Gender Differences and miRNAs Expression in Cancer: Implications on Prognosis and Susceptibility
Abstract
1. Introduction
1.1. General Considerations on miRNAs
1.2. Sex Differences, miRNAs, and Cancer
2. Hematological Malignancies
2.1. Thyroid Cancer
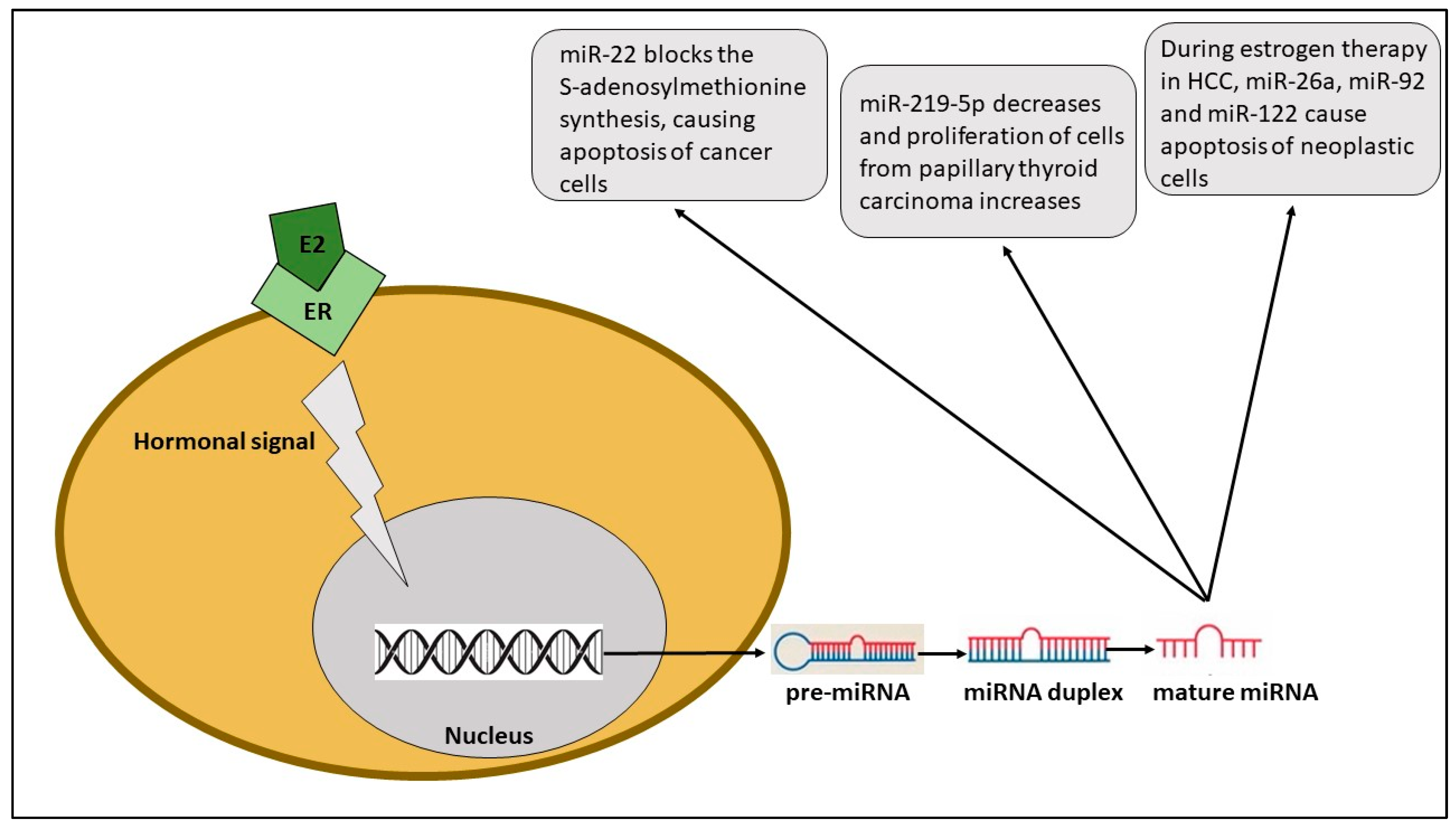
2.2. Hepatocellular Carcinoma
2.3. Colorectal Cancer
2.4. Gastric Cancer
2.5. Lung Cancer
2.6. Melanoma
2.7. Breast Cancer
2.8. Glioblastoma
2.9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allegra, A.; Murdaca, G.; Gammeri, L.; Ettari, R.; Gangemi, S. Alarmins and MicroRNAs, a New Axis in the Genesis of Respiratory Diseases: Possible Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 1783. [Google Scholar] [CrossRef]
- Amato, G.; Vita, F.; Quattrocchi, P.; Minciullo, P.L.; Pioggia, G.; Gangemi, S. Involvement of miR-142 and miR-155 in Non-Infectious Complications of CVID. Molecules 2020, 25, 4760. [Google Scholar] [CrossRef]
- Kraczkowska, W.; Stachowiak, L.; Pławski, A.; Jagodziński, P.P. Circulating miRNA as potential biomarkers for diabetes mellitus type 2: Should we focus on searching for sex differences? J. Appl. Genet. 2022, 63, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Hasakova, K.; Bezakova, J.; Vician, M.; Reis, R.; Zeman, M.; Herichova, I. Gender-Dependent Expression of Leading and Passenger Strand of miR-21 and miR-16 in Human Colorectal Cancer and Adjacent Colonic Tissues. Physiol. Res. 2017, 66, S575–S582. [Google Scholar] [CrossRef] [PubMed]
- Umansky, S. Aging and aging-associated diseases: A microRNA-based endocrine regulation hypothesis. Aging 2018, 10, 2557–2569. [Google Scholar] [CrossRef]
- Warnefors, M.; Mössinger, K.; Halbert, J.; Studer, T.; VandeBerg, J.L.; Lindgren, I.; Fallahshahroudi, A.; Jensen, P.; Kaessmann, H. Sex-biased microRNA expression in mammals and birds reveals underlying regulatory mechanisms and a role in dosage compensation. Genome Res. 2017, 27, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Bezler, A.; Braukmann, F.; West, S.M.; Duplan, A.; Conconi, R.; Schütz, F.; Gönczy, P.; Piano, F.; Gunsalus, K.; Miska, E.A.; et al. Tissue- and sex-specific small RNAomes reveal sex differences in response to the environment. PLOS Genet. 2019, 15, e1007905. [Google Scholar] [CrossRef]
- Bellenghi, M.; Puglisi, R.; Pontecorvi, G.; De Feo, A.; Carè, A.; Mattia, G. Sex and Gender Disparities in Melanoma. Cancers 2020, 12, 1819. [Google Scholar] [CrossRef]
- Otmani, K.; Rouas, R.; Lewalle, P. OncomiRs as noncoding RNAs having functions in cancer: Their role in immune suppression and clinical implications. Front. Immunol. 2022, 13, 913951. [Google Scholar] [CrossRef]
- Caserta, S.; Innao, V.; Musolino, C.; Allegra, A. Immune checkpoint inhibitors in multiple myeloma: A review of the literature. Pathol. Res. Pract. 2020, 216, 153114. [Google Scholar] [CrossRef]
- Carè, A.; Bellenghi, M.; Matarrese, P.; Gabriele, L.; Salvioli, S.; Malorni, W. Sex disparity in cancer: Roles of microRNAs and related functional players. Cell Death Differ. 2018, 25, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Torrón, R.; García-Puga, M.; Emparanza, J.-I.; Maneiro, M.; Cobo, A.-M.; Poza, J.-J.; Espinal, J.-B.; Zulaica, M.; Ruiz, I.; Martorell, L.; et al. Cancer risk in DM1 is sex-related and linked to miRNA-200/141 downregulation. Neurology 2016, 87, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Tomeva, E.; Krammer, U.D.B.; Switzeny, O.J.; Haslberger, A.G.; Hippe, B. Sex-Specific miRNA Differences in Liquid Biopsies from Subjects with Solid Tumors and Healthy Controls. Epigenomes 2023, 7, 2. [Google Scholar] [CrossRef]
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Sadia, H.; Qadri, Q.R.; Raza, S.; Irshad, A.; Akbar, A.; et al. Correction: Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int. 2022, 22, 256. [Google Scholar] [CrossRef]
