Cripto Is Targeted by miR-1a-3p in a Mouse Model of Heart Development
Abstract
1. Introduction
2. Results
2.1. Complementarity Prediction between miRNA-1 and Cripto
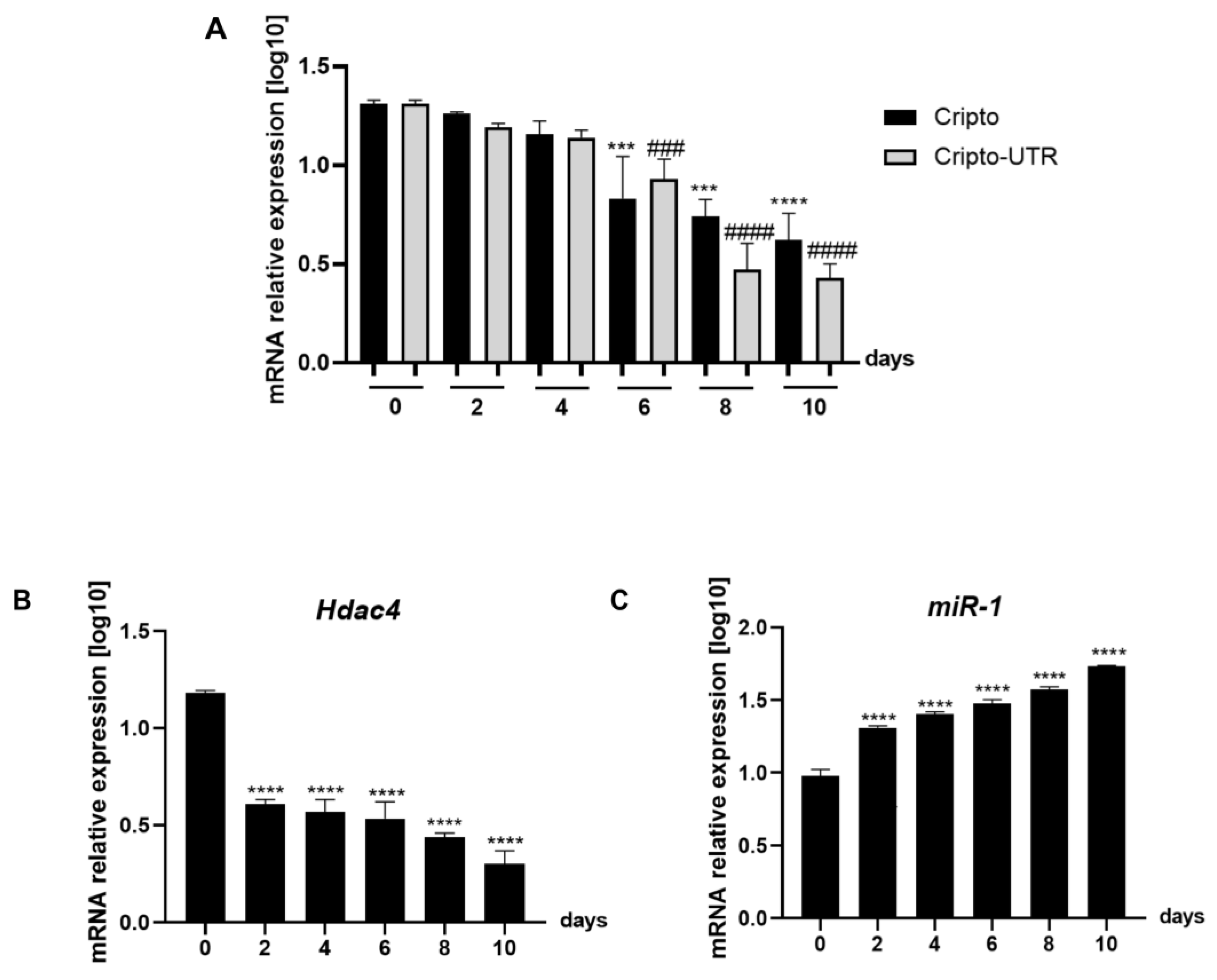
2.2. Expression Level of miRNA-1 and Cripto in Mouse EBs during Cardiac Differentiation
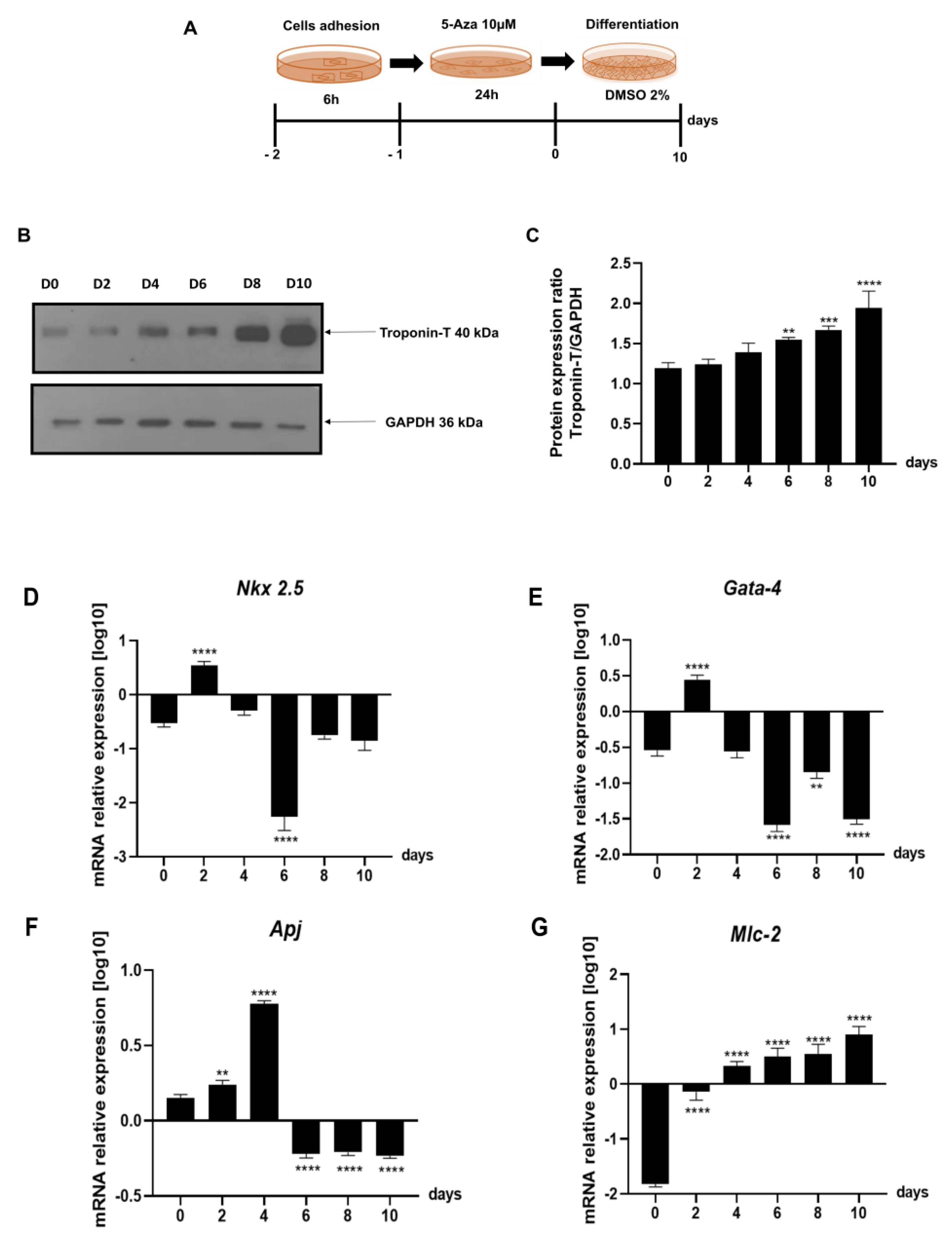
2.3. Evaluation of Gene Expression of the Leading Agents of Cardiomyogenesis in P19 Cells Undergoing Cardiac Differentiation
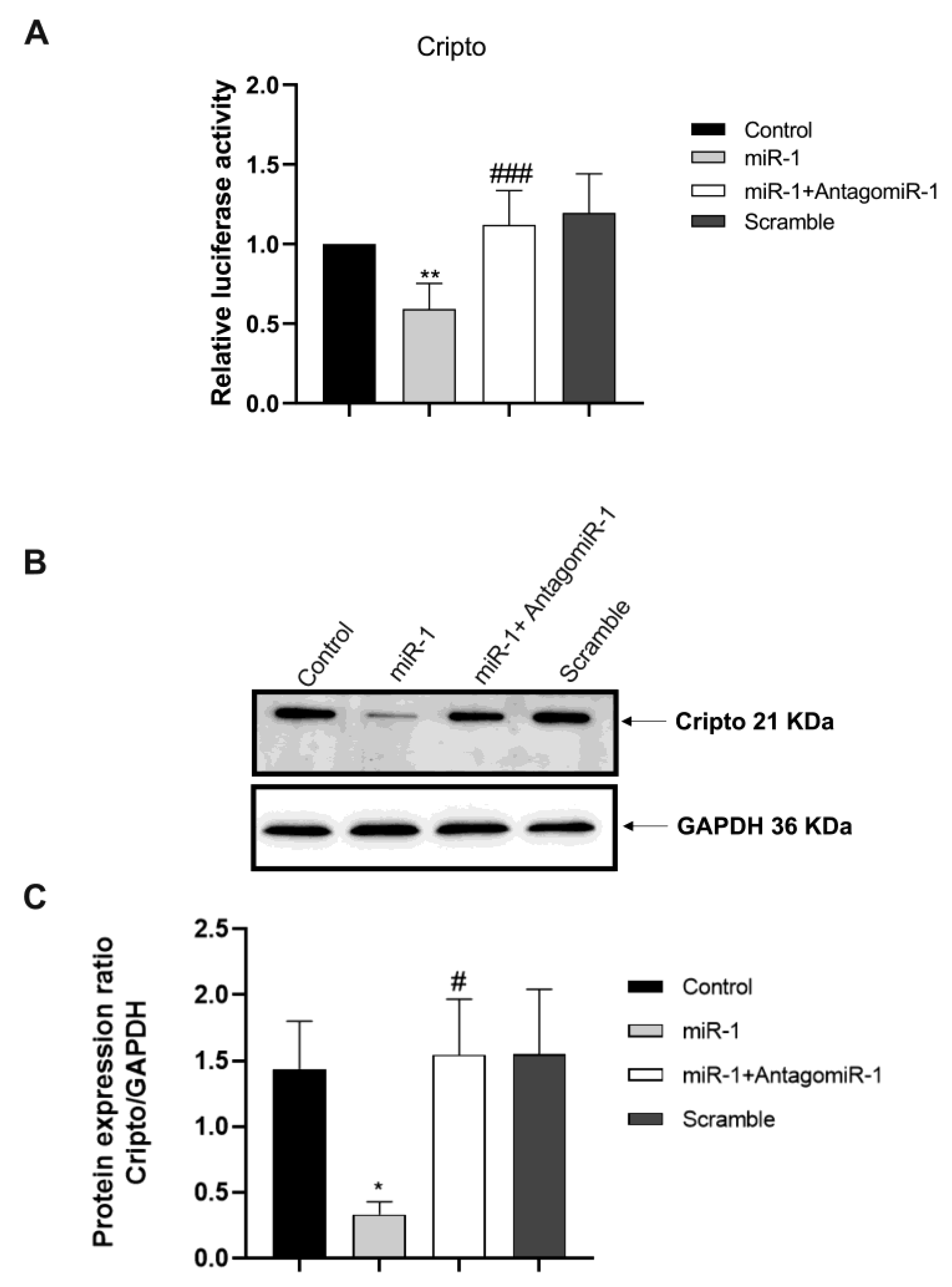
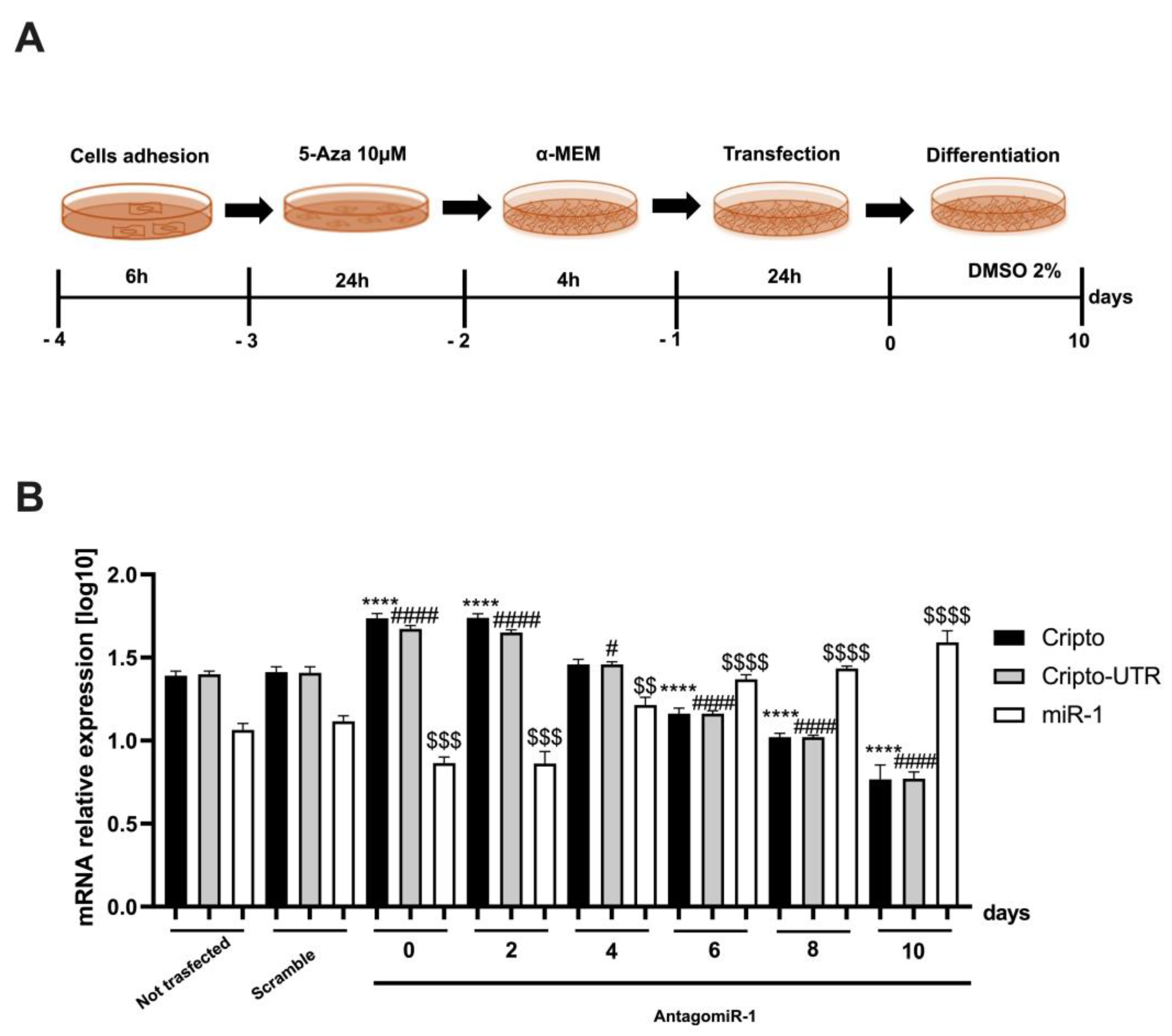
2.4. Silencing the miR-1 Gene
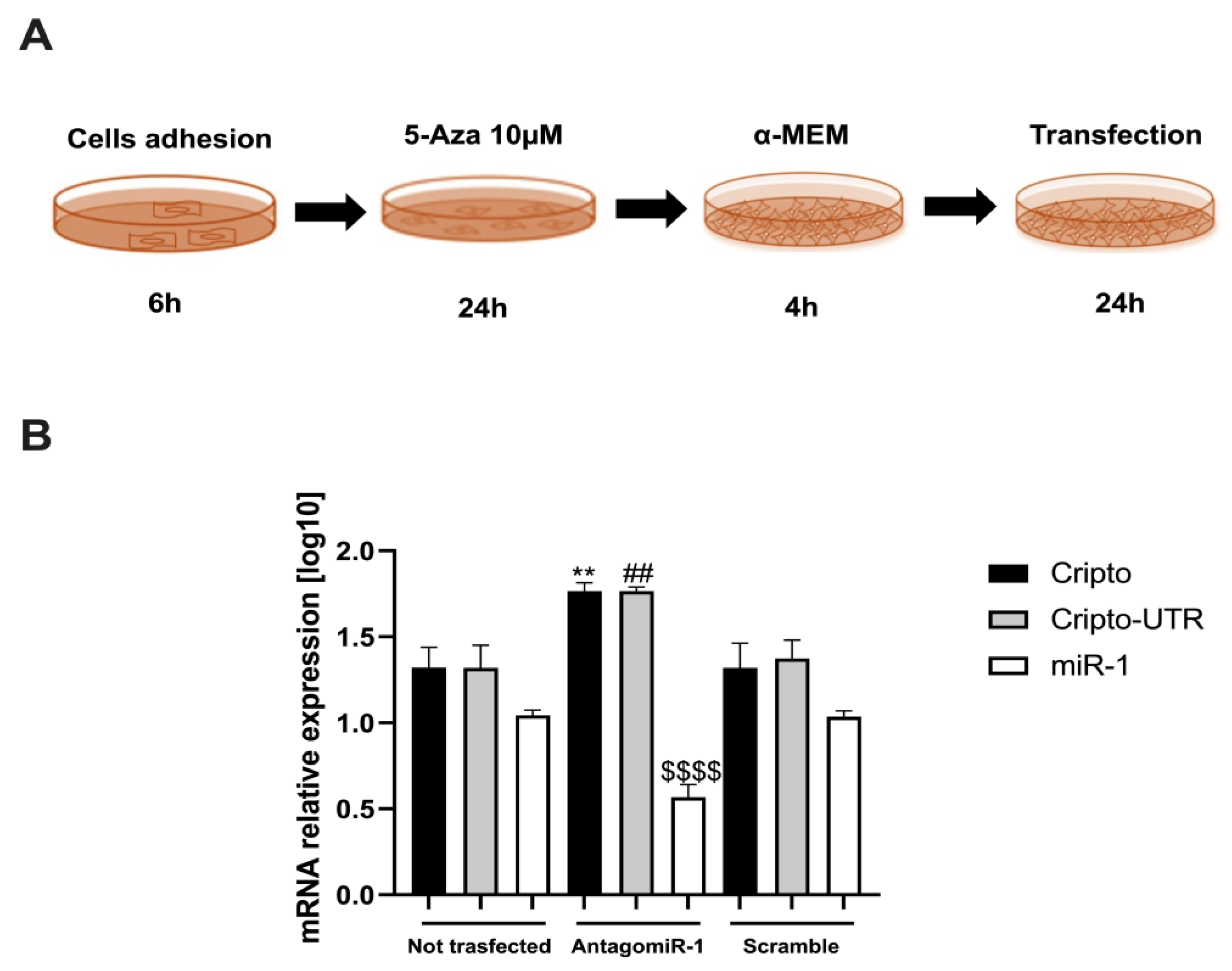
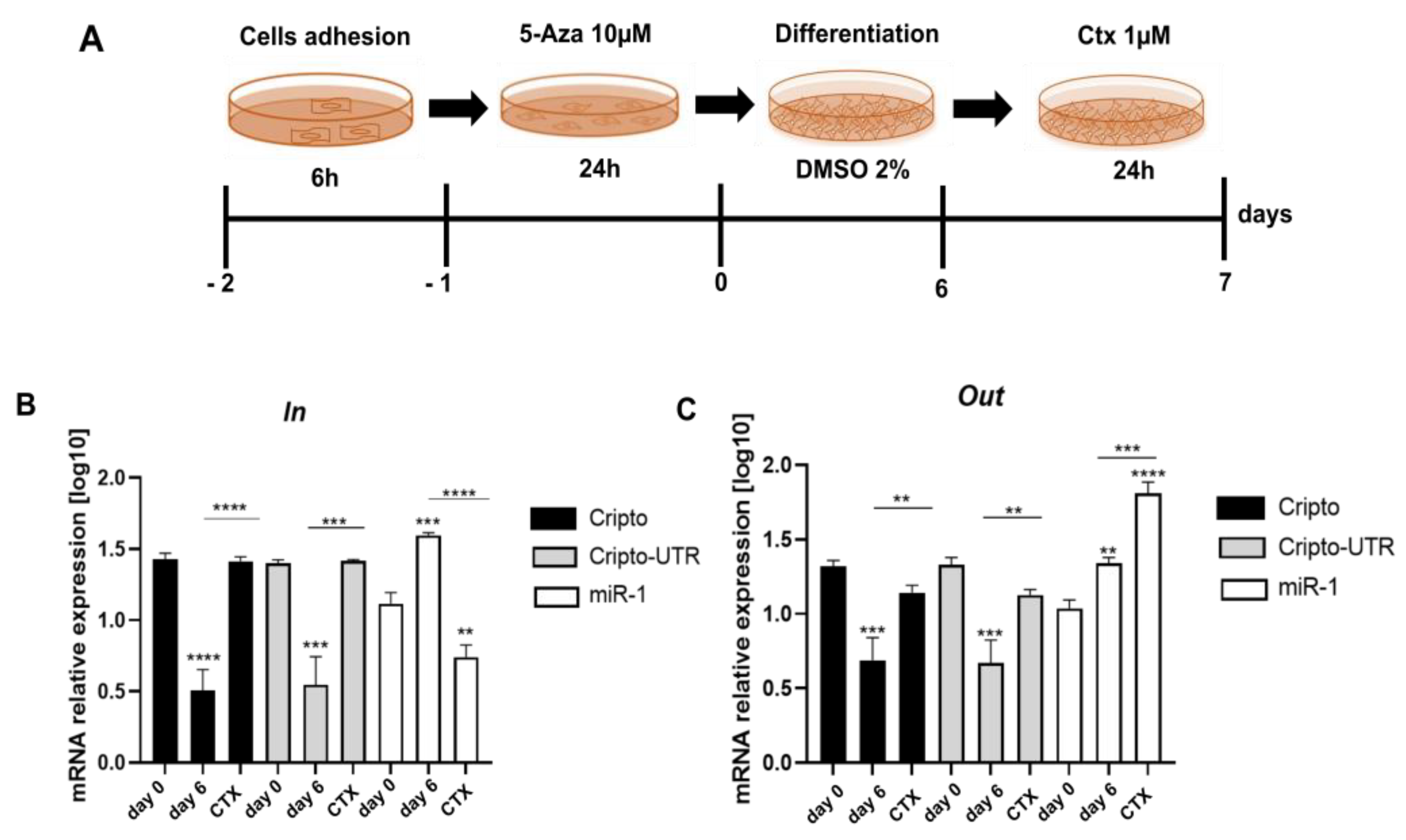
2.5. Monitoring the Relationship between miR-1 and Cripto during CTX Damage
2.6. Correlation between Cripto and miR-1
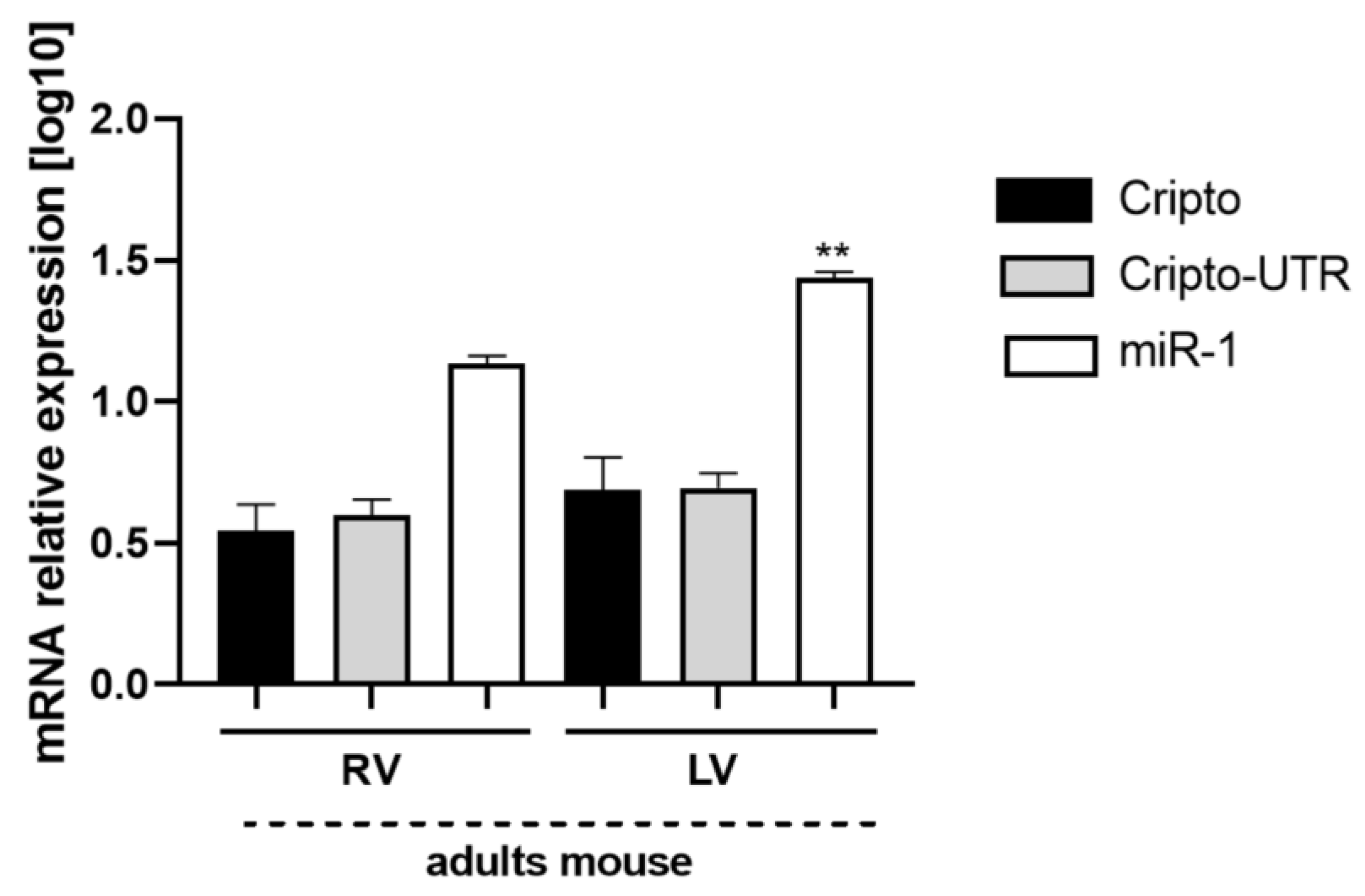
2.7. Evaluation of Cripto and miR-1 in Adult Mouse Heart Biopsies
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.2. Synthesis of miR-1 and Anti-miR-1 Antisense Inhibitor (Antagomir-1)
4.3. Luciferase Assay
4.4. ES Differentiation
4.5. Cell Cultures
4.6. AntagomiR-1 Transfection
4.7. Cardiomyocyte Treatments and Analysis
4.8. Ex Vivo Mouse Heart Tissues
4.9. RNA Extraction and cDNA Synthesis
4.10. Gene Expression by Real-Time qPCR
4.11. Protein Extraction
4.12. Western Blot Analysis
4.13. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar]
- Zhazykbayeva, S.; Pabel, S.; Mügge, A.; Sossalla, S.; Hamdani, N. The molecular mechanisms associated with the physiological responses to inflammation and oxidative stress in cardiovascular diseases. Biophys. Rev. 2020, 12, 947–968. [Google Scholar]
- Meilhac, S.M.; Lescroart, F.; Blanpain, C.; Buckingham, M.E. Cardiac cell lineages that form the heart. Cold Spring Harb. Perspect. Med. 2014, 9, a013888. [Google Scholar] [CrossRef]
- Buijtendijk, M.F.J.; Barnett, P.; van den Hoff, M.J.B. Development of the human heart. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 7–22. [Google Scholar]
