Anti-Fibrotic Effects of RF Electric Currents
Abstract
:1. Introduction
2. Results
2.1. Extracellular Matrix Production
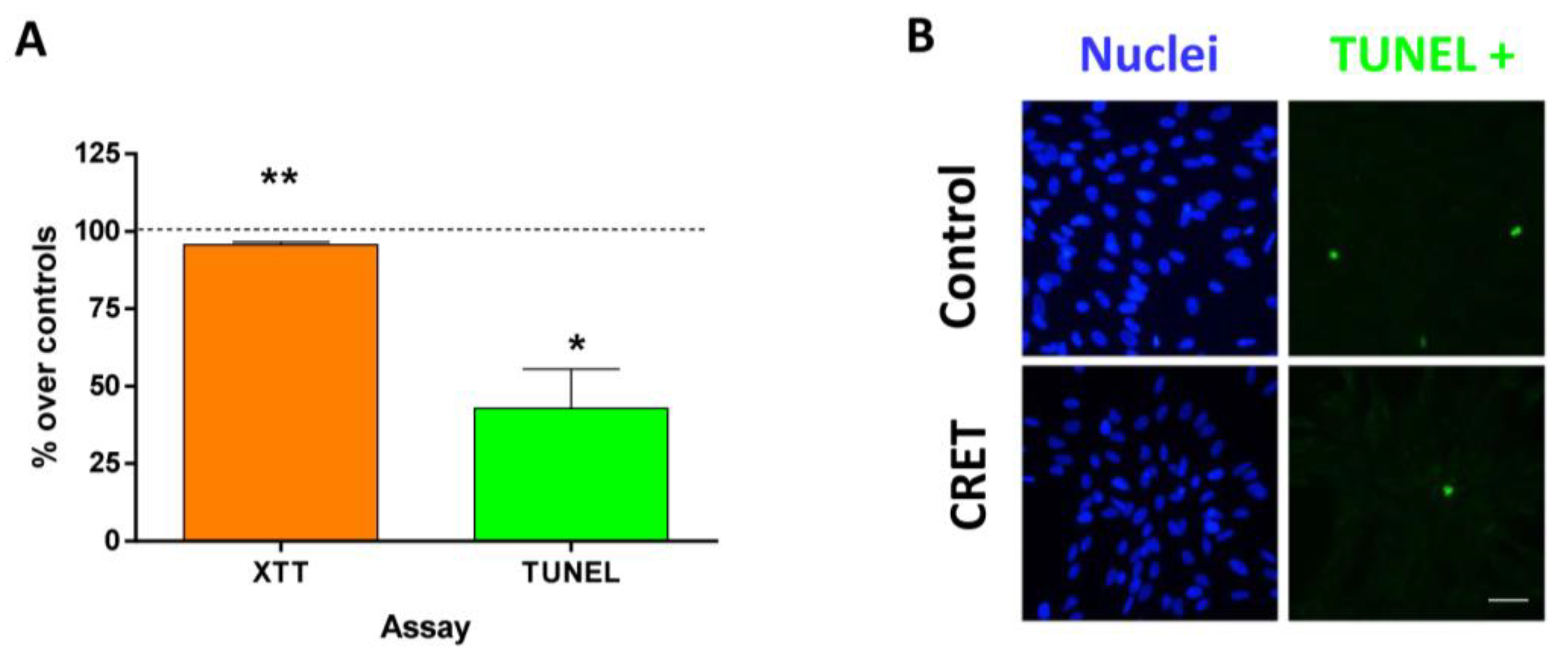
2.2. Cell Proliferation and Death
2.3. Cell Migration
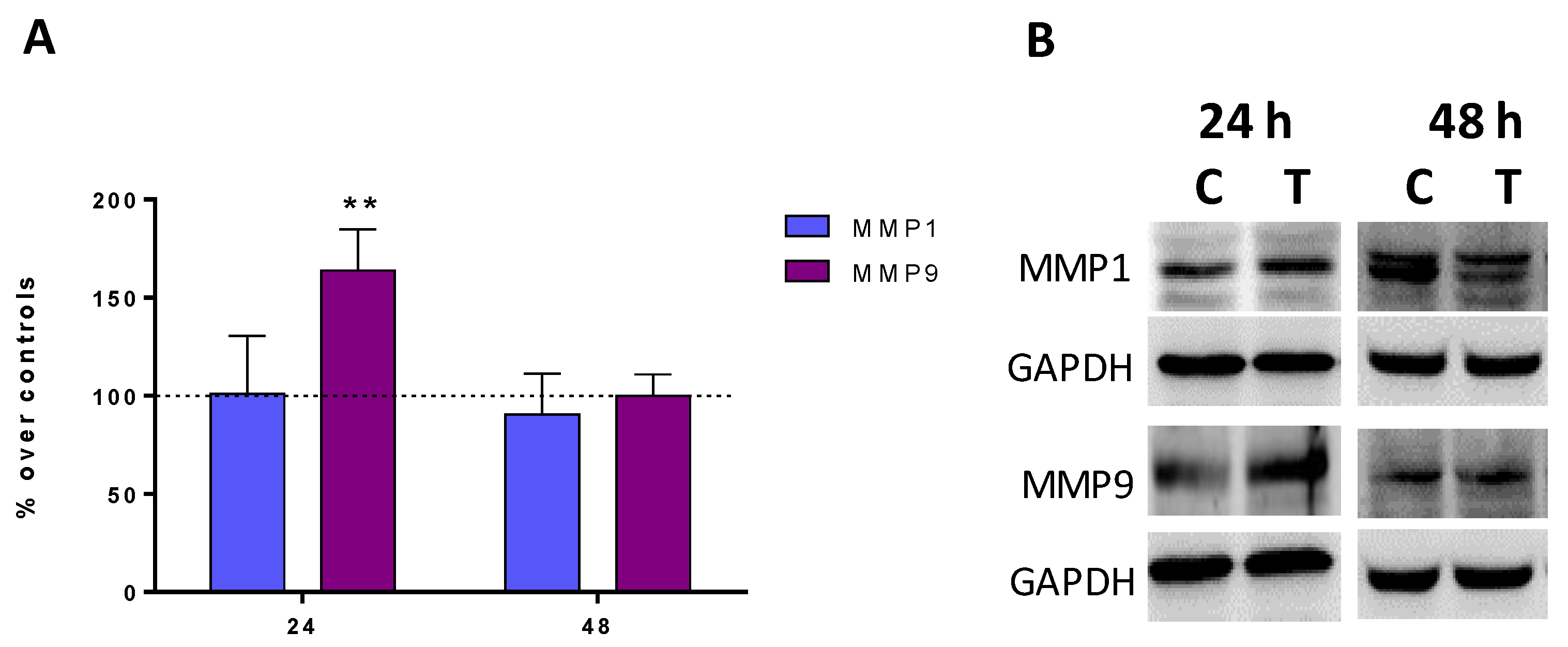
2.4. Expression of Metalloproteinases MMP1 and MMP9
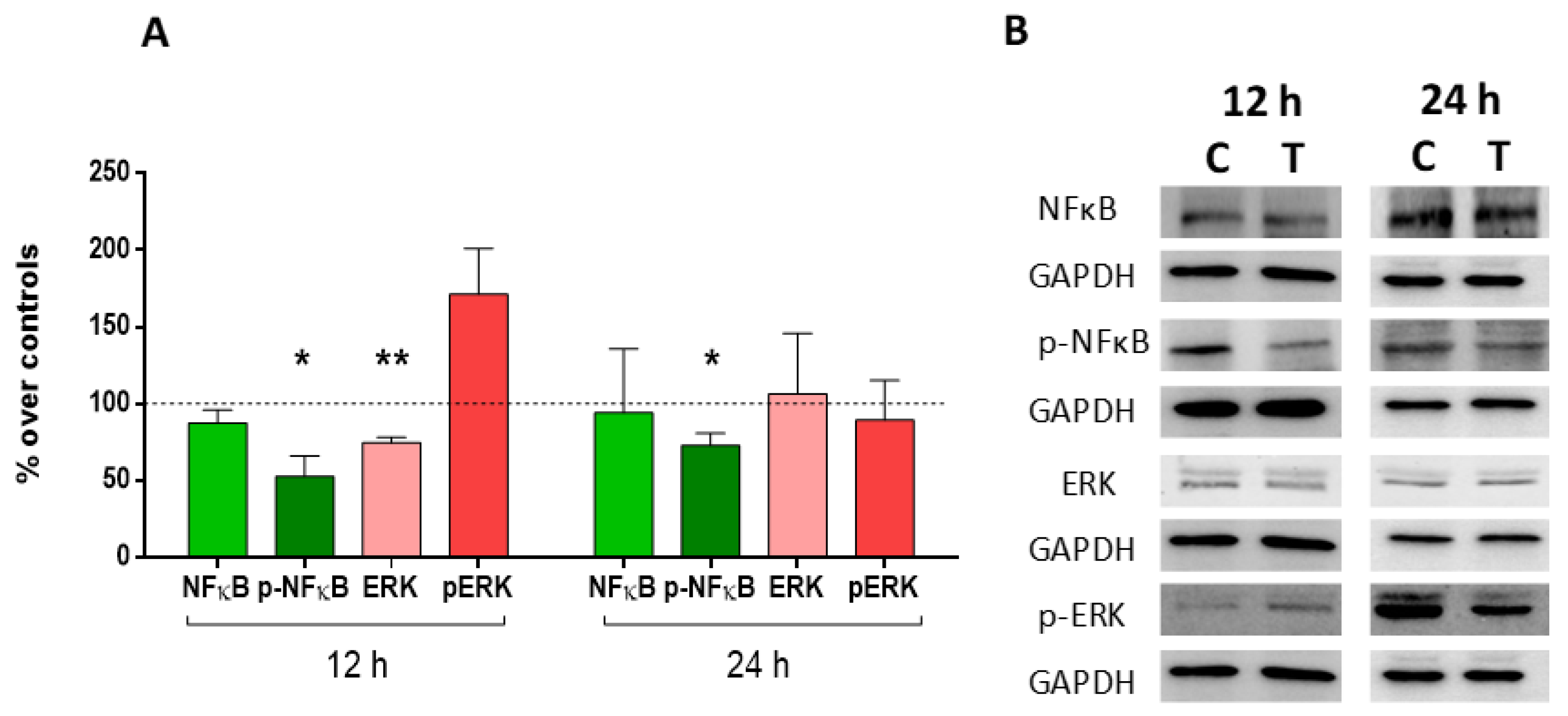
2.5. Expression and Activation of MAP-Kinase ERK1/2 and Nuclear Factor (NF)-Kappa B p65
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Electric Treatment
4.3. XTT Proliferation Assay
4.4. TUNEL Assay
4.5. Wound Assay
4.6. Immunofluorescence for α-SMA, Collagen Type I and Collagen Type III
4.7. Immunoblot for α-SMA, MMP1, MMP9, ERK, P-ERK, NF-κB and p-NF-κB
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, P.; Nunan, R. Cellular and Molecular Mechanisms of Repair in Acute and Chronic Wound Healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Toledano-Macías, E.; Naranjo, A.; de Andrés-Zamora, M.; Úbeda, A. In Vitro Stimulation with Radiofrequency Currents Promotes Proliferation and Migration in Human Keratinocytes and Fibroblasts. Electromagn. Biol. Med. 2021, 40, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guarino, M.; Bacci, S.; Pérez González, L.A.; Bermejo-Martínez, M.; Cecilia-Matilla, A.; Hernández-Bule, M.L. The Role of Physical Therapies in Wound Healing and Assisted Scarring. Int. J. Mol. Sci. 2023, 24, 7487. [Google Scholar] [CrossRef]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-ΚB Signaling Is Key in the Wound Healing Processes of Silk Fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jang, Y.J. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int. J. Mol. Sci. 2018, 19, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.P.; Marttala, J.; Macarak, E.; Rosenbloom, J.; Uitto, J. Keloids: The Paradigm of Skin Fibrosis—Pathomechanisms and Treatment. Matrix Biol. 2016, 51, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Vinaik, R.; Barayan, D.; Auger, C.; Abdullahi, A.; Jeschke, M.G. Regulation of Glycolysis and the Warburg Effect in Wound Healing. JCI Insight 2020, 5, e138949. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Zhao, W.-Y.; Cao, Y.; Liu, Y.-Q.; Sun, Q.; Shi, P.; Cai, J.-Q.; Shen, X.Z.; Tan, W.-Q. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front. Immunol. 2020, 11, 603187. [Google Scholar] [CrossRef]
