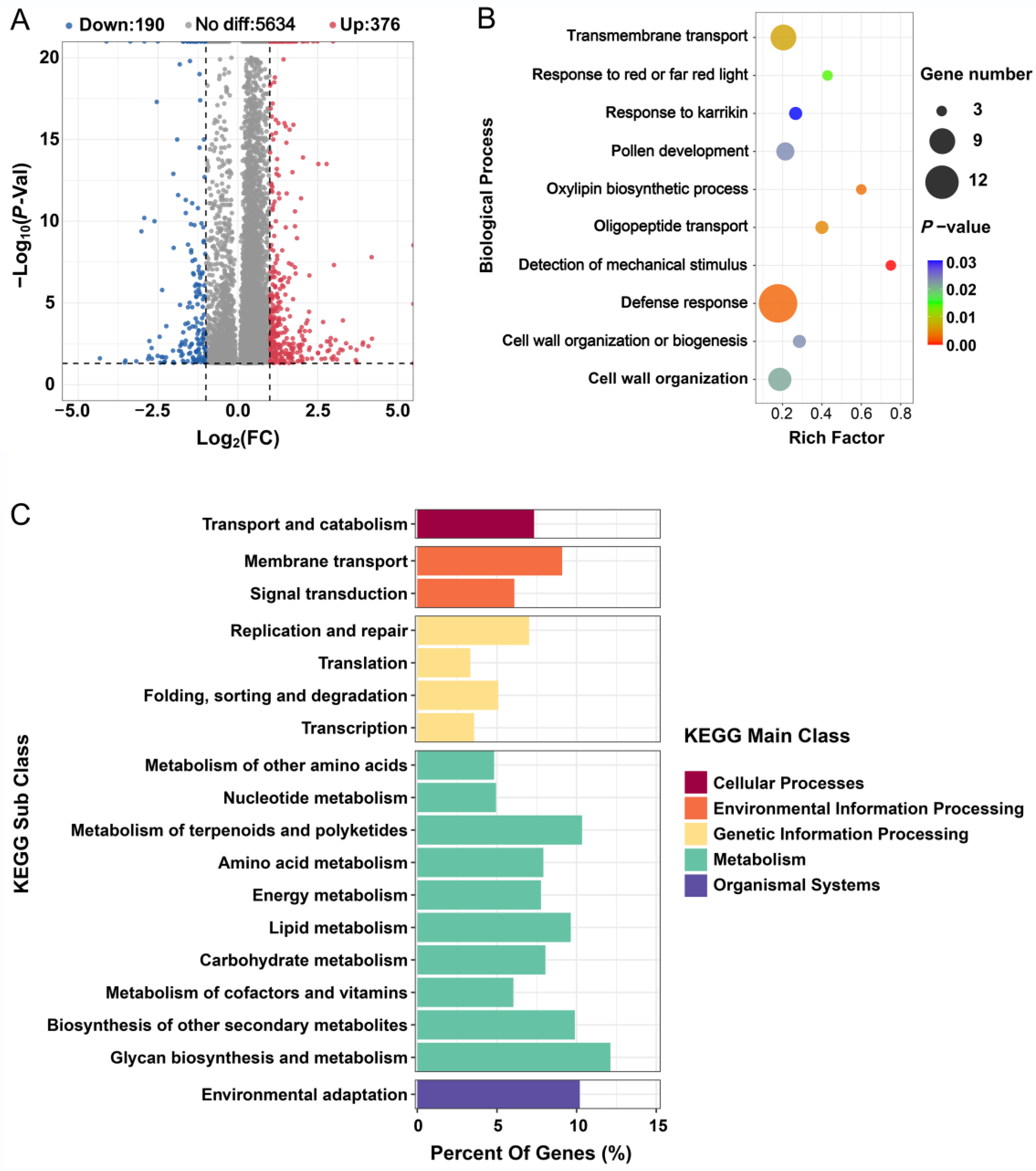
2.3. m6A Methylation Is Affected by Salt Stress
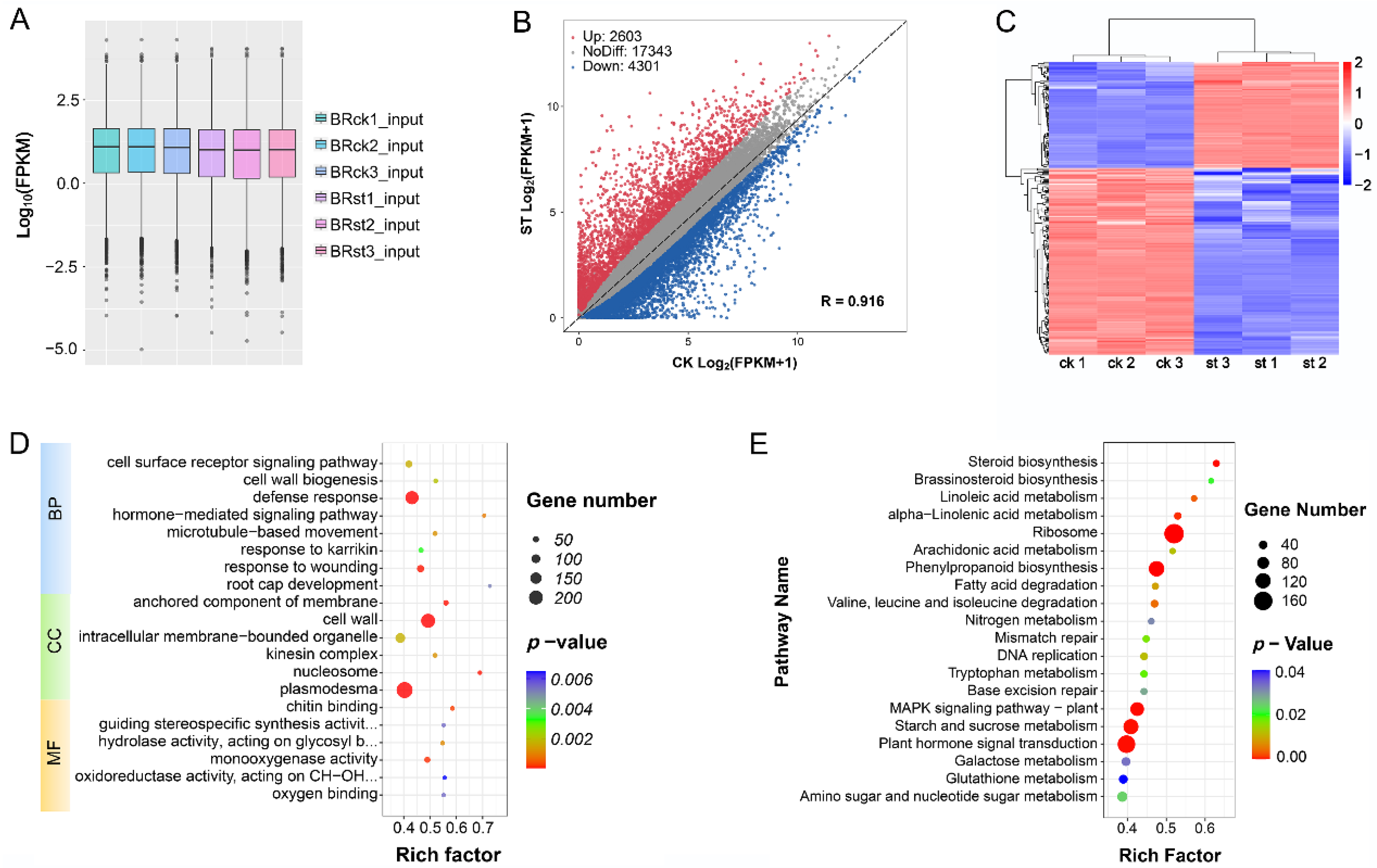
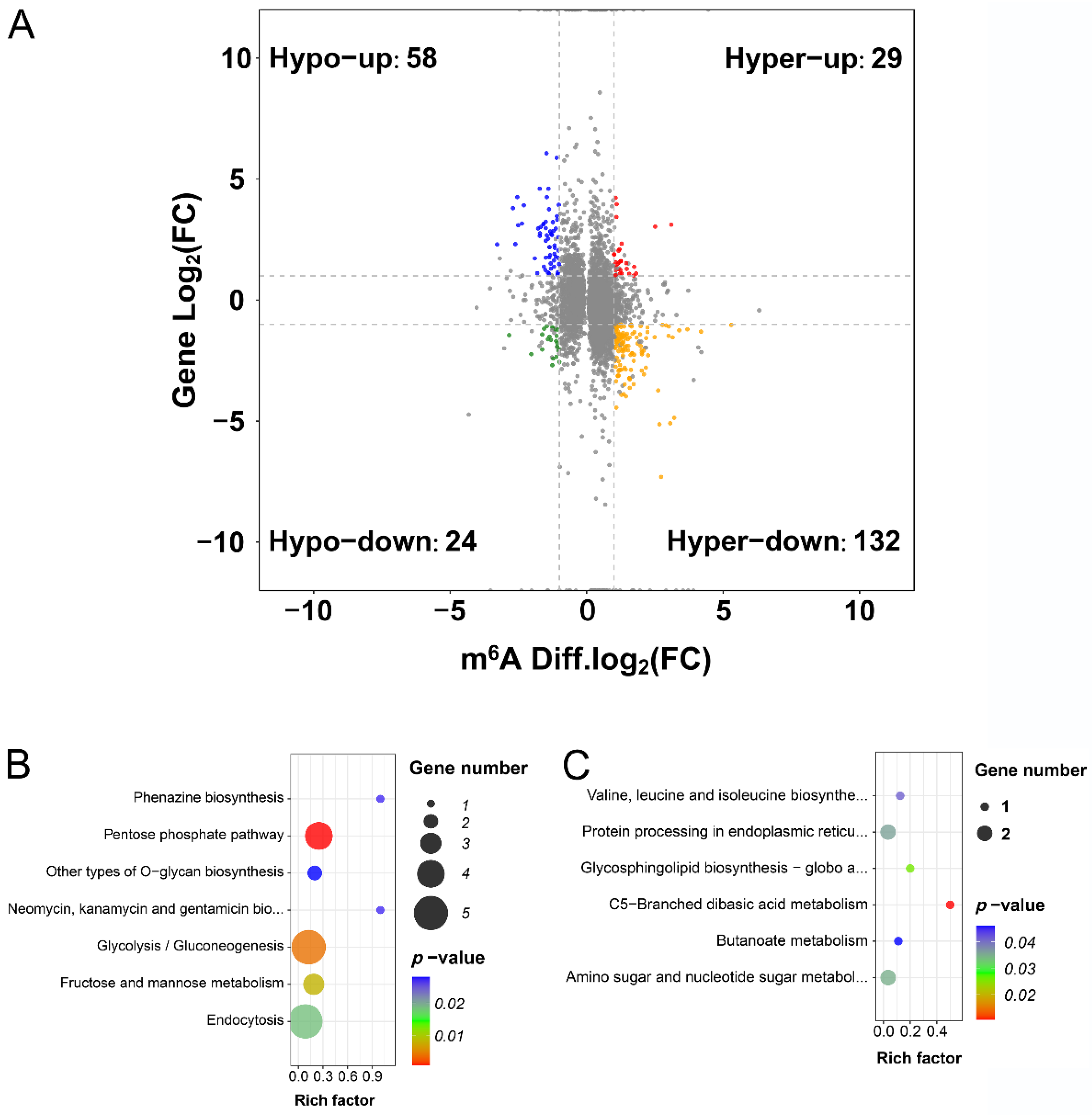
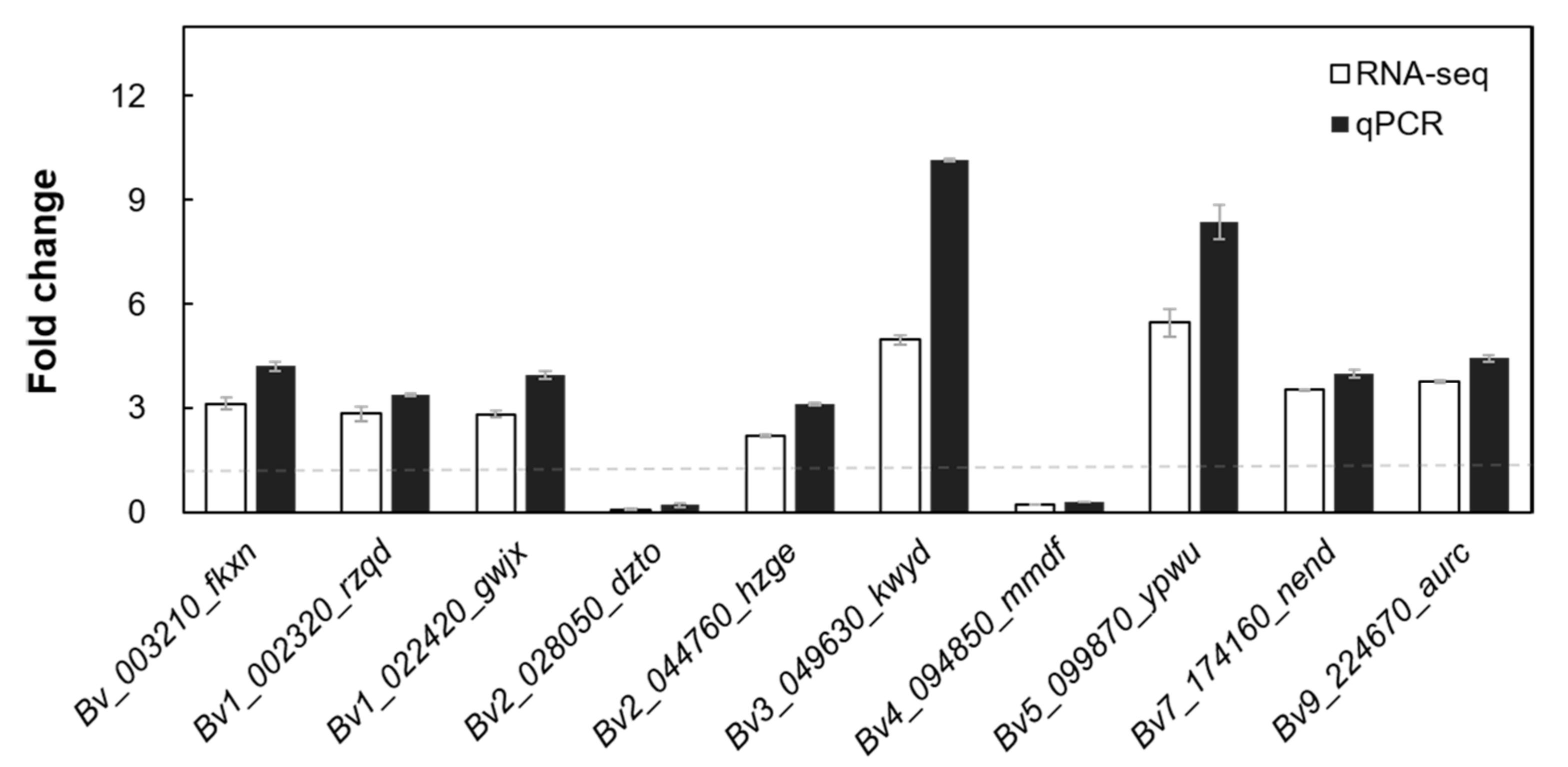
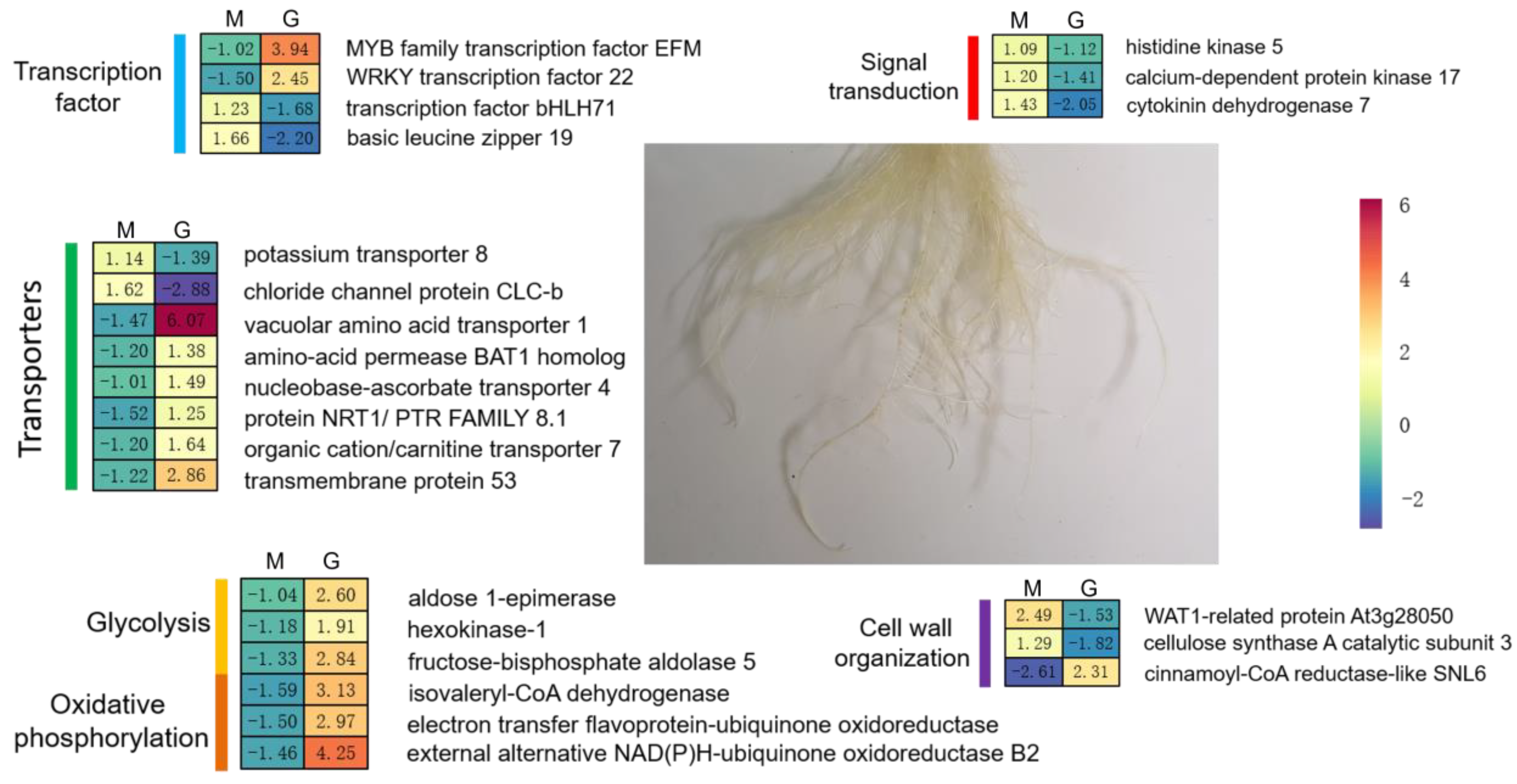
2.4. Conjoint Analysis of Changes in Gene Expression and Methylation Levels
2.5. Changes in RNA Methylation-Related Genes in Response to Salt Stress
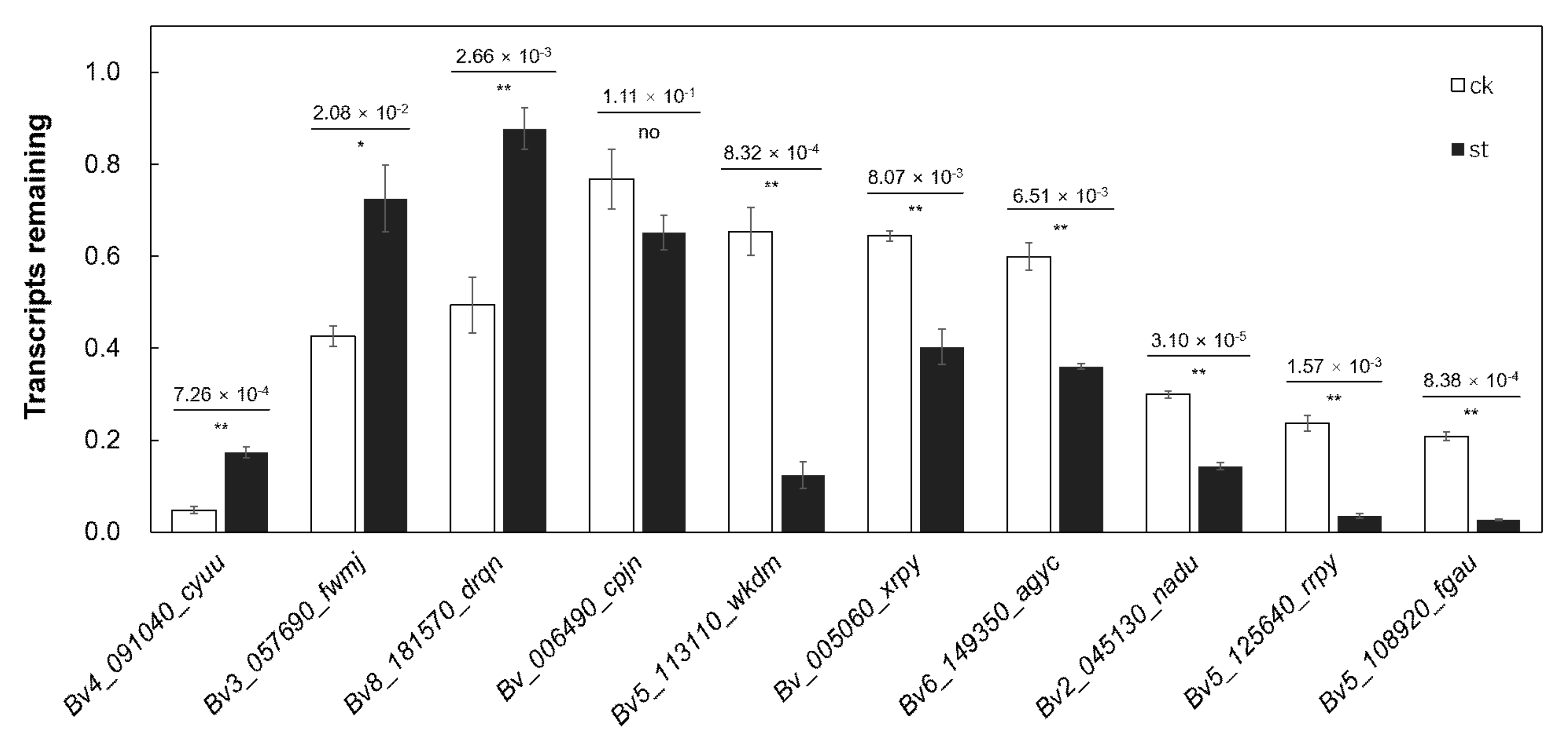
2.6. m6A Modification Regulates mRNA Abundance by Regulating the Stability of Salt-Tolerant Transcripts
3. Discussion
4. Materials and Methods
4.1. Cultivation and Treatment of Beet Seedlings
4.2. Measurement of Physiologic Indicators and Ion Content
4.3. RNA Extraction, Library Construction, and Sequencing
4.4. Analysis of Sequencing Data
4.5. Transcript Stability Time Course
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Gilliham, M. Salinity tolerance of crops-what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, L.; Chude, V.; Yagi, K.; Krasilnikov, P.; Panah, S.K.A.; Mendonça-Santos, M.d.L.; Pennock, D.; McKenzie, N. Status of the World’s Soil Resources (SWSR)—Main Report. In Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils; FAO: Rome, Italy, 2015. [Google Scholar]
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sörensen, T.R.; Stracke, R.; Reinhardt, R.; et al. The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Wheeler, R.M.; Levine, L.H.; Stutte, G.W. Glycine betaine accumulation, ionic and water relations of red-beet at contrasting levels of sodium supply. J. Plant Physiol. 2001, 158, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Wheeler, R.M.; Stutte, G.W.; Levine, L.H. Low potassium enhances sodium uptake in red-beet under moderate saline conditions. J. Plant Nutr. 2000, 23, 1449–1470. [Google Scholar] [CrossRef]