- Durocher, D.; Charron, F.; Warren, R.; Schwartz, R.J.; Nemer, M. The cardiac transcription factors Nkx2-5 and GATA-4 are mutual cofactors. EMBO J. 1997, 16, 5687–5696. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.N.; Liu, J.; Shang, C.; Woods, C.; Chang, A.C.; Zhao, M.; Charo, D.N.; Grunwald, Z.; Huang, Y.; Seo, K.; et al. Apelin and APJ orchestrate complex tissue-specific control of cardiomyocyte hypertrophy and contractility in the hypertrophy-heart failure transition. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H348–H356. [Google Scholar]
- Sheikh, F.; Lyon, R.C.; Chen, J. Functions of myosin light chain-2 (MYL2) in cardiac muscle and disease. Gene 2015, 569, 14–20. [Google Scholar]
- Gomes, A.V.; Potter, J.D.; Szczesna-Cordary, D. The role of troponins in muscle contraction. IUBMB Life 2002, 6, 323–333. [Google Scholar] [CrossRef]
- Sharma, S.; Jackson, P.G.; Makan, J. Cardiac troponins. J. Clin. Pathol. 2004, 10, 1025–1026. [Google Scholar] [CrossRef]
- Parisi, S.; D’Andrea, D.; Lago, C.T.; Adamson, E.D.; Persico, M.G.; Minchiotti, G. Nodal-dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J. Cell Biol. 2003, 163, 303–314. [Google Scholar]
- Yan, Y.T.; Liu, J.J.; Luo, Y.; Chaosu, E.; Haltiwanger, R.S.; Abate-Shen, C.; Shen, M.M. Dual roles of Cripto as a ligand and co-receptor in the nodal signaling pathway. Mol. Cell Biol. 2002, 22, 4439–4449. [Google Scholar] [CrossRef] [PubMed]
- Minchiotti, G.; Parisi, S.; Liguori, G.L.; D’Andrea, D.; Persico, M.G. Role of the EGF-CFC gene cripto in cell differentiation and embryo development. Gene 2002, 287, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zinski, J.; Tajer, B.; Mullins, M.C. TGF-β Family Signaling in Early Vertebrate Development. Cold Spring Harb. Perspect. Biol. 2018, 10, a033274. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, E.; Parish, C.L.; Ponticelli, S.; Marasco, D.; Ribeiro, D.; Ruvo, M.; De Falco, S.; Arenas, E.; Minchiotti, G. A small synthetic cripto blocking Peptide improves neural induction, dopaminergic differentiation, and functional integration of mouse embryonic stem cells in a rat model of Parkinson’s disease. Stem Cells 2010, 28, 1326–1337. [Google Scholar] [CrossRef]
- Ravisankar, V.; Singh, T.P.; Manoj, N. Molecular evolution of the EGF-CFC protein family. Gene 2011, 482, 143–150. [Google Scholar] [CrossRef]
- Fiorenzano, A.; Pascale, E.; D’Aniello, C.; Acampora, D.; Bassalert, C.; Russo, F.; Andolfi, G.; Biffoni, M.; Francescangeli, F.; Zeuner, A.; et al. Cripto is essential to capture mouse epiblast stem cell and human embryonic stem cell pluripotency. Nat. Commun. 2016, 7, 12589. [Google Scholar] [CrossRef]
