Effect of Systemic Inflammation in the CNS: A Silent History of Neuronal Damage
Abstract
1. Introduction
2. Local Infections Generate Systemic Inflammatory Response Syndrome
3. Endothelial Damage
4. CNS and Systemic Inflammation
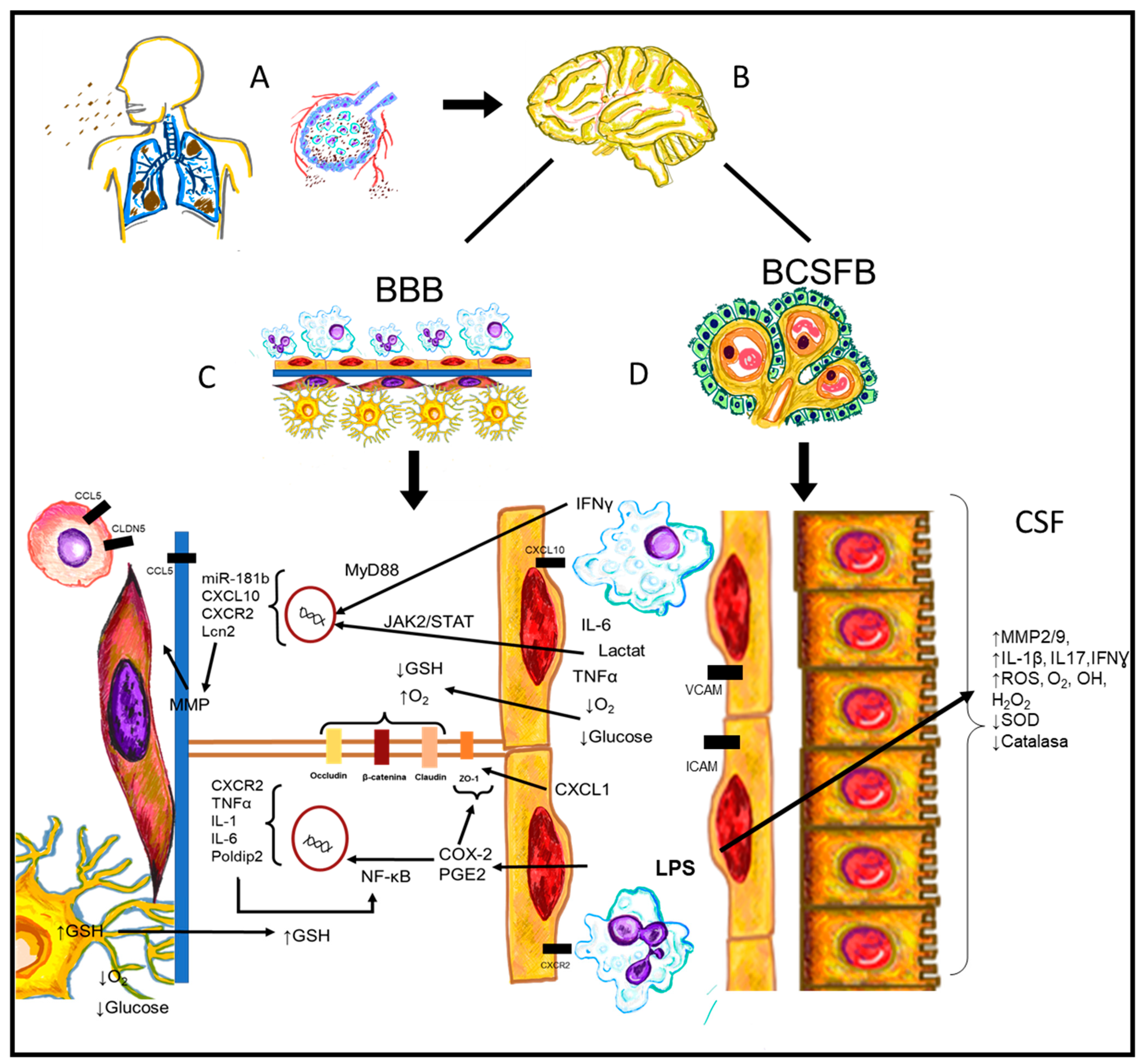
5. CVO and Systemic Inflammation
6. CNS Endothelium and Systemic Inflammation
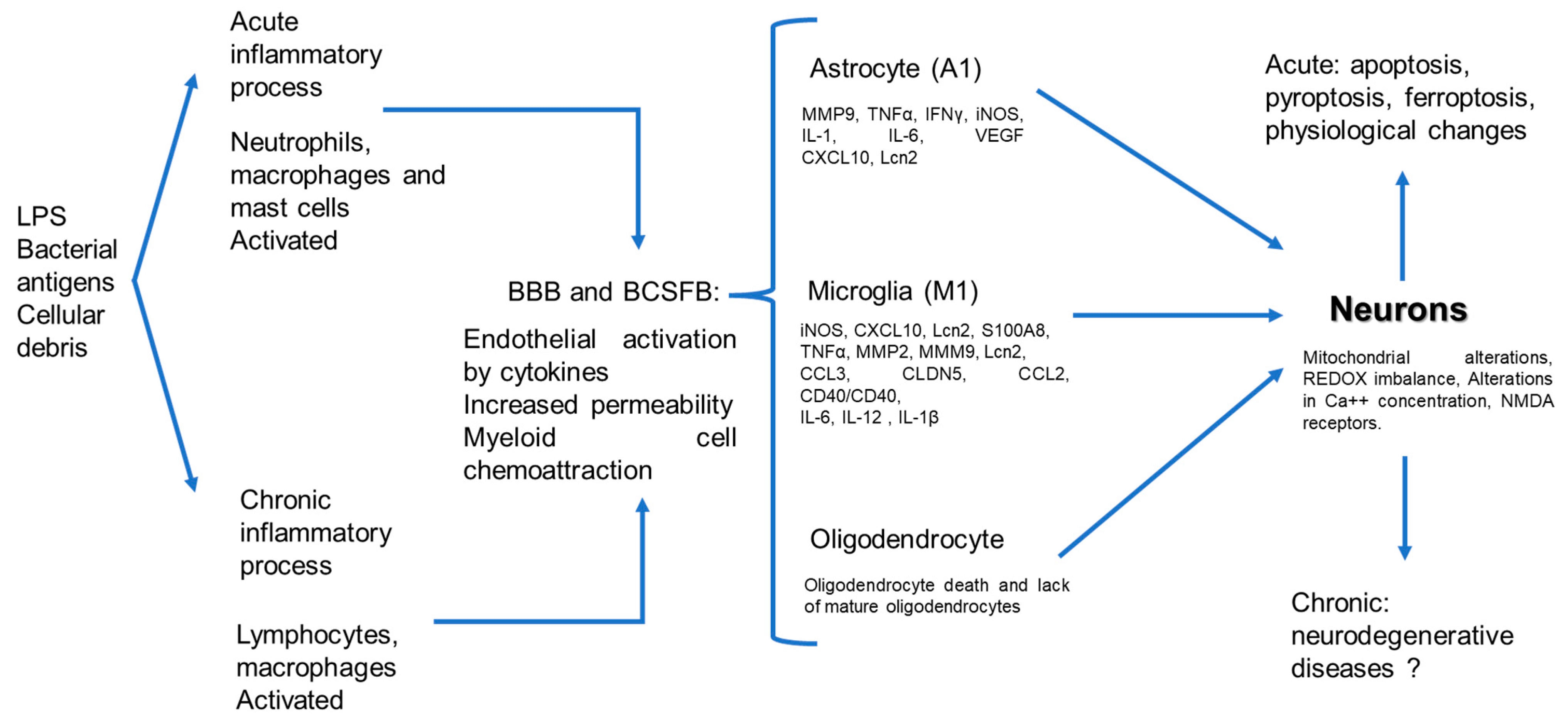
7. Glial Cells and Systemic Inflammation
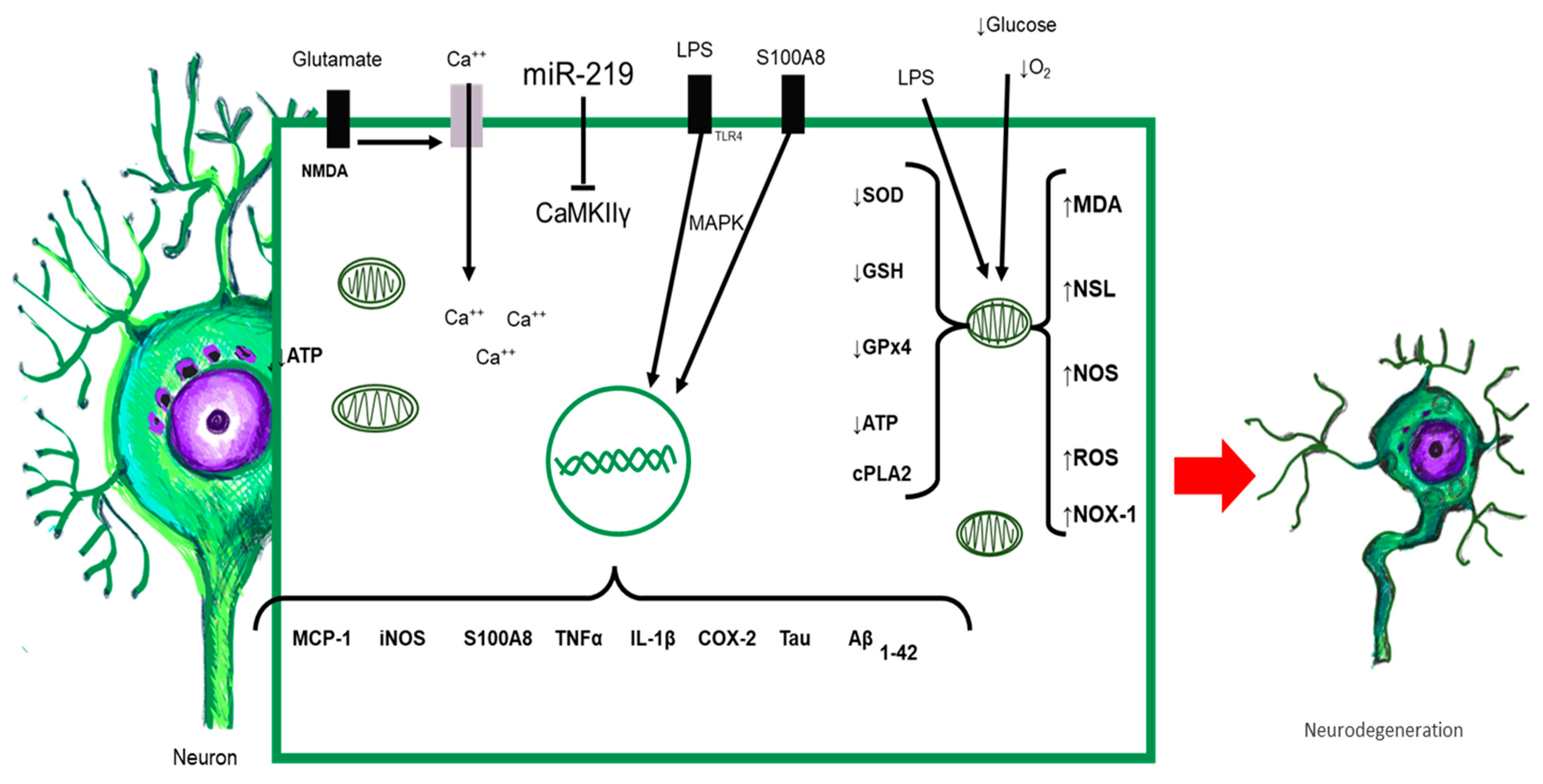
8. Neurons and Systemic Inflammation
9. Clinical Data of Sepsis and Neurological Damage
10. Systemic Inflammation and Neurodegeneration
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karin, M.; Lawrence, T.; Nizet, V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell 2006, 124, 823–835. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA J. Am. Med. Assoc 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA J. Am. Med. Assoc. 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef]
- Sonneville, R.; Verdonk, F.; Rauturier, C.; Klein, I.F.; Wolff, M.; Annane, D.; Chretien, F.; Sharshar, T. Understanding brain dysfunction in sepsis. Ann Intensive Care 2013, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Danielski, L.G.; Giustina, A.D.; Badawy, M.; Barichello, T.; Quevedo, J.; Dal-Pizzol, F.; Petronilho, F. Brain Barrier Breakdown as a Cause and Consequence of Neuroinflammation in Sepsis. Mol. Neurobiol. 2018, 55, 1045–1053. [Google Scholar] [CrossRef]
- Michels, M.; Steckert, A.V.; Quevedo, J.; Barichello, T.; Dal-Pizzol, F. Mechanisms of long-term cognitive dysfunction of sepsis: From blood-borne leukocytes to glial cells. Intensive Care Med. Exp. 2015, 3, 1–13. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Ma, D.; Jin, S.; Li, E.; Doi, Y.; Parajuli, B.; Noda, M.; Sonobe, Y.; Mizuno, T.; Suzumura, A. The neurotoxic effect of astrocytes activated with toll-like receptor ligands. J. Neuroimmunol. 2013, 254, 10–18. [Google Scholar] [CrossRef]
- Lehnardt, S.; Lehmann, S.; Kaul, D.; Tschimmel, K.; Hoffmann, O.; Cho, S.; Krueger, C.; Nitsch, R.; Meisel, A.; Weber, J.R. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J. Neuroimmunol. 2007, 190, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Yamasaki, D.; Arce-Soto, E.; Ramírez, K.; Fornaguera-Trías, J.; Mora-Gallegos, A. The role of microglia in the neuroinflammatory signaling and neuroimmune response. Rev. Electron. Neurobiol. 2016, 7, 101016. [Google Scholar]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. 2017, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.; Tuominen, H.; Vakkala, M.; Liisanantti, J.H.; Karttunen, T.; Syrjälä, H.; Ala-Kokko, T.I. Brain tight junction protein expression in sepsis in an autopsy series. Crit. Care. 2020, 24, 1–7. [Google Scholar] [CrossRef]
- Kingsley, S.M.K.; Bhat, B.V. Could stem cells be the future therapy for sepsis? Blood Rev. 2016, 30, 439–452. [Google Scholar] [CrossRef]
- Gomez, H.G.; Rugeles, M.T.; Jaimes, F.A. Key immunological characteristics in the pathophysiology of sepsis. Infectio 2015, 19, 40–46. [Google Scholar] [CrossRef]
- Verstrepen, L.; Bekaert, T.; Chau, T.L.; Tavernier, J.; Chariot, A.; Beyaert, R. TLR-4, IL-1R and TNF-R signaling to NF-κB: Variations on a common theme. Cell. Mol. Life Sci. 2008, 65, 2964–2978. [Google Scholar] [CrossRef]
- Wright, S.D. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 1999, 189, 605–609. [Google Scholar] [CrossRef]
- Durán Giménez-Rico, H.J.; Aller Reyero, M.A.; Lorente Ruigómez, L.; Durán Giménez-Rico, L.; Arias Pérez, J.; Durán Sacristán, H. Sepsis and septic shock: A turmoil of inflammatory mediators with difficult therapeutic management. An. Med. Interna 2002, 19, 35–43. [Google Scholar]
- Huang, M.; Cai, S.; Su, J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019, 20, 1–31. [Google Scholar]
- Dinarello, C.A. Cytokines as mediators in the pathogenesis of septic shock. Curr. Top. Microbiol. Immunol. 1996, 216, 133–165. [Google Scholar]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2016, 2, 1–21. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The Healing Power of Neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef]
- Roth, J.; Vogl, T.; Sorg, C.; Sunderkötter, C. Phagocyte-specific S100 proteins: A novel group of proinflammatory molecules. Trends Immunol. 2003, 24, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Bresnick, A.R.; Weber, D.J.; Zimmer, D.B. S100 proteins in cancer. Nat. Rev. Cancer 2015, 15, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Talley, S.; Valiauga, R.; Anderson, L.; Cannon, A.R.; Choudhry, M.A.; Campbell, E.M. DSS-induced inflammation in the colon drives a proinflammatory signature in the brain that is ameliorated by prophylactic treatment with the S100A9 inhibitor paquinimod. J. Neuroinflamm. 2021, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Burwinkel, F.; Van den Bos, C.; Goebeler, M.; Vollmer, E.; Sorg, C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood 1993, 82, 1875–1883. [Google Scholar] [CrossRef]
- Goyette, J.; Geczy, C.L. Inflammation-associated S100 proteins: New mechanisms that regulate function. Amino Acids 2011, 41, 821–842. [Google Scholar] [CrossRef]
