Sigma-1 Receptor Agonist Fluvoxamine Ameliorates Fibrotic Response of Trabecular Meshwork Cells
Abstract
:1. Introduction
2. Results
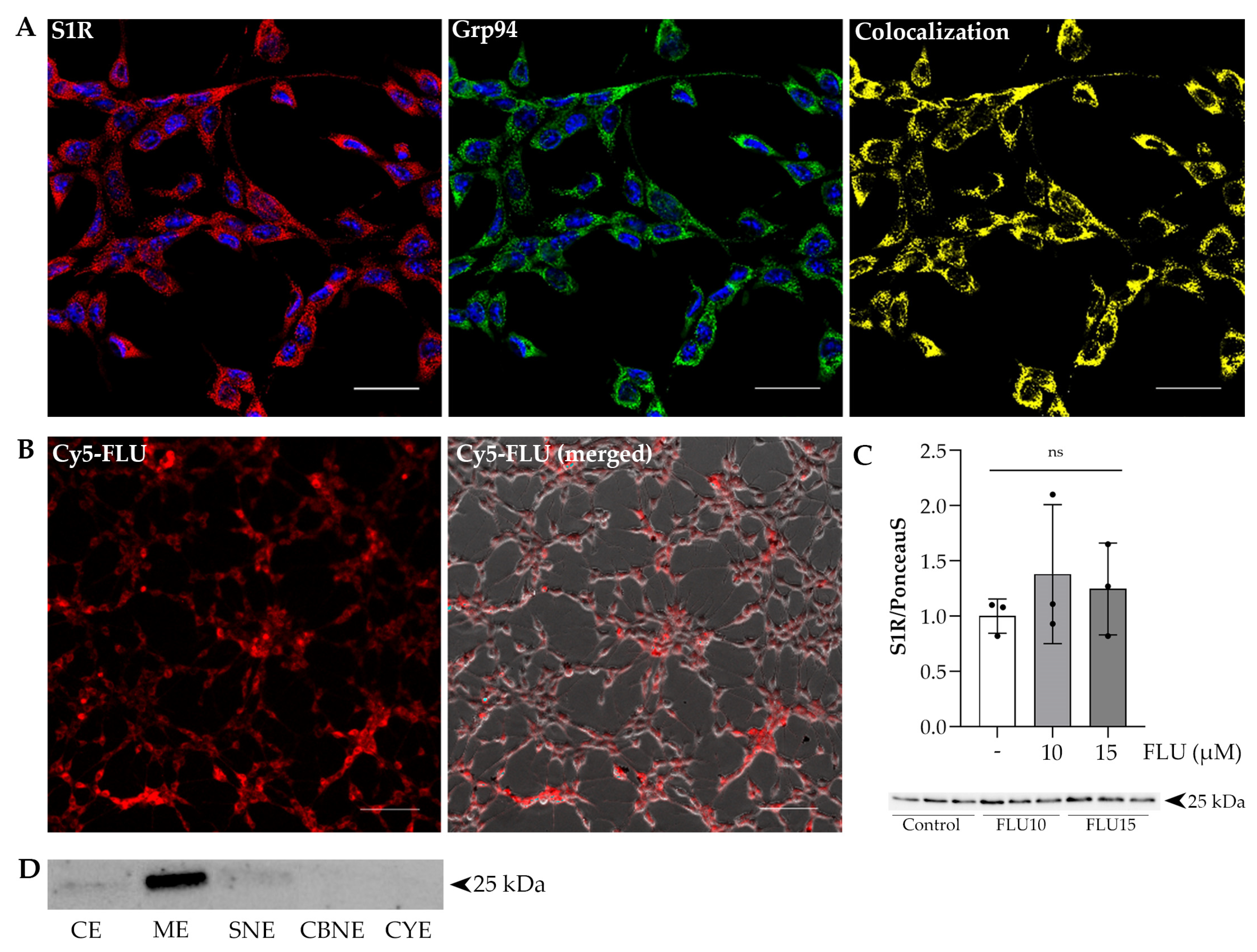
2.1. S1R Resides in the Endoplasmic Reticulum (ER) Membrane and in the Cytoplasm of Human Trabecular Meshwork Cells (HTM5), and Its Selective and Specific Agonist Fluvoxamine (FLU) Enters the Cells In Vivo
2.2. FLU Ameliorates the PDGF-Induced F-Actin Enhancement in Human Trabecular Meshwork (HTM5) Cells
2.3. FLU and Other Specific S1R Agonists Mitigate the PDGF-Induced Cell Proliferation in HTM5 Cells
2.4. S1R Knockout (S1R−/−) Primary Mouse TM (pMsTM) Cells Are More Prone to Fibrotic-like Changes
2.4.1. The Absence of S1R Results in More Pronounced Cytoskeletal Rearrangements in Response to PDGF, Whereas FLU Is Effective Only in Primary TM Cultures Isolated from Wild-Type Mice (WT)
2.4.2. S1R−/− Cells Respond with a Higher α-SMA Level to PDGF
2.5. FLU Decreases the Production of ECM Components in HTM5
2.6. FLU Decreases PDGF-Induced Cell Migration of HTM5
2.7. FLU Elevates the Level of Cathepsin K and Facilitates Nitric Oxide (NO) Release in HTM5 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Animals and Primary Mouse Trabecular Meshwork (pMsTM) Cell Isolation
4.3. Human Cell Culture
4.4. Immunocytochemistry
4.5. Cell Proliferation Assay
4.6. Cell Migration Assay
4.7. NO Measurements
4.8. Subcellular Fractionation
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniels, C.E.; Lasky, J.A.; Limper, A.H.; Mieras, K.; Gabor, E.; Schroeder, D.R.; Imatinib, I.P.F.S.I. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am. J. Respir. Crit. Care Med. 2010, 181, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar] [PubMed]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Imrie, C.; Tatham, A.J. Glaucoma: The patient’s perspective. Br. J. Gen. Pract. 2016, 66, e371–e373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leske, M.C.; Connell, A.M.; Wu, S.Y.; Hyman, L.G.; Schachat, A.P. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch. Ophthalmol. 1995, 113, 918–924. [Google Scholar] [CrossRef]