- Shiau, J.-P.; Chuang, Y.-T.; Yen, C.-Y.; Chang, F.-R.; Yang, K.-H.; Hou, M.-F.; Tang, J.-Y.; Chang, H.-W. Modulation of AKT Pathway-Targeting miRNAs for Cancer Cell Treatment with Natural Products. Int. J. Mol. Sci. 2023, 24, 3688. [Google Scholar] [CrossRef]
- Caserta, S.; Zaccuri, A.M.; Innao, V.; Musolino, C.; Allegra, A. Immune thrombocytopenia: Options and new perspectives. Blood Coagul. Fibrinolysis 2021, 32, 427–433. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F.; Allegra, A.; Di Chio, C.; Previti, S.; Zappalà, M.; Ettari, R. Monoclonal Antibodies: The Greatest Resource to Treat Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 3136. [Google Scholar] [CrossRef]
- Musolino, C.; Oteri, G.; Allegra, A.; Mania, M.; D’ascola, A.; Avenoso, A.; Innao, V.; Allegra, A.G.; Campo, S. Altered microRNA expression profile in the peripheral lymphoid compartment of multiple myeloma patients with bisphosphonate-induced osteonecrosis of the jaw. Ann. Hematol. 2018, 97, 1259–1269. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Giordano, L.; Musolino, C.; Gangemi, S. Targeting Redox Regulation as a Therapeutic Opportunity against Acute Leukemia: Pro-Oxidant Strategy or Antioxidant Approach? Antioxidants 2022, 11, 1696. [Google Scholar] [CrossRef]
- Taverna, S.; Tonacci, A.; Ferraro, M.; Cammarata, G.; Cuttitta, G.; Bucchieri, S.; Pace, E.; Gangemi, S. High Mobility Group Box 1: Biological Functions and Relevance in Oxidative Stress Related Chronic Diseases. Cells 2022, 11, 849. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, X.; Liang, Y. Sex differences in normal and malignant hematopoiesis. Blood Sci. 2022, 4, 185–191. [Google Scholar] [CrossRef]
- Matarrese, P.; Mattia, G.; Pagano, M.T.; Pontecorvi, G.; Ortona, E.; Malorni, W.; Carè, A. The Sex-Related Interplay between TME and Cancer: On the Critical Role of Estrogen, MicroRNAs and Autophagy. Cancers 2021, 13, 3287. [Google Scholar] [CrossRef]
- Anonymous. Correction to: Hormonally Regulated Myogenic miR-486 Influences Sex-specific Differences in Cancer-induced Skeletal Muscle Defects. Endocrinology 2022, 163, bqac120, Erratum in: Endocrinology 2021, 162, 35939403. [Google Scholar] [CrossRef]
- Ruiz-Pozo, V.A.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Zambrano, A.K. Differential microRNA expression for diagnosis and prognosis of papillary thyroid cancer. Front. Med. 2023, 10, 1139362. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhang, D.; Tao, L.; Xu, N.; Lu, X.; Wang, J.; He, G.; Tang, Q.; Huang, K.; Shen, S.; Chu, J. CircRNA circTIAM1 promotes papillary thyroid cancer progression through the miR-646/HNRNPA1 signaling pathway. Cell Death Discov. 2022, 8, 21. [Google Scholar] [CrossRef]
- Celano, M.; Rosignolo, F.; Maggisano, V.; Pecce, V.; Iannone, M.; Russo, D.; Bulotta, S. MicroRNAs as Biomarkers in Thyroid Carcinoma. Int. J. Genom. 2017, 2017, 6496570. [Google Scholar] [CrossRef] [PubMed]
- Rogucki, M.; Sidorkiewicz, I.; Niemira, M.; Dzięcioł, J.B.; Buczyńska, A.; Adamska, A.; Siewko, K.; Kościuszko, M.; Maliszewska, K.; Wójcicka, A.; et al. Expression Profile and Diagnostic Significance of MicroRNAs in Papillary Thyroid Cancer. Cancers 2022, 14, 2679. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, M.; Chorti, A.G.; Chatzikyriakidou, A.; Giannoulis, K.; Bakkar, S.; Papavramidis, T.S. MicroRNAs in Papillary Thyroid Cancer: What Is New in Diagnosis and Treatment. Front. Oncol. 2022, 11, 755097. [Google Scholar] [CrossRef]
- Huang, C.; Cai, Z.; Huang, M.; Mao, C.; Zhang, Q.; Lin, Y.; Zhang, X.; Tang, B.; Chen, Y.; Wang, X.; et al. miR-219–5p Modulates Cell Growth of Papillary Thyroid Carcinoma by Targeting Estrogen Receptor α. J. Clin. Endocrinol. Metab. 2015, 100, E204–E213. [Google Scholar] [CrossRef]