- Hofer, S.; Uhle, F.; Fleming, T.; Hell, C.; Schmoch, T.; Bruckner, T. RAGE-mediated inflammation in patients with septic shock. J. Surg. Res. 2016, 202, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Dubois, C.; Marcé, D.; Faivre, V.; Lukaszewicz, A.C.; Junot, C.; Fenaille, F.; Simon, S.; Becher, F.; Morel, N.; Payen, D. High plasma level of S100A8/S100A9 and S100A12 at admission indicates a higher risk of death in septic shock patients. Sci. Rep. 2019, 9, 15660. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E.; Agresti, A. HMG proteins: Dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 2005, 15, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Antoine, D.J.; Andersson, U.; Tracey, K.J. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 2013, 93, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Foglio, E.; Pontemezzo, E.; Germani, A.; Russo, M.A.; Limana, F. HMGB1 and repair: Focus on the heart. Pharmacol. Ther. 2019, 196, 160–182. [Google Scholar] [CrossRef]
- Chen, R.; Huang, Y.; Quan, J.; Liu, J.; Wang, H.; Billiar, T.R.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Sundén-Cullberg, J.; Norrby-Teglund, A.; Rouhiainen, A.; Rauvala, H.; Herman, G.; Tracey, K.J.; Lee, M.L.; Andersson, J.; Tokics, L.; Treutiger, C.J. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit. Care Med. 2005, 33, 564–573. [Google Scholar] [CrossRef]
- Yoo, H.; Im, Y.; Ko, R.E.; Lee, J.Y.; Park, J.; Jeon, K. Association of plasma level of high-mobility group box-1 with necroptosis and sepsis outcomes. Sci. Rep. 2021, 11, 9512. [Google Scholar] [CrossRef]
- Peek, V.; Harden, L.M.; Damm, J.; Aslani, F.; Leisengang, S.; Roth, J.; Gerstberger, R.; Meurer, M.; von Köckritz-Blickwede, M.; Schulz, S.; et al. LPS Primes Brain Responsiveness to High Mobility Group Box-1 Protein. Pharmaceuticals 2021, 14, 558. [Google Scholar] [CrossRef]
- Czapski, G.A.; Zhao, Y.; Lukiw, W.J.; Strosznajder, J.B. Acute systemic inflammatory response alters transcription profile of genes related to immune response and ca2+ homeostasis in hippocampus; relevance to neurodegenerative disorders. Int. J. Mol. Sci. 2020, 21, 7838. [Google Scholar] [CrossRef]
- Denstaedt, S.J.; Spencer-Segal, J.L.; Newstead, M.; Laborc, K.; Zeng, X.; Standiford, T.J.; Singer, B.H. Persistent Neuroinflammation and Brain-Specific Immune Priming in a Novel Survival Model of Murine Pneumosepsis. Shock 2020, 54, 78–86. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Thrombus formation in vivo. J. Clin. Investig. 2005, 115, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, G.E.; Atkinson, B.T.; Frampton, J.; Watson, S.P. Thrombin-induced conversion of fibrinogen to fibrin results in rapid platelet trapping which is not dependent on platelet activation or GPIb. Br. J. Pharmacol. 2003, 138, 574–583. [Google Scholar] [CrossRef]
- Lundblad, R.L.; White, G.C. The interaction of thrombin with blood platelets. Platelets 2005, 16, 373–385. [Google Scholar] [CrossRef]
- Pin Gutiérrez, E.; Sánchez Díaz, J.S.; Martínez Rodríguez, E.A.; García Méndez, R.C.; Peniche Moguel, K.G.; Calyeca Sánchez, M.V. Classification of septic shock on the basis of unmeasured ions. Med. Crít. Col. Mex. Med. Crít. 2018, 32, 13–19. [Google Scholar]
- Fels, J.; Jeggle, P.; Liashkovich, I.; Peters, W.; Oberleithner, H. Nanomechanics of vascular endothelium. Cell Tissue Res. 2014, 355, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Kolka, C.M.; Bergman, R.N. The barrier within: Endothelial transport of hormones. Physiology 2012, 27, 237–247. [Google Scholar] [CrossRef]
- Wiesinger, A.; Peters, W.; Chappell, D.; Kentrup, D.; Reuter, S.; Pavenstädt, H.; Oberleithner, H.; Kümpers, P. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS ONE 2013, 8, e80905. [Google Scholar]
- Stubbs, D.J.; Yamamoto, A.K.; Menon, D.K. Imaging in sepsis-associated encephalopathy—Insights and opportunities. Nat. Rev. Neurol. 2013, 9, 551–561. [Google Scholar] [CrossRef]
- Semmler, A.; Hermann, S.; Mormann, F.; Weberpals, M.; Paxian, S.A.; Okulla, T.; Schäfers, M.; Kummer, M.P.; Klockgether, T.; Heneka, M.T. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J. Neuroinflamm. 2008, 5, 38. [Google Scholar] [CrossRef]
- Heming, N.; Mazeraud, A.; Verdonk, F.; Bozza, F.A.; Chrétien, F.; Sharshar, T. Neuroanatomy of sepsis-associated encephalopathy. Crit. Care 2017, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Johanson, C.E.; Duncan, J.A.; Stopa, E.G.; Baird, A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus—CSF route. Pharm. Res. 2005, 22, 1011–1037. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Chow, B.W.; Nuñez, V.; Kaplan, L.; Granger, A.J.; Bistrong, K.; Zucker, H.L.; Kumar, P.; Sabatini, B.L.; Gu, C. Caveolae in CNS arterioles mediate neurovascular coupling. Nature. 2020, 579, 106–110. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–24. [Google Scholar] [CrossRef]
- Gutierrez, E.G.; Banks, W.A.; Kastin, A.J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 1993, 47, 169–176. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Gutierrez, E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994, 179, 53–56. [Google Scholar] [CrossRef]