- Guardiola, O.; Lafuste, P.; Brunelli, S.; Iaconis, S.; Touvier, T.; Mourikis, P.; De Bock, K.; Lonardo, E.; Andolfi, G.; Bouché, A.; et al. Cripto regulates skeletal muscle regeneration and modulates satellite cell determination by antagonising myostatin. Proc. Natl. Acad. Sci. USA 2012, 109, E3231–E3240. [Google Scholar] [CrossRef]
- Prezioso, C.; Iaconis, S.; Andolfi, G.; Zentilin, L.; Iavarone, F.; Guardiola, O.; Minchiotti, G. Conditional Cripto overexpression in satellite cells promotes myogenic commitment and enhances early regeneration. Front. Cell Dev. Biol. 2015, 3, 31. [Google Scholar] [CrossRef]
- Islas, J.F.; Moreno-Cuevas, J.E. A MicroRNA Perspective on Cardiovascular Development and Diseases: An Update. Int. J. Mol. Sci. 2018, 9, 2075. [Google Scholar] [CrossRef]
- Forini, F.; Pitto, L. Editorial for Special Issue: “MicroRNA in Cardiac Health and Disease”. Int. J. Mol. Sci. 2022, 23, 15567. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Wang, Z.; Wu, J. MicroRNA-1 in Cardiac Diseases and Cancers. Korean J. Physiol. Pharmacol. 2014, 18, 359–363. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Mitchelson, K.R.; Qin, W.Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J. Biol. Chem. 2015, 3, 162–208. [Google Scholar] [CrossRef]
- Ivey, K.N.; Muth, A.; Arnold, J.; King, F.W.; Yeh, R.F.; Fish, J.E.; Hsiao, E.C.; Schwartz, R.J.; Conklin, B.R.; Bernstein, H.S.; et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell 2008, 2, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Jin, L.; Zhang, F.; Sarnow, P.; Kay, M.A. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 144–150. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, G.; Zhao, T.C. HDAC4: Mechanism of regulation and biological functions. Epigenomics 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Barile, L.; Messina, E.; Giacomello, A.; Marbán, E. Endogenous cardiac stem cells. Prog. Cardiovasc. Dis. 2007, 50, 31–48. [Google Scholar] [CrossRef]
- Evans, T. Embryonic Stem Cells as a Model for Cardiac Development and Disease. Drug Discov. Today Dis. Models 2008, 5, 147–155. [Google Scholar] [CrossRef][Green Version]
