Oxido-Reduction Potential as a Method to Determine Oxidative Stress in Semen Samples
Abstract
:1. Introduction
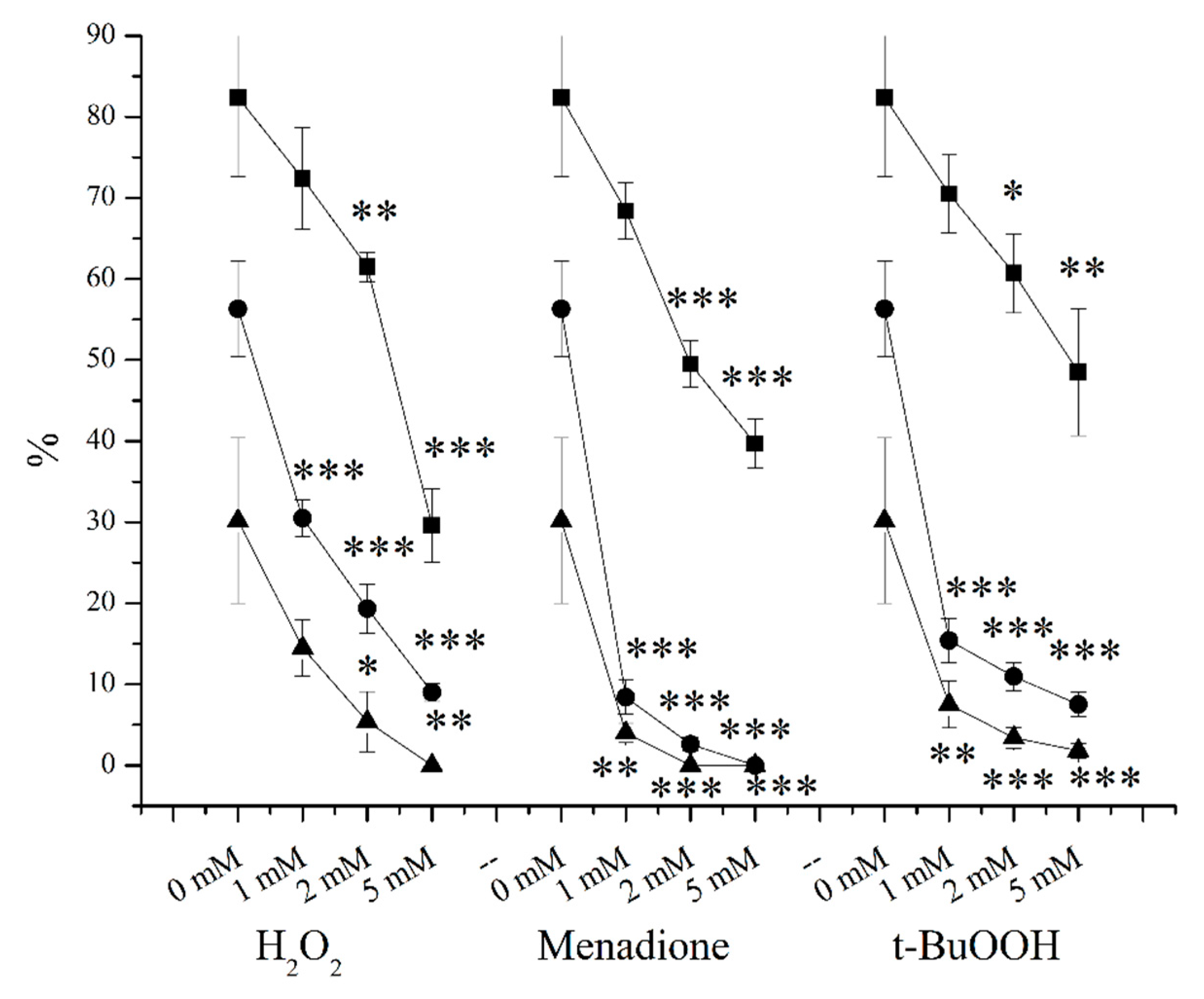
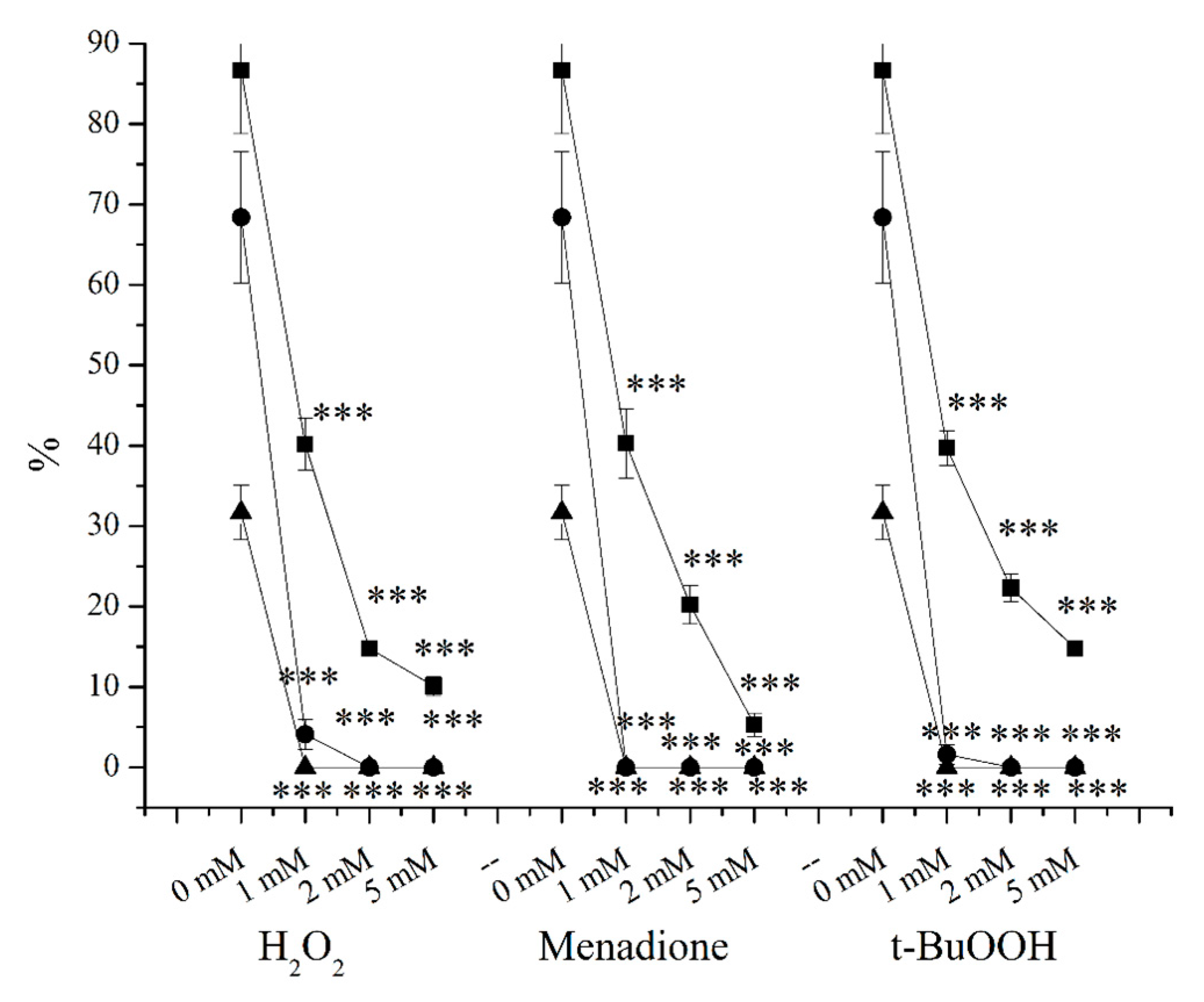
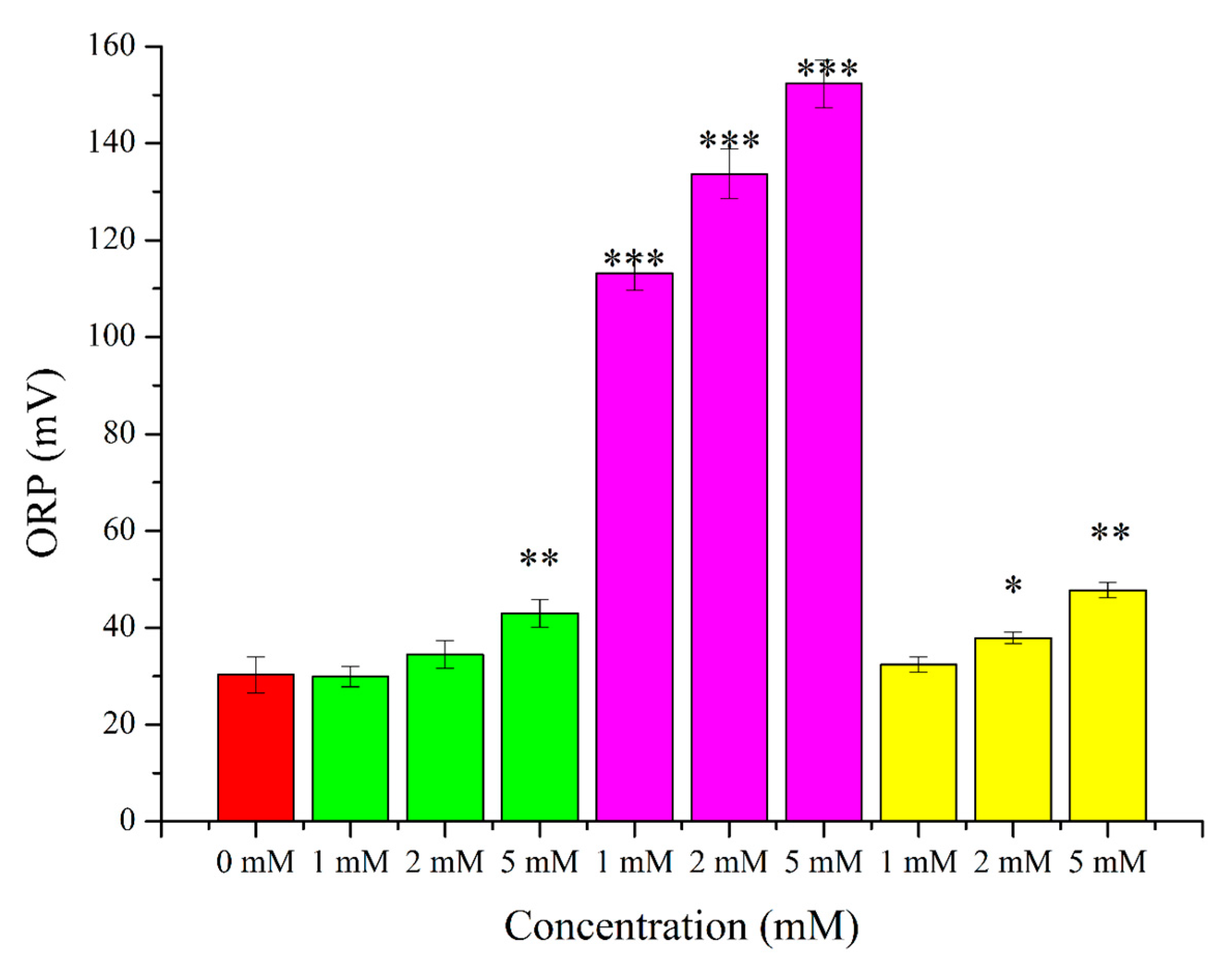
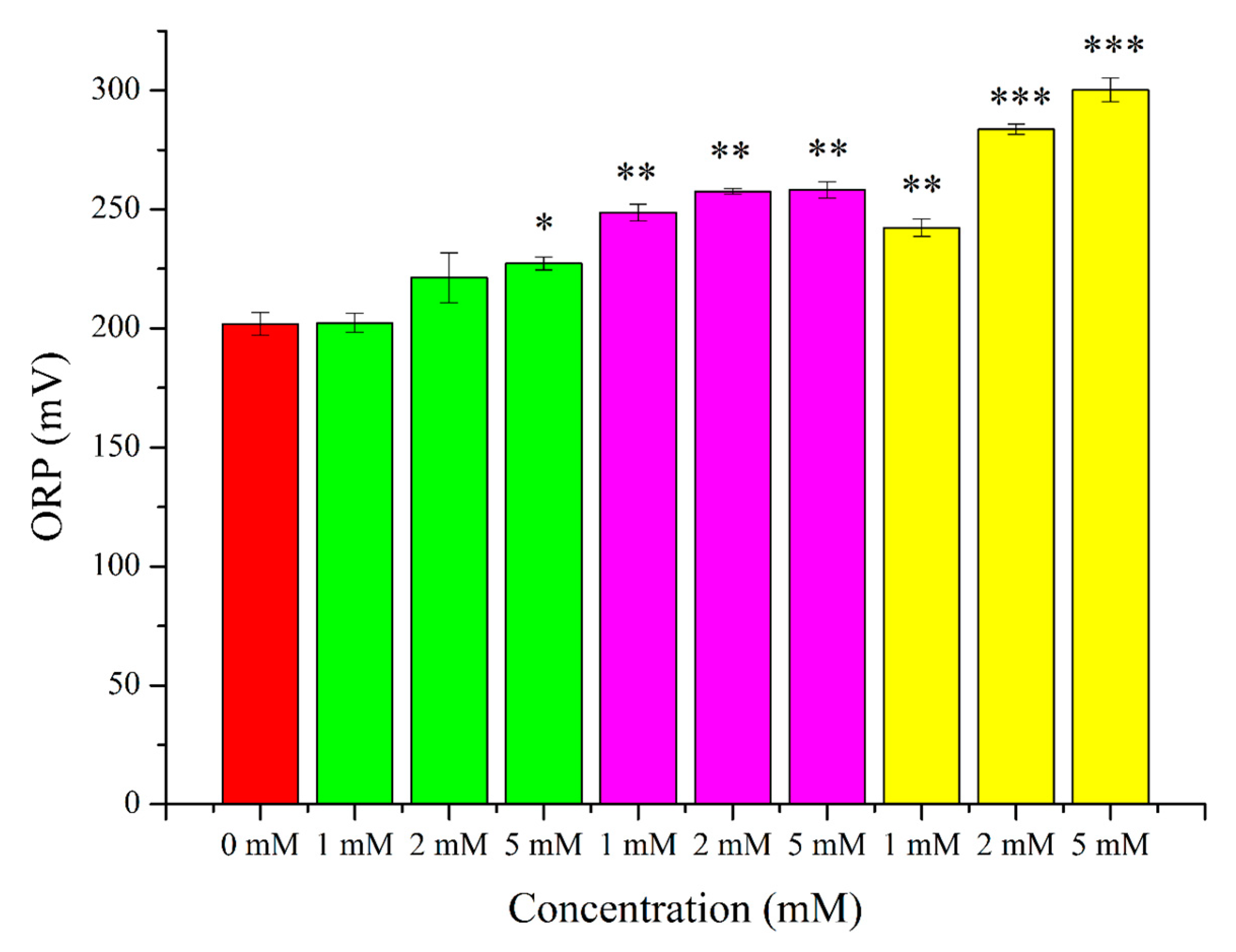
2. Results
3. Discussion
3.1. OS in Andrology
3.2. O2●− Induction by Menadione and Its Significance
3.3. Hydrogen Peroxide
3.4. Lipid Peroxidation Induction by t-BuOOH
3.5. Effects of OS-Inducers on Parameters of Spermatozoa and ORP Levels
4. Materials and Methods
4.1. Retrospective Analysis of ORP and Sperm Parameter Measurements
4.2. Sample Collection for Treatments
4.3. Artificial ROS Induction
4.4. Measurements of Basic Sperm Parameters and Their Oxido-Reduction State
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thonneau, P.; Marchand, S.; Tallec, A.; Ferial, M.L.; Ducot, B.; Lansac, J.; Lopes, P.; Tabaste, J.M.; Spira, A. Incidence and Main Causes of Infertility in a Resident Population (1,850,000) of Three French Regions (1988–1989). Hum. Reprod. 1991, 6, 811–816. [Google Scholar] [CrossRef]
- Hirsh, A. Male Subfertility. BMJ 2003, 327, 669–672. [Google Scholar] [CrossRef]
- Siddiq, F.M.; Sigman, M. A New Look at the Medical Management of Infertility. Urol. Clin. N. Am. 2002, 29, 949–963. [Google Scholar] [CrossRef]
- Greenberg, S.H.; Lipshultz, L.I.; Wein, A.J. Experience with 425 Subfertile Male Patients. J. Urol. 1978, 119, 507–510. [Google Scholar] [CrossRef]
- Hamada, A.; Esteves, S.C.; Nizza, M.; Agarwal, A. Unexplained Male Infertility: Diagnosis and Management. Int. Braz. J. Urol. 2012, 38, 576–594. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Saalu, L.C. The Incriminating Role of Reactive Oxygen Species in Idiopathic Male Infertility: An Evidence Based Evaluation. Pakistan J. Biol. Sci. PJBS 2010, 13, 413–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iommiello, V.M.; Albani, E.; Di Rosa, A.; Marras, A.; Menduni, F.; Morreale, G.; Levi, S.L.; Pisano, B.; Levi-Setti, P.E. Ejaculate Oxidative Stress Is Related with Sperm DNA Fragmentation and Round Cells. Int. J. Endocrinol. 2015, 2015, 321901. [Google Scholar] [CrossRef]
- Ozmen, B.; Koutlaki, N.; Youssry, M.; Diedrich, K.; Al-Hasani, S. DNA Damage of Human Spermatozoa in Assisted Reproduction: Origins, Diagnosis, Impacts and Safety. Reprod. Biomed. Online 2007, 14, 384–395. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Scarlata, E. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: The Protection of Mammalian Spermatozoa against Oxidative Stress. Reproduction 2022, 164, F67–F78. [Google Scholar] [CrossRef]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative Breakdown of Phospholipids in Human Spermatozoa, Spermicidal Properties of Fatty Acid Peroxides, and Protective Action of Seminal Plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Kowalczyk, A. The Role of the Natural Antioxidant Mechanism in Sperm Cells. Reprod. Sci. 2022, 29, 1387–1394. [Google Scholar] [CrossRef]
