Antimicrobial Effect of Ocimum gratissimum L. Essential Oil on Shewanella putrefaciens: Insights Based on the Cell Membrane and External Structure
Abstract
1. Introduction
2. Results and Discussion
2.1. Major Compounds Detected in OGEO
2.2. Minimum Bactericidal Concentration (MBC) and Minimum Inhibitory Concentration (MIC) Value of OGEO
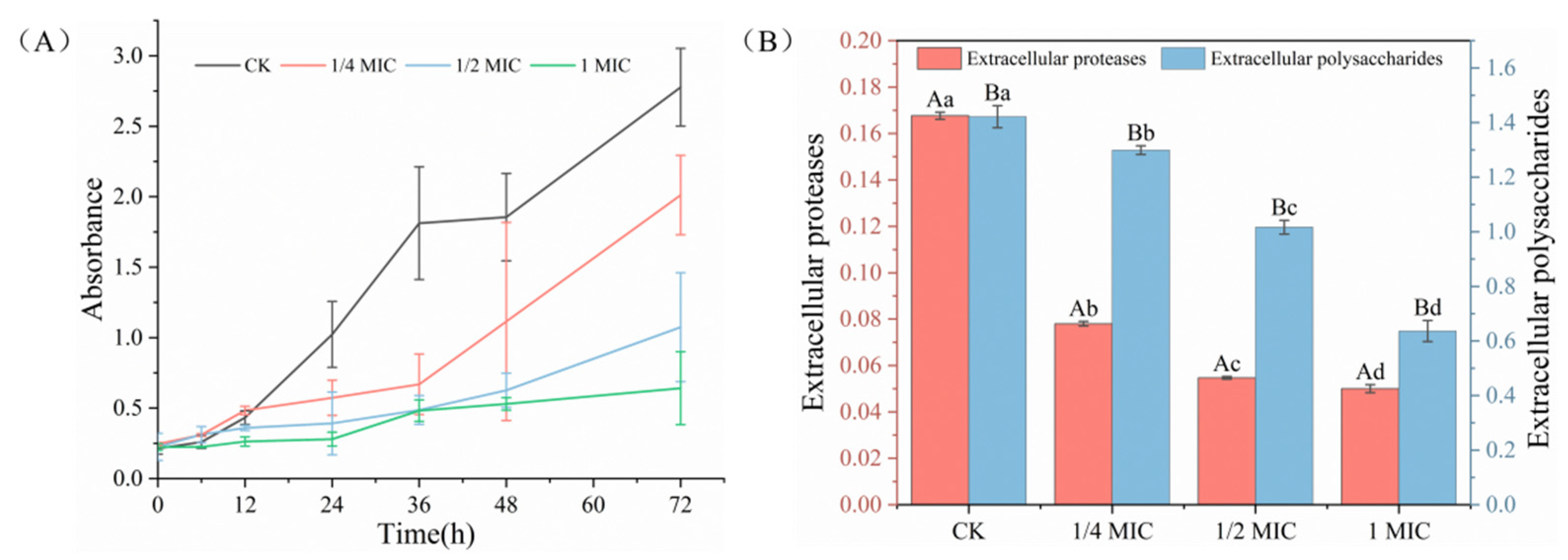
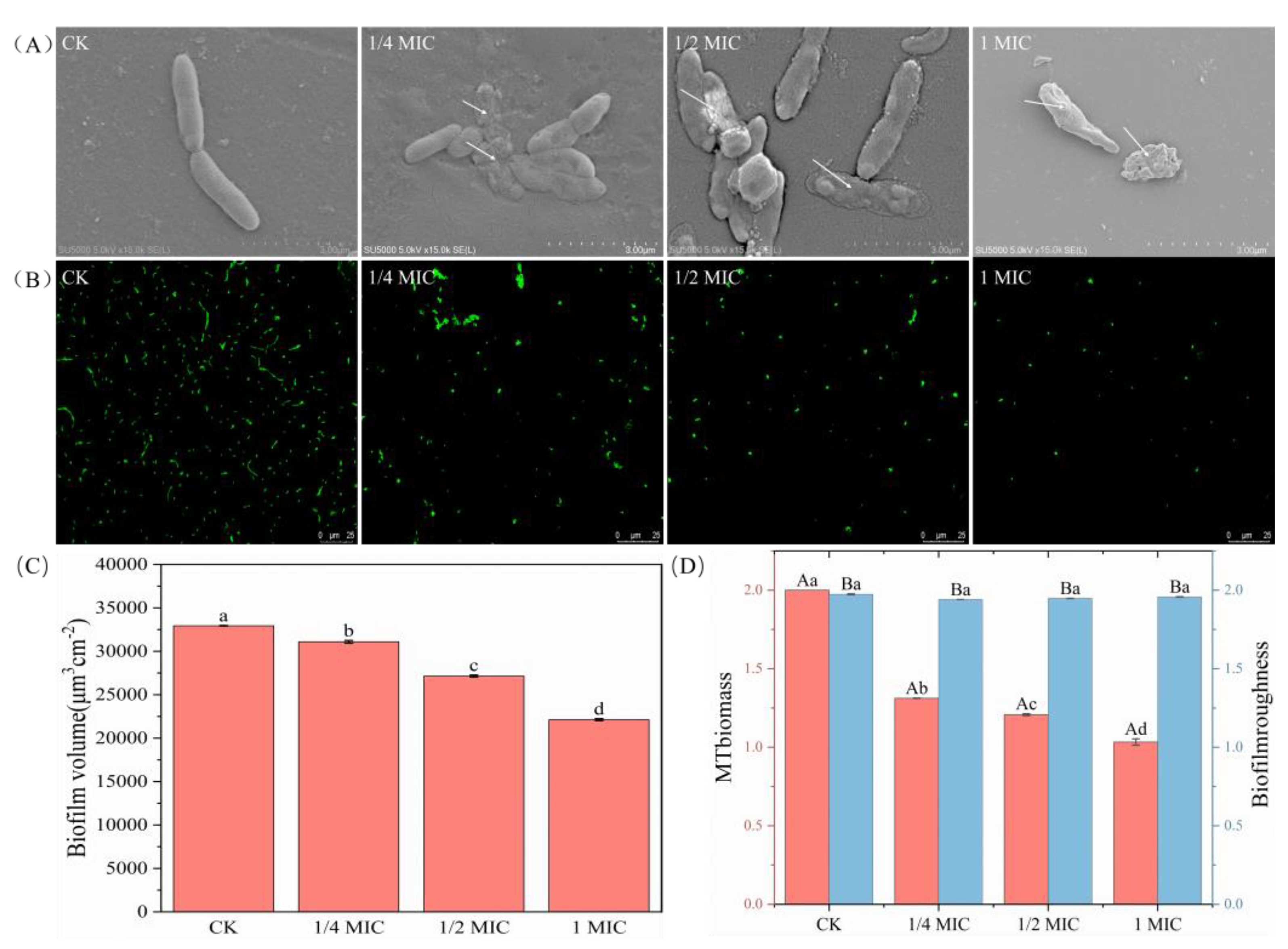
2.3. Inhibition of Biofilm Formation Ability
2.4. Inhibition of Extracellular Polymeric Substances
2.5. Inhibition of Motility
2.6. Hemolytic Activity Analysis
2.7. ATPase Activity Analysis
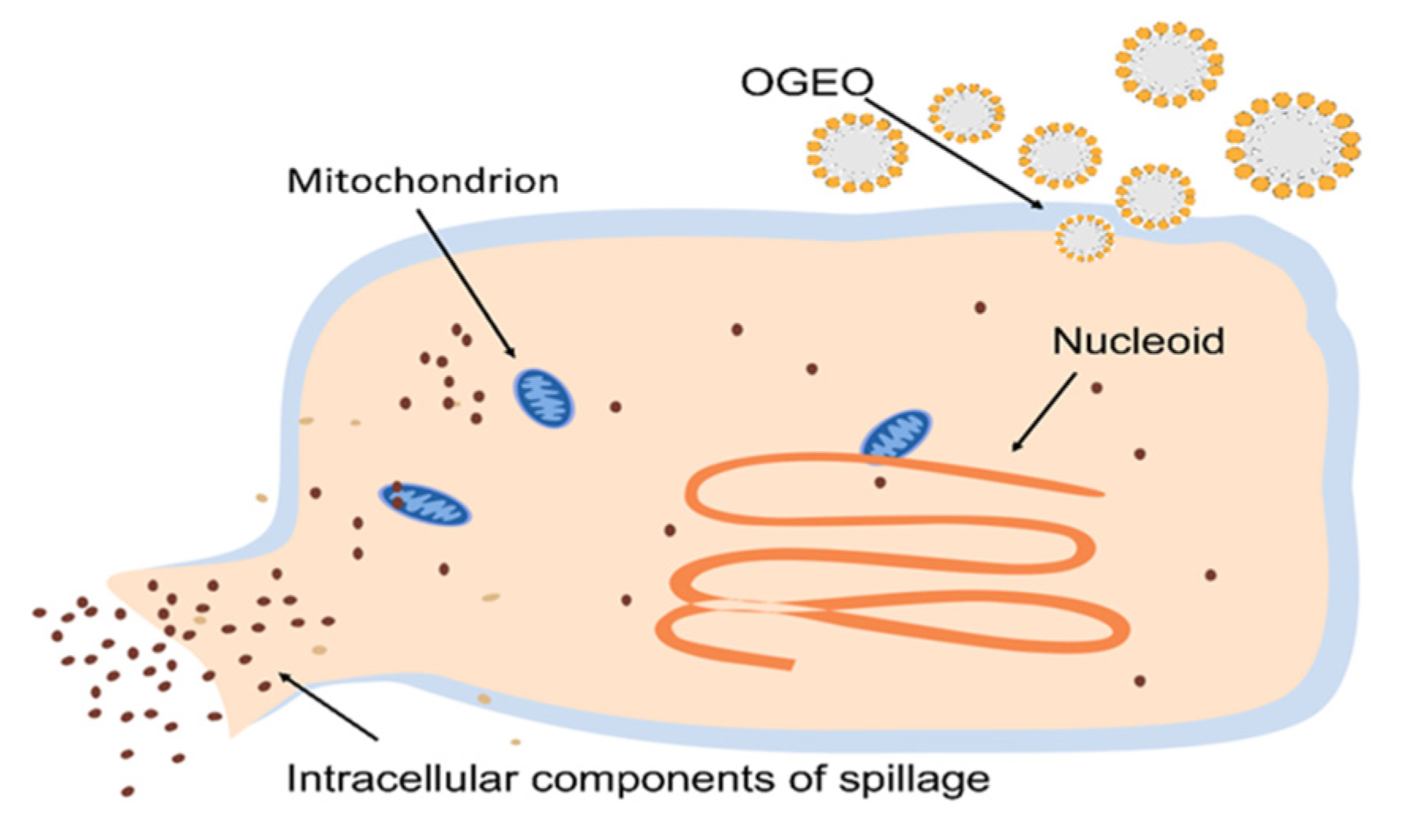
2.8. SEM and CLSM
2.9. FTIR Analysis
2.10. XTT
3. Materials and Methods
3.1. GC-MS Analysis
3.2. Bacterial Culture
3.3. MIC and MBC Measurements
3.4. Quantification of Biofilms Using Crystal Violet Staining
3.5. Extracellular Polysaccharide (EPS) Measurements
3.6. Extracellular Protease (EP) Production Measurements
3.7. Swimming and Swarming Motility Analysis
3.8. Hemolytic Activity Test
3.9. Determination of Extracellular ATPase Concentration
3.10. Scanning Electron Microscopy (SEM) Analysis
3.11. Confocal Laser Scanning Microscopy (CLSM) Analysis
3.12. XTT Content Measurements
3.13. Fourier Transform Infrared (FTIR) Spectroscopy
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bono, G.; Okpala, C.O.R.; Vitale, S.; Ferrantelli, V.; Noto, A.D.; Costa, A.; Di Bella, C.; Monaco, D.L. Effects of different ozonized slurry-ice treatments and superchilling storage (-1 degrees C) on microbial spoilage of two important pelagic fish species. Food Sci. Nutr. 2017, 5, 1049–1056. [Google Scholar] [CrossRef]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef]
- Wright, M.H.; Matthews, B.; Arnold, M.S.J.; Greene, A.C.; Cock, I.E. The prevention of fish spoilage by high antioxidant Australian culinary plants: Shewanella putrefaciens growth inhibition. Int. J. Food Sci. Technol. 2016, 51, 801–813. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, X.; Liu, J.; Zhu, J.; Chen, J. Interactions between fish isolates Pseudomonas fluorescens and Staphylococcus aureus in dual-species biofilms and sensitivity to carvacrol. Food Microbiol. 2020, 91, 103506. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Arsene, M.M.J.; Jorelle, A.B.; Sarra, S.; Viktorovna, P.I.; Davares, A.K.; Ingrid, N.K.; Steve, A.A.; Andreevna, S.L.; Vyacheslavovna, Y.N.; Carime, B.Z. Short review on the potential alternatives to antibiotics in the era of antibiotic resistance. J. Appl. Pharm. Sci. 2021, 12, 29–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, J.; Xie, Y.F.; Guo, Y.H.; Cheng, Y.L.; Qian, H.; Yao, W.R. Essential oil components inhibit biofilm formation in Erwinia carotovora and Pseudomonas fluorescens via anti-quorum sensing activity. LWT-Food Sci. Technol. 2018, 92, 133–139. [Google Scholar] [CrossRef]
- Melo, R.S.; Albuquerque Azevedo, Á.M.; Gomes Pereira, A.M.; Rocha, R.R.; Bastos Cavalcante, R.M.; Carneiro Matos, M.N.; Ribeiro Lopes, P.H.; Gomes, G.A.; Soares Rodrigues, T.H.; Santos, H.S.d. Chemical composition and antimicrobial effectiveness of Ocimum gratissimum L. essential oil against multidrug-resistant isolates of Staphylococcus aureus and Escherichia coli. Molecules 2019, 24, 3864. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, S.K.; Pandiyan, R.; Natesan, S.; Chindam, S.; Gouti, A.K.; Sugumaran, A. Fabrication of basil oil Nanoemulsion loaded gellan gum hydrogel—Evaluation of its antibacterial and anti-biofilm potential. J. Drug Deliv. Sci. Technol. 2022, 68, 103129. [Google Scholar] [CrossRef]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of bactericidal action of Eugenol against Escherichia coli. J. Herb. Med. 2021, 26, 100406. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Sorensen, A.; Setzer, W.N. Volatile Constituents and Antimicrobial Activity of Naio (Myoporum Sandwicense A. Gray), a Native Hawaiian Tree. Compounds 2023, 3, 142–152. [Google Scholar] [CrossRef]
- Bassole, I.H.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebie, R.C.; Dicko, M.H. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef]
- Li, Q.X.; Chang, C.L. Chapter 25—Basil (Ocimum basilicum L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 231–238. [Google Scholar] [CrossRef]
- Fandohan, P.; Gnonlonfin, B.; Laleye, A.; Gbenou, J.D.; Darboux, R.; Moudachirou, M. Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem. Toxicol. 2008, 46, 2493–2497. [Google Scholar] [CrossRef]