- Lingzhi, Z.; Meirong, L.; Xiaobing, F. Biological Approaches for Hypertrophic Scars. Int. Wound J. 2020, 17, 405–418. [Google Scholar] [CrossRef]
- Jiang, D.; Rinkevich, Y. Scars or Regeneration?—Dermal Fibroblasts as Drivers of Diverse Skin Wound Responses. Int. J. Mol. Sci. 2020, 21, 617. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-S.; Yosipovitch, G.; Chan, Y.-H.; Goh, C.-L. Pruritus, Pain, and Small Nerve Fiber Function in Keloids: A Controlled Study. J. Am. Acad. Dermatol. 2004, 51, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Goldberg, D.J. Update on the Treatment of Scars. J. Drugs Dermatol. 2019, 18, 550–555. [Google Scholar] [PubMed]
- Arno, A.I.; Gauglitz, G.G.; Barret, J.P.; Jeschke, M.G. Up-to-Date Approach to Manage Keloids and Hypertrophic Scars: A Useful Guide. Burns 2014, 40, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, N.M.; Melo, P.R.; Crema, V.O.; Mendonça, A.C. Effects of Radiofrequency Procedure on Hypertrophic Scar Due to Burns. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Meshkinpour, A.; Ghasri, P.; Pope, K.; Lyubovitsky, J.G.; Risteli, J.; Krasieva, T.B.; Kelly, K.M. Treatment of Hypertrophic Scars and Keloids with a Radiofrequency Device: A Study of Collagen Effects. Lasers Surg. Med. 2005, 37, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedr, M.M.; Mahmoud, W.H.; Sallam, F.A.; Elmelegy, N. Comparison of Nd:YAG Laser and Combined Intense Pulsed Light and Radiofrequency in the Treatment of Hypertrophic Scars: A Prospective Clinico-Histopathological Study. Ann. Plast. Surg. 2020, 84, 518–524. [Google Scholar] [CrossRef]
- Trelles, M.A.; Martínez-Carpio, P.A. Clinical and Histological Results in the Treatment of Atrophic and Hypertrophic Scars Using a Combined Method of Radiofrequency, Ultrasound, and Transepidermal Drug Delivery. Int. J. Dermatol. 2016, 55, 926–933. [Google Scholar] [CrossRef]