- Smith, T.B.; Dun, M.D.; Smith, N.D.; Curry, B.J.; Connaughton, H.S.; Aitken, R.J. The Presence of a Truncated Base Excision Repair Pathway in Human Spermatozoa That Is Mediated by OGG1. J. Cell Sci. 2013, 126, 1488–1497. [Google Scholar] [CrossRef] [Green Version]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of Sperm Oxidative Stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, H.W.G. Andrology: Seminal Leukocytes: Passengers, Terrorists or Good Samaritans? Hum. Reprod. 1995, 10, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive Oxygen Species as Mediators of Sperm Capacitation and Pathological Damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [Green Version]
- Fisher, H.M.; Aitken, R.J. Comparative Analysis of the Ability of Precursor Germ Cells and Epididymal Spermatozoa to Generate Reactive Oxygen Metabolites. J. Exp. Zool. 1997, 277, 390–400. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive Role of Reactive Oxygen Species in Mammalian Sperm Capacitation: Triggering and Modulation of Phosphorylation Events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef]
- Ávila, C.; Vinay, J.I.; Arese, M.; Saso, L.; Rodrigo, R. Antioxidant Intervention against Male Infertility: Time to Design Novel Strategies. Biomedicines 2022, 10, 3058. [Google Scholar] [CrossRef]
- Aitken, R.J. The Amoroso Lecture. The Human Spermatozoon—A Cell in Crisis? J. Reprod. Fertil. 1999, 115, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary Evidence on the Physiological Role of Reactive Oxygen Species in Human Sperm Function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Majzoub, A. Laboratory Tests for Oxidative Stress. Indian J. Urol. 2017, 33, 199–206. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampiao, F. Free Radicals Generation in an in Vitro Fertilization Setting and How to Minimize Them. World J. Obstet. Gynecol. 2012, 1, 29. [Google Scholar] [CrossRef]
- Agarwal, A.; Roychoudhury, S.; Bjugstad, K.B.; Cho, C.-L. Oxidation-Reduction Potential of Semen: What Is Its Role in the Treatment of Male Infertility? Ther. Adv. Urol. 2016, 8, 302–318. [Google Scholar] [CrossRef] [Green Version]
- Castleton, P.; Gyawali, P.; Mathews, N.; Mutuku, S.M.; Sharkey, D.J.; McPherson, N.O. MiOXSYS(®) and OxiSperm(®) II Assays Appear to Provide No Clinical Utility for Determining Oxidative Stress in Human Sperm—Results from Repeated Semen Collections. Andrology 2022. Early View. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Griffin, S.; Munch, G.; Pasinetti, G.M. Amyloid Beta-Peptide and Amyloid Pathology Are Central to the Oxidative Stress and Inflammatory Cascades under Which Alzheimer’s Disease Brain Exists. J. Alzheimer’s Dis. 2002, 4, 193–201. [Google Scholar] [CrossRef]
- Schapira, A.H.V. Mitochondria in the Aetiology and Pathogenesis of Parkinson’s Disease. Lancet Neurol. 2008, 7, 97–109. [Google Scholar] [CrossRef]