- Hashemi, H.; Mohammadi, M.; Zandvakil, N.; Khabazkhoob, M.; Emamian, M.H.; Shariati, M.; Fotouhi, A. Prevalence and risk factors of glaucoma in an adult population from Shahroud, Iran. J. Curr. Ophthalmol. 2019, 31, 366–372. [Google Scholar] [CrossRef]
- Suzuki, Y.; Iwase, A.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. Risk factors for open-angle glaucoma in a Japanese population: The Tajimi Study. Ophthalmology 2006, 113, 1613–1617. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Izumchenko, E.; Kanherkar, R.R.; Teka, M.; Cantor, C.; Manaye, K.; Sidransky, D.; West, M.D.; Makarev, E.; Csoka, A.B. Pro-fibrotic pathway activation in trabecular meshwork and lamina cribrosa is the main driving force of glaucoma. Cell. Cycle 2016, 15, 1643–1652. [Google Scholar] [CrossRef]
- Jayanetti, V.; Sandhu, S.; Lusthaus, J.A. The Latest Drugs in Development That Reduce Intraocular Pressure in Ocular Hypertension and Glaucoma. J. Exp. Pharm. 2020, 12, 539–548. [Google Scholar] [CrossRef]
- Liu, P.; Wang, F.; Song, Y.; Wang, M.; Zhang, X. Current situation and progress of drugs for reducing intraocular pressure. Ther. Adv. Chronic Dis. 2022, 13, 20406223221140392. [Google Scholar] [CrossRef]
- Kaufman, P.L.; Mohr, M.E.; Riccomini, S.P.; Rasmussen, C.A. Glaucoma Drugs in the Pipeline. Asia Pac. J. Ophthalmol. 2018, 7, 345–351. [Google Scholar] [CrossRef]
- Klinkhammer, B.M.; Floege, J.; Boor, P. PDGF in organ fibrosis. Mol. Asp. Med. 2018, 62, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.M.; Pokrovskaya, O.; O’Brien, C.J. The Function of Matricellular Proteins in the Lamina Cribrosa and Trabecular Meshwork in Glaucoma. J. Ocul. Pharm. Ther. 2015, 31, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Patent-Novel use of sigma-1 receptor agonist compounds. Available online: https://patents.google.com/patent/US20190209575A1/en (accessed on 31 May 2023).