- Huang, D.; Rao, D.; Jin, Q.; Lai, M.; Zhang, J.; Lai, Z.; Shen, H.; Zhong, T. Role of CD147 in the development and diagnosis of hepatocellular carcinoma. Front. Immunol. 2023, 14, 1149931. [Google Scholar] [CrossRef]
- Javed; Yadav, S. Advanced therapeutics avenues in hepatocellular carcinoma: A novel paradigm. Med. Oncol. 2023, 40, 239. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Oliveri, R.S.; Wetterslev, J.; Gluud, C. Hepatocellular carcinoma. Lancet 2012, 380, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. Interna-tional validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Medavaram, S.; Zhang, Y. Emerging therapies in advanced hepatocellular carcinoma. Exp. Hematol. Oncol. 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer Statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef]
- Venook, A.P.; Papandreou, C.; Furuse, J.; de Guevara, L.L. The Incidence and Epidemiology of Hepatocellular Carcinoma: A Global and Regional Perspective. Oncologist 2010, 15, 5–13. [Google Scholar] [CrossRef]
- Bosetti, C.; Turati, F.; La Vecchia, C. Hepatocellular carcinoma epidemiology. Best Prac. Res. Clin. Gastroenterol. 2014, 28, 753–770. [Google Scholar] [CrossRef]
- Bai, P.-S.; Hou, P.; Kong, Y. Hepatitis B virus promotes proliferation and metastasis in male Chinese hepatocellular carcinoma patients through the LEF-1/miR-371a-5p/SRCIN1/pleiotrophin/Slug pathway. Exp. Cell Res. 2018, 370, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-L.; Yeh, K.-H.; Liu, W.-H.; Chen, C.-L.; Chen, D.-S.; Chen, P.-J.; Yeh, S.-H. Elevated p53 promotes the processing of miR-18a to decrease estrogen receptor-α in female hepatocellular carcinoma. Int. J. Cancer 2014, 136, 761–770. [Google Scholar] [CrossRef]
- Huang, F.-Y.; Wong, D.K.-H.; Seto, W.-K.; Lai, C.-L.; Yuen, M.-F. Estradiol induces apoptosis via activation of miRNA-23a and p53: Implication for gender difference in liver cancer development. Oncotarget 2015, 6, 34941–34952. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Caserta, S.; Genovese, S.; Pioggia, G.; Gangemi, S. Gender Differences in Oxidative Stress in Relation to Cancer Susceptibility and Survival. Antioxidants 2023, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Vincze, Á. Endoscopic diagnosis and treatment in gastric cancer: Current evidence and new perspectives. Front. Surg. 2023, 10, 1122454. [Google Scholar] [CrossRef]
- Pan, D.; Li, Z.; Lin, X.; Li, L. Transcriptome sequencing and miRNA-mRNA network construction in exosome of macrophage M2 in stomach adenocarcinoma. World J. Surg. Oncol. 2023, 21, 193. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; He, X.; Zhu, Y.; Jiang, X.; Chen, Y.; Wang, Y.; Huang, L.; Shang, R.; Dong, Z.; et al. Deep learning system compared with expert endoscopists in predicting early gastric cancer and its invasion depth and differentiation status (with videos). Gastrointest. Endosc. 2021, 95, 92–104.e3. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, M.; Li, X.; Zhang, X.; Wang, Q.; Liu, L.; Yang, M.; Yang, D.; Guo, Y.; Zhang, Q.; et al. Interrogation of gender disparity uncovers androgen receptor as the transcriptional activator for oncogenic miR-125b in gastric cancer. Cell Death Dis. 2021, 12, 441. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, H. Immunotherapy resistance in non-small-cell lung cancer: From mechanism to clinical strategies. Front. Immunol. 2023, 14, 1129465. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Goldstraw, P.; Ball, D.; Jett, J.R.; Le Chevalier, T.; Lim, E.; Nicholson, A.G.; Shepherd, A.F. Non-small-cell lung cancer. Lancet 2011, 378, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. IMpower010 Investigators. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2015, 387, 1540–1550. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Rao, D.-Y.; Huang, D.-F.; Si, M.-Y.; Lu, H.; Tang, Z.-X.; Zhang, Z.-X. Role of exosomes in non-small cell lung cancer and EGFR-mutated lung cancer. Front. Immunol. 2023, 14, 1142539. [Google Scholar] [CrossRef]
