The Impact of Hypromellose on Pharmaceutical Properties of Alginate Microparticles as Novel Drug Carriers for Posaconazole
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microparticle Characterization
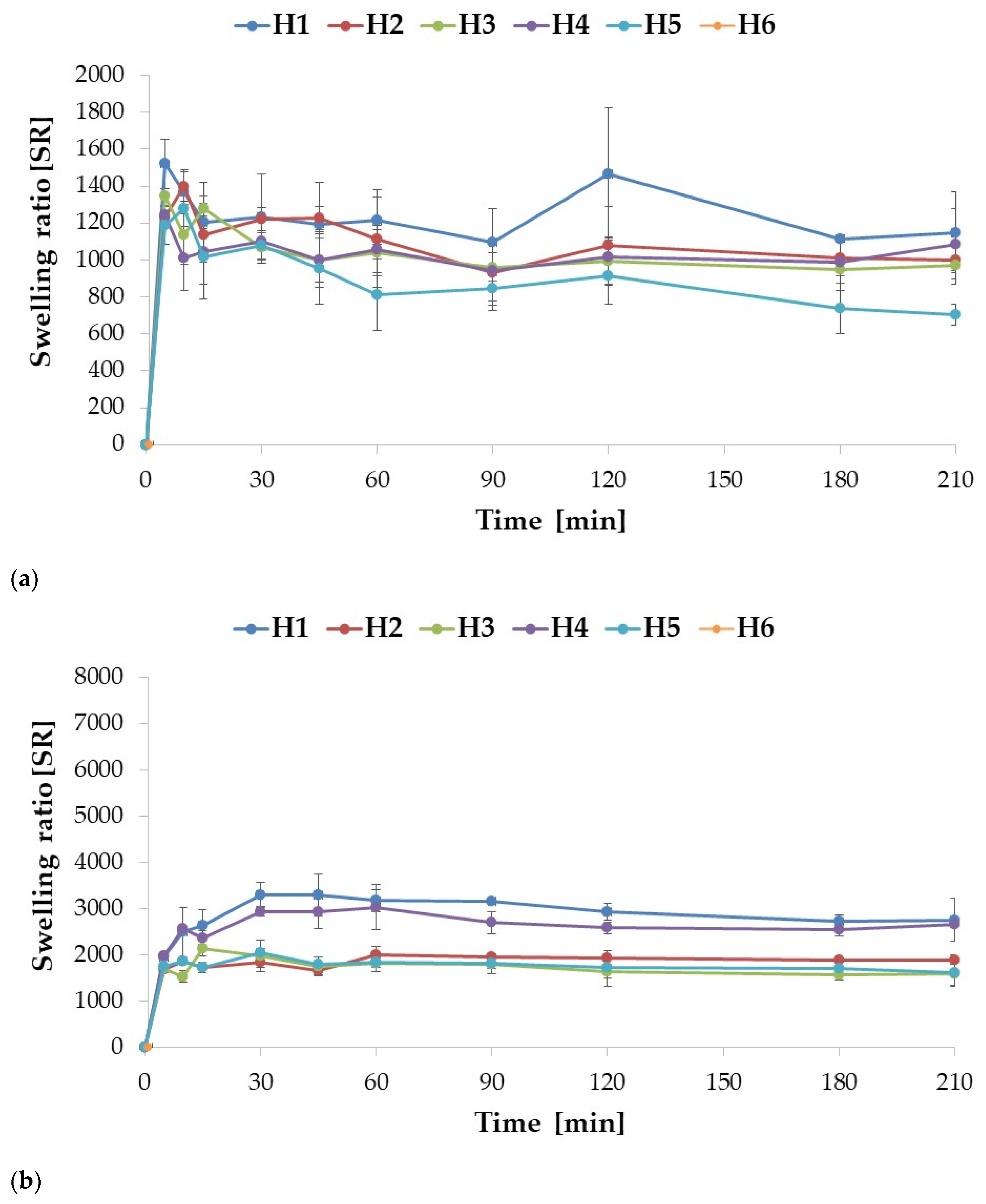
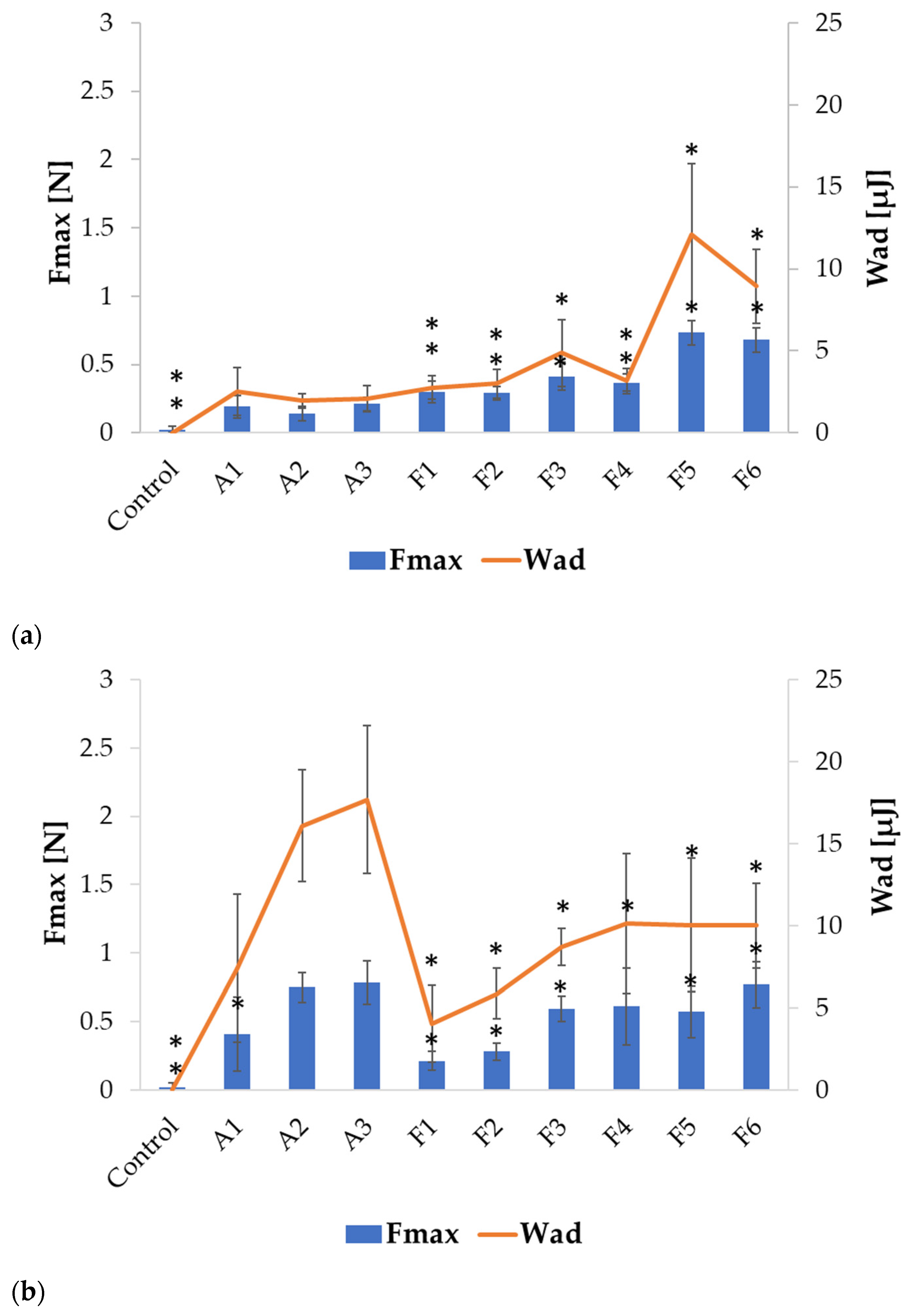
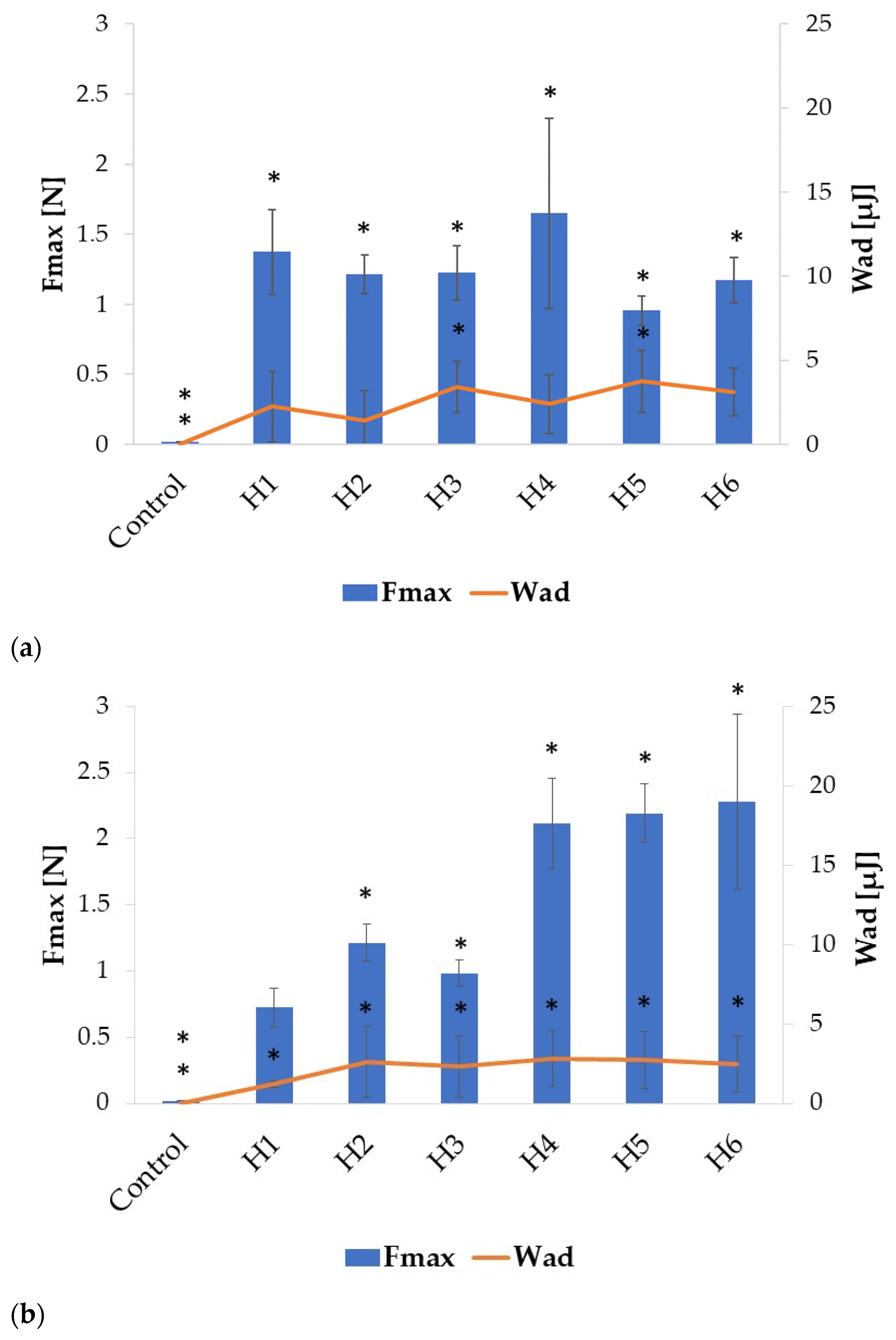
2.2. Swelling and Mucoadhesion
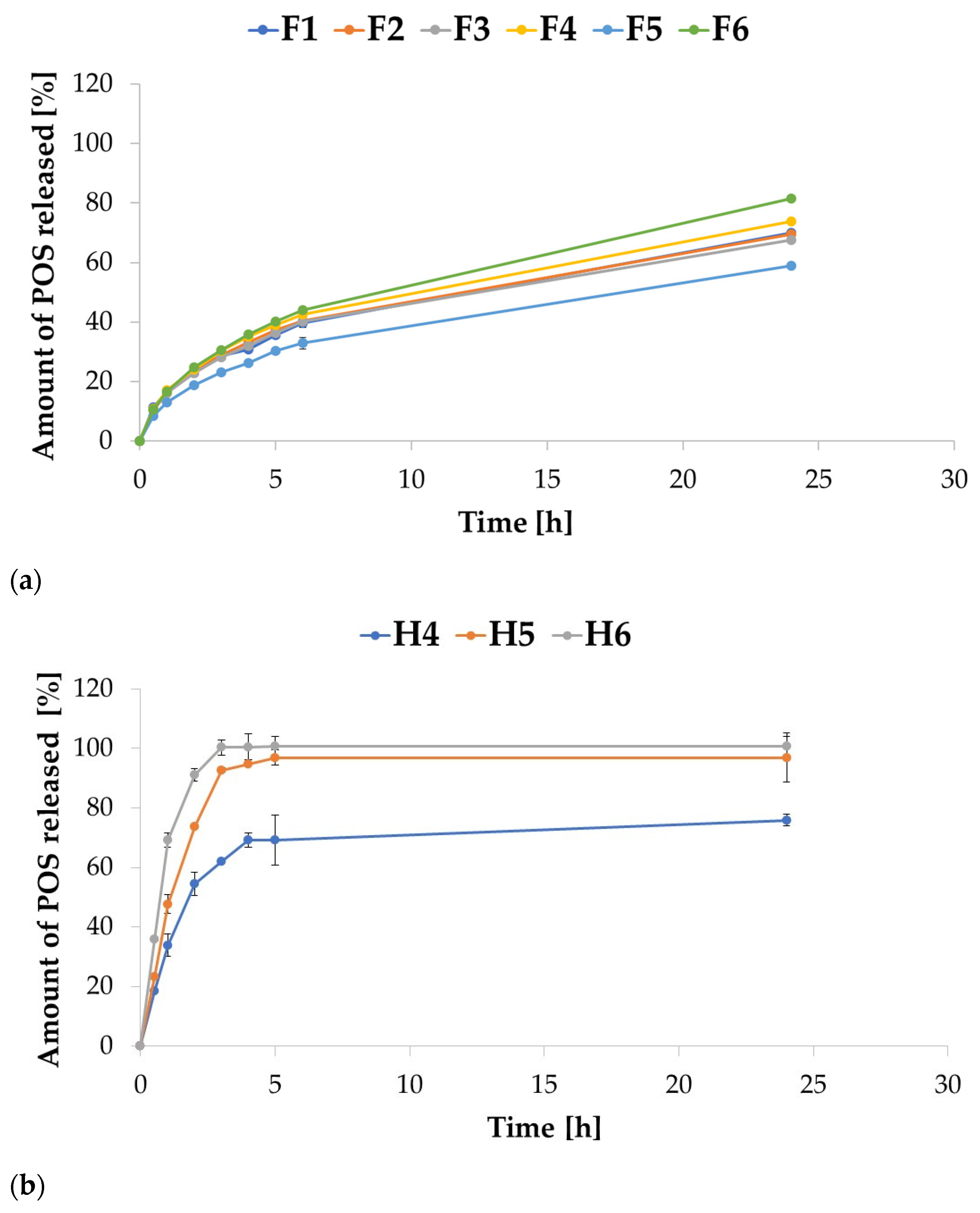
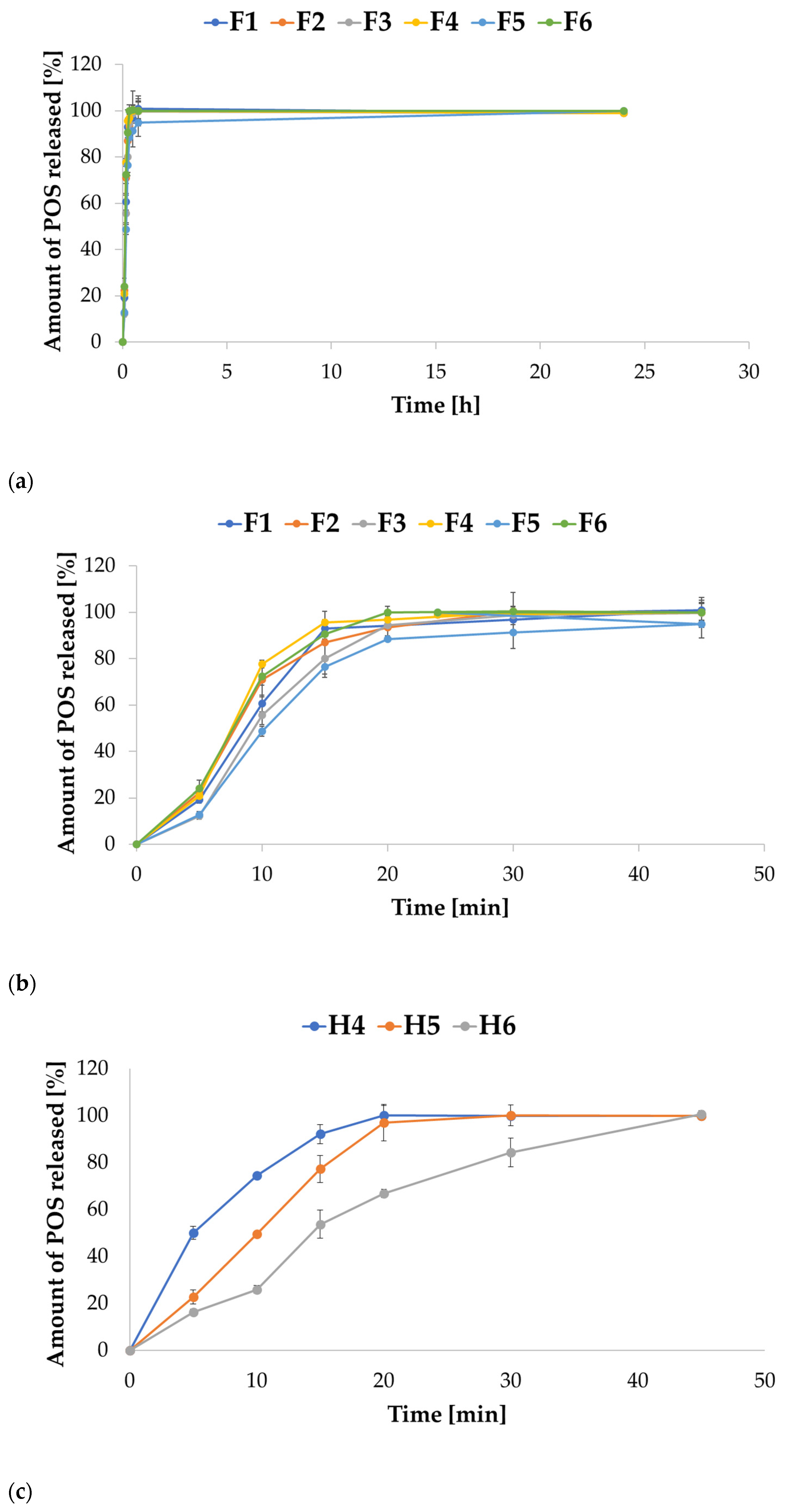
2.3. In Vitro POS Release
2.4. DSC Analysis
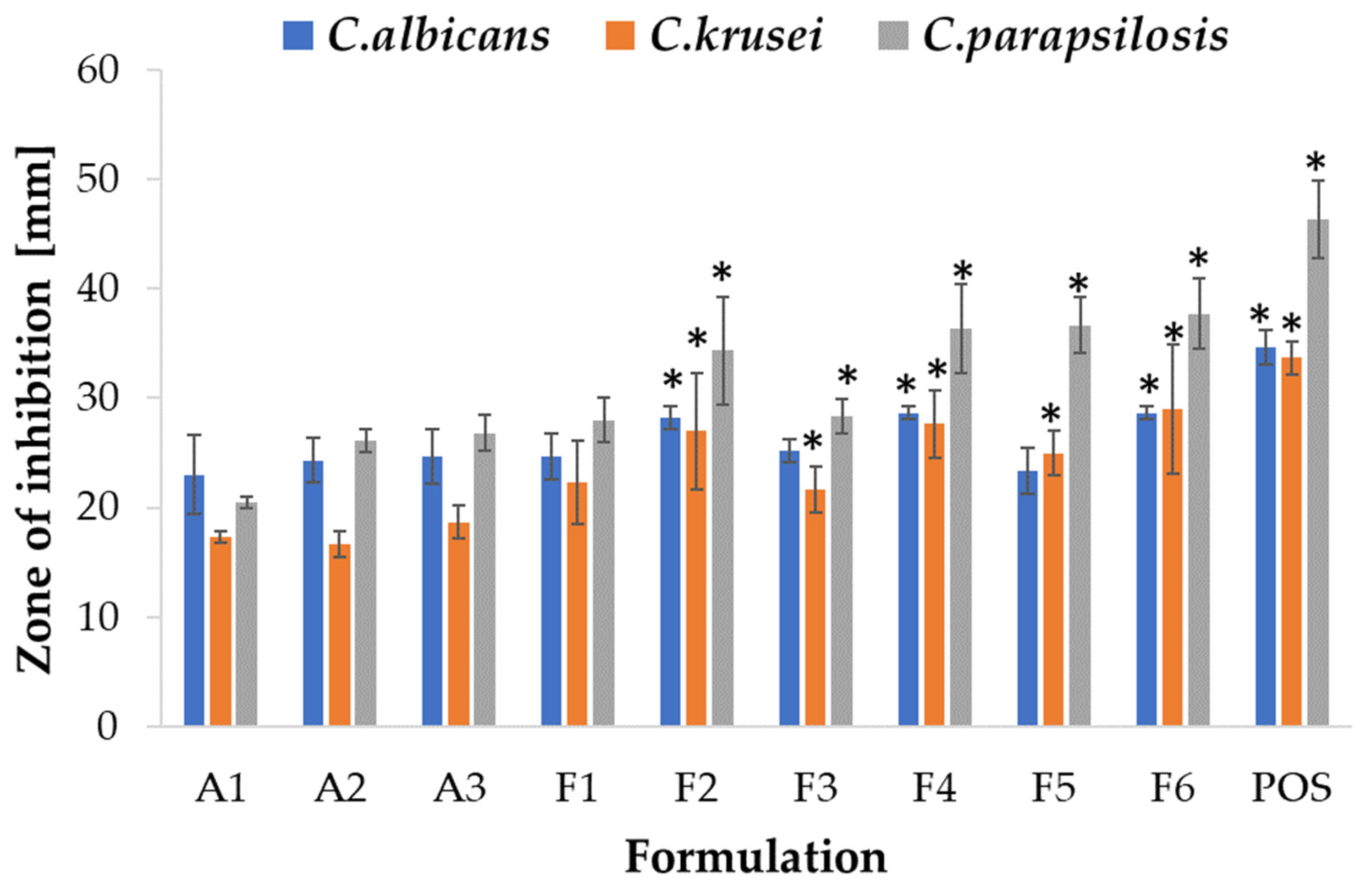
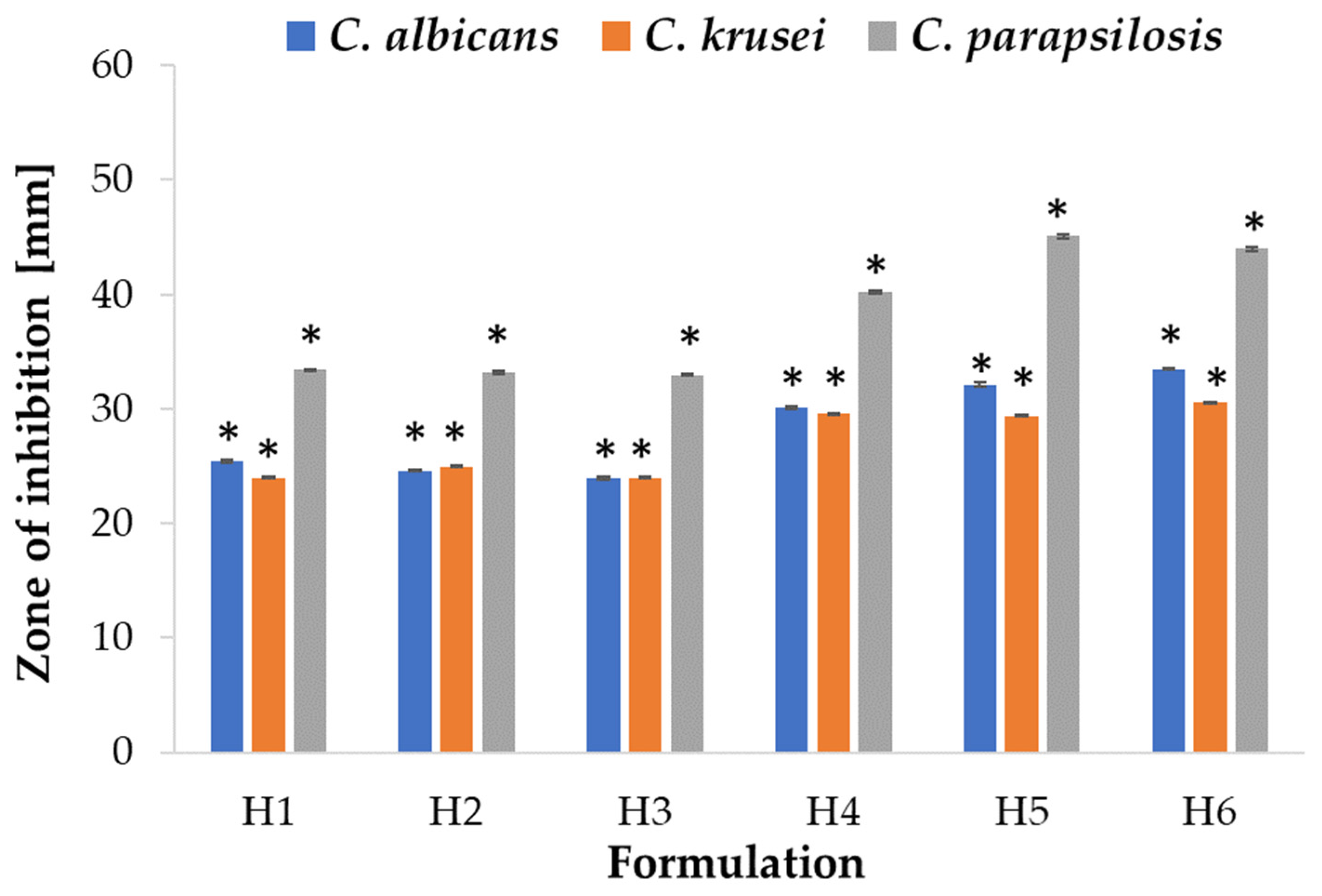
2.5. Antifungal Activity
3. Materials and Methods
3.1. Materials
3.2. ALG and ALG/HPMC Solutions Preparation
3.3. Viscosity Measurements
3.4. Microparticle Preparation
3.5. Moisture Presence
3.6. Assessment of Microparticles Morphology
3.7. Estimation of POS Loading, Encapsulation Efficacy, and Production Yield
3.8. POS Spectrophotometry Analysis
3.9. Swelling Capacity