- Torella, D.; Ellison, G.M.; Méndez-Ferrer, S.; Ibanez, B.; Nadal-Ginard, B. Resident human cardiac stem cells: Role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3 (Suppl. S1), S8–S13. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genom. Res. 2009, 1, 92–105. [Google Scholar] [CrossRef]
- Minchiotti, G.; Parisi, S.; Persico, M.G. Cripto signaling in differentiating embryonic stem cells. Methods Mol. Biol. 2006, 329, 151–169. [Google Scholar] [PubMed]
- Qian, Q.; Qian, H.; Zhang, X.; Zhu, W.; Yan, Y.; Ye, S.; Peng, X.; Li, W.; Xu, Z.; Sun, L.; et al. 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extracellular regulated kinase. Stem Cells Dev. 2012, 1, 67–75. [Google Scholar] [CrossRef]
- Makino, S.; Fukuda, K.; Miyoshi, S.; Konishi, F.; Kodama, H.; Pan, J.; Sano, M.; Takahashi, T.; Hori, S.; Abe, H.; et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Investig. 1999, 5, 697–705. [Google Scholar] [CrossRef]
- Lim, C.K.; Efthymios, M.; Tan, W.; Autio, M.I.; Tiang, Z.; Li, P.Y.; Foo, R.S.Y. Dimethyl sulfoxide (DMSO) enhances direct cardiac reprogramming by inhibiting the bromodomain of coactivators CBP/p300. J. Mol. Cell Cardiol. 2021, 160, 15–26. [Google Scholar] [CrossRef]
- Jasmin Spray, D.C.; Campos de Carvalho, A.C.; Mendez-Otero, R. Chemical induction of cardiac differentiation in p19 embryonal carcinoma stem cells. Stem Cells Dev. 2010, 3, 403–412. [Google Scholar] [CrossRef] [PubMed]
- van der Heyden, M.A.; van Kempen, M.J.; Tsuji, Y.; Rook, M.B.; Jongsma, H.J.; Opthof, T. P19 embryonal carcinoma cells: A suitable model system for cardiac electrophysiological differentiation at the molecular and functional level. Cardiovasc. Res. 2003, 2, 410–422. [Google Scholar] [CrossRef]
- Wang, H.X.; Lau, S.Y.; Huang, S.J.; Kwan, C.Y.; Wong, T.M. Cobra venom cardiotoxin induces perturbations of cytosolic calcium homeostasis and hypercontracture in adult rat ventricular myocytes. J. Mol. Cell Cardiol. 1997, 10, 2759–2770. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Luo, X.; Murohara, T.; Yang, B.; Dobrev, D.; Nattel, S. MicroRNA regulation and cardiac calcium signaling: Role in cardiac disease and therapeutic potential. Circ. Res. 2014, 4, 689–705. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 3, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Monda, E.; D’Argenio, V.; Casaburi, G.; Mazzaccara, C.; Ranieri, A.; Fimiani, F.; Barretta, F.; et al. Multidisciplinary In-Depth Investigation in a Young Athlete Suffering from Syncope Caused by Myocardial Bridge. Diagnostics 2021, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Belostotskaya, G.; Hendrikx, M.; Galagudza, M.; Suchkov, S. How to Stimulate Myocardial Regeneration in Adult Mammalian Heart: Existing Views and New Approaches. Biomed. Res. Int. 2020, 2020, 7874109. [Google Scholar] [CrossRef]
- Lucci, V.; De Marino, E.; Tagliaferri, D.; Amente, S.; Pollice, A.; Calabrò, V.; Vivo, M.; Falco, G.; Angrisano, T. Identification of Cdk8 and Cdkn2d as New Prame-Target Genes in 2C-like Embryonic Stem Cells. Genes 2022, 13, 1745. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Moscarella, E.; Caiazza, M.; Gragnano, F.; Ranieri, A.; D’Alicandro, G.; Tinto, N.; et al. Dietary Thiols: A Potential Supporting Strategy against Oxidative Stress in Heart Failure and Muscular Damage during Sports Activity. Int. J. Environ. Res. Public Health 2020, 17, 9424. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, Y. Using bioinformatics for drug target identification from the genome. Am. J. Pharmacogenom. 2005, 6, 387–396. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, W.; Jia, J.; Lin, T.; Xiao, G.; Wang, S.; Lin, X.; Liu, Y.; Chen, L.; Qin, Y.; et al. Ectopic expression of Cripto-1 in transgenic mouse embryos causes hemorrhages, fatal cardiac defects and embryonic lethality. Sci. Rep. 2016, 6, 34501. [Google Scholar] [CrossRef]
- Malizia, A.P.; Wang, D.Z. MicroRNAs in cardiomyocyte development. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 183–190. [Google Scholar] [CrossRef]
- Ragozzino, E.; Brancaccio, M.; Di Costanzo, A.; Scalabrì, F.; Andolfi, G.; Wanderlingh, L.G.; Patriarca, E.J.; Minchiotti, G.; Altamura, S.; Summa, V.; et al. 6-Bromoindirubin-3′-oxime intercepts GSK3 signalling to promote and enhance skeletal muscle differentiation affecting miR-206 expression in mice. Sci. Rep. 2019, 9, 18091. [Google Scholar] [CrossRef]