- Khadela, A.; Postwala, H.; Rana, D.; Dave, H.; Ranch, K.; Boddu, S.H.S. A review of recent advances in the novel therapeutic targets and immunotherapy for lung cancer. Med. Oncol. 2023, 40, 152. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, Z.; Aramini, B.; Lin, D.; Li, X.; Fan, J. Potential biomarkers for immunotherapy in non-small-cell lung cancer. Cancer Metastasis Rev. 2023, 1–15. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Moosavi-Movahedi, F.; Saso, L.; Habibi-Rezaei, M.; Khatibi, A.; Hong, J.; Moosavi-Movahedi, A.A. Modulation of Nrf2/HO-1 by Natural Compounds in Lung Cancer. Antioxidants 2023, 12, 735. [Google Scholar] [CrossRef]
- Skjefstad, K.; Johannessen, C.; Grindstad, T.; Kilvaer, T.; Paulsen, E.-E.; Pedersen, M.; Donnem, T.; Andersen, S.; Bremnes, R.; Richardsen, E.; et al. A gender specific improved survival related to stromal miR-143 and miR-145 expression in non-small cell lung cancer. Sci. Rep. 2018, 8, 8549. [Google Scholar] [CrossRef] [PubMed]
- Arafat, H.M.; Omar, J.; Shafii, N.; Naser, I.A.; Al Laham, N.A.; Muhamad, R.; Al-Astani, T.A.D.; Shaqaliah, A.J.; Shamallakh, O.M.; Shamallakh, K.M.; et al. The association between breast cancer and consumption of dairy products: A systematic review. Ann. Med. 2023, 55, 2198256. [Google Scholar] [CrossRef]
- Ryoo, J.-A.; Kim, S.Y. Incidental Extramammary Findings on Preoperative Breast MRI in Breast Cancer Patients: A Pictorial Essay. J. Korean Soc. Radiol. 2023, 84, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Leithner, D.; Wengert, G.; Helbich, T.; Thakur, S.; Ochoa-Albiztegui, R.; Morris, E.; Pinker, K. Clinical role of breast MRI now and going forward. Clin. Radiol. 2017, 73, 700–714. [Google Scholar] [CrossRef]
- Gao, Y.; Ibidapo, O.; Toth, H.K.; Moy, L. Delineating Extramammary Findings at Breast MR Imaging. Radiographics 2017, 37, 10–31. [Google Scholar] [CrossRef]
- Yang, S.M.; Kim, S.H.; Kang, B.J.; Song, B.J. Extramammary findings on breast MRI: Prevalence and imaging characteristics favoring malignancy detection: A retrospective analysis. World J. Surg. Oncol. 2016, 14, 119. [Google Scholar] [CrossRef]
- Bassett, L.W.; Dhaliwal, S.G.; Eradat, J.; Khan, O.; Farria, D.F.; Brenner, R.J.; Sayre, J.W. National Trends and Practices in Breast MRI. Am. J. Roentgenol. 2008, 191, 332–339. [Google Scholar] [CrossRef]
- Jung, S.; Kim, Y.A.; Lee, D.; Joo, J.; Back, J.H.; Kong, S.; Lee, E.S. Clinical impact of follow-up imaging on mortality in Korean breast cancer patients: A national cohort study. Cancer Med. 2021, 10, 6480–6491. [Google Scholar] [CrossRef]
- Suh, H.J.; Choi, J.S.; Ko, K. Extra-Mammary Findings Detected on Breast Magnetic Resonance Imaging: A Pictorial Essay. Korean J. Radiol. 2014, 15, 423–429. [Google Scholar] [CrossRef]
- Laurent, F.; Latrabe, V.; Lecesne, R.; Zennaro, H.; Airaud, J.Y.; Rauturier, J.F.; Drouillard, J. Mediastinal masses: Diagnostic approach. Eur. Radiol. 1998, 8, 1148–1159. [Google Scholar] [CrossRef]
- Alduk, A.; Prutki, M.; Stern-Padovan, R. Incidental extra-mammary findings in breast MRI. Clin. Radiol. 2015, 70, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Brakohiapa, E.; Botwe, B.O.; Sarkodie, B.D.; Ofori, E.K.; Coleman, J. Radiographic determination of cardiomegaly using cardiothoracic ratio and transverse cardiac diameter: Can one size fit all? Part one. Pan Afr. Med. J. 2017, 27, 201. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.Y.; Johnson, W.; Karamlou, K.; Khaki, A.; Komanapalli, C.; Walts, D.; Mahin, D.; Johnson, N. The evaluation and treatment implications of isolated pulmonary nodules in patients with a recent history of breast cancer. Am. J. Surg. 2006, 191, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chung, S.B.; Kim, M.S. Prevalence and Anatomy of Aberrant Right Subclavian Artery Evaluated by Computed Tomographic Angiography at a Single Institution in Korea. J. Korean Neurosurg. Soc. 2019, 62, 175–182. [Google Scholar] [CrossRef]