- Nicoletti, G.; Perugini, P.; Bellino, S.; Capra, P.; Malovini, A.; Jaber, O.; Tresoldi, M.; Faga, A. Scar Remodeling with the Association of Monopolar Capacitive Radiofrequency, Electric Stimulation, and Negative Pressure. Photomed. Laser Surg. 2017, 35, 246–258. [Google Scholar] [CrossRef] [Green Version]
- Meyer, P.F.; de Oliveira, P.; Silva, F.K.B.A.; da Costa, A.C.S.; Pereira, C.R.A.; Casenave, S.; Valentim Silva, R.M.; Araújo-Neto, L.G.; Santos-Filho, S.D.; Aizamaque, E.; et al. Radiofrequency Treatment Induces Fibroblast Growth Factor 2 Expression and Subsequently Promotes Neocollagenesis and Neoangiogenesis in the Skin Tissue. Lasers Med. Sci. 2017, 32, 1727–1736. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Paíno, C.L.; Trillo, M.Á.; Úbeda, A. Electric Stimulation at 448 KHz Promotes Proliferation of Human Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2014, 34, 1741–1755. [Google Scholar] [CrossRef]
- Hernandez Bule, M.L.; Angeles Trillo, M.; Martinez Garcia, M.A.; Abilahoud, C.; Ubeda, A. Chondrogenic Differentiation of Adipose-Derived Stem Cells by Radiofrequency Electric Stimulation. J. Stem Cell Res. Ther. 2017, 7, 12. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Emerging and Novel Therapies for Keloids: A Compendious Review. Sultan Qaboos Univ. Med. J. 2021, 21, e22–e33. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.-Q.; Wang, X.-F.; Zhao, W.-Y.; Ding, S.-L.; Shi, B.-H.; Xia, Y.; Yang, H.; Wu, L.-H.; Li, C.-Y.; Tan, W.-Q. Angiotensin-Converting Enzyme Inhibitor Reduces Scar Formation by Inhibiting Both Canonical and Noncanonical TGF-Β1 Pathways. Sci. Rep. 2018, 8, 3332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-S.; Wu, P.-H.; Fang, A.-H.; Lan, C.-C.E. FK506 Inhibits the Enhancing Effects of Transforming Growth Factor (TGF)-Β1 on Collagen Expression and TGF-β/Smad Signalling in Keloid Fibroblasts: Implication for New Therapeutic Approach. Br. J. Dermatol. 2012, 167, 532–541. [Google Scholar] [CrossRef]
- Wang, C.-J.; Ko, J.-Y.; Chou, W.-Y.; Cheng, J.-H.; Kuo, Y.-R. Extracorporeal Shockwave Therapy for Treatment of Keloid Scars. Wound Repair Regen. 2018, 26, 69–76. [Google Scholar] [CrossRef]