- Mhatre, M.; Floyd, R.A.; Hensley, K. Oxidative Stress and Neuroinflammation in Alzheimer’s Disease and Amyotrophic Lateral Sclerosis: Common Links and Potential Therapeutic Targets. J. Alzheimer’s Dis. 2004, 6, 147–157. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative Stress and Hypertension: Possibility of Hypertension Therapy with Antioxidants. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 358–367. [Google Scholar]
- Teuber, J.P.; Essandoh, K.; Hummel, S.L.; Madamanchi, N.R.; Brody, M.J. NADPH Oxidases in Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction. Antioxidants 2022, 11, 1822. [Google Scholar] [CrossRef]
- Nomani, H.; Bayat, G.; Sahebkar, A.; Fazelifar, A.F.; Vakilian, F.; Jomezade, V.; Johnston, T.P.; Mohammadpour, A.H. Atrial Fibrillation in β-Thalassemia Patients with a Focus on the Role of Iron-Overload and Oxidative Stress: A Review. J. Cell. Physiol. 2019, 234, 12249–12266. [Google Scholar] [CrossRef] [PubMed]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rudich, A. Positive and Negative Regulation of Insulin Signaling by Reactive Oxygen and Nitrogen Species. Physiol. Rev. 2009, 89, 27–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117. [Google Scholar] [CrossRef] [Green Version]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative Stress in Chronic Kidney Disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Gao, Y.; Li, H.; Wang, X.; Jin, M.; Shen, Z.; Yang, D.; Zhang, X.; Wei, Z.; Chen, Z.; et al. Association between Oxidative Stress, Mitochondrial Function of Peripheral Blood Mononuclear Cells and Gastrointestinal Cancers. J. Transl. Med. 2023, 21, 107. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, E.V.; Gavriliuk, L.A.; Pokrovsky, V.S. Oxidative Stress and Redox-Dependent Signaling in Prostate Cancer. Biochemistry 2022, 87, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Gurer-Orhan, H.; Ince, E.; Konyar, D.; Saso, L.; Suzen, S. The Role of Oxidative Stress Modulators in Breast Cancer. Curr. Med. Chem. 2018, 25, 4084–4101. [Google Scholar] [CrossRef]
- Spector, A. Oxidative Stress-Induced Cataract: Mechanism of Action. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 1173–1182. [Google Scholar] [CrossRef]
- Aitken, R.J.; West, K.; Buckingham, D. Leukocytic Infiltration into the Human Ejaculate and Its Association with Semen Quality, Oxidative Stress, and Sperm Function. J. Androl. 1994, 15, 343–352. [Google Scholar] [PubMed]
- Aitken, R.J. A Free Radical Theory of Male Infertility. Reprod. Fertil. Dev. 1994, 6, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Braga, P.C.; Martins, A.D.; Silva, B.M.; Alves, M.G.; Oliveira, P.F. Antioxidants Present in Reproductive Tract Fluids and Their Relevance for Fertility. Antioxidants 2021, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Salonia, C.A.; Bettocchi, P.; Capogrosso, J.; Carvalho, G.; Corona, G.; Hatzichristodoulou, T.H.; Jones, A.; Kadioglu, J.I.; Martinez-Salamanca, S.; Minhas, E.C.; et al. EAU Guidelines on Sexual and Reproductive Health; EAU Guidelines Office: Arnhem, The Netherlands, 2023; ISBN 978-94-92671-19-6. [Google Scholar]