- Xu, Z.; Lei, Y.; Qin, H.; Zhang, S.; Li, P.; Yao, K. Sigma-1 Receptor in Retina: Neuroprotective Effects and Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 7572. [Google Scholar] [CrossRef]
- Mueller, B.H.; Park, Y.; Ma, H.-Y.; Dibas, A.; Ellis, D.Z.; Clark, A.F.; Yorio, T. Sigma-1 receptor stimulation protects retinal ganglion cells from ischemia-like insult through the activation of extracellular-signal-regulated kinases 1/2. Exp. Cell. Res. 2014, 128, 156–169. [Google Scholar] [CrossRef]
- Vogler, S.; Winters, H.; Pannicke, T.; Wiedemann, P.; Reichenbach, A.; Bringmann, A. Sigma-1 receptor activation inhibits osmotic swelling of rat retinal glial (Müller) cells by transactivation of glutamatergic and purinergic receptors. Neurosci. Lett. 2016, 610, 13–18. [Google Scholar] [CrossRef]
- Smith, S.B.; Wang, J.; Cui, X.; Mysona, B.A.; Zhao, J.; Bollinger, K.E. Sigma 1 receptor: A novel therapeutic target in retinal disease. Prog. Retin. Eye Res. 2018, 67, 130–149. [Google Scholar] [CrossRef]
- Zhao, J.; Gonsalvez, G.B.; Mysona, B.A.; Smith, S.B.; Bollinger, K.E. Sigma 1 Receptor Contributes to Astrocyte-Mediated Retinal Ganglion Cell Protection. Invest. Ophthalmol. Vis. Sci. 2022, 63, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mysona, B.A.; Qureshi, A.; Kim, L.; Fields, T.; Gonsalvez, G.B.; Smith, S.B.; Bollinger, K.E. (+)-Pentazocine Reduces NMDA-Induced Murine Retinal Ganglion Cell Death Through a σR1-Dependent Mechanism. Invest. Ophthalmol. Vis. Sci. 2016, 57, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Ola, M.S.; Moore, P.; El-Sherbeny, A.; Roon, P.; Agarwal, N.; Sarthy, V.P.; Casellas, P.; Ganapathy, V.; Smith, S.B. Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Brain Res. Mol. Brain Res. 2001, 95, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Saul, A.; Roon, P.; Smith, S.B. Activation of the molecular chaperone, sigma 1 receptor, preserves cone function in a murine model of inherited retinal degeneration. Proc. Natl. Acad. Sci. USA 2016, 113, E3764–E3772. [Google Scholar] [CrossRef]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Cell. Res. 2017, 158, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polansky, J.R.; Fauss, D.J.; Zimmerman, C.C. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye 2000, 14 Pt 3B, 503–514. [Google Scholar] [CrossRef]
- Steely, H.T.; Browder, S.L.; Julian, M.B.; Miggans, S.T.; Wilson, K.L.; Clark, A.F. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2242–2250. [Google Scholar]
- Wang, J.; Rong, Y.; Liu, Y.; Zhu, M.; Chen, W.; Chen, Z.; Guo, J.; Deng, C.; Manyande, A.; Wang, P.; et al. The effect of ET1-CTGF mediated pathway on the accumulation of extracellular matrix in the trabecular meshwork and its contribution to the increase in IOP. Int. Ophthalmol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, R.; Abdullah, C.S.; Morshed, M.; Remex, N.S.; Bhuiyan, M.S. Sigmar1’s Molecular, Cellular, and Biological Functions in Regulating Cellular Pathophysiology. Front. Physiol. 2021, 12, 705575. [Google Scholar] [CrossRef]
- Pattabiraman, P.P.; Vuda, S.S.; Heo, J.; Reddy, H.; Toris, C.B.; Rhee, D.J. Clusterin-Cathepsin K Signaling in Trabecular Meshwork Outflow Pathway Regulates Intraocular Pressure. Invest. Ophthalmol. Vis. Sci. 2019, 60, 5157. [Google Scholar]
- Soundararajan, A.; Ghag, S.A.; Vuda, S.S.; Wang, T.; Pattabiraman, P.P. Cathepsin K Regulates Intraocular Pressure by Modulating Extracellular Matrix Remodeling and Actin-Bundling in the Trabecular Meshwork Outflow Pathway. Cells 2021, 10, 2864. [Google Scholar] [CrossRef]