- Choi, S.C.; Yoon, J.; Shim, W.J.; Ro, Y.M.; Lim, D.S. 5-azacytidine induces cardiac differentiation of P19 embryonic stem cells. Exp. Mol. Med. 2004, 36, 515–523. [Google Scholar] [CrossRef]
- Li, T.; He, Z.; Zhang, X.; Tian, M.; Jiang, K.; Cheng, G.; Wang, Y. The status of MAPK cascades contributes to the induction and activation of Gata4 and Nkx2.5 during the stepwise process of cardiac differentiation. Cell Signal 2019, 54, 17–26. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Jiang, K.; Liu, J.; Liu, Z. Dural effects of oxidative stress on cardiomyogenesis via Gata4 transcription and protein ubiquitination. Cell Death Dis. 2018, 2, 246. [Google Scholar] [CrossRef]
- D’Aniello, C.; Lonardo, E.; Iaconis, S.; Guardiola, O.; Liguoro, A.M.; Liguori, G.L.; Autiero, M.; Carmeliet, P.; Minchiotti, G. G protein-coupled receptor APJ and its ligand apelin act downstream of Cripto to specify embryonic stem cells toward the cardiac lineage through extracellular signal-regulated kinase/p70S6 kinase signalling pathway. Circ. Res. 2009, 105, 231–238. [Google Scholar] [CrossRef]
- Peterlin, A.; Počivavšek, K.; Petrovič, D.; Peterlin, B. The Role of microRNAs in Heart Failure: A Systematic Review. Front. Cardiovasc. Med. 2020, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Koudstaal, S.; Jansen Of Lorkeers, S.J.; Gaetani, R.; Gho, J.M.; van Slochteren, F.J.; Sluijter, J.P.; Doevendans, P.A.; Ellison, G.M.; Chamuleau, S.A. Concise review: Heart regeneration and the role of cardiac stem cells. Stem Cells Transl. Med. 2013, 2, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, E.; Medzikovic, L.; Ruffenach, G.; Eghbali, M. Role of miRNA-1 and miRNA-21 in Acute Myocardial Ischemia-Reperfusion Injury and Their Potential as Therapeutic Strategy. Int. J. Mol. Sci. 2022, 23, 1512. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Ding, C.; Yin, P.; He, L.; Xu, Q.; Wu, Z.; Shi, Y.; Su, L. MiR-1a-3p mitigates isoproterenol-induced heart failure by enhancing the expression of mitochondrial ND1 and COX1. Exp. Cell Res. 2019, 1, 87–97. [Google Scholar] [CrossRef]