- Torpol, K.; Wiriyacharee, P.; Sriwattana, S.; Sangsuwan, J.; Prinyawiwatkul, W. Antimicrobia activity of garlic (Allium sativum L.) and holy basil (Ocimum sanctum L.) essential oils applied by liquid vs. vapour phases. Int. J. Food Sci. Technol. 2018, 53, 2119–2128. [Google Scholar] [CrossRef]
- Srivastava, S.; Lal, R.K.; Maurya, R.; Mishra, A.; Yadav, A.K.; Pandey, G.; Rout, P.K.; Chanotiya, C.S. Chemical diversity of essential oil among basil genotypes (Ocimum viride Willd.) across the years. Ind. Crops Prod. 2021, 173, 114153. [Google Scholar] [CrossRef]
- Joshi, R. Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J. Pharm. Sci. 2013, 75, 457. [Google Scholar] [CrossRef]
- Tangpao, T.; Chung, H.-H.; Sommano, S.R. Aromatic profiles of essential oils from five commonly used Thai basils. Foods 2018, 7, 175. [Google Scholar] [CrossRef]
- Matasyoh, L.G.; Matasyoh, J.C.; Wachira, F.N.; Kinyua, M.G.; Muigai, A.W.T.; Mukiama, T.K. Antimicrobial activity of essential oils of Ocimum gratissimum L. from different populations of Kenya. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 187–193. [Google Scholar] [CrossRef]
- Saha, S.; Dhar, T.; Sengupta, C.; Ghosh, P. Biological activities of essential oils and methanol extracts of five Ocimum species against pathogenic bacteria. Czech J. Food Sci. 2013, 31, 195–202. [Google Scholar] [CrossRef]
- Mutlu-ingok, A.; Firtin, B.; Karbancioglu-guler, F. Chemical composition and comparative antibacterial properties of basil essential oil against clinical and standard strains of campylobacter spp. ACTA Pharm. Sci. 2019, 57, 183. [Google Scholar] [CrossRef]
- Mihajilov-Krstev, T.; Radnović, D.; Kitić, D.; Zlatković, B.; Ristić, M.; Branković, S. Chemical composition and antimicrobial activity of Satureja hortensis L. essential oil. Open Life Sci. 2009, 4, 411–416. [Google Scholar] [CrossRef]
- Mith, H.; Yayi-Ladékan, E.; Sika Kpoviessi, S.D.; Yaou Bokossa, I.; Moudachirou, M.; Daube, G.; Clinquart, A. Chemical Composition and Antimicrobial Activity of Essential Oils of Ocimum basilicum, Ocimum canum and Ocimum gratissimumin Function of Harvesting Time. J. Essent. Oil Bear. Plants 2016, 19, 1413–1425. [Google Scholar] [CrossRef]