- Reina-Torres, E.; De Ieso, M.L.; Pasquale, L.R.; Madekurozwa, M.; van Batenburg-Sherwood, J.; Overby, D.R.; Stamer, W.D. The vital role for nitric oxide in intraocular pressure homeostasis. Prog. Retin. Eye Res. 2021, 83, 100922. [Google Scholar] [CrossRef] [PubMed]
- Wiederholt, M.; Sturm, A.; Lepple-Wienhues, A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest. Ophthalmol. Vis. Sci. 1994, 35, 2515–2520. [Google Scholar] [PubMed]
- Dismuke, W.M.; Liang, J.; Overby, D.R.; Stamer, W.D. Concentration-related effects of nitric oxide and endothelin-1 on human trabecular meshwork cell contractility. Exp. Cell. Res. 2014, 120, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Ye, N.; Qin, W.; Tian, S.; Xu, Q.; Wold, E.A.; Zhou, J.; Zhen, X.-C. Small Molecules Selectively Targeting Sigma-1 Receptor for the Treatment of Neurological Diseases. J. Med. Chem. 2020, 63, 15187–15217. [Google Scholar] [CrossRef]
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 862. [Google Scholar] [CrossRef] [Green Version]
- Albayrak, Y.; Hashimoto, K. Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders. Adv. Exp. Med. Biol. 2017, 964, 153–161. [Google Scholar] [CrossRef]
- Omi, T.; Tanimukai, H.; Kanayama, D.; Sakagami, Y.; Tagami, S.; Okochi, M.; Morihara, T.; Sato, M.; Yanagida, K.; Kitasyoji, A.; et al. Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell. Death Dis. 2014, 5, e1332. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Tsai, S.-Y.; Mori, T.; Fujimoto, M.; Su, T.-P. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert. Opin. Ther. Targets 2011, 15, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Mavlyutov, T.A.; Guo, L.-W. Peeking into Sigma-1 Receptor Functions Through the Retina. Adv. Exp. Med. Biol. 2017, 964, 285–297. [Google Scholar] [CrossRef]
- Meng, B.; Li, H.; Sun, X.; Qu, W.; Yang, B.; Cheng, F.; Shi, L.; Yuan, H. σ-1 receptor stimulation protects against pressure-induced damage through InsR-MAPK signaling in human trabecular meshwork cells. Mol. Med. Rep. 2017, 16, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.G.; Sinha, N.R.; Mohan, R.R.; Webel, A.D. Novel Therapies for the Prevention of Fibrosis in Glaucoma Filtration Surgery. Biomedicines 2023, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, P.P.; Epstein, D.L.; Rao, P.V. Regulation of Adherens Junctions in Trabecular Meshwork Cells by Rac GTPase and their influence on Intraocular Pressure. J. Ocul. Biol. 2013, 1, 2. [Google Scholar] [CrossRef]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-Smooth Muscle Actin Expression Upregulates Fibroblast Contractile Activity. Mol. Biol. Cell. 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattabiraman, P.P.; Rao, P.V. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am. J. Physiol. Cell. Physiol. 2010, 298, C749–C763. [Google Scholar] [CrossRef] [Green Version]
- Nettesheim, A.; Shim, M.S.; Dixon, A.; Raychaudhuri, U.; Gong, H.; Liton, P.B. Cathepsin B Localizes in the Caveolae and Participates in the Proteolytic Cascade in Trabecular Meshwork Cells. Potential New Drug Target for the Treatment of Glaucoma. J. Clin. Med. 2020, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, P.P.; Toris, C.B. The exit strategy: Pharmacological modulation of extracellular matrix production and deposition for better aqueous humor drainage. Eur. J. Pharmacol. 2016, 787, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Stamer, W.D.; Bertrand, J.; Read, A.T.; Marando, C.M.; Ethier, C.R.; Overby, D.R. Role of nitric oxide in murine conventional outflow physiology. Am. J. Physiol. Cell. Physiol. 2015, 309, C205–C214. [Google Scholar] [CrossRef] [Green Version]