- Cui, H.S.; Hong, A.R.; Kim, J.-B.; Yu, J.H.; Cho, Y.S.; Joo, S.Y.; Seo, C.H. Extracorporeal Shock Wave Therapy Alters the Expression of Fibrosis-Related Molecules in Fibroblast Derived from Human Hypertrophic Scar. Int. J. Mol. Sci. 2018, 19, 124. [Google Scholar] [CrossRef] [Green Version]
- Soares-Lopes, L.R.; Soares-Lopes, I.M.; Filho, L.L.; Alencar, A.P.; da Silva, B.B. Morphological and Morphometric Analysis of the Effects of Intralesional Tamoxifen on Keloids. Exp. Biol. Med. 2017, 242, 926–929. [Google Scholar] [CrossRef] [Green Version]
- Aoki, M.; Miyake, K.; Ogawa, R.; Dohi, T.; Akaishi, S.; Hyakusoku, H.; Shimada, T. SiRNA Knockdown of Tissue Inhibitor of Metalloproteinase-1 in Keloid Fibroblasts Leads to Degradation of Collagen Type I. J. Investig. Dermatol. 2014, 134, 818–826. [Google Scholar] [CrossRef] [Green Version]
- Perry, D.; Colthurst, J.; Giddings, P.; McGrouther, D.A.; Morris, J.; Bayat, A. Treatment of Symptomatic Abnormal Skin Scars with Electrical Stimulation. J. Wound Care 2010, 19, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, G.; Cornaglia, A.I.; Faga, A.; Scevola, S. The Biological Effects of Quadripolar Radiofrequency Sequential Application: A Human Experimental Study. Photomed. Laser Surg. 2014, 32, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.-M.; Chen, Y.-S.; Harn, H.-J. The Versatile Role of Matrix Metalloproteinase for the Diverse Results of Fibrosis Treatment. Molecules 2019, 24, 4188. [Google Scholar] [CrossRef] [Green Version]
- Eto, H.; Suga, H.; Aoi, N.; Kato, H.; Doi, K.; Kuno, S.; Tabata, Y.; Yoshimura, K. Therapeutic Potential of Fibroblast Growth Factor-2 for Hypertrophic Scars: Upregulation of MMP-1 and HGF Expression. Lab. Investig. 2012, 92, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ma, Y.; Gao, Z.; Yang, J. Human Adipose-Derived Stem Cells Inhibit Bioactivity of Keloid Fibroblasts. Stem Cell Res. Ther. 2018, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Kuan-hong, S.; Hong-gang, W. Pressure Therapy Upregulates Matrix Metalloproteinase Expression and Downregulates Collagen Expression in Hypertrophic Scar Tissue. Chin. Med. J. 2013, 126, 3321–3324. [Google Scholar]