- Pi, Z.; Stevanato, P.; Sun, F.; Yang, Y.; Sun, X.; Zhao, H.; Geng, G.; Yu, L. Proteomic changes induced by potassium deficiency and potassium substitution by sodium in sugar beet. J. Plant Res. 2016, 129, 527–538. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Kader, A.; Lindberg, S. Uptake of sodium in quince, sugar beet, and wheat protoplasts determined by the fluorescent sodium-binding dye benzofuran isophthalate. J. Plant Physiol. 2005, 162, 421–428. [Google Scholar] [CrossRef]
- Skorupa, M.; Gołębiewski, M.; Kurnik, K.; Niedojadło, J.; Kęsy, J.; Klamkowski, K.; Wójcik, K.; Treder, W.; Tretyn, A.; Tyburski, J. Salt stress vs. salt shock-the case of sugar beet and its halophytic ancestor. BMC Plant Biology 2019, 19, 57. [Google Scholar] [CrossRef]
- Skorupa, M.; Gołebiewski, M.; Domagalski, K.; Kurnika, K.; Nahia, K.A.; Złoch, M.; Tretyn, A.; Tyburski, J. Transcriptomic profiling of the salt stress response in excised leaves of the halophyte Beta vulgaris ssp. maritima. Plant Sci. 2016, 243, 56–70. [Google Scholar] [CrossRef]
- Lv, X.; Jin, Y.; Wang, Y. De novo transcriptome assembly and identification of salt-responsive genes in sugar beet M14. Comput. Biol. Chem. 2018, 75, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Dai, C.; Liu, T.; Cheng, D.; Luo, C. Whole-Transcriptome RNA Sequencing Reveals the Global Molecular Responses and CeRNA Regulatory Network of mRNAs, lncRNAs, miRNAs and circRNAs in Response to Salt Stress in Sugar Beet (Beta vulgaris). Int. J. Mol. Sci. 2020, 22, 289. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Nie, X.; Yan, Z.; Weining, S. N6-methyladenosine regulatory machinery in plants: Composition, function and evolution. Plant Biotechnol. J. 2019, 17, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.-M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.-L.; Song, S.-H.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Fu, Y.; Dominissini, D.; Rechavi, G.; He, C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat. Rev. Genet. 2014, 15, 293–306. [Google Scholar] [CrossRef]
- Yang, Y.; Hsu, P.J.; Chen, Y.-S.; Yang, Y.-G. Dynamic transcriptomic m(6)A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef]
- Yang, D.; Qiao, J.; Wang, G.; Lan, Y.; Li, G.; Guo, X.; Xi, J.; Ye, D.; Zhu, S.; Chen, W.; et al. N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic. Acids. Res. 2018, 46, 3906–3920. [Google Scholar] [CrossRef]
- Kramer, M.C.; Janssen, K.A.; Palos, K.; Nelson, A.D.L.; Vandivier, L.E.; Garcia, B.A.; Lyons, E.; Beilstein, M.A.; Gregory, B.D. N6-methyladenosine and RNA secondary structure affect transcript stability and protein abundance during systemic salt stress in Arabidopsis. Plant Direct 2020, 4, e00239. [Google Scholar] [CrossRef]
- Hu, J.; Cai, J.; Park, S.J.; Lee, K.; Li, Y.; Chen, Y.; Yun, J.-Y.; Xu1, T.; Kang, H. N6-Methyladenosine mRNA methylation is important for salt stress tolerance in Arabidopsis. Plant J. 2021, 106, 1759–1775. [Google Scholar] [CrossRef]