- Kikuchi, D.S.; Campos, A.C.P.; Qu, H.; Forrester, S.J.; Pagano, R.L.; Lassègue, B.; Sadikot, R.T.; Griendling, K.K.; Hernandes, M.S. Poldip2 mediates blood-brain barrier disruption in a model of sepsis-associated encephalopathy. J. Neuroinflamm. 2019, 16, 241. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G.; et al. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflamm. 2015, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Dohgu, S.; Takata, F.; Eto, T.; Ishikawa, N.; Kodama, K.B.; Nakagawa, S.; Yamauchi, A.; Kataoka, Y. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell. Mol. Neurobiol. 2009, 29, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Frister, A.; Schmidt, C.; Schneble, N.; Brodhun, M.; Gonnert, F.A.; Bauer, M.; Hirsch, E.; Müller, J.P.; Wetzker, R.; Bauer, R. Phosphoinositide 3-kinase γ affects LPS-induced disturbance of blood-brain barrier via lipid kinase-independent control of cAMP in microglial cells. NeuroMolecular Med. 2014, 16, 704–713. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinosa, J.V.; Santana-Martínez, R.A.; Maldonado, P.D.; Zetter, M.; Becerril-Villanueva, E.; Pérez-Sánchez, G.; Pavón, L.; Mata-Espinosa, D.; Barrios-Payán, J.; López-Torres, M.O.; et al. Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male BALB/c Mice. Int. J. Mol. Sci. 2020, 21, 9483. [Google Scholar] [CrossRef]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgärtner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-Term Neuroinflammation Induced by Influenza A Virus Infection and the Impact on Hippocampal Neuron Morphology and Function. Long-term neuroinflammation induced by influenza a virus infection and the impact on hippocampal neuron morphology and function. J. Neurosci. 2018, 38, 3060–3080. [Google Scholar] [CrossRef]
- Sweis, R.; Ortiz, J.; Biller, J. Neurology of Sepsis. Curr. Neurol. Neurosci. Rep. 2016, 16, 21. [Google Scholar] [CrossRef]
- Azabou, E.; Magalhaes, E.; Braconnier, A.; Yahiaoui, L.; Moneger, G.; Heming, N.; Annane, D.; Mantz, J.; Chrétien, F.; Durand, M.C.; et al. Groupe d’Explorations Neurologiques en Réanimation (GENER). Early Standard Electroencephalogram Abnormalities Predict Mortality in Septic Intensive Care Unit Patients. PLoS ONE 2015, 10, e0139969. [Google Scholar] [CrossRef]
- Sonneville, R.; de Montmollin, E.; Poujade, J.; Garrouste-Orgeas, M.; Souweine, B.; Darmon, M.; Mariotte, E.; Argaud, L.; Barbier, F.; Goldgran-Toledano, D.; et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 2017, 43, 1075–1084. [Google Scholar] [CrossRef]
- Warford, J.; Lamport, A.C.; Kennedy, B.; Easton, A.S. Human Brain Chemokine and Cytokine Expression in Sepsis: A Report of Three Cases. Can. J. Neurol. Sci. 2017, 44, 96–104. [Google Scholar] [CrossRef]
- Sarin, H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes Res. 2010, 2, 14. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.F. Choroid plexus in the central nervous system: Biology and physiopathology. J. Neuropathol. Exp. Neurol. 2000, 59, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Paulus, W. Choroid plexus: Biology and pathology. Acta Neuropathol. 2010, 119, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Solár, P.; Zamani, A.; Kubíčková, L.; Dubový, P.; Joukal, M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.J.; McCarthy, L.E.; Borison, H.L. Electron microscopic study on the epiplexus (Kolmer) cells of the cat choroid plexus. Z. Für Zellforsch. Und Mikrosk. Anat. 1970, 110, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, E.W. The origin of (Kolmer’s) epiplexus cells—A combined histomorphological and histochemical study. Histochemistry 1975, 44, 103–104. [Google Scholar] [CrossRef]
- Bors, L.; Tóth, K.; Tóth, E.Z.; Bajza, Á.; Csorba, A.; Szigeti, K.; Máthé, D.; Perlaki, G.; Orsi, G.; Tóth, G.K.; et al. Age-dependent changes at the blood-brain barrier. A Comparative structural and functional study in young adult and middle aged rats. Brain Res. Bull. 2018, 139, 269–277. [Google Scholar] [CrossRef]
- Huber, J.D.; Egleton, R.D.; Davis, T.P. Molecular physiology and pathophysiology of tight junctions in the blood -brain barrier. Trends Neurosci. 2001, 24, 719–725. [Google Scholar] [CrossRef]
- Parikh, V.; Tucci, V.; Galwankar, S. Infections of the nervous system. Int. J. Crit. Illn. Inj. Sci. 2012, 2, 1–52. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Garibay, C.; Salinas-Lara, C.; Gómez-López, M.A.; Soto-Rojas, L.O.; Castillón-Benavides, N.K.; Castillón-Benavides, O.J.; Hernández-Campos, M.E.; Hernández-Pando, R.; Marquina-Castillo, B.; Flores-Barrada, M.A.; et al. Mycobacterium tuberculosis Infection Induces BCSFB Disruption but No BBB Disruption In Vivo: Implications in the Pathophysiology of Tuberculous Meningitis. Int. J. Mol. Sci. 2022, 23, 6436. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Herkenham, M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 2005, 25, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, M.; Szczepkowska, A.; Herman, A.P.; Pellicer-Rubio, M.T.; Jałyński, M.; Skipor, J. Melatonin from slow-release implants did not influence the gene expression of the lipopolysaccharide receptor complex in the choroid plexus of seasonally anoestrous adult ewes subjected or not to a systemic inflammatory stimulus. Small Rumin. Res. 2017, 147, 1–7. [Google Scholar] [CrossRef]
- Lacroix, S.; Feinstein, D.; Rivest, S. The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol. 1998, 8, 625–640. [Google Scholar] [CrossRef]