3.10. Mucoadhesive Properties
3.11. In Vitro POS Release
3.12. Mathematical Modeling of POS Release Profile
3.13. Thermal Analysis
3.14. Antifungal Activity
3.15. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janbon, G.; Quintin, J.; Lanternier, F.; d’Enfert, C. Studying Fungal Pathogens of Humans and Fungal Infections: Fungal Diversity and Diversity of Approaches. Genes Immun. 2019, 20, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A Global View on Fungal Infections in Humans and Animals: Opportunistic Infections and Microsporidioses. J. Appl. Microbiol. 2021, 131, 2095–2113. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, A.; Santos, H.; Franco-Duarte, R.; Sampaio, P. Fungal Infections Diagnosis—Past, Present and Future. Res. Microbiol. 2022, 173, 103915. [Google Scholar] [CrossRef]
- Taipaleenmäki, E.; Städler, B. Recent Advancements in Using Polymers for Intestinal Mucoadhesion and Mucopenetration. Macromol. Biosci. 2020, 20, 1900342. [Google Scholar] [CrossRef]
- Cook, M.T.; Khutoryanskiy, V.V. Mucoadhesion and Mucosa-Mimetic Materials—A Mini-Review. Int. J. Pharm. 2015, 495, 991–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidovich-Pinhas, M.; Bianco-Peled, H. Mucoadhesion: A Review of Characterization Techniques. Expert Opin. Drug Deliv. 2010, 7, 259–271. [Google Scholar] [CrossRef]
- Smart, J. The Basics and Underlying Mechanisms of Mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-Known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Kruk, K.; Winnicka, K. Alginates Combined with Natural Polymers as Valuable Drug Delivery Platforms. Mar. Drugs 2022, 21, 11. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose—A Traditional Pharmaceutical Excipient with Modern Applications in Oral and Oromucosal Drug Delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.L. Hypromellose. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical press: London, UK, 2009; pp. 326–329. [Google Scholar]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The Use of Hypromellose in Oral Drug Delivery. J. Pharm. Pharmacol. 2010, 57, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Timmins, P.; Pygall, S.; Melia, C.D. Hydrophilic Matrix Tablets for Oral Controlled Release; American Association of Pharmaceutical Scientists; AAPS Advances in the Pharmaceutical Sciences Series; Springer: New York, NY, USA, 2014. [Google Scholar]
- Wen, H.; Park, K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Khan, S.A.; Ahmad, M.; Murtaza, G.; Aamir, M.N.; Kousar, R.; Rasool, F.; Shahiq-u-Zaman. In Vitro-in Vivo Correlation Study on Nimesulide Loaded Hydroxypropylmethylcellulose Microparticles. Yao Xue Xue Bao 2010, 45, 772–777. [Google Scholar] [PubMed]
- Kousar, R.; Ahmad, M.; Murtaza, G.; Khan, S.A.; Karim, S.; Hussain, I. Pharmacokinetic Study of Hydroxypropylmethylcellulose Microparticles Loaded with Cimetidine. Adv. Clin. Exp. Med. 2013, 22, 41–45. [Google Scholar] [PubMed]
- Mahmoudian, M.; Ganji, F. Vancomycin-Loaded HPMC Microparticles Embedded within Injectable Thermosensitive Chitosan Hydrogels. Prog. Biomater. 2017, 6, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czyrski, A.; Resztak, M.; Świderski, P.; Brylak, J.; Główka, F.K. The Overview on the Pharmacokinetic and Pharmacodynamic Interactions of Triazoles. Pharmaceutics 2021, 13, 1961. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Posaconaloze EMA Assessment Report. 2019. Available online: https://www.google.com/url?sa=i&rct=j&q=&esrc=s&source=web&cd=&ved=0CAIQw7AJahcKEwiwyLTli5r_AhUAAAAAHQAAAAAQAg&url=https%3A%2F%2Fwww.ema.europa.eu%2Fen%2Fdocuments%2Fassessment-report%2Fposaconazole-accord-epar-assessment-report_en.pdf&psig=AOvVaw0RNTVdytxsai5r6xGw8238&ust=1685434721649831 (accessed on 29 May 2023).