- Shoaib, Y.; Hu, J.; Manduzio, S.; Kang, H. Alpha-ketoglutarate-dependent dioxygenase homolog 10B, an N(6)-methyladenosine mRNA demethylase, plays a role in salt stress and abscisic acid responses in Arabidopsis thaliana. Physiol. Plant. 2021, 173, 1078–1089. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, T.; Qi, Y.; Song, J.; Han, Z.; Ma, C. Evolution of the RNA N6-Methyladenosine Methylome Mediated by Genomic Duplication. Plant Physiol. 2020, 182, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lv, Z.; Diao, S.; Liu, H.; Duan, A.; He, C.; Zhang, J. Unique features of the m6A methylome and its response to drought stress in sea buckthorn (Hippophae rhamnoides Linn.). RNA Biol. 2021, 18 (Suppl. 2), 794–803. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-H.; Song, P.; Wang, Y.; Lu, Z.; Tang, Q.; Yu, Q.; Xiao, Y.; Zhang, X.; Duan, H.-C.; Jia, G. The m6A Reader ECT2 Controls Trichome Morphology by Affecting mRNA Stability in Arabidopsis. Plant Cell 2018, 30, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, J.; Li, J.; Cheng, D.; Dai, C. Genome-wide sequence identification and expression analysis of N6 -methyladenosine demethylase in sugar beet (Beta vulgaris L.) under salt stress. PeerJ 2022, 10, e12719. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ Tolerance and Na+ Transport in Higher Plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Timson, D.J.; Reece, R.J. Identification and characterisation of human aldose 1-epimerase. FEBS Lett. 2003, 543, 21–24. [Google Scholar] [CrossRef]
- Claeyssen, E.r.; Rivoal, J. Isozymes of plant hexokinase: Occurrence, properties and functions. Phytochemistry 2007, 68, 709–731. [Google Scholar] [CrossRef]
- Giegé, P.; Heazlewood, J.L.; Roessner-Tunali, U.; Millar, A.H.; Fernie, A.R.; Leaver, C.J.; Sweetlove, L.J. Enzymes of glycolysis are functionally associated with the mitochondrion in Arabidopsis cells. Plant Cell 2003, 15, 2140–2151. [Google Scholar] [CrossRef]