- Quan, N.; Whiteside, M.; Kim, L.; Herkenham, M. Induction of inhibitory factor κBα mRNA in the central nervous system after peripheral lipopolysaccharide administration: An in situ hybridization histochemistry study in the rat. Proc. Natl. Acad. Sci. USA 1997, 94, 10985–10990. [Google Scholar] [CrossRef]
- Stridh, L.; Ek, C.J.; Wang, X.; Nilsson, H.; Mallard, C. Regulation of Toll-Like Receptors in the Choroid Plexus in the Immature Brain After Systemic Inflammatory Stimuli. Transl. Stroke Res. 2013, 4, 220–227. [Google Scholar] [CrossRef]
- Laflamme, N.; Echchannaoui, H.; Landmann, R.; Rivest, S. Cooperation between toll-like receptor 2 and 4 in the brain of mice challenged with cell wall components derived from gram-negative and gram-positive bacteria. Eur. J. Immunol. 2003, 33, 1127–1138. [Google Scholar] [CrossRef]
- Laflamme, N.; Rivest, S. Toll-like receptor 4: The missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 2001, 15, 155–163. [Google Scholar] [CrossRef]
- Wang, D.; Duan, H.; Feng, J.; Xiang, J.; Feng, L.; Liu, D.; Chen, X.; Jing, L.; Liu, Z.; Zhang, D.; et al. Soluble CD146, a cerebrospinal fluid marker for neuroinflammation, promotes blood-brain barrier dysfunction. Theranostics 2020, 10, 231–246. [Google Scholar] [CrossRef]
- Chiu, P.S.; Lai, S.C. Matrix Metalloproteinase-9 Leads to Claudin-5 Degradation via the NF-κB Pathway in BALB/c Mice with Eosinophilic Meningoencephalitis Caused by Angiostrongylus cantonensis. PLoS ONE 2013, 8, e53370. [Google Scholar] [CrossRef]
- Goldim, M.P.; Danielski, L.G.; Rodrigues, J.F.; Joaquim, L.; Garbossa, L.; de Oliveira Junior, A.N.; Metzker, K.L.L.; Giustina, A.D.; Cardoso, T.; Barichello, T.; et al. Oxidative stress in the choroid plexus contributes to blood-cerebrospinal fluid barrier disruption during sepsis development. Microvasc. Res. 2019, 123, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Fortunato, J.J.; Vitali, A.M.; Feier, G.; Reinke, A.; Moreira, J.C.; Quevedo, J.; Dal-Pizzol, F. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit. Care Med. 2006, 34, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.D.; Frank, M.G.; Tracey, K.J.; Watkins, L.R.; Maier, S.F. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male sprague dawley rats: A priming stimulus of microglia and the NLRP3 inflammasome. J. Neurosci. 2015, 35, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ganong, W.F. Circumventricular organs: Definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000, 27, 422–427. [Google Scholar] [CrossRef]
- Roth, J.; Harré, E.M.; Rummel, C.; Gerstberger, R.; Hübschle, T. Signaling the brain in systemic inflammation: Role of sensory circumventricular organs. Front. Biosci. 2004, 9, 290–300. [Google Scholar] [CrossRef]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Wen, Z.; Xu, L.; Chen, X.; Xu, W.; Yin, Z.; Gao, X.; Xiong, S. Autoantibody Induction by DNA-Containing Immune Complexes Requires HMGB1 with the TLR2/MicroRNA-155 Pathway. J. Immunol. 2013, 190, 5411–5422. [Google Scholar] [CrossRef] [PubMed]
- Murayama, S.; Kurganov, E.; Miyata, S. Activation of microglia and macrophages in the circumventricular organs of the mouse brain during TLR2-induced fever and sickness responses. J. Neuroimmunol. 2019, 334, 576973. [Google Scholar] [CrossRef]
- Tian, J.; Avalos, A.M.; Mao, S.Y.; Chen, B.; Senthil, K.; Wu, H.; Parroche, P.; Drabic, S.; Golenbock, D.; Sirois, C.; et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007, 8, 487–496. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
- Huang, S.F.; Othman, A.; Koshkin, A.; Fischer, S.; Fischer, D.; Zamboni, N.; Ono, K.; Sawa, T.; Ogunshola, O.O. Astrocyte glutathione maintains endothelial barrier stability. Redox Biol. 2020, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Hsieh, H.-L.; Shih, R.-H.; Chi, P.-L.; Cheng, S.-E.; Yang, C.-M. Up-regulation of COX-2/PGE2 by endothelin-1 via MAPK-dependent NF-κB pathway in mouse brain microvascular endothelial cells. Cell Commun. Signal. 2013, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Ohira, S.; Isse, K.; Ozaki, S.; Zen, Y.; Sato, Y.; Nakanuma, Y. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Lab. Investig. 2003, 83, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, X.; Zhai, L.; Wang, H.; Sun, M.; Song, C.; Wang, T.; Qian, Z. CXCR2 antagonist attenuates neutrophil transmigration into brain in a murine model of LPS induced neuroinflammation. Biochem. Biophys. Res. Commun. 2020, 529, 839–845. [Google Scholar] [CrossRef]
- Chen, S.L.; Cai, G.X.; Ding, H.G.; Liu, X.Q.; Wang, Z.H.; Jing, Y.W.; Han, Y.L.; Jiang, W.Q.; Wen, M.Y. JAK/STAT signaling pathway-mediated microRNA-181b promoted blood-brain barrier impairment by targeting sphingosine-1-phosphate receptor 1 in septic rats. Ann. Transl. Med. 2020, 8, 1458. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, X.; Dong, R.; Sun, L.; Chen, L.; Zhang, Z.; Peng, M. MicroRNA-181b-5p attenuates early postoperative cognitive dysfunction by suppressing hippocampal neuroinflammation in mice. Cytokine 2019, 120, 41–53. [Google Scholar] [CrossRef]