- Pathania, R.; Kaushik, R.; Khan, M.A. Essential oil nanoemulsions and their antimicrobial and food applications. Curr. Res. Nutr. Food Sci. J. 2018, 6, 626–643. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.R. Microcolony and biofilm formation as a survival strategy for bacteria. J. Theor. Biol. 2008, 251, 24–34. [Google Scholar] [CrossRef]
- De Martino, L.; Amato, G.; Caputo, L.; Nazzaro, F.; Scognamiglio, M.R.; De Feo, V. Variations in composition and bioactivity of Ocimum basilicum cv ‘Aroma 2’ essential oils. Ind. Crops Prod. 2021, 172, 114068. [Google Scholar] [CrossRef]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef]
- Sahal, G.; Woerdenbag, H.J.; Hinrichs, W.L.J.; Visser, A.; Tepper, P.G.; Quax, W.J.; van der Mei, H.C.; Bilkay, I.S. Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J. Ethnopharmacol. 2020, 246, 112188. [Google Scholar] [CrossRef]
- Tribedi, P.; Sil, A.K. Cell surface hydrophobicity: A key component in the degradation of polyethylene succinate by Pseudomonas spp. AKS2. J. Appl. Microbiol. 2014, 116, 295–303. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, J.; Xie, Y.; Guo, Y.; Yu, H.; Cheng, Y.; Qian, H.; Shi, R.; Yao, W. Quorum-sensing inhibition by hexanal in biofilms formed by Erwinia carotovora and Pseudomonas fluorescens. LWT 2019, 109, 145–152. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Lu, H.; Zhu, J.; Kumar, V.; Liu, X. Transcriptomic analysis of the food spoilers Pseudomonas fluorescens reveals the antibiofilm of carvacrol by interference with intracellular signaling processes. Food Control 2021, 127, 108115. [Google Scholar] [CrossRef]
- Chimnoi, N.; Reuk-Ngam, N.; Chuysinuan, P.; Khlaychan, P.; Khunnawutmanotham, N.; Chokchaichamnankit, D.; Thamniyom, W.; Klayraung, S.; Mahidol, C.; Techasakul, S. Characterization of essential oil from Ocimum gratissimum leaves: Antibacterial and mode of action against selected gastroenteritis pathogens. Microb. Pathog. 2018, 118, 290–300. [Google Scholar] [CrossRef]
- Martínez, A.; Manrique-Moreno, M.; Klaiss-Luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of Essential Oils on Growth Inhibition, Biofilm Formation and Membrane Integrity of Escherichia coli and Staphylococcus aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef]