- Gou, D.; Liu, R.; Shan, X.; Deng, H.; Chen, C.; Xiang, J.; Liu, Y.; Gao, Q.; Li, Z.; Huang, A.; et al. Gluconeogenic enzyme PCK1 supports S-adenosylmethionine biosynthesis and promotes H3K9me3 modification to suppress hepatocellular carcinoma progression. J. Clin. Investig. 2023, 133, e161713. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M. Advances in Electrochemical Biosensor Technologies for the Detection of Nucleic Acid Breast Cancer Biomarkers. Sensors 2023, 23, 4128. [Google Scholar] [CrossRef]
- Pinto, R.; Pilato, B.; Ottini, L.; Lambo, R.; Simone, G.; Paradiso, A.; Tommasi, S. Different methylation and MicroRNA expression pattern in male and female familial breast cancer. J. Cell. Physiol. 2013, 228, 1264–1269. [Google Scholar] [CrossRef]
- Li, E.W.; Bai, Y. Computational Identification of Sex-Biased Biomarker MicroRNAs and Genes Associated with Immune Infiltration in Breast Cancer. Genes 2021, 12, 570. [Google Scholar] [CrossRef]
- Singh, H. Role of Molecular Targeted Therapeutic Drugs in Treatment of Glioblastoma: A Review Article. Glob. Med. Genet. 2023, 10, 42–47. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, A.K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncology 2022, 24, v1–v95. [Google Scholar] [CrossRef]
- Kamran, N.; Candolfi, M.; Baker, G.J.; Ayala, M.M.; Dzaman, M.; Lowenstein, P.R.; Castro, M.G. Gene Therapy for the Treatment of Neurological Disorders: Central Nervous System Neoplasms. Methods Mol. Bol. 2016, 1382, 467–482. [Google Scholar] [CrossRef]
- Rønning, P.A.; Helseth, E.; Meling, T.R.; Johannesen, T.B. A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro-Oncology 2012, 14, 1178–1184. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Snuderl, M.; Fazlollahi, L.; Le, L.P.; Nitta, M.; Zhelyazkova, B.H.; Davidson, C.J.; Akhavanfard, S.; Cahill, D.P.; Aldape, K.D.; Betensky, R.A.; et al. Mosaic Amplification of Multiple Receptor Tyrosine Kinase Genes in Glioblastoma. Cancer Cell 2011, 20, 810–817. [Google Scholar] [CrossRef]
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.M.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavaré, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Pointer, K.B.; Kuo, J.S.; Dempsey, R.J. Mutational Analysis Reveals the Origin and Therapy-Driven Evolution of Recurrent Glioma. Neurosurgery 2014, 75, N9–N10. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Zheng, S.; Amini, S.S.; Virk, S.M.; Mikkelsen, T.; Brat, D.J.; Grimsby, J.; Sougnez, C.; Muller, F.; Hu, J.; et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015, 25, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, I.-H.; Cho, H.J.; Park, C.-K.; Jung, Y.-S.; Kim, Y.; Nam, S.H.; Kim, B.S.; Johnson, M.D.; Kong, D.-S.; et al. Spatiotemporal Evolution of the Primary Glioblastoma Genome. Cancer Cell 2015, 28, 318–328. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Jill, P.; Alexe, G.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.L.R.; Lemos, F.F.B.; Marques, H.S.; Luz, M.S.; Silva, L.G.d.O.; dos Santos, C.F.S.M.; Evangelista, K.d.C.; Calmon, M.S.; Loureiro, M.S.; de Melo, F.F. Immunotherapy in glioblastoma treatment: Current state and future prospects. World J. Clin. Oncol. 2023, 14, 138–159. [Google Scholar] [CrossRef]
- Yazdi, A.H.; Zarrinpour, V.; Moslemi, E.; Forghanifard, M.M. A Signature of Three microRNAs Is a Potential Diagnostic Biomarker for Glioblastoma. Iran. Biomed. J. 2022, 26, 301–312. [Google Scholar] [CrossRef]