- Fritz, M.; Klawonn, A.M.; Jaarola, M.; Engblom, D. Interferon-ɣ mediated signaling in the brain endothelium is critical for inflammation-induced aversion. Brain Behav. Immun. 2018, 67, 54–58. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Zhang, H.; Li, S.; Li, J.; Liu, H.; Cheng, Q. The role of lipocalin 2 in brain injury and recovery after ischemic and hemorrhagic stroke. Front. Mol. Neurosci. 2022, 15, 930526. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, G.A.; Vindedal, G.F.; Skare, Ø.; Nagelhus, E.A. Evidence that pericytes regulate aquaporin-4 polarization in mouse cortical astrocytes. Brain Struct. Funct. 2014, 219, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Dohgu, S.; Matsumoto, J.; Takahashi, H.; Machida, T.; Wakigawa, T.; Harada, E.; Miyaji, H.; Koga, M.; Nishioku, T.; et al. Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-α, releasing matrix metalloproteinase-9 and migrating in vitro. J. Neuroinflamm. 2011, 8, 106. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.M.; Montagne, A.; Kisler, K.; Dai, Z.; Wang, Y.; Huuskonen, M.T.; Sagare, A.P.; Lazic, D.; Sweeney, M.D.; Kong, P.; et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019, 7, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Kovac, A.; Erickson, M.A.; Banks, W.A. Brain microvascular pericytes are immunoactive in culture: Cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J. Neuroinflammation 2011, 8, 1–9. [Google Scholar] [CrossRef]
- Liu, C.Y.; Yang, Y.; Ju, W.N.; Wang, X.; Zhang, H.L. Emerging roles of astrocytes in neuro-vascular unit and the tripartite synapse with emphasis on reactive gliosis in the context of alzheimer’s disease. Front. Cell. Neurosci. 2018, 12, 193. [Google Scholar] [CrossRef]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Dual roles of astrocyte-derived factors in regulation of blood-brain barrier function after brain damage. Int. J. Mol. Sci. 2019, 20, 571. [Google Scholar] [CrossRef]
- Jang, E.; Kim, J.H.; Lee, S.; Kim, J.H.; Seo, J.W.; Jin, M.; Lee, M.G.; Jang, I.S.; Lee, W.H.; Suk, K. Phenotypic Polarization of Activated Astrocytes: The Critical Role of Lipocalin-2 in the Classical Inflammatory Activation of Astrocytes. J. Immunol. 2013, 191, 5204–5219. [Google Scholar] [CrossRef]
- Gouix, E.; Buisson, A.; Nieoullon, A.; Kerkerian-Le, G.L.; Tauskela, J.S.; Blondeau, N.; Had-Aissouni, L. Oxygen glucose deprivation-induced astrocyte dysfunction provokes neuronal death through oxidative stress. Pharmacol. Res. 2014, 87, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Tan, S.; Lin, Y.; Liao, S.; Zhang, B.; Chen, X.; Wang, J.; Deng, Z.; Zeng, Q.; Zhang, L.; et al. The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J. Neuroinflamm. 2019, 16, 242. [Google Scholar] [CrossRef]
- Domínguez, R.O. Prof. Pío del Río-Hortega: From oligodendroglia to demyelination-remyelination. Previous history and exile in Argentina 1940 to 1945. Neurol. Argent. 2016, 8, 61–64. [Google Scholar] [CrossRef]
- Kabba, J.A.; Xu, Y.; Christian, H.; Ruan, W.; Chenai, K.; Xiang, Y.; Zhang, L.; Saavedra, J.M.; Pang, T. Microglia: Housekeeper of the Central Nervous System. Cell. Mol. Neurobiol. 2018, 38, 53–71. [Google Scholar] [CrossRef]
- Gopinath, A.; Collins, A.; Khoshbouei, H.; Streit, W.J. Microglia and other myeloid cells in central nervous system health and disease. J. Pharmacol. Exp. Ther. 2020, 375, 154–160. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Michels, M.; Danieslki, L.G.; Vieira, A.; Florentino, D.; Dall’Igna, D.; Galant, L.; Sonai, B.; Vuolo, F.; Mina, F.; Pescador, B.; et al. CD40-CD40 Ligand Pathway is a Major Component of Acute Neuroinflammation and Contributes to Long-term Cognitive Dysfunction after Sepsis. Mol. Med. 2015, 21, 219–226. [Google Scholar] [CrossRef]
- Jang, E.; Lee, S.; Kim, J.H.; Kim, J.H.; Seo, J.W.; Lee, W.H.; Mori, K.; Nakao, K.; Suk, K. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 2013, 27, 1176–1190. [Google Scholar] [CrossRef]
- Bauer, A.T.; Bürgers, H.F.; Rabie, T.; Marti, H.H. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J. Cereb. Blood Flow Metab. 2010, 30, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Zhou, Y.; Qiu, L.B.; Ding, G.R.; Pang, X.F. Altered expression of matrix metalloproteinases and tight junction proteins in rats following PEMF-induced BBB permeability change. Biomed. Environ. Sci. 2012, 25, 197–202. [Google Scholar]
- Ha, J.S.; Choi, H.R.; Kim, I.S.; Kim, E.A.; Cho, S.W.; Yang, S.J. Hypoxia-induced S100A8 expression activates microglial inflammation and promotes neuronal apoptosis. Int. J. Mol. Sci. 2021, 22, 1205. [Google Scholar] [CrossRef]
- Ma, L.; Sun, P.; Zhang, J.C.; Zhang, Q.; Yao, S.L. Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling pathways in BV-2 microglial cells. Int. J. Mol. Med. 2017, 40, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xu, L.; Wang, Y.; Zhou, N.; Zhen, F.; Zhang, Y.; Qu, X.; Fan, H.; Liu, S.; Chen, Y.; et al. S100A8/A9 induces microglia activation and promotes the apoptosis of oligodendrocyte precursor cells by activating the NF-κB signaling pathway. Brain Res. Bull. 2018, 143, 234–245. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Yan, B. Clash of the Cytokine Titans: Counter-regulation of interleukin-1 and type i interferon-mediated inflammatory responses. Cell. Mol. Immunol. 2017, 14, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, I.E.; Lewen, A.; Galow, L.V.; Cesetti, T.; Scheffel, J.; Regen, T.; Hanisch, U.K.; Kann, O. TLR4-activated microglia require IFN-γ to induce severe neuronal dysfunction and death in situ. Proc. Natl. Acad. Sci. USA 2016, 113, 212–217. [Google Scholar] [CrossRef]