- Ahmed, S.O.; Zedan, H.H.; Ibrahim, Y.M. Quorum sensing inhibitory effect of bergamot oil and aspidosperma extract against Chromobacterium violaceum and Pseudomonas aeruginosa. Arch. Microbiol. 2021, 203, 4663–4675. [Google Scholar] [CrossRef]
- Vrenna, G.; Artini, M.; Ragno, R.; Relucenti, M.; Fiscarelli, E.V.; Tuccio Guarna Assanti, V.; Papa, R.; Selan, L. Anti-Virulence Properties of Coridothymus capitatus Essential Oil against Pseudomonas aeruginosa Clinical Isolates from Cystic Fibrosis Patients. Microorganisms 2021, 9, 2257. [Google Scholar] [CrossRef]
- Mehmood, T.; Afzal, A.; Anwar, F.; Iqbal, M.; Afzal, M.; Qadir, R. Variations in the Composition, Antibacterial and Haemolytic Activities of Peel Essential Oils from Unripe and Ripened Citrus limon (L.) Osbeck Fruit. J. Essent. Oil Bear. Plants 2019, 22, 159–168. [Google Scholar] [CrossRef]
- Pandey, A.; Naik, M.; Dubey, S.K. Hemolysin, Protease, and EPS Producing PathogenicAeromonas hydrophilaStrain An4 Shows Antibacterial Activity against Marine Bacterial Fish Pathogens. J. Mar. Biol. 2010, 2010, 563205. [Google Scholar] [CrossRef]
- Khashe, S.; Janda, J.M. Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens. J. Clin. Microbiol. 1998, 36, 783–787. [Google Scholar] [CrossRef]
- Soltani, M.; Ghodratnama, M.; Ebrahimzadeh-Mosavi, H.A.; Nikbakht-Brujeni, G.; Mohamadian, S.; Ghasemian, M. Shirazi thyme (Zataria multiflora Boiss) and Rosemary (Rosmarinus officinalis) essential oils repress expression of sagA, a streptolysin S-related gene in Streptococcus iniae. Aquaculture 2014, 430, 248–252. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Holley, R. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006, 111, 170–174. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mizan, M.F.R.; Ha, A.J.-w.; Park, S.H.; Ha, S.-D. Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020, 91, 103500. [Google Scholar] [CrossRef]
- Han, J.; Chen, F.; Gao, C.; Zhang, Y.; Tang, X. Environmental stability and curcumin release properties of Pickering emulsion stabilized by chitosan/gum arabic nanoparticles. Int. J. Biol. Macromol. 2020, 157, 202–211. [Google Scholar] [CrossRef]
- Almanaa, T.N.; Alharbi, N.S.; Ramachandran, G.; Kanisha Chelliah, C.; Rajivgandhi, G.; Manoharan, N.; Kadaikunnan, S.; Khaled, J.M.; Alanzi, K.F. Anti-biofilm effect of Nerium oleander essential oils against biofilm forming Pseudomonas aeruginosa on urinary tract infections. J. King Saud Univ. Sci. 2021, 33, 101340. [Google Scholar] [CrossRef]