- Zheng, L.; Guo, C.-Y.; Chen, C.-S.; Xiao, J.-C.; Hu, H.-T.; Cheng, H.-T.; Zong, D.-W.; Jiang, L.; Li, H.-L. Sorafenib improves lipiodol deposition in transarterial chemoembolization of Chinese patients with hepatocellular carcinoma: A long-term, retrospective study. Oncotarget 2017, 8, 97613–97622. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Jiang, X.; Yokosuka, O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World J. Gastroenterol. 2014, 20, 9229–9236. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Shousha, W.G.; Shalby, A.B.; El-Mezayen, A.H.; Ismaiel, N.N.; Mahmoud, N.S. Implications of Sex Hormone Receptor Gene Expression in the Predominance of Hepatocellular Carcinoma in Males: Role of Natural Products. Asian Pac. J. Cancer Prev. 2015, 16, 4949–4954. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, X.; Lin, Y.; Tan, G.; Zhong, W. Androgen receptor in hepatocarcinogenesis: Recent developments and perspectives. Oncol. Lett. 2015, 9, 1983–1988. [Google Scholar] [CrossRef]
- Kanda, T.; Yokosuka, O. The androgen receptor as an emerging target in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2015, 2, 91–99. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z. Interplay of estrogen receptors and FOXA factors in the liver cancer. Mol. Cell. Endocrinol. 2015, 418, 334–339. [Google Scholar] [CrossRef]
- Ringelhan, M.; O’Connor, T.; Protzer, U.; Heikenwalder, M. The direct and indirect roles of HBV in liver cancer: Prospective markers for HCC screening and potential therapeutic targets. J. Pathol. 2014, 235, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; Onorato, L.; Iodice, V.; Starace, M.; Minichini, C.; Farella, N.; Liorre, G.; Filippini, P.; Sagnelli, E.; de Stefano, G. Occult HBV infection in HCC and cirrhotic tissue of HBsAg-negative patients: A virological and clinical study. Oncotarget 2016, 7, 62706–62714. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Yang, X.-N.; Jazag, A.; Pan, J.-S.; Hu, T.-H.; Liu, J.-J.; Guleng, B.; Ren, J.-L. HBsAg Inhibits the Translocation of JTB into Mitochondria in HepG2 Cells and Potentially Plays a Role in HCC Progression. PLoS ONE 2012, 7, e36914. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.-L.; Zhang, Y.-G.; Liu, J.; Zeng, Y.; Wu, H. MicroRNAs Associated with HBV Infection And HBV-related HCC. Theranostics 2014, 4, 1176–1192. [Google Scholar] [CrossRef]
- Gabriele, L.; Buoncervello, M.; Ascione, B.; Bellenghi, M.; Matarrese, P.; Carè, A. The gender perspective in cancer research and therapy: Novel insights and on-going hypotheses. Ann. Dell’istituto Super. Sanita 2016, 52, 213–222. [Google Scholar]
- Giampietri, C.; Petrungaro, S.; Filippini, A.; Ziparo, E. Sex-related differences in death control of somatic cells. J. Cell. Mol. Med. 2013, 17, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Penaloza, C.; Estevez, B.; Orlanski, S.; Sikorska, M.; Walker, R.; Smith, C.; Smith, B.; Lockshin, R.A.; Zakeri, Z. Sex of the cell dictates its response: Differential gene expression and sensitivity to cell death inducing stress in male and female cells. FASEB J. 2009, 23, 1869–1879. [Google Scholar] [CrossRef]
- Malorni, W.; Straface, E.; Matarrese, P.; Ascione, B.; Coinu, R.; Canu, S.; Galluzzo, P.; Marino, M.; Franconi, F. Redox state and gender differences in vascular smooth muscle cells. FEBS Lett. 2008, 582, 635–642. [Google Scholar] [CrossRef]
- Maselli, A.; Matarrese, P.; Straface, E.; Canu, S.; Franconi, F.; Malorni, W. Cell sex: A new look at cell fate studies. FASEB J. 2008, 23, 978–984. [Google Scholar] [CrossRef]
- Lista, P.; Straface, E.; Brunelleschi, S.; Franconi, F.; Malorni, W. On the role of autophagy in human diseases: A gender perspective. J. Cell. Mol. Med. 2011, 15, 1443–1457. [Google Scholar] [CrossRef]
- Completed Clinical Trials about miRNAs in Different Types of Cancer. Available online: Clinicaltrials.gov (accessed on 7 July 2023).