- Manabe, T.; Rácz, I.; Schwartz, S.; Oberle, L.; Santarelli, F.; Emmrich, J.V.; Neher, J.J.; Heneka, M.T. Systemic inflammation induced the delayed reduction of excitatory synapses in the CA3 during ageing. J. Neurochem. 2021, 159, 525–542. [Google Scholar] [CrossRef]
- Neher, J.J.; Neniskyte, U.; Zhao, J.-W.; Bal-Price, A.; Tolkovsky, A.M.; Brown, G.C. Inhibition of Microglial Phagocytosis Is Sufficient to Prevent Inflammatory Neuronal Death. J. Immunol. 2011, 186, 4973–4983. [Google Scholar] [CrossRef] [PubMed]
- Semmler, A.; Okulla, T.; Sastre, M.; Dumitrescu-Ozimek, L.; Heneka, M.T. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J. Chem. Neuroanat. 2005, 30, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Olympiou, M.; Sargiannidou, I.; Markoullis, K.; Karaiskos, C.; Kagiava, A.; Kyriakoudi, S.; Abrams, C.K.; Kleopa, K.A. Systemic inflammation disrupts oligodendrocyte gap junctions and induces ER stress in a model of CNS manifestations of X-linked Charcot-Marie-Tooth disease. Acta Neuropathol. Commun. 2016, 4, 95. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, H.J.; Lee, K.; Lim, J.; Hwang, J.K.; Kim, C.R.; Kim, H.A.; Kim, H.S.; Park, H.K. Effects of Lipopolysaccharide on Oligodendrocyte Differentiation at Different Developmental Stages: An In Vitro Study. J. Korean Med. Sci. 2021, 36, e332. [Google Scholar] [CrossRef]
- Carlson, N.G.; Bellamkonda, S.; Schmidt, L.; Redd, J.; Huecksteadt, T.; Weber, L.M.; Davis, E.; Wood, B.; Maruyama, T.; Rose, J.W. The role of the prostaglandin E2 receptors in vulnerability of oligodendrocyte precursor cells to death. J. Neuroinflamm. 2015, 12, 101. [Google Scholar] [CrossRef]
- Shiow, L.R.; Favrais, G.; Schirmer, L.; Schang, A.L.; Cipriani, S.; Andres, C.; Wright, J.N.; Nobuta, H.; Fleiss, B.; Gressens, P.; et al. Reactive astrocyte COX2-PGE2 production inhibits oligodendrocyte maturation in neonatal white matter injury. Glia 2017, 65, 2024–2037. [Google Scholar] [CrossRef]
- Pang, Y.; Cai, Z.; Rhodes, P.G. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Dev. Brain Res. 2003, 140, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Buser, J.R.; Maire, J.; Riddle, A.; Gong, X.; Nguyen, T.; Nelson, K.; Luo, N.L.; Ren, J.; Struve, J.; Sherman, L.S.; et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 2012, 71, 93–109. [Google Scholar] [CrossRef]
- Czapski, G.A.; Gajkowska, B.; Strosznajder, J.B. Systemic administration of lipopolysaccharide induces molecular and morphological alterations in the hippocampus. Brain Res. 2010, 1356, 85–94. [Google Scholar] [CrossRef]
- Czapski, G.A.; Cakala, M.; Chalimoniuk, M.; Gajkowska, B.; Strosznajder, J.B. Role of nitric oxide in the brain during lipopolysaccharide-evoked systemic inflammation. J. Neurosci. Res. 2007, 85, 1694–1703. [Google Scholar] [CrossRef]
- He, Y.; Zhou, A.; Jiang, W. Toll-like receptor 4-mediated signaling participates in apoptosis of hippocampal neurons. Neural Regen. Res. 2013, 8, 2744–2753. [Google Scholar] [PubMed]
- Flores-Martínez, Y.M.; Fernández-Parrilla, M.A.; Ayala-Dávila, J.; Reyes-Corona, D.; Blanco-Álvarez, V.M.; Soto-Rojas, L.O.; Luna-Herrera, C.; González-Barrios, J.A.; Leon-Chavez, B.A.; Gutierrez-Castillo, M.E.; et al. Acute Neuroinflammatory Response in the Substantia Nigra Pars Compacta of Rats after a Local Injection of Lipopolysaccharide. J. Immunol. Res. 2018, 2018, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.E.; Liu, L.; Wang, Y.C.; Wang, C.T.; Zheng, Q.; Liu, Q.X.; Li, Z.F.; Bai, X.J.; Liu, X.H. Caspase-1 inhibitor exerts brain-protective effects against sepsis-associated encephalopathy and cognitive impairments in a mouse model of sepsis. Brain Behav. Immun. 2019, 8, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Arzeta, I.E.; Soto-Rojas, L.O.; Flores-Martinez, Y.M.; Delgado-Minjares, K.M.; Gatica-Garcia, B.; Mascotte-Cruz, J.U.; Nava, P.; Aparicio-Trejo, O.E.; Reyes-Corona, D.; Martínez-Dávila, I.A.; et al. LPS Triggers Acute Neuroinflammation and Parkinsonism Involving NLRP3 Inflammasome Pathway and Mitochondrial CI Dysfunction in the Rat. Int. J. Mol. Sci. 2023, 24, 4628. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Chen, Y.; Li, Y.; Zhang, Y.; Qi, H.; Xu, W. Hippocampal neuronal ferroptosis involved in cognitive dysfunction in rats with sepsis-related encephalopathy through the Nrf2/GPX4 signaling pathway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 1389–1394. [Google Scholar]
- Xie, Z.; Xu, M.; Xie, J.; Liu, T.; Xu, X.; Gao, W.; Li, Z.; Bai, X.; Liu, X. Inhibition of Ferroptosis Attenuates Glutamate Excitotoxicity and Nuclear Autophagy in a CLP Septic Mouse Model. Shock 2022, 57, 694–702. [Google Scholar] [CrossRef]
- Kang, Y.; Tiziani, S.; Park, G.; Kaul, M.; Paternostro, G. Cellular protection using Flt3 and PI3Kα inhibitors demonstrates multiple mechanisms of oxidative glutamate toxicity. Nat. Commun. 2014, 5, 3672. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Green, J.A.; Dholakia, S.; Janczar, K.; Ong, C.W.; Moores, R.; Fry, J.; Elkington, P.T.; Roncaroli, F.; Friedland, J.S. Mycobacterium tuberculosis-infected human monocytes down-regulate microglial MMP-2 secretion in CNS tuberculosis via TNFα, NFκB, p38 and caspase 8 dependent pathways. J. Neuroinflamm. 2011, 8, 46. [Google Scholar] [CrossRef]