- Yu, H.J.; Pei, J.X.; Qiu, W.Q.; Mei, J.; Xie, J. The Antimicrobial Effect of Melissa officinalis L. Essential Oil on Vibrio parahaemolyticus: Insights Based on the Cell Membrane and External Structure. Front. Microbiol. 2022, 13, 812792. [Google Scholar] [CrossRef]
- Arsene, M.M.J.; Viktorovna, P.I.; Alla, M.V.; Mariya, M.A.; Sergei, G.V.; Cesar, E.; Davares, A.K.L.; Parfait, K.; Wilfrid, K.N.; Nikolay, T.S.; et al. Optimization of Ethanolic Extraction of Enantia chloranta Bark, Phytochemical Composition, Green Synthesis of Silver Nanoparticles, and Antimicrobial Activity. Fermentation 2022, 8, 530. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Qiu, W.; Mei, J.; Xie, J. Effect of different slaughter/stunning methods on stress response, quality indicators and susceptibility to oxidation of large yellow croaker (Larimichthys crocea). Plants 2023, 12, 1720. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria—Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Liu, T.; Kang, J.M.; Liu, L. Thymol as a critical component of Thymus vulgaris L. essential oil combats Pseudomonas aeruginosa by intercalating DNA and inactivating biofilm. LWT-Food Sci. Technol. 2021, 136, 110354. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Cho, K.-H.; Lee, J.-H.; Lee, J. Antibiofilm Activities of Cinnamaldehyde Analogs against Uropathogenic Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 7225. [Google Scholar] [CrossRef]
- Ghaderi, L.; Aliahmadi, A.; Ebrahimi, S.N.; Rafati, H. Effective Inhibition and eradication of Pseudomonas aeruginosa biofilms by Satureja khuzistanica essential oil nanoemulsion. J. Drug Deliv. Sci. Technol. 2021, 61, 102260. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Z.; Li, G.; Fu, F.; Liang, Z.; Zhu, H.; Shan, Y. Antimicrobial and antibiofilm efficacy and mechanism of essential oil from Citrus Changshan-huyou Y. B. chang against Listeria monocytogenes. Food Control 2019, 105, 256–264. [Google Scholar] [CrossRef]
- Pei, J.; Yu, H.; Qiu, W.; Mei, J.; Xie, J. Antimicrobial Effect of Epigallocatechin Gallate Against Shewanella putrefaciens ATCC 8071: A Study Based on Cell Membrane and Biofilm. Curr. Microbiol. 2022, 79, 297. [Google Scholar] [CrossRef]