- Zhong, X.; Zimmers, T.A. Sex Differences in Cancer Cachexia. Curr. Osteoporos. Rep. 2020, 18, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Iwasaki, A. Sex differences in immune responses. Science 2021, 371, 347–348. [Google Scholar] [CrossRef]
- Small, E.M.; O’Rourke, J.R.; Moresi, V.; Sutherland, L.B.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc. Natl. Acad. Sci. USA 2010, 107, 4218–4223. [Google Scholar] [CrossRef]
- Holstein, I.; Singh, A.K.; Pohl, F.; Misiak, D.; Braun, J.; Leitner, L.; Hüttelmaier, S.; Posern, G. Post-transcriptional regulation of MRTF-A by miRNAs during myogenic differentiation of myoblasts. Nucleic Acids Res. 2020, 48, 8927–8942. [Google Scholar] [CrossRef]
- Cui, C.; Yang, W.; Shi, J.; Zhou, Y.; Yang, J.; Cui, Q.; Zhou, Y. Identification and Analysis of Human Sex-biased MicroRNAs. Genom. Proteom. Bioinform. 2018, 16, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Heffler, E.; Allegra, A.; Pioggia, G.; Picardi, G.; Musolino, C.; Gangemi, S. MicroRNA Profiling in Asthma: Potential Biomarkers and Therapeutic Targets. Am. J. Respir. Cell Mol. Biol. 2017, 57, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Lampignano, R.; Kloten, V.; Krahn, T.; Schlange, T. Integrating circulating miRNA analysis in the clinical management of lung cancer: Present or future? Mol. Asp. Med. 2020, 72, 100844. [Google Scholar] [CrossRef]
- Zhou, M.; Hara, H.; Dai, Y.; Mou, L.; Cooper, D.K.C.; Wu, C.; Cai, Z. Circulating Organ-Specific MicroRNAs Serve as Biomarkers in Organ-Specific Diseases: Implications for Organ Allo- and Xeno-Transplantation. Int. J. Mol. Sci. 2016, 17, 1232. [Google Scholar] [CrossRef]
| Cancer | miRNAs | UP/DOWN Regulated | Mechanism of Action | Onset/Prognosis/Response to Therapy Involvement | References |
|---|---|---|---|---|---|
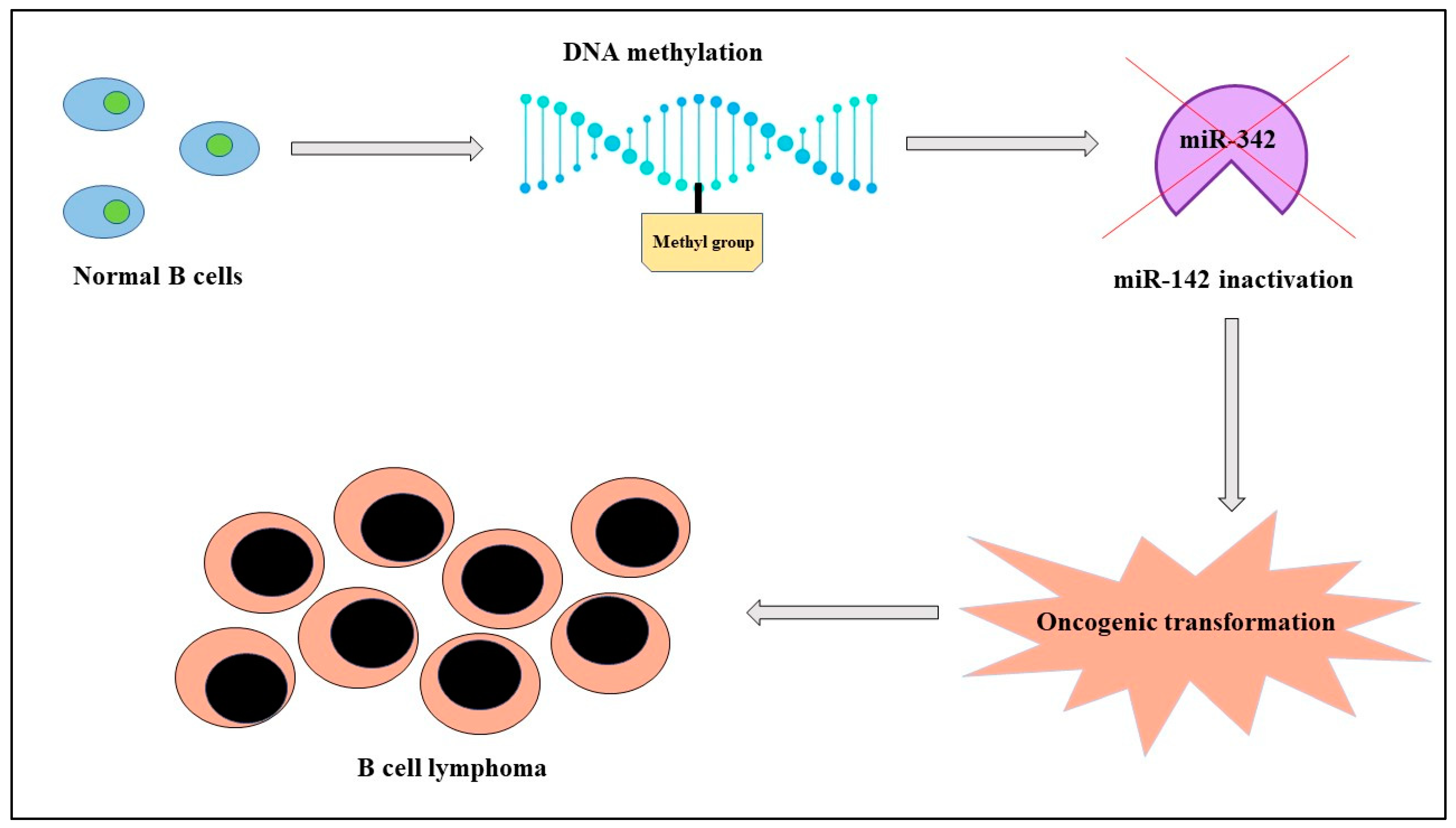
| B-cell lymphoma | miR-142 | DOWN | Altered migration of cells | Poor prognosis | [22] |
| Papillary thyroid cancer | miR-21 miR-26a miR-181a miR-181b miR-219 miR-221 miR-222 miR-245 | UP | Modulation in protein p27Kip1 expression | Onset | [30] |
| Hepatocellular carcinoma | miR-371a-5p | UP | Transition from G to S phase of cell cycle | Onset | [41] |
| Colorectal cancer | miR-16 miR-22 miR-142-3p | miR-22 UP; miR-16 and miR-142-3p DOWN | Inhibition of autophagy (miR-16, miR-142-3p) and inhibition of estrogen activity | Onset (miR-16, miR-142-3p) and better response to therapy (miR-22) | [8] |
| Gastric cancer | miR-125 | UP | Block of apoptosis | Onset | [48] |
| Lung cancer | miR-143 miR-145 miR-153-3p | UP | Apoptosis induction | Good prognosis | [22] |
| Melanoma | miR-23a miR-221 miR-222 | UP | Block of cell proliferation | Onset | [8] |
| Breast cancer | miR-17 miR-21 miR-124 | UP | BRCA1 inactivation | Onset | [80,81] |
| Glioblastoma | hsa-miR-1909-5p hsa-let-7c-5p miR-206-5p | hsa-miR-1909-5p and hsa-let-7c-5p UP; miR-206-5p DOWN | Promotion of cell migration and invasion (hsa-miR-1909-5p, hsa-let-7c-5p) and modulation of apoptosis (miR-206-5p) | Onset | [85] |
| Study Title | Conditions | Study Type | Inclusion Criteria | Sex | Age | NCT Number |