- Dismuke, W.M.; Mbadugha, C.C.; Ellis, D.Z. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am. J. Physiol. Cell. Physiol. 2008, 294, C1378–C1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavet, M.E.; Vittitow, J.L.; Impagnatiello, F.; Ongini, E.; Bastia, E. Nitric Oxide (NO): An Emerging Target for the Treatment of Glaucoma. Invest. Ophthalmol. Vis. Sci. 2014, 55, 5005–5015. [Google Scholar] [CrossRef] [Green Version]
- Aliancy, J.; Stamer, W.D.; Wirostko, B. A Review of Nitric Oxide for the Treatment of Glaucomatous Disease. Ophthalmol. Ther. 2017, 6, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Schmetterer, L.; Polak, K. Role of Nitric Oxide in the Control of Ocular Blood Flow. Prog. Retin. Eye Res. 2001, 20, 823–847. [Google Scholar] [CrossRef]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Oku, H.; Sugiyama, T.; Yang, Y.; Ikeda, T. Evidence that Nitric Oxide Is Involved in Autoregulation in Optic Nerve Head of Rabbits. Invest. Ophthalmol. Vis. Sci. 2002, 43, 784–789. [Google Scholar]
- Hayashi, K.; Michiue, H.; Yamada, H.; Takata, K.; Nakayama, H.; Wei, F.-Y.; Fujimura, A.; Tazawa, H.; Asai, A.; Ogo, N.; et al. Fluvoxamine, an anti-depressant, inhibits human glioblastoma invasion by disrupting actin polymerization. Sci. Rep. 2016, 6, 23372. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Liu, Y.; Wordinger, R.J.; Clark, A.F. A magnetic bead-based method for mouse trabecular meshwork cell isolation. Invest. Ophthalmol. Vis. Sci. 2013, 54, 3600–3606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, I.H.; Shade, D.L.; Clark, A.F.; Steely, H.T.; DeSantis, L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr. Eye Res. 1994, 13, 51–63. [Google Scholar] [CrossRef]
- Huygens Deconvolution: Restore Microscopy Images. Available online: https://svi.nl/Huygens-Deconvolution (accessed on 5 July 2023).







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodrea, J.; Tran, M.N.; Besztercei, B.; Medveczki, T.; Szabo, A.J.; Őrfi, L.; Kovacs, I.; Fekete, A. Sigma-1 Receptor Agonist Fluvoxamine Ameliorates Fibrotic Response of Trabecular Meshwork Cells. Int. J. Mol. Sci. 2023, 24, 11646. https://doi.org/10.3390/ijms241411646
Hodrea J, Tran MN, Besztercei B, Medveczki T, Szabo AJ, Őrfi L, Kovacs I, Fekete A. Sigma-1 Receptor Agonist Fluvoxamine Ameliorates Fibrotic Response of Trabecular Meshwork Cells. International Journal of Molecular Sciences. 2023; 24(14):11646. https://doi.org/10.3390/ijms241411646
Chicago/Turabian StyleHodrea, Judit, Minh Ngoc Tran, Balazs Besztercei, Timea Medveczki, Attila J. Szabo, Laszlo Őrfi, Illes Kovacs, and Andrea Fekete. 2023. "Sigma-1 Receptor Agonist Fluvoxamine Ameliorates Fibrotic Response of Trabecular Meshwork Cells" International Journal of Molecular Sciences 24, no. 14: 11646. https://doi.org/10.3390/ijms241411646
APA StyleHodrea, J., Tran, M. N., Besztercei, B., Medveczki, T., Szabo, A. J., Őrfi, L., Kovacs, I., & Fekete, A. (2023). Sigma-1 Receptor Agonist Fluvoxamine Ameliorates Fibrotic Response of Trabecular Meshwork Cells. International Journal of Molecular Sciences, 24(14), 11646. https://doi.org/10.3390/ijms241411646