- Colpi, G.M.; Francavilla, S.; Haidl, G.; Link, K.; Behre, H.M.; Goulis, D.G.; Krausz, C.; Giwercman, A. European Academy of Andrology Guideline Management of Oligo-Astheno-Teratozoospermia. Andrology 2018, 6, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; Vermeulen, N.; et al. ESHRE Guideline: Recurrent Pregnancy Loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar] [CrossRef]
- Harlev, A.; Agarwal, A.; Gunes, S.O.; Shetty, A.; du Plessis, S.S. Smoking and Male Infertility: An Evidence-Based Review. World J. Men’s Health 2015, 33, 143–160. [Google Scholar] [CrossRef] [Green Version]
- Maneesh, M.; Jayalekshmi, H.; Dutta, S.; Chakrabarti, A.; Vasudevan, D.M. Effect of Chronic Ethanol Administration on Testicular Antioxidant System and Steroidogenic Enzyme Activity in Rats. Indian J. Exp. Biol. 2005, 43, 445–449. [Google Scholar]
- Abarikwu, S.O.; Duru, Q.C.; Chinonso, O.V.; Njoku, R.-C. Antioxidant Enzymes Activity, Lipid Peroxidation, Oxidative Damage in the Testis and Epididymis, and Steroidogenesis in Rats after Co-Exposure to Atrazine and Ethanol. Andrologia 2016, 48, 548–557. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, K.V.; Nirala, J.; Murmu, N.N.; Meena, R.; Rajamani, P. Oxidative Stress-Mediated Alterations on Sperm Parameters in Male Wistar Rats Exposed to 3G Mobile Phone Radiation. Andrologia 2019, 51, e13201. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Behari, J. Evidence for Mobile Phone Radiation Exposure Effects on Reproductive Pattern of Male Rats: Role of ROS. Electromagn. Biol. Med. 2012, 31, 213–222. [Google Scholar] [CrossRef]
- Erogul, O.; Oztas, E.; Yildirim, I.; Kir, T.; Aydur, E.; Komesli, G.; Irkilata, H.C.; Irmak, M.K.; Peker, A.F. Effects of Electromagnetic Radiation from a Cellular Phone on Human Sperm Motility: An in Vitro Study. Arch. Med. Res. 2006, 37, 840–843. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Tavassoli, A.; Ranjbari, F.; Moammaiee, P. Effects of Laptop Computers’ Electromagnetic Field on Sperm Quality. J. Reprod. Fertil. 2010, 2010, 251–258. [Google Scholar]
- Zhang, G.; Jiang, F.; Chen, Q.; Yang, H.; Zhou, N.; Sun, L.; Zou, P.; Yang, W.; Cao, J.; Zhou, Z.; et al. Associations of Ambient Air Pollutant Exposure with Seminal Plasma MDA, Sperm MtDNA Copy Number, and MtDNA Integrity. Environ. Int. 2020, 136, 105483. [Google Scholar] [CrossRef]