- Austin, E.; Koo, E.; Jagdeo, J. The Cellular Response of Keloids and Hypertrophic Scars to Botulinum Toxin A: A Comprehensive Literature Review. Dermatol. Surg. 2018, 44, 149–157. [Google Scholar] [CrossRef]
- Sasaki, T.; Holeyfield, K.C.; Uitto, J. Doxorubicin-Induced Inhibition of Prolyl Hydroxylation during Collagen Biosynthesis in Human Skin Fibroblast Cultures. Relevance to Imparied Wound Healing. J. Clin. Investig. 1987, 80, 1735–1741. [Google Scholar] [CrossRef]
- Yeo, D.C.; Balmayor, E.R.; Schantz, J.-T.; Xu, C. Microneedle Physical Contact as a Therapeutic for Abnormal Scars. Eur. J. Med. Res. 2017, 22, 28. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Huang, R.-L.; Zheng, Y.; Liu, M.; Huo, R. Bone Marrow Derived Mesenchymal Stem Cells Inhibit the Proliferative and Profibrotic Phenotype of Hypertrophic Scar Fibroblasts and Keloid Fibroblasts through Paracrine Signaling. J. Dermatol. Sci. 2016, 83, 95–105. [Google Scholar] [CrossRef]
- Lin, W.-C.; Liou, S.-H.; Kotsuchibashi, Y. Development and Characterisation of the Imiquimod Poly(2-(2-Methoxyethoxy)Ethyl Methacrylate) Hydrogel Dressing for Keloid Therapy. Polymers 2017, 9, 579. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Liu, X.; Jin, Z. Adipose-derived Mesenchymal Stem Cells-sourced Exosomal microRNA-7846-3p Suppresses Proliferation and Pro-angiogenic Role of Keloid Fibroblasts by Suppressing Neuropilin 2. J. Cosmet. Dermatol. 2023, ahead of print. [CrossRef]
- Linge, C.; Richardson, J.; Vigor, C.; Clayton, E.; Hardas, B.; Rolfe, K.J. Hypertrophic Scar Cells Fail to Undergo a Form of Apoptosis Specific to Contractile Collagen—The Role of Tissue Transglutaminase. J. Investig. Dermatol. 2005, 125, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, A.; Syed, F.; McGrouther, D.A.; Colthurst, J.; Paus, R.; Bayat, A. A Novel in Vitro Assay for Electrophysiological Research on Human Skin Fibroblasts: Degenerate Electrical Waves Downregulate Collagen I Expression in Keloid Fibroblasts. Exp. Dermatol. 2011, 20, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the Skin1. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine Signaling in the Skin with a Special Focus on the Epidermal Neuropeptides. Am. J. Physiol.-Cell Physiol. 2022, 323, C1757–C1776. [Google Scholar] [CrossRef] [PubMed]