- BCS Classification. Available online: https://www.pharmaspecialists.com/p/available-bcs-classification-of-drugs-2.html#gsc.tab=0 (accessed on 29 May 2023).
- Lipp, H.-P. Clinical Pharmacodynamics and Pharmacokinetics of the Antifungal Extended-Spectrum Triazole Posaconazole: An Overview: Pharmacodynamics and Pharmacokinetics of the Antifungal Posaconazole. Br. J. Clin. Pharmacol. 2010, 70, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Krekels, E.H.J.; Verweij, P.E.; Buil, J.B.; Knibbe, C.A.J.; Brüggemann, R.J.M. Pharmacokinetics and Pharmacodynamics of Posaconazole. Drugs 2020, 80, 671–695. [Google Scholar] [CrossRef] [Green Version]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Szekalska, M.; Citkowska, A.; Wróblewska, M.; Winnicka, K. The Impact of Gelatin on the Pharmaceutical Characteristics of Fucoidan Microspheres with Posaconazole. Materials 2021, 14, 4087. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, D.R.; Walsh, D.; O’Connell, P.; Mugheirbi, N.A.; Worku, Z.A.; Bolas-Fernandez, F.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Optimising the in Vitro and in Vivo Performance of Oral Cocrystal Formulations via Spray Coating. Eur. J. Pharm. Biopharm. 2018, 124, 13–27. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Z.; Zhang, Y.; Zhang, F.; Wang, Y.; Zhao, Z. Preparation and Evaluation of Posaconazole-Loaded Enteric Microparticles in Rats. Drug Dev. Ind. Pharm. 2017, 43, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Bowey, K.; Neufeld, R.J. Systemic and Mucosal Delivery of Drugs within Polymeric Microparticles Produced by Spray Drying. BioDrugs 2010, 24, 359–377. [Google Scholar] [CrossRef]
- Szekalska, M.; Wróblewska, M.; Sosnowska, K.; Winnicka, K. Influence of Sodium Alginate on Hypoglycemic Activity of Metformin Hydrochloride in the Microspheres Obtained by the Spray Drying. Int. J. Polym. Sci. 2016, 2016, 8635408. [Google Scholar] [CrossRef] [Green Version]
- Salama, A.H. Spray Drying as an Advantageous Strategy for Enhancing Pharmaceuticals Bioavailability. Drug Deliv. Transl. Res. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Mugheirbi, N.A.; O’Connell, P.; Serrano, D.R.; Healy, A.M.; Taylor, L.S.; Tajber, L. A Comparative Study on the Performance of Inert and Functionalized Spheres Coated with Solid Dispersions Made of Two Structurally Related Antifungal Drugs. Mol. Pharm. 2017, 14, 3718–3728. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Rojewska, M.; Hyla, K.; Zembrzuska, J.; Prochaska, K. Surface and Swelling Properties of Mucoadhesive Blends and Their Ability to Release Fluconazole in a Mucin Environment. Colloids Surf. B Biointerfaces 2018, 172, 586–593. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, K.; Ranjan, A.; Bhowmik, B.B. A Novel Vaginal Drug Delivery System: Anti-HIV Bioadhesive Film Containing Abacavir. J. Mater. Sci. Mater. Med. 2014, 25, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Shoukat, M.; Asif, M.; Khalid, S.H.; Asghar, S.; Munir, M.U.; Irfan, M.; Rasul, A.; Qari, S.H.; Qumsani, A.T.; et al. Assessing the Synergistic Activity of Clarithromycin and Therapeutic Oils Encapsulated in Sodium Alginate Based Floating Microbeads. Microorganisms 2022, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Syed, M.A.; Abbas, N.; Hanif, S.; Arshad, M.S.; Bukhari, N.I.; Hussain, K.; Akhlaq, M.; Ahmad, Z. Development of an ANN Optimized Mucoadhesive Buccal Tablet Containing Flurbiprofen and Lidocaine for Dental Pain. Acta Pharm. 2016, 66, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, S.; Yousefi, G.; Ansari, A.; Mohammadi-Samani, S. Formulation and in Vitro Evaluation of a Fast-Disintegrating/Sustained Dual Release Bucoadhesive Bilayer Tablet of Captopril for Treatment of Hypertension Crises. Res. Pharm. Sci. 2016, 11, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okeke, O.C.; Boateng, J.S. Composite HPMC and Sodium Alginate Based Buccal Formulations for Nicotine Replacement Therapy. Int. J. Biol. Macromol. 2016, 91, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Alginate-Based Composite Sponges as Gastroretentive Carriers for Curcumin-Loaded Self-Microemulsifying Drug Delivery Systems. Sci. Pharm. 2017, 85, 11. [Google Scholar] [CrossRef] [Green Version]
- Yong, C.S.; Jung, J.-H.; Rhee, J.-D.; Kim, C.-K.; Choi, H.-G. Physicochemical Characterization and Evaluation of Buccal Adhesive Tablets Containing Omeprazole. Drug Dev. Ind. Pharm. 2001, 27, 447–455. [Google Scholar] [CrossRef]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2019, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Chiu, M.; Prenner, E. Differential Scanning Calorimetry: An Invaluable Tool for a Detailed Thermodynamic Characterization of Macromolecules and Their Interactions. J. Pharm. Bioallied Sci. 2011, 3, 39. [Google Scholar] [CrossRef]
- Dudek, G.; Turczyn, R. New Type of Alginate/Chitosan Microparticle Membranes for Highly Efficient Pervaporative Dehydration of Ethanol. RSC Adv. 2018, 8, 39567–39578. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, W.W.; Fletcher, J.; Khoder, M.; Alany, R.G. Solid Dispersions of Gefitinib Prepared by Spray Drying with Improved Mucoadhesive and Drug Dissolution Properties. AAPS PharmSciTech 2022, 23, 48. [Google Scholar] [CrossRef]
- Lim, H.-T.; Balakrishnan, P.; Oh, D.H.; Joe, K.H.; Kim, Y.R.; Hwang, D.H.; Lee, Y.-B.; Yong, C.S.; Choi, H.-G. Development of Novel Sibutramine Base-Loaded Solid Dispersion with Gelatin and HPMC: Physicochemical Characterization and Pharmacokinetics in Beagle Dogs. Int. J. Pharm. 2010, 397, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Spadari, C.D.C.; Lopes, L.B.; Ishida, K. Potential Use of Alginate-Based Carriers as Antifungal Delivery System. Front. Microbiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Mohammad, I.S.; Fan, L.; Zhao, Z.; Nurunnabi, M.; Sallam, M.A.; Wu, J.; Chen, Z.; Yin, L.; He, W. Delivery Strategies of Amphotericin B for Invasive Fungal Infections. Acta Pharm. Sin. B 2021, 11, 2585–2604. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antifungal Susceptibility Testing of Yeasts. Available online: https://clsi.org/media/3680/m60ed2_sample.pdf (accessed on 29 May 2023).