- Li, D.; Yin, D.; Han, X. Methyl Tert-Butyl Ether (MTBE)-Induced Cytotoxicity and Oxidative Stress in Isolated Rat Spermatogenic Cells. J. Appl. Toxicol. 2007, 27, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and Male Infertility: Mechanisms and Management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, Y.; Wang, C.; Liang, M.; Liao, Y.; Hu, K. Role of Oxidative Stress in Varicocele. Front. Genet. 2022, 13, 850114. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-D.; Jeng, S.-Y.; Lee, T.-H. Increased Expression of Hypoxia-Inducible Factor-1alpha in the Internal Spermatic Vein of Patients with Varicocele. J. Urol. 2006, 175, 1045–1048; discussion 1048. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Hamada, A.; Esteves, S.C. Insight into Oxidative Stress in Varicocele-Associated Male Infertility: Part 1. Nat. Rev. Urol. 2012, 9, 678–690. [Google Scholar] [CrossRef]
- Nago, M.; Arichi, A.; Omura, N.; Iwashita, Y.; Kawamura, T.; Yumura, Y. Aging Increases Oxidative Stress in Semen. Investig. Clin. Urol. 2021, 62, 233–238. [Google Scholar] [CrossRef]
- Aitken, R.J.; Harkiss, D.; Knox, W.; Paterson, M.; Irvine, D.S. A Novel Signal Transduction Cascade in Capacitating Human Spermatozoa Characterised by a Redox-Regulated, CAMP-Mediated Induction of Tyrosine Phosphorylation. J. Cell Sci. 1998, 111 Pt 5, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K. Timing of Fertilization in Mammals: Sperm Cholesterol/Phospholipid Ratio as a Determinant of the Capacitation Interval. Proc. Natl. Acad. Sci. USA 1981, 78, 7560–7564. [Google Scholar] [CrossRef]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of Mouse Spermatozoa. I. Correlation between the Capacitation State and Protein Tyrosine Phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef]
- Agarwal, A.; Qiu, E.; Sharma, R. Laboratory Assessment of Oxidative Stress in Semen. Arab J. Urol. 2018, 16, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Kashou, A.H.; Sharma, R.; Agarwal, A. Assessment of Oxidative Stress in Sperm and Semen. Methods Mol. Biol. 2013, 927, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Buckingham, D.W.; West, K.M. Reactive Oxygen Species and Human Spermatozoa: Analysis of the Cellular Mechanisms Involved in Luminol- and Lucigenin-Dependent Chemiluminescence. J. Cell. Physiol. 1992, 151, 466–477. [Google Scholar] [CrossRef]
- Aitken, R.J.; Buckingham, D. Enhanced Detection of Reactive Oxygen Species Produced by Human Spermatozoa with 7-Dimethyl Amino-Naphthalin-1, 2-Dicarbonic Acid Hydrazide. Int. J. Androl. 1992, 15, 211–219. [Google Scholar] [CrossRef]
- Agarwal, A.; Ahmad, G.; Sharma, R. Reference Values of Reactive Oxygen Species in Seminal Ejaculates Using Chemiluminescence Assay. J. Assist. Reprod. Genet. 2015, 32, 1721–1729. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Gil-Guzman, E.; Mahran, A.M.; Rakesh; Nelson, D.R.; Thomas, A.J.J.; Agarwa, A. Quality Control of Reactive Oxygen Species Measurement by Luminol-Dependent Chemiluminescence Assay. J. Androl. 2001, 22, 568–574. [Google Scholar] [PubMed]
- Garcia-Segura, S.; Ribas-Maynou, J.; Lara-Cerrillo, S.; Garcia-Peiró, A.; Castel, A.B.; Benet, J.; Oliver-Bonet, M. Relationship of Seminal Oxidation-Reduction Potential with Sperm DNA Integrity and PH in Idiopathic Infertile Patients. Biology 2020, 9, 262. [Google Scholar] [CrossRef]