|---|---|---|---|---|---|---|
| Studying Genes in Samples From Younger Patients With Ovarian or Testicular Sex Cord Stromal Tumors | Childhood Germ Cell Tumor; Leydig Cell Tumor; Ovarian Cancer | Observational |
Fixed and frozen tissue samples from the ATBR01 B1 tissue bank and from the International Pleuropulmonary Blastoma Registry, Children’s Hospital of Boston, and Massachusetts General Hospital | All | up to 120 Years | NCT01572467 |
| Exosomal microRNA in Predicting the Aggressiveness of Prostate Cancer in Chinese Patients | Prostate Cancer | Observational | Patients clinically diagnosed to have localized Prostate Cancer and planned for radical prostatectomy; no prior systemic therapy for Prostate Cancer used, including hormonal or chemotherapy | Male | 45 Years and older | NCT03911999 |
| The Long Noncoding MALAT1 as a Potential Salivary Diagnostic Biomarker in Oral Squamous Cell Carcinoma Through Targeting mi RNA 124 | Oral Cancer Biomarkers | Observational | Patients with Oral Squamous Cell Carcinoma and healthy controls of both sexes | All | Child, Adult, Older Adult | NCT05708209 |
| Elucidating the Genetic Basis of the Pleuropulmonary Blastoma (PPB) Familial Cancer Syndrome | Pleuropulmonary Blastoma; Cystic Nephroma; Sertoli-Leydig Cell Tumor of Ovary | Observational | Patients diagnosed with pleuropulmonary blastoma, cystic nephroma, embryonal rhabdomyosarcoma of uterine cervix, ovarian Sertoli–Leydig tumor or gynandroblastoma, pineoblastoma, pituitary blastoma, nasal chondromesenchymal hamartoma, medulloepithelioma, Wilms tumor, germline or mosaic DICER1 mutation | All | 1 Day to 95 Years | NCT00565903 |
| STI.VI. Study: How to Improve Lifestyles in Screening Contexts | Lifestyle Risk Reduction; Weight Changes, Body; Breast Cancer | Interventional |
49- to 55-year-old women invited to mammography screening; 58- to 61-year-old people (both sexes) invited to colorectal screening | All | 49 Years to 61 Years | NCT03118882 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caserta, S.; Gangemi, S.; Murdaca, G.; Allegra, A. Gender Differences and miRNAs Expression in Cancer: Implications on Prognosis and Susceptibility. Int. J. Mol. Sci. 2023, 24, 11544. https://doi.org/10.3390/ijms241411544
Caserta S, Gangemi S, Murdaca G, Allegra A. Gender Differences and miRNAs Expression in Cancer: Implications on Prognosis and Susceptibility. International Journal of Molecular Sciences. 2023; 24(14):11544. https://doi.org/10.3390/ijms241411544
Chicago/Turabian StyleCaserta, Santino, Sebastiano Gangemi, Giuseppe Murdaca, and Alessandro Allegra. 2023. "Gender Differences and miRNAs Expression in Cancer: Implications on Prognosis and Susceptibility" International Journal of Molecular Sciences 24, no. 14: 11544. https://doi.org/10.3390/ijms241411544
APA StyleCaserta, S., Gangemi, S., Murdaca, G., & Allegra, A. (2023). Gender Differences and miRNAs Expression in Cancer: Implications on Prognosis and Susceptibility. International Journal of Molecular Sciences, 24(14), 11544. https://doi.org/10.3390/ijms241411544