- Carter, C.; Pan, S.; Zouhar, J.; Avila, E.L.; Girke, T.; Raikhel, N.V. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 2004, 16, 3285–3303. [Google Scholar] [CrossRef] [PubMed]
- Barkla, B.J.; Vera-Estrella, R.; Herna´ndez-Coronado, M.; Pantoja, O. Quantitative proteomics of the tonoplast reveals a role for glycolytic enzymes in salt tolerance. Plant Cell 2009, 21, 4044–4058. [Google Scholar] [CrossRef] [PubMed]
- Rich, P.R.; Maréchal, A. The mitochondrial respiratory chain. Essays Biochem. 2010, 47, 1–23. [Google Scholar] [PubMed]
- Watmough, N.J.; Frerman, F.E. The electron transfer flavoprotein: Ubiquinone oxidoreductases. Biochim. Biophys. Acta 2010, 1797, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Izumi, M.; Wada, S.; Makino, A.; Ishida, H. Vacuolar Protein Degradation via Autophagy Provides Substrates to Amino Acid Catabolic Pathways as an Adaptive Response to Sugar Starvation in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 1363–1376. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Sharma, A.; Prakash, S.; Chattopadhyay, D. Killing two birds with a single stone-genetic manipulation of cytokinin oxidase/dehydrogenase (CKX) genes for enhancing crop productivity and amelioration of drought stress response. Front. Genet. 2022, 13, 941595. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Z.; Gu, Y.; Li, W.; Wang, W.; Yuan, X.; Zhang, Y.; Yuan, M.; Du, J.; Zhao, Q. Genome-wide identification of the soybean cytokinin oxidase/dehydrogenase gene family and its diverse roles in response to multiple abiotic stress. Front. Plant Sci. 2023, 14, 1163219. [Google Scholar] [CrossRef]