- Henkel, R.; Morris, A.; Vogiatzi, P.; Saleh, R.; Sallam, H.; Boitrelle, F.; Garrido, N.; Arafa, M.; Gül, M.; Rambhatla, A.; et al. Predictive Value of Seminal Oxidation-Reduction Potential Analysis for Reproductive Outcomes of ICSI. Reprod. Biomed. Online 2022, 45, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Gille, G.; Sigler, K. Oxidative Stress and Living Cells. Folia Microbiol. 1995, 40, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous Mechanisms of Reactive Oxygen Species (ROS) Generation. Postepy Hig. Med. Dosw. 2016, 70, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Ayad, B.; Omolaoye, T.S.; Louw, N.; Ramsunder, Y.; Skosana, B.T.; Oyeipo, P.I.; Du Plessis, S.S. Oxidative Stress and Male Infertility: Evidence from a Research Perspective. Front. Reprod. Health 2022, 4, 822257. [Google Scholar] [CrossRef]
- Fukui, M.; Choi, H.J.; Zhu, B.T. Rapid Generation of Mitochondrial Superoxide Induces Mitochondrion-Dependent but Caspase-Independent Cell Death in Hippocampal Neuronal Cells That Morphologically Resembles Necroptosis. Toxicol. Appl. Pharmacol. 2012, 262, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999; ISBN 9780198500452. [Google Scholar]
- Aitken, R.J.; Smith, T.B.; Lord, T.; Kuczera, L.; Koppers, A.J.; Naumovski, N.; Connaughton, H.; Baker, M.A.; De Iuliis, G.N. On Methods for the Detection of Reactive Oxygen Species Generation by Human Spermatozoa: Analysis of the Cellular Responses to Catechol Oestrogen, Lipid Aldehyde, Menadione and Arachidonic Acid. Andrology 2013, 1, 192–205. [Google Scholar] [CrossRef]
- Zhu, Z.; Zeng, Y.; Zeng, W. Cysteine Improves Boar Sperm Quality via Glutathione Biosynthesis during the Liquid Storage. Anim. Biosci. 2022, 35, 166–176. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R.; Long, J.A. Mitochondrial Function and Reactive Oxygen Species Action in Relation to Boar Motility. Theriogenology 2008, 70, 1209–1215. [Google Scholar] [CrossRef]
- Goodla, L.; Morrell, J.M.; Yusnizar, Y.; Stålhammar, H.; Johannisson, A. Quality of Bull Spermatozoa after Preparation by Single-Layer Centrifugation. J. Dairy Sci. 2014, 97, 2204–2212. [Google Scholar] [CrossRef] [Green Version]
- Saran, M.; Bors, W. Direct and Indirect Measurements of Oxygen Radicals. Klin. Wochenschr. 1991, 69, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Oya, Y.; Yamamoto, K. The Biological Activity of Hydrogen Peroxide. IV. Enhancement of Its Clastogenic Actions by Coadministration of L-Histidine. Mutat. Res. 1988, 198, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chaki, S.P.; Misro, M.M. Assessment of Human Sperm Function after Hydrogen Peroxide Exposure. Development of a Vaginal Contraceptive. Contraception 2002, 66, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Pujianto, D.A.; Oktarina, M.; Sharma Sharaswati, I.A.; Yulhasri. Hydrogen Peroxide Has Adverse Effects on Human Sperm Quality Parameters, Induces Apoptosis, and Reduces Survival. J. Hum. Reprod. Sci. 2021, 14, 121–128. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Harvey, K.; Stillwell, W. Anticancer Properties of Oxidation Products of Docosahexaenoic Acid. Chem. Phys. Lipids 2008, 153, 47–56. [Google Scholar] [CrossRef]