- Sharshar, T.; Gray, F.; Lorin de la Grandmaison, G.; Hopkinson, N.S.; Ross, E.; Dorandeu, A.; Orlikowski, D.; Raphael, J.C.; Gajdos, P.; Annane, D. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 2003, 362, 1799–1805. [Google Scholar] [CrossRef]
- Sharshar, T.; Annane, D.; De La Gradmaison, G.L.; Brouland, J.P.; Hopkinson, N.S.; Gray, F. The Neuropathology of Septic Shock. Brain Pathol. 2004, 14, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef]
- Huerta, P.T.; Robbiati, S.; Huerta, T.S.; Sabharwal, A.; Berlin, R.A.; Frankfurt, M.; Volpe, B.T. Preclinical models of overwhelming sepsis implicate the neural system that encodes contextual fear memory. Mol. Med. 2016, 22, 789–799. [Google Scholar] [CrossRef]
- Gunther, M.L.; Morandi, A.; Krauskopf, E.; Pandharipande, P.; Girard, T.D.; Jackson, J.C.; Thompson, J.; Shintani, A.K.; Geevarghese, S.; Miller, R.R., 3rd; et al. Visions Investigation, Visualizing Icu Survivors Neuroradiological Sequelae. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: The VISIONS cohort magnetic resonance imaging study. Crit. Care Med. 2012, 40, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Orhun, G.; Tüzün, E.; Bilgiç, B.; Ergin Özcan, P.; Sencer, S.; Barburoğlu, M.; Esen, F. Brain Volume Changes in Patients with Acute Brain Dysfunction Due to Sepsis. Neurocrit. Care. 2020, 32, 459–468. [Google Scholar] [CrossRef]
- Beyer, M.M.S.; Lonnemann, N.; Remus, A.; Latz, E.; Heneka, M.T.; Korte, M. Enduring Changes in Neuronal Function upon Systemic Inflammation Are NLRP3 Inflammasome Dependent. J. Neurosci. 2020, 40, 5480–5494. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Ai, S.; Ouyang, W.; Le, Y.; Tong, J. Sepsis-induced selective loss of NMDA receptors modulates hippocampal neuropathology in surviving septic mice. PLoS ONE 2017, 12, e0188273. [Google Scholar] [CrossRef]
- Verma, A.; Azhar, G.; Zhang, X.; Patyal, P.; Kc, G.; Sharma, S.; Che, Y.; Wei, J.Y.P. P. gingivalis-LPS Induces Mitochondrial Dysfunction Mediated by Neuroinflammation through Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 950. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Zhou, R.; Li, Y.; Gao, Y.; Tu, D.; Wilson, B.; Song, S.; Feng, J.; Hong, J.S.; et al. A novel role of NLRP3-generated IL-1β in the acute-chronic transition of peripheral lipopolysaccharide-elicited neuroinflammation: Implications for sepsis-associated neurodegeneration. J. Neuroinflamm. 2020, 17, 64. [Google Scholar] [CrossRef]
- Fu, H.Q.; Yang, T.; Xiao, W.; Fan, L.; Wu, Y.; Terrando, N.; Wang, T.L. Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS ONE 2014, 9, e106331. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.H.; Dickson, R.P.; Denstaedt, S.J.; Newstead, M.W.; Kim, K.; Falkowski, N.R.; Erb-Downward, J.R.; Schmidt, T.M.; Huffnagle, G.B.; Standiford, T.J. Bacterial Dissemination to the Brain in Sepsis. Am J Respir Crit Care Med. 2018, 197, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.H.; Newstead, M.W.; Zeng, X.; Cooke, C.L.; Thompson, R.C.; Singer, K.; Ghantasala, R.; Parent, J.M.; Murphy, G.G.; Iwashyna, T.J.; et al. Cecal Ligation and Puncture Results in Long-Term Central Nervous System Myeloid Inflammation. PLoS ONE 2016, 11, e0149136. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Mohammed, N.A.; Sleem, A.A. The effects of trimetazidine on lipopolysaccharide-induced oxidative stress in mice. EXCLI J. 2011, 10, 162–172. [Google Scholar]
- Manfredini, A.; Constantino, L.; Pinto, M.C.; Michels, M.; Burger, H.; Kist, L.W.; Silva, M.C.; Gomes, L.M.; Dominguini, D.; Steckert, A.; et al. Mitochondrial dysfunction is associated with long-term cognitive impairment in an animal sepsis model. Clin. Sci. 2019, 133, 1993–2004. [Google Scholar] [CrossRef]
- Morales-Martínez, A.; Martínez-Gómez, P.A.; Martinez-Fong, D.; Villegas-Rojas, M.M.; Pérez-Severiano, F.; Del Toro-Colín, M.A.; Delgado-Minjares, K.M.; Blanco-Alvarez, V.M.; Leon-Chavez, B.A.; Aparicio-Trejo, O.E.; et al. Oxidative Stress and Mitochondrial Complex I Dysfunction Correlate with Neurodegeneration in an α-Synucleinopathy Animal Model. Int. J. Mol. Sci. 2022, 23, 11394. [Google Scholar] [CrossRef]
- Hirai, K.; Aliev, G.; Nunomura, A.; Fujioka, H.; Russell, R.L.; Atwood, C.S.; Johnson, A.B.; Kress, Y.; Vinters, H.V.; Tabaton, M.; et al. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001, 21, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Kelley, K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007, 21, 153–160. [Google Scholar] [CrossRef]
- Adam, N.; Kandelman, S.; Mantz, J.; Chretien, F.; Sharshar, T. Sepsis-induced brain dysfunction. Expert Rev. Anti Infect. Ther. 2013, 11, 211–221. [Google Scholar] [CrossRef]
- Iacobone, E.; Bailly-Salin, J.; Polito, A.; Friedman, D.; Stevens, R.D.; Sharshar, T. Sepsis-associated encephalopathy and its differential diagnosis. Crit. Care Med. 2009, 37, S331–S336. [Google Scholar] [CrossRef]
- Polito, A.; Eischwald, F.; Maho, A.L.; Polito, A.; Azabou, E.; Annane, D.; Chrétien, F.; Stevens, R.D.; Carlier, R.; Sharshar, T. Pattern of brain injury in the acute setting of human septic shock. Crit. Care. 2013, 17, R204. [Google Scholar] [CrossRef]