- Thatcher, J.D. Transport proteins. Sci. Signal. 2013, 6, tr3. [Google Scholar] [CrossRef]
- Sun, T.; Pei, T.; Yang, L.; Zhang, Z.; Li, M.; Liu, Y.; Ma, F.; Liu, C. Exogenous application of xanthine and uric acid and nucleobase-ascorbate transporter MdNAT7 expression regulate salinity tolerance in apple. BMC Plant Biol. 2021, 21, 52. [Google Scholar] [CrossRef]
- Voiniciuc, C.; Pauly, M.; Usadel, B. Monitoring Polysaccharide Dynamics in the Plant Cell Wall. Plant Physiol. 2018, 176, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2014, 5, 771. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Isayenkov, S.V. The regulation of plant cell wall organisation under salt stress. Front. Plant Sci. 2023, 14, 1118313. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.J.; Kramer, M.C.; Gosai, S.J.; Yu, X.; Vandivier, L.E.; Nelson, A.D.L.; Anderson, Z.D.; Beilstein, M.A.; Fray, R.G.; Lyons, E.; et al. N6-Methyladenosine Inhibits Local Ribonucleolytic Cleavage to Stabilize mRNAs in Arabidopsis. Cell Rep. 2018, 25, 1146–1157.e3. [Google Scholar] [CrossRef]
- Cui, C.; Ma, Z.; Wan, H.; Gao, J.; Zhou, B. GhALKBH10 negatively regulates salt tolerance in cotton. Plant Physiol. Biochem. 2022, 192, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, X.; Li, J.; Song, Y.; Song, J.; Wang, F.; Liu, L.; Zhang, X.; Sui, N. Analysis of N6-methyladenosine reveals a new important mechanism regulating the salt tolerance of sweet sorghum. Plant Sci. 2021, 304, 110801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, Q.; Cao, S.; Tian, Y.; Han, K.; Sun, Y.; Li, J.; Yang, Q.; Ji, Q.; Sederoff, R.; et al. Genome-wide identification of the AlkB homologs gene family, PagALKBH9B and PagALKBH10B regulated salt stress response in Populus. Front. Plant Sci. 2022, 13, 994154. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Xu, Z.; Jiang, S.; Shi, Y.; Xie, H.; Wang, S.; Hua, J.; Wu, Y. m6A mRNA modification promotes chilling tolerance and modulates gene translation efficiency in Arabidopsis. Plant Physiol. 2023, 192, 1466–1482. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Cheng, D.; Dai, C.; Liu, T.; Wang, C.; Luo, C. iTRAQ protein profile analysis of sugar beet under salt stress: Different coping mechanisms in leaves and roots. BMC Plant Biol. 2020, 20, 347. [Google Scholar] [CrossRef]
- Shi, S.; Cui, J.; Lu, Z.; Cheng, D.; Luo, C. Screening of tolerance to NaCl in sugar beet germplasms. China Beet Sugar 2008, 4, 7–9. [Google Scholar]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]

| Gene_Id | Type | Homologous Gene | log2 (FC) | p-Value | Up/Down |
|---|---|---|---|---|---|
| Bv5_103730_enox | writer | AtMTA | −0.33 | 0.17 | no dif. |
| Bv3_054970_hfjn | writer | AtMTB | −0.39 | 0.00 | no dif. |
| Bv5_117690_arcy | writer | AtFKBP12 | 0.15 | 0.00 | no dif. |
| Bv5_110090_noir | writer | AtVIR | −0.86 | 0.00 | no dif. |
| Bv5_121260_sckp | writer | AtHAKAI | −0.30 | 0.44 | no dif. |
| Bv6_150770_huzh | eraser | AtALKBH1A | −1.01 | 0.00 | down |
| Bv7_157650_ryeg | eraser | AtALKBH1D | 0.24 | 0.00 | no dif. |
| Bv7_169620_pkhc | eraser | AtALKBH1D | −0.45 | 0.06 | no dif. |
| Bv8_184320_kacr | eraser | AtALKBH2 | 0.08 | 0.05 | no dif. |
| Bv5_102160_pgse | eraser | AtALKBH8A | −0.63 | 0.01 | no dif. |
| Bv3_051230_eskg | eraser | AtALKBH9A | 0.71 | 0.00 | no dif. |