- Girotti, A.W. Lipid Hydroperoxide Generation, Turnover, and Effector Action in Biological Systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [CrossRef]
- Halliwell, B.; Chirico, S. Lipid Peroxidation: Its Mechanism, Measurement, and Significance. Am. J. Clin. Nutr. 1993, 57, 715S–724S; discussion 724S–725S. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Hong, Y.; Johnson, M.K.; Maier, R.J. Lipid Peroxidation as a Source of Oxidative Damage in Helicobacter Pylori: Protective Roles of Peroxiredoxins. Biochim. Biophys. Acta 2006, 1760, 1596–1603. [Google Scholar] [CrossRef]
- Howlett, N.G.; Avery, S. V Induction of Lipid Peroxidation during Heavy Metal Stress in Saccharomyces Cerevisiae and Influence of Plasma Membrane Fatty Acid Unsaturation. Appl. Environ. Microbiol. 1997, 63, 2971–2976. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef] [Green Version]
- Moazamian, R.; Polhemus, A.; Connaughton, H.; Fraser, B.; Whiting, S.; Gharagozloo, P.; Aitken, R.J. Oxidative Stress and Human Spermatozoa: Diagnostic and Functional Significance of Aldehydes Generated as a Result of Lipid Peroxidation. Mol. Hum. Reprod. 2015, 21, 502–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboua, Y.; Brooks, N. Impact of Organic Hydroperoxides on Rat Testicular Tissue and Epididymal Sperm. Afr. J. Biotechnol. 2009, 8, 6416–6424. [Google Scholar] [CrossRef] [Green Version]
- Kumar, T.R.; Muralidhara. Induction of Oxidative Stress by Organic Hydroperoxides in Testis and Epididymal Sperm of Rats in Vivo. J. Androl. 2007, 28, 77–85. [Google Scholar] [CrossRef]
- Wu, P.Y.; Scarlata, E.; O’Flaherty, C. Long-Term Adverse Effects of Oxidative Stress on Rat Epididymis and Spermatozoa. Antioxidants 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Bisconti, M.; Grosjean, P.; Arcolia, V.; Simon, J.-F.; Hennebert, E. Influence of Two Widely Used Solvents, Ethanol and Dimethyl Sulfoxide, on Human Sperm Parameters. Int. J. Mol. Sci. 2023, 24, 505. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E.; Behre, H.M.; Nieschlag, S. (Eds.) Andrology: Male Reproductive Health and Dysfunction, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-540-78354-1. [Google Scholar]
- Juyena, N.S.; Stelletta, C. Seminal Plasma: An Essential Attribute to Spermatozoa. J. Androl. 2012, 33, 536–551. [Google Scholar] [CrossRef] [Green Version]
- Ben Ali, H.; Guerin, J.F.; Pinatel, M.C.; Mathieu, C.; Boulieu, D.; Tritar, B. Relationship between Semen Characteristics, Alpha-Glucosidase and the Capacity of Spermatozoa to Bind to the Human Zona Pellucida. Int. J. Androl. 1994, 17, 121–126. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021.