| NO. | Main Compounds | Retention Time (min) | Retention Index | Peak Area (%) | CAS |
|---|---|---|---|---|---|
| 1 | Heptane, 2,2,4,6,6-pentamethyl- | 7.478 | 1197 | 0.23 | 13475-82-6 |
| 2 | Cyclotetrasiloxane, octamethyl- | 7.825 | 1227 | 0.17 | 556-67-2 |
| 3 | Heptane, 4-ethyl-2,2,6,6-tetramethyl- | 7.887 | 1233 | 0.03 | 62108-31-0 |
| 4 | 2,2,4,4, -Tetramethyloctane | 8.112 | 1252 | 0.07 | 62183-79-3 |
| 5 | β-pinene | 8.25 | 1263 | 0.45 | 17301-28-9 |
| 6 | Linalool | 9.358 | 1360 | 1.09 | 78-70-6 |
| 7 | Cyclopentasiloxane, decamethyl- | 10.08 | 1426 | 0.74 | 541-02-6 |
| 8 | Estragole | 10.962 | 1509 | 4.87 | 140-67-0 |
| 10 | α-Cubebene | 13.000 | 1718 | 0.06 | 17699-14-8 |
| 11 | Eugenol | 13.161 | 1735 | 51.88 | 97-53-0 |
| 12 | 3-Allyl-6-methoxyphenol | 13.381 | 1758 | 3.15 | 501-19-9 |
| 13 | Caryophyllene | 13.976 | 1817 | 9.57 | 87-44-5 |
| 14 | trans-α-Bergamotene | 14.109 | 1829 | 0.56 | 13474-59-4 |
| 15 | Humulen | 14.34 | 1851 | 0.93 | 24405-93-4 |
| 16 | 1,4,7,-Cycloundecatriene, 1,5,9,9-tetramethyl-, (1Z,4Z,7Z)- | 14.406 | 1857 | 2.34 | 400822-79-9 |
| 17 | (+)-δ-Cadinene | 14.662 | 1881 | 0.74 | 483-76-1 |
| 18 | Cycloheptasiloxane, tetradecamethyl- | 14.792 | 1893 | 1.32 | 107-50-6 |
| 19 | δ-cadinene | 15.238 | 1931 | 0.25 | 483-76-1 |
| 20 | 4-allyl-2-methoxyphenyl acetate | 15.445 | 1950 | 0.13 | 93-28-7 |
| 21 | Caryophyllene oxide | 16.027 | 1999 | 1.05 | 1139-30-6 |
| 22 | Humulene | 16.203 | 2014 | 3.23 | 6753-98-6 |
| 23 | Cyclooctasiloxane, hexadecamethyl- | 16.785 | 2061 | 0.08 | 556-68-3 |
| 24 | Androstan-17-one, 3-ethyl-3-hydroxy-, (5 α)- | 16.887 | 2070 | 2.37 | 57344-99-7 |
| 25 | Patchouli alcohol | 16.947 | 2074 | 3.35 | 5986-55-0 |
| 26 | Undecane, 3,6-dimethyl- | 17.3118 | 2298 | 0.09 | 18172-67-3 |
| 27 | β-myrcene | 19.5734 | 2473 | 0.02 | 123-35-3 |
| 28 | Bikaverin | 25.054 | 2665 | 0.76 | 33390-21-5 |
| 29 | 3-Hexen-1-ol, (Z)- | 27.6961 | 2795 | 0.49 | 928-96-1 |
| 30 | Acetic acid | 29.3855 | 2933 | 2.89 | 64-19-7 |
| 31 | 5-Methyl-2-furfural | 32.8607 | 3197 | 0.01 | 620-02-0 |
| 32 | Phthalic acid, mono(o-methylbenzyl) ester | 34.5087 | 3376 | 0.50 | 4619-49-2 |