| Bv4_083160_sqec | eraser | AtALKBH6 | 0.08 | 0.00 | no dif. |
| Bv6_130050_njrf | eraser | AtALKBH5 | −0.27 | 0.78 | no dif. |
| Bv7_164580_swwm | eraser | AtALKBH8B | −0.35 | 0.35 | no dif. |
| Bv7_179400_uxaj | eraser | AtALKBH10A | −2.93 | 0.00 | down |
| Bv2_036020_nmug | reader | AtECT10 | 0.22 | 0.00 | no dif. |
| Bv3_056220_tirq | reader | AtECT3 | −0.37 | 0.00 | no dif. |
| Bv3_059680_euso | reader | AtECT6 | 0.12 | 0.00 | no dif. |
| Bv5_101530_jzsk | reader | AtECT12 | −0.27 | 0.82 | no dif. |
| Bv8_181150_pemc | reader | AtECT11 | −0.33 | 0.01 | no dif. |
| Bv8_187630_mced | reader | AtCPSF30 | −0.22 | 0.65 | no dif. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Pang, Q.; Yan, X. Unique Features of the m6A Methylome and Its Response to Salt Stress in the Roots of Sugar Beet (Beta vulgaris). Int. J. Mol. Sci. 2023, 24, 11659. https://doi.org/10.3390/ijms241411659
Li J, Pang Q, Yan X. Unique Features of the m6A Methylome and Its Response to Salt Stress in the Roots of Sugar Beet (Beta vulgaris). International Journal of Molecular Sciences. 2023; 24(14):11659. https://doi.org/10.3390/ijms241411659
Chicago/Turabian StyleLi, Junliang, Qiuying Pang, and Xiufeng Yan. 2023. "Unique Features of the m6A Methylome and Its Response to Salt Stress in the Roots of Sugar Beet (Beta vulgaris)" International Journal of Molecular Sciences 24, no. 14: 11659. https://doi.org/10.3390/ijms241411659
APA StyleLi, J., Pang, Q., & Yan, X. (2023). Unique Features of the m6A Methylome and Its Response to Salt Stress in the Roots of Sugar Beet (Beta vulgaris). International Journal of Molecular Sciences, 24(14), 11659. https://doi.org/10.3390/ijms241411659