- Andonegui, G.; Zelinski, E.L.; Schubert, C.L.; Knight, D.; Craig, L.A.; Winston, B.W.; Spanswick, S.C.; Petri, B.; Jenne, C.N.; Sutherland, J.C.; et al. Targeting infammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight. 2018, 3, 1–20. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Eskind, A.; Inaba, H.; Hudson, M.M.; Pui, C.H.; Krull, K.R.; Wolf, J. Association of Bacteremic Sepsis With Long-term Neurocognitive Dysfunction in Pediatric Patients With Acute Lymphoblastic Leukemia. JAMA Pediatr. 2018, 172, 1092–1095. [Google Scholar] [CrossRef]
- Semmler, A.; Widmann, C.N.; Okulla, T.; Urbach, H.; Kaiser, M.; Widman, G.; Mormann, F.; Weide, J.; Fliessbach, K.; Hoeft, A.; et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J. Neurol. Neurosurg. Psychiatry 2013, 84, 62–69. [Google Scholar] [CrossRef]
- Pantzaris, N.D.; Platanaki, C.; Tsiotsios, K.; Koniari, I.; Velissaris, D. The Use of Electroencephalography in Patients with Sepsis: A Review of the Literature. J. Transl. Intern. Med. 2021, 9, 12–16. [Google Scholar] [CrossRef]
- De Araújo, B.E.S.; da Silva Fontana, R.; de Magalhães-Barbosa, M.C.; Lima-Setta, F.; Paravidino, V.B.; Riveiro, P.M.; Pulcheri, L.B.; dos Santos Salú, M.; Genuíno-Oliveira, M.B.; Robaina, J.R.; et al. Clinical Features, Electroencephalogram, and Biomarkers in Pediatric Sepsis-Associated Encephalopathy. Sci. Rep. 2022, 12, 10673. [Google Scholar] [CrossRef] [PubMed]
- Bircak-Kuchtova, B.; Chung, H.Y.; Wickel, J.; Ehler, J.; Geis, C. Neurofilament Light Chains to Assess Sepsis-Associated Encephalopathy: Are We on the Track toward Clinical Implementation? Crit. Care 2023, 27, 214. [Google Scholar] [CrossRef]
- Ehler, J.; Saller, T.; Wittstock, M.; Rommer, P.S.; Chappell, D.; Zwissler, B.; Grossmann, A.; Richter, G.; Reuter, D.A.; Nöldge-Schomburg, G.; et al. Diagnostic Value of NT-ProCNP Compared to NSE and S100B in Cerebrospinal Fluid and Plasma of Patients with Sepsis-Associated Encephalopathy. Neurosci. Lett. 2019, 692, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Lv, Z.; Wang, R.; Shu, H.; Yuan, S.; Yu, Y.; Shang, Y. Sepsis-Induced Brain Dysfunction: Pathogenesis, Diagnosis, and Treatment. Oxidative Med. Cell. Longev. 2022, 2022, 1328729. [Google Scholar] [CrossRef]
- Walker, K.A. Inflammation and neurodegeneration: Chronicity matters. Aging 2018, 11, 3–4. [Google Scholar] [CrossRef]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.A.; Harris, E.A. Herpes Simplex Virus Type 1 and Other Pathogens are Key Causative Factors in Sporadic Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 48, 319–353. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Herrero, M.; Soto-Rojas, L.O.; Harrington, C.R.; Flores-Martinez, Y.M.; Villegas-Rojas, M.M.; León-Aguilar, A.M.; Martínez-Gómez, P.A.; Campa-Córdoba, B.B.; Apátiga-Pérez, R.; Corniel-Taveras, C.N.; et al. Elucidating the Neuropathologic Mechanisms of SARS-CoV-2 Infection. Front. Neurol. 2021, 12, 660087. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Caggiu, E.; Arru, G.; Hosseini, S.; Niegowska, M.; Sechi, G.; Zarbo, I.R.; Sechi, L.A. Inflammation, Infectious Triggers, and Parkinson’s Disease. Front. Neurol. 2019, 10, 122. [Google Scholar] [CrossRef]
- Kim, J.S.; Chen, M.H.; Wang, H.E.; Lu, C.L.; Wang, Y.P.; Zhang, B. Inflammatory Bowel Disease and Neurodegenerative Diseases. Gut Liver 2023, 17, 1–10. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Ruggiero, M.; Panaro, M.A. Inflammaging and Brain: Curcumin and Its Beneficial Potential as Regulator of Microglia Activation. Molecules 2022, 27, 341. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán Solano, M.V.; Salinas Lara, C.; Sánchez-Garibay, C.; Soto-Rojas, L.O.; Escobedo-Ávila, I.; Tena-Suck, M.L.; Ortíz-Butrón, R.; Choreño-Parra, J.A.; Romero-López, J.P.; Meléndez Camargo, M.E. Effect of Systemic Inflammation in the CNS: A Silent History of Neuronal Damage. Int. J. Mol. Sci. 2023, 24, 11902. https://doi.org/10.3390/ijms241511902
Millán Solano MV, Salinas Lara C, Sánchez-Garibay C, Soto-Rojas LO, Escobedo-Ávila I, Tena-Suck ML, Ortíz-Butrón R, Choreño-Parra JA, Romero-López JP, Meléndez Camargo ME. Effect of Systemic Inflammation in the CNS: A Silent History of Neuronal Damage. International Journal of Molecular Sciences. 2023; 24(15):11902. https://doi.org/10.3390/ijms241511902
Chicago/Turabian StyleMillán Solano, Mara Verónica, Citlaltepetl Salinas Lara, Carlos Sánchez-Garibay, Luis O. Soto-Rojas, Itzel Escobedo-Ávila, Martha Lilia Tena-Suck, Rocío Ortíz-Butrón, José Alberto Choreño-Parra, José Pablo Romero-López, and María Estela Meléndez Camargo. 2023. "Effect of Systemic Inflammation in the CNS: A Silent History of Neuronal Damage" International Journal of Molecular Sciences 24, no. 15: 11902. https://doi.org/10.3390/ijms241511902
APA StyleMillán Solano, M. V., Salinas Lara, C., Sánchez-Garibay, C., Soto-Rojas, L. O., Escobedo-Ávila, I., Tena-Suck, M. L., Ortíz-Butrón, R., Choreño-Parra, J. A., Romero-López, J. P., & Meléndez Camargo, M. E. (2023). Effect of Systemic Inflammation in the CNS: A Silent History of Neuronal Damage. International Journal of Molecular Sciences, 24(15), 11902. https://doi.org/10.3390/ijms241511902