| 33 | α-Amorphene | 36.0394 | 3487 | 0.01 | 23515-88-0 |
| 34 | γ-Cadinenema | 38.3287 | 3658 | 0.73 | 39029-41-9 |
| 35 | Safrole | 39.6732 | 3720 | 0.46 | 94-59-7 |
| 36 | Phenol, 2-methoxy-4-(2-propenyl)- | 43.4243 | 4089 | 0.58 | 97-53-0 |
| 37 | Patchouli alcohol | 44.3276 | 4173 | 0.74 | 5986-55-0 |
| 38 | 1-Cyclopentenecarboxylic acid, 2-methyl-3-vinyl-, 4′-fluorophenyl ester | 45.1205 | 4298 | 0.11 | 1000158-81-1 |
| 39 | Chavicol | 45.6515 | 4367 | 0.01 | 501-92-8 |
| 40 | Isoaromadendrene epoxide | 46.5961 | 4449 | 0.06 | 1000159-36-6 |
| 41 | 6-Methoxy-3-methylbenzofuran | 48.0786 | 4667 | 0.03 | 29040-52-6 |
| 42 | Triacontane | 48.1958 | 4713 | 3.85 | 638-68-6 |
| Index | CK | 1/4× MIC | 1/2× MIC | 1× MIC |
|---|---|---|---|---|
| EPS | - | −0.9256 | −0.9909 | −0.9966 |
| EP | - | −0.9997 | −0.9998 | −0.9997 |
| Samples | Swimming (mm) | Swarming (mm) |
|---|---|---|
| CK | 80.58 ± 0.47 | 58.42 ± 0.64 |
| 1/4 MIC | 52.14 ± 0.34 | 35.25 ± 0.20 |
| 1/2 MIC | 16.37 ± 0.25 | 22.98 ± 0.92 |
| 1 MIC | 9.34 0.11 | 14.73 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Zhang, C.; Mei, J.; Xie, J. Antimicrobial Effect of Ocimum gratissimum L. Essential Oil on Shewanella putrefaciens: Insights Based on the Cell Membrane and External Structure. Int. J. Mol. Sci. 2023, 24, 11066. https://doi.org/10.3390/ijms241311066
Xie Y, Zhang C, Mei J, Xie J. Antimicrobial Effect of Ocimum gratissimum L. Essential Oil on Shewanella putrefaciens: Insights Based on the Cell Membrane and External Structure. International Journal of Molecular Sciences. 2023; 24(13):11066. https://doi.org/10.3390/ijms241311066
Chicago/Turabian StyleXie, Yao, Chi Zhang, Jun Mei, and Jing Xie. 2023. "Antimicrobial Effect of Ocimum gratissimum L. Essential Oil on Shewanella putrefaciens: Insights Based on the Cell Membrane and External Structure" International Journal of Molecular Sciences 24, no. 13: 11066. https://doi.org/10.3390/ijms241311066
APA StyleXie, Y., Zhang, C., Mei, J., & Xie, J. (2023). Antimicrobial Effect of Ocimum gratissimum L. Essential Oil on Shewanella putrefaciens: Insights Based on the Cell Membrane and External Structure. International Journal of Molecular Sciences, 24(13), 11066. https://doi.org/10.3390/ijms241311066