- Bianco, C.; Rangel, M.C.; Castro, N.P.; Nagaoka, T.; Rollman, K.; Gonzales, M.; Salomon, D.S. Role of Cripto-1 in stem cell maintenance and malignant progression. Am. J. Pathol. 2010, 177, 532–540. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Y.; Wang, X.; Li, S.; Yu, T.; Duan, W.; Wu, J.; Wen, Z.; Jiao, Y.; Sun, Z.; et al. Circulating miR-1 as a potential predictor of left ventricular remodelling following acute ST-segment myocardial infarction using cardiac magnetic resonance. Quant. Imaging Med. Surg. 2020, 10, 1490–1503. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Chen, L.; Chen, K.; Zhou, J.; Song, J. MiR-1-3p that correlates with left ventricular function of HCM can serve as a potential target and differentiate HCM from DCM. J. Transl. Med. 2018, 1, 161. [Google Scholar] [CrossRef]
- Brancaccio, M.; Giachino, C.; Iazzetta, A.M.; Cordone, A.; De Marino, E.; Affinito, O.; Vivo, M.; Calabrò, V.; Pollice, A.; Angrisano, T. Integrated Bioinformatics Analysis Reveals Novel miRNA as Biomarkers Associated with Preeclampsia. Genes 2022, 13, 1781. [Google Scholar] [CrossRef]
- Krishnan, A.; Samtani, R.; Dhanantwari, P.; Lee, E.; Yamada, S.; Shiota, K.; Donofrio, M.T.; Leatherbury, L.; Lo, C.W. A detailed comparison of mouse and human cardiac development. Pediatr. Res. 2014, 6, 500–507. [Google Scholar] [CrossRef]
- McGinnis, S.; Madden, T.L. BLAST: At the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004, 32, W20–W25. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, X.; Ren, J.; Li, X.; Gao, X.; Lu, C.; Zhang, Y.; Sun, H.; Wang, Y.; Wang, H.; et al. miR-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS ONE 2012, 11, e50515. [Google Scholar] [CrossRef]
- Ishii, H.; Zahra, M.H.; Takayanagi, A.; Seno, M. A Novel Artificially Humanized Anti-Cripto-1 Antibody Suppressing Cancer Cell Growth. Int. J. Mol. Sci. 2021, 4, 1709. [Google Scholar] [CrossRef]
- Fico, A.; Manganelli, G.; Simeone, M.; Guido, S.; Minchiotti, G.; Filosa, S. High-throughput screening-compatible single-step protocol to differentiate embryonic stem cells in neurons. Stem Cells Dev. 2008, 17, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Brattelid, T.; Aarnes, E.K.; Helgeland, E.; Guvaåg, S.; Eichele, H.; Jonassen, A.K. Normalization strategy is critical for the outcome of miRNA expression analyses in the rat heart. Physiol. Genom. 2011, 10, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, D.; Mazzone, P.; Noviello, T.M.R.; Addeo, M.; Angrisano, T.; Del Vecchio, L.; Visconte, F.; Ruggieri, V.; Russi, S.; Caivano, A.; et al. Retinoic Acid Induces Embryonic Stem Cells (ESCs) Transition to 2 Cell-Like State Through a Coordinated Expression of Dux and Duxbl1. Front. Cell Dev. Biol. 2020, 7, 385. [Google Scholar] [CrossRef] [PubMed]