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total Antioxidant Capacity-Relevance, Methods and Clinical Implications. Andrologia 2021, 53, e13624. [Google Scholar] [CrossRef]
- Heise, A.; Kähn, W.; Volkmann, D.H.; Thompson, P.N.; Gerber, D. Influence of Seminal Plasma on Fertility of Fresh and Frozen-Thawed Stallion Epididymal Spermatozoa. Anim. Reprod. Sci. 2010, 118, 48–53. [Google Scholar] [CrossRef]
- Hernández, M.; Roca, J.; Calvete, J.J.; Sanz, L.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Vázquez, J.M.; Martínez, E.A. Cryosurvival and in Vitro Fertilizing Capacity Postthaw Is Improved When Boar Spermatozoa Are Frozen in the Presence of Seminal Plasma from Good Freezer Boars. J. Androl. 2007, 28, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Castleton, P.E.; Deluao, J.C.; Sharkey, D.J.; McPherson, N.O. Measuring Reactive Oxygen Species in Semen for Male Preconception Care: A Scientist Perspective. Antioxidants 2022, 11, 264. [Google Scholar] [CrossRef]
- Majzoub, A.; Agarwal, A. Systematic Review of Antioxidant Types and Doses in Male Infertility: Benefits on Semen Parameters, Advanced Sperm Function, Assisted Reproduction and Live-Birth Rate. Arab J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan, V.; Showell, M.G. Antioxidants for Male Subfertility. Cochrane Database Syst. Rev. 2019, 3, CD007411. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-P.; Yang, X.-S.; Wu, T. The Effect of Antioxidants on Sperm Quality Parameters and Pregnancy Rates for Idiopathic Male Infertility: A Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 810242. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.Z.; Hansen, K.R.; Barnhart, K.T.; Cedars, M.I.; Legro, R.S.; Diamond, M.P.; Krawetz, S.A.; Usadi, R.; Baker, V.L.; Coward, R.M.; et al. The Effect of Antioxidants on Male Factor Infertility: The Males, Antioxidants, and Infertility (MOXI) Randomized Clinical Trial. Fertil. Steril. 2020, 113, 552–560. [Google Scholar] [CrossRef]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The Excessive Use of Antioxidant Therapy: A Possible Cause of Male Infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef] [Green Version]
- Kopa, Z.; Keszthelyi, M.; Sofikitis, N. Administration of Antioxidants in the Infertile Male: When It May Have a Beneficial Effect? Curr. Pharm. Des. 2021, 27, 2665–2668. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y.J.R.; Hazout, A.; Panteix, G.; Robert, F.; Rollet, J.; Cohen-Bacrie, P.; Chapuis, F.; Clément, P.; Benkhalifa, M. Antioxidants to Reduce Sperm DNA Fragmentation: An Unexpected Adverse Effect. Reprod. Biomed. Online 2007, 14, 418–421. [Google Scholar] [CrossRef]
- Agarwal, A.; Leisegang, K.; Majzoub, A.; Henkel, R.; Finelli, R.; Panner Selvam, M.K.; Tadros, N.; Parekh, N.; Ko, E.Y.; Cho, C.L.; et al. Utility of Antioxidants in the Treatment of Male Infertility: Clinical Guidelines Based on a Systematic Review and Analysis of Evidence. World J. Men’s Health 2021, 39, 233–290. [Google Scholar] [CrossRef]
- Thijssen, A.; Klerkx, E.; Huyser, C.; Bosmans, E.; Campo, R.; Ombelet, W. Influence of Temperature and Sperm Preparation on the Quality of Spermatozoa. Reprod. Biomed. Online 2014, 28, 436–442. [Google Scholar] [CrossRef] [Green Version]
| n | Concentration M/mL | Motility % | Progressive Motility % | Viability % | ORP mV/106/mL | ORP mV | DNA Fragmentation % | |
|---|---|---|---|---|---|---|---|---|
| <16 M/mL | 69 | 8.3 ± 4.1 | 25.8 ± 14.1 | 19.7 ± 13.1 | 60.5 ± 8.7 | 8.5 ± 13.9 | 38.5 ± 16.6 | 20.5 ± 10.6 |
| >16 M/mL | 90 | 61.6 ± 58.8 *** | 37.4 ± 17.8 *** | 28.8 ± 15.8 *** | 67.4 ± 11.6 *** | 1.0 ± 0.9 ** | 40.6 ± 25.5 | 16.8 ± 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balló, A.; Czétány, P.; Busznyákné, K.S.; Márk, L.; Mike, N.; Török, A.; Szántó, Á.; Máté, G. Oxido-Reduction Potential as a Method to Determine Oxidative Stress in Semen Samples. Int. J. Mol. Sci. 2023, 24, 11981. https://doi.org/10.3390/ijms241511981
Balló A, Czétány P, Busznyákné KS, Márk L, Mike N, Török A, Szántó Á, Máté G. Oxido-Reduction Potential as a Method to Determine Oxidative Stress in Semen Samples. International Journal of Molecular Sciences. 2023; 24(15):11981. https://doi.org/10.3390/ijms241511981
Chicago/Turabian StyleBalló, András, Péter Czétány, Kinga Székvári Busznyákné, László Márk, Nóra Mike, Attila Török, Árpád Szántó, and Gábor Máté. 2023. "Oxido-Reduction Potential as a Method to Determine Oxidative Stress in Semen Samples" International Journal of Molecular Sciences 24, no. 15: 11981. https://doi.org/10.3390/ijms241511981
APA StyleBalló, A., Czétány, P., Busznyákné, K. S., Márk, L., Mike, N., Török, A., Szántó, Á., & Máté, G. (2023). Oxido-Reduction Potential as a Method to Determine Oxidative Stress in Semen Samples. International Journal of Molecular Sciences, 24(15), 11981. https://doi.org/10.3390/ijms241511981