- Zbytek, B.; Pfeffer, L.; Slominski, A. Corticotropin-Releasing Hormone Stimulates NF-KappaB in Human Epidermal Keratinocytes. J. Endocrinol. 2004, 181, R1–R7. [Google Scholar] [CrossRef] [Green Version]
- Khodeneva, N.; Sugimoto, M.A.; Davan-Wetton, C.S.A.; Montero-Melendez, T. Melanocortin Therapies to Resolve Fibroblast-Mediated Diseases. Front. Immunol. 2023, 13, 1084394. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Trillo, M.A.; Cid, M.A.; Leal, J.; Ubeda, A. In Vitro Exposure to 0.57-MHz Electric Currents Exerts Cytostatic Effects in HepG2 Human Hepatocarcinoma Cells. Int. J. Oncol. 2007, 30, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Bule, M.L.; Trillo, M.Á.; Úbeda, A. Molecular Mechanisms Underlying Antiproliferative and Differentiating Responses of Hepatocarcinoma Cells to Subthermal Electric Stimulation. PLoS ONE 2014, 9, e84636. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Martínez-Botas, J.; Trillo, M.Á.; Paíno, C.L.; Úbeda, A. Antiadipogenic Effects of Subthermal Electric Stimulation at 448 KHz on Differentiating Human Mesenchymal Stem Cells. Mol. Med. Rep. 2016, 13, 3895–3903. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Bule, M.L.; Toledano-Macías, E.; Pérez-González, L.A.; Martínez-Pascual, M.A.; Fernández-Guarino, M. Anti-Fibrotic Effects of RF Electric Currents. Int. J. Mol. Sci. 2023, 24, 10986. https://doi.org/10.3390/ijms241310986
Hernández-Bule ML, Toledano-Macías E, Pérez-González LA, Martínez-Pascual MA, Fernández-Guarino M. Anti-Fibrotic Effects of RF Electric Currents. International Journal of Molecular Sciences. 2023; 24(13):10986. https://doi.org/10.3390/ijms241310986
Chicago/Turabian StyleHernández-Bule, María Luisa, Elena Toledano-Macías, Luis Alfonso Pérez-González, María Antonia Martínez-Pascual, and Montserrat Fernández-Guarino. 2023. "Anti-Fibrotic Effects of RF Electric Currents" International Journal of Molecular Sciences 24, no. 13: 10986. https://doi.org/10.3390/ijms241310986