- Jaykaran, J. “Mean ± SEM” or “Mean (SD)”? Indian J. Pharmacol. 2010, 5, 329. [Google Scholar] [CrossRef] [PubMed]



| Variables | ρ | |||||
|---|---|---|---|---|---|---|
| Days | ||||||
| 0 | 2 | 4 | 6 | 8 | 10 | |
| Cripto vs. miR-1 | 0.16 | 0.27 | 0.48 | −0.29 | −0.70 | −0.99 |
| Cripto-UTR vs. miR-1 | 0.20 | 0.38 | 0.56 | −0.19 | −0.60 | −0.99 |
| Gene | Primer for 5′-3′ | Primer rev 5′-3′ |
|---|---|---|
| Gapdh | GGTGAAGGTCGGTGTGAACG | CTCGCTCCTGGAAGATGGTG |
| Cripto-utr | GACAGACAGGCCTACACAGA | TCGCTACATAGACCAGGCTG |
| Cripto | TGGACGCAACTGTGAACATG | TTGAGGTCCTGGTCCATCAC |
| Nkx 2.5 | CAGAACCGTCGCTACAAGTG | GGTAGGGGTAGGCGTTGTAG |
| Gata-4 | GTTACCTGGCTCTGGGACTT | GTGGGTGATGAGGACAAGGA |
| Apj | CCAGTGTCTTTTGCCTCACC | CTGAGTTTGAAGTGGCCACC |
| Mlc-2 | ATCAAAGAGGCTCCAGGTCC | GTCAGCATCTCCCGGACATA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angrisano, T.; Varrone, F.; Ragozzino, E.; Fico, A.; Minchiotti, G.; Brancaccio, M. Cripto Is Targeted by miR-1a-3p in a Mouse Model of Heart Development. Int. J. Mol. Sci. 2023, 24, 12251. https://doi.org/10.3390/ijms241512251
Angrisano T, Varrone F, Ragozzino E, Fico A, Minchiotti G, Brancaccio M. Cripto Is Targeted by miR-1a-3p in a Mouse Model of Heart Development. International Journal of Molecular Sciences. 2023; 24(15):12251. https://doi.org/10.3390/ijms241512251
Chicago/Turabian StyleAngrisano, Tiziana, Francesca Varrone, Elvira Ragozzino, Annalisa Fico, Gabriella Minchiotti, and Mariarita Brancaccio. 2023. "Cripto Is Targeted by miR-1a-3p in a Mouse Model of Heart Development" International Journal of Molecular Sciences 24, no. 15: 12251. https://doi.org/10.3390/ijms241512251
APA StyleAngrisano, T., Varrone, F., Ragozzino, E., Fico, A., Minchiotti, G., & Brancaccio, M. (2023). Cripto Is Targeted by miR-1a-3p in a Mouse Model of Heart Development. International Journal of Molecular Sciences, 24(15), 12251. https://doi.org/10.3390/ijms241512251