- Owen, D.H.; Katz, D.F. A Vaginal Fluid Simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Lee, J.-A.; Che, H.-Y. In Vitro Antifungal Activity of Equol against Candida Albicans. Mycobiology 2010, 38, 328–330. [Google Scholar] [CrossRef] [Green Version]

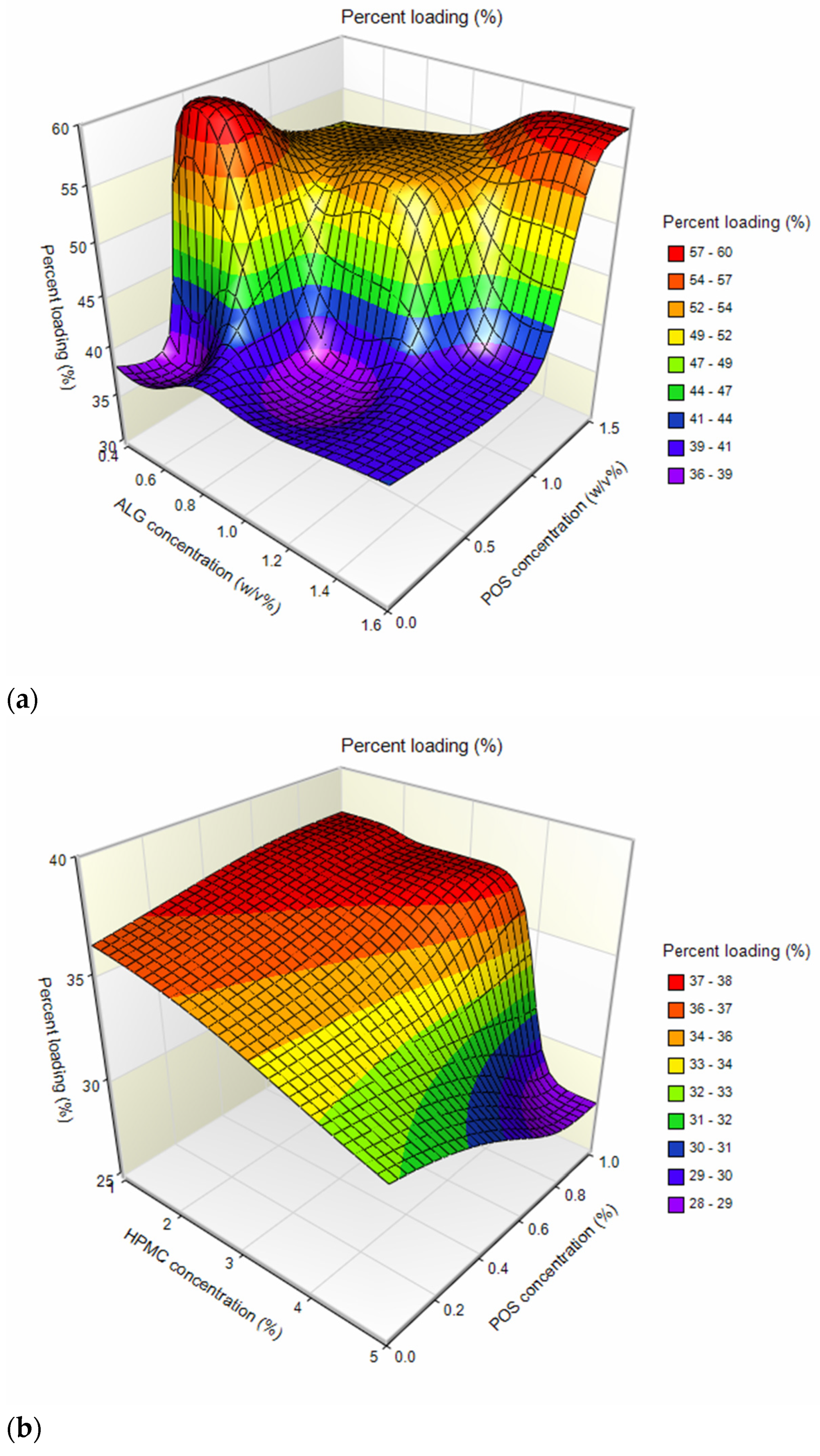



| Formulation | Particle Size (µm) | Percent Loading (%) | Encapsulation Efficiency (%) | Production Yield (%) | Moisture Content (%) |
|---|---|---|---|---|---|
| A1 | 10.03 ± 3.84 | - | - | 63.99 ± 2.93 | 10.59 ± 1.09 * |
| A2 | 10.69 ± 3.49 | - | - | 63.49 ± 2.77 | 13.28 ± 0.92 |
| A3 | 13.42 ± 5.50 | - | - | 64.47 ± 6.51 | 14.23 ± 1.53 |
| F1 | 12.66 ± 4.80 | 36.21 ± 2.95 | 118.73 ± 5.44 | 53.38 ± 4.14 | 13.12 ± 1.97 |
| F2 | 13.73 ± 4.63 | 59.68 ± 2.72 * | 108.64 ± 8.84 * | 53.23 ± 2.65 | 8.97 ± 0.49 * |
| F3 | 12.81 ± 5.86 | 37.46 ± 1.41 | 112.38 ± 4.22 | 57.72 ± 4.33 | 11.24 ± 2.22 |
| F4 | 15.27 ± 5.76 | 54.30 ± 2.59 * | 108.59 ± 5.18 * | 56.55 ± 4.11 | 9.10 ± 1.48 * |
| F5 | 11.70 ± 4.81 | 39.89 ± 1.14 | 119.67 ± 3.41 | 53.35 ± 2.95 | 8.93 ± 1.16 * |
| F6 | 13.19 ± 4.01 | 58.39 ± 2.24 * | 116.78 ± 4.48 | 46.62 ± 3.84 * | 10.20 ± 0.81 |
| H1 | 10.53 ± 3.22 | - | - | 54.06 ± 5.77 | 7.54 ± 0.32 * |
| H2 | 14.00 ± 4.27 | - | - | 51.61 ± 4.69 | 11.87 ± 0.70 |
| H3 | 15.25 ± 5.12 | - | - | 52.03 ± 4.58 | 10.54 ± 2.28 |
| H4 | 12.23 ± 4.13 | 37.89 ± 2.39 | 113.77 ± 7.18 | 62.46 ± 4.56 | 9.89 ± 2.18 |
| H5 | 14.08 ± 3.96 | 37.41 ± 3.09 | 112.34 ± 9.28 | 59.43 ± 1.90 | 7.60 ± 2.79 |
| H6 | 15.64 ± 4.48 | 27.60 ± 2.84 * | 138.00 ± 14.21 * | 60.05 ± 1.18 | 13.44 ± 0.35 * |
| Formulation | Zero-Order Kinetics | First-Order Kinetics | Higuchi Model | Hixson–Crowell Model | Korsmeyer–Peppas Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K | R2 | K | R2 | K | R2 | K | R2 | K | n | |
| 0.1 M HCl (pH 1.2) | |||||||||||
| F1 | 0.91 | 2.25 | 0.98 | 0.04 | 0.99 | 15.42 | 0.98 | 113.48 | 0.99 | 0.54 | 0.47 |
| F2 | 0.88 | 2.22 | 0.97 | 0.04 | 0.99 | 17.43 | 0.98 | 115.33 | 0.99 | 0.55 | 0.49 |
| F3 | 0.88 | 2.15 | 0.96 | 0.04 | 0.99 | 16.68 | 0.98 | 118.49 | 0.99 | 0.55 | 0.48 |
| F4 | 0.88 | 2.37 | 0.98 | 0.05 | 0.99 | 18.31 | 0.98 | 107.89 | 0.99 | 0.56 | 0.49 |
| F5 | 0.90 | 1.93 | 0.96 | 0.03 | 0.99 | 13.99 | 0.99 | 130.85 | 0.99 | 0.54 | 0.50 |
| F6 | 0.91 | 2.72 | 0.99 | 0.07 | 0.99 | 19.23 | 0.97 | 91.96 | 0.99 | 0.59 | 0.53 |
| H4 | 0.35 | 1.52 | 0.47 | 0.04 | 0.55 | 11.32 | 0.98 | 104.69 | 0.75 | 0.51 | 0.34 |
| H5 | 0.24 | 1.73 | 0.37 | 0.03 | 0.43 | 13.77 | 0.95 | 44.00 | 0.66 | 0.54 | 0.36 |
| H6 | 0.89 | 24.06 | 0.99 | 1.32 | 0.93 | 61.39 | 0.97 | 4.66 | 0.93 | 0.73 | 0.57 |
| SVF (pH 4.2) | |||||||||||
| F1 | 0.55 | 1.63 | 0.88 | 0.10 | 0.69 | 16.58 | 0.76 | 10.84 | 0.60 | 0.20 | 0.14 |
| F2 | 0.56 | 1.52 | 0.99 | 0.18 | 0.70 | 15.51 | 0.77 | 8.92 | 0.58 | 0.20 | 0.12 |
| F3 | 0.62 | 1.85 | 0.99 | 0.15 | 0.76 | 18.59 | 0.89 | 29.87 | 0.61 | 0.18 | 0.17 |
| F4 | 0.45 | 1.42 | 0.98 | 0.27 | 0.60 | 14.84 | 0.87 | 17.76 | 0.51 | 0.21 | 0.12 |
| F5 | 0.64 | 1.75 | 0.86 | 0.07 | 0.76 | 17.60 | 0.96 | 60.44 | 0.64 | 0.18 | 0.17 |
| F6 | 0.88 | 4.92 | 0.85 | 0.43 | 0.94 | 34.02 | 0.86 | 25.76 | 0.86 | 0.22 | 0.27 |
| H4 | 0.95 | 3.37 | 0.94 | 0.26 | 0.99 | 22.90 | 0.73 | 7.06 | 0.99 | 0.61 | 0.51 |
| H5 | 0.99 | 5.01 | 0.89 | 0.21 | 0.99 | 33.64 | 0.93 | 49.65 | 0.99 | 1.10 | 1.06 |
| H6 | 0.92 | 2.14 | 0.93 | 0.11 | 0.97 | 20.01 | 0.97 | 2.45 | 0.95 | 0.82 | 0.88 |
| Formulation | ALG Concentration (w/v%) | HPMC Concentration (w/v%) | ALG:POS Ratio |
|---|---|---|---|
| A1 | 0.5 | - | - |
| A2 | 1 | - | - |
| A3 | 1.5 | - | - |
| F1 | 0.5 | - | 1:0.5 |
| F2 | 0.5 | - | 1:1 |
| F3 | 1 | - | 1:0.5 |
| F4 | 1 | - | 1:1 |
| F5 | 1.5 | - | 1:0.5 |
| F6 | 1.5 | - | 1:1 |
| H1 | 1 | 1 | - |
| H2 | 1 | 3 | - |
| H3 | 1 | 5 | - |
| H4 | 1 | 1 | 1:1 |
| H5 | 1 | 3 | 1:1 |
| H6 | 1 | 5 | 1:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, K.; Szekalska, M.; Basa, A.; Winnicka, K. The Impact of Hypromellose on Pharmaceutical Properties of Alginate Microparticles as Novel Drug Carriers for Posaconazole. Int. J. Mol. Sci. 2023, 24, 10793. https://doi.org/10.3390/ijms241310793
Kruk K, Szekalska M, Basa A, Winnicka K. The Impact of Hypromellose on Pharmaceutical Properties of Alginate Microparticles as Novel Drug Carriers for Posaconazole. International Journal of Molecular Sciences. 2023; 24(13):10793. https://doi.org/10.3390/ijms241310793
Chicago/Turabian StyleKruk, Katarzyna, Marta Szekalska, Anna Basa, and Katarzyna Winnicka. 2023. "The Impact of Hypromellose on Pharmaceutical Properties of Alginate Microparticles as Novel Drug Carriers for Posaconazole" International Journal of Molecular Sciences 24, no. 13: 10793. https://doi.org/10.3390/ijms241310793







