Changes of the Protein CoAlation Pattern in Response to Oxidative Stress and Capacitation in Human Spermatozoa
Abstract
:1. Introduction
2. Results
2.1. Protein CoAlation Pattern Differentially Changed during In Vitro Induced Oxidative Stress in Human Spermatozoa
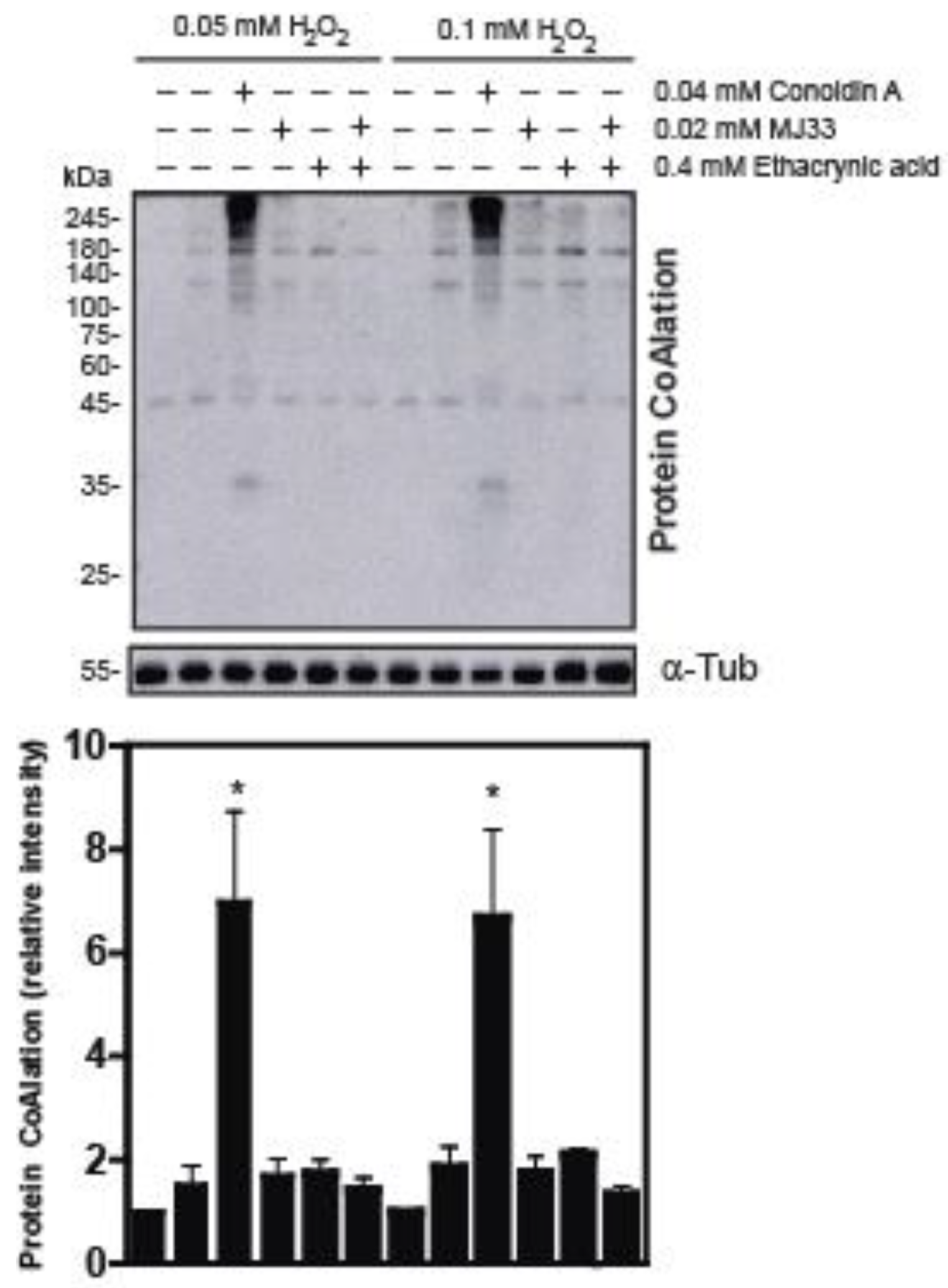
2.2. The Inhibition of 2-Cys Peroxiredoxins Increases the Level of Protein CoAlation in Spermatozoa
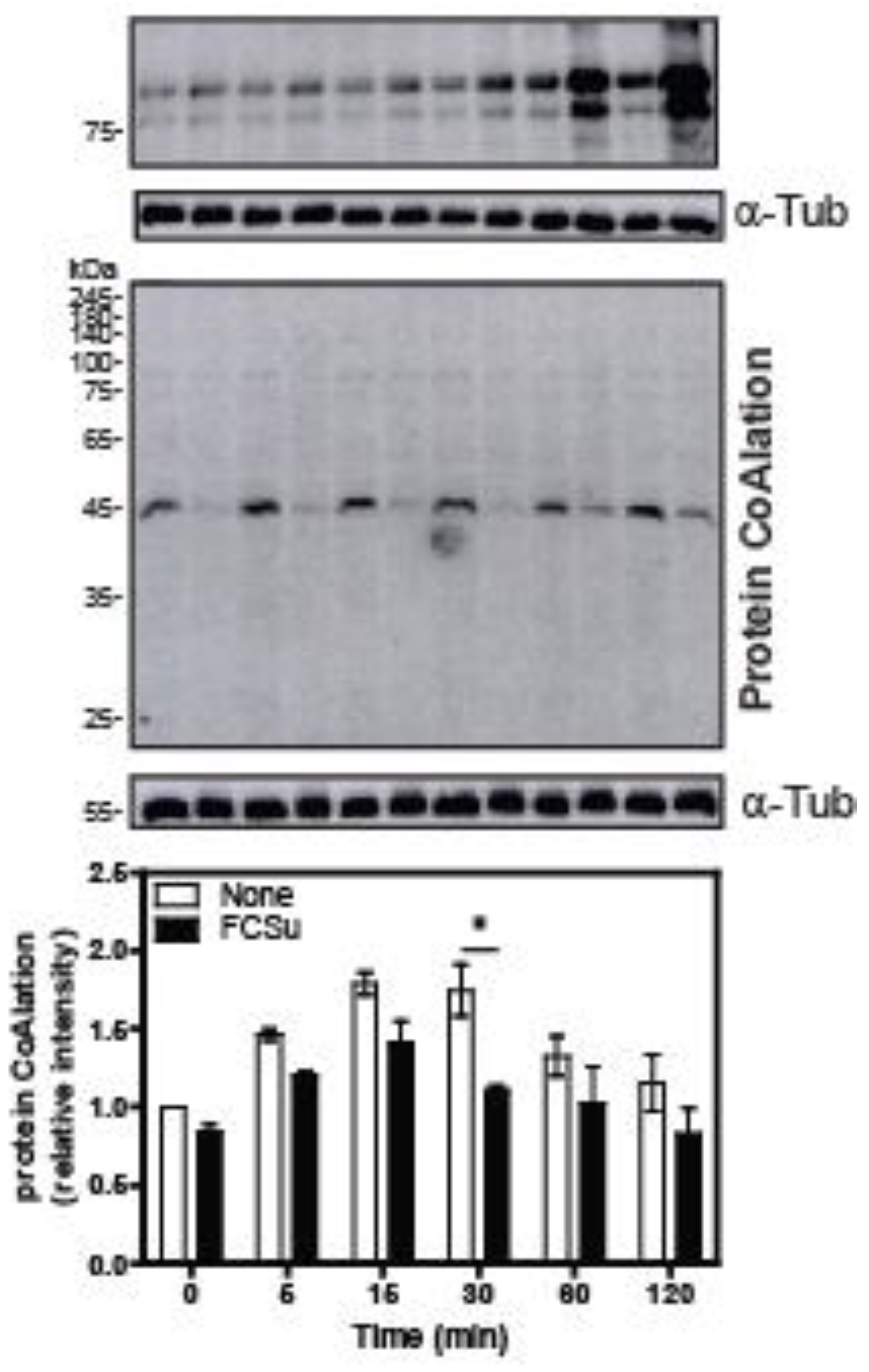
2.3. Protein CoAlation Pattern during Time-Course Sperm Capacitation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sperm Sample Preparation
4.3. Induction of In Vitro Oxidative Stress by Hydrogen Peroxide, Diamide and Tert-Butyl Hydroperoxide
4.4. Inhibition of PRDXs in Spermatozoa Treated with H2O2 to Promote In Vitro Oxidative Stress
4.5. Sperm Viability and Motility Analysis
4.6. Profile of Protein CoAlation during Time-Course Sperm Capacitation
4.7. SDS PAGE and Immunoblotting
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Infertility Prevalence Estimates, 1990–2021; WHO: Geneva, Switzerland, 2023.
- Aitken, R.J.; Baker, M.A. Oxidative stress and male reproductive biology. Reprod. Fertil. Dev. 2004, 16, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Flaherty, C.; Scarlata, E. Oxidative Stress and Reproductive Function: The protection of mammalian spermatozoa against oxidative stress. Reproduction 2022, 164, F67–F78. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.; Smith, T.; Jobling, M.; Baker, M.; De Iuliis, G. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; O’Flaherty, C. Peroxiredoxin 6 activates maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yumura, Y.; Takeshima, T.; Kawahara, T.; Sanjo, H.; Kuroda, S.N.; Asai, T.; Mori, K.; Kondou, T.; Uemura, H.; Iwasaki, A. Reactive oxygen species measured in the unprocessed semen samples of 715 infertile patients. Reprod. Med. Biol. 2017, 16, 354–363. [Google Scholar] [CrossRef] [Green Version]
- De Jonge, C. Biological basis for human capacitation-revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef]
- Leclerc, P.; de Lamirande, E.; Gagnon, C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol. Reprod. 1996, 55, 684–692. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Moawad, A.; Morielli, T.; Fernandez, M.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.W.; Dodia, C.; Feinstein, S.I.; Jain, M.K.; Fisher, A.B. 1-Cys Peroxiredoxin, a Bifunctional Enzyme with Glutathione Peroxidase and Phospholipase A2 Activities. J. Biol. Chem. 2000, 275, 28421–28427. [Google Scholar] [CrossRef] [Green Version]
- Gong, S.; San Gabriel, M.; Zini, A.; Chan, P.; O’Flaherty, C. Low Amounts and High Thiol Oxidation of Peroxiredoxins in Spermatozoa from Infertile Men. J. Androl. 2012, 33, 1342–1351. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Souza, A.R. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol. Reprod. 2011, 84, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Gout, I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem. Soc. Trans. 2018, 46, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Baković, J.; Yu, B.Y.K.; Silva, D.; Chew, S.P.; Kim, S.; Ahn, S.-H.; Palmer, L.; Aloum, L.; Stanzani, G.; Malanchuk, O.; et al. A key metabolic integrator, coenzyme A, modulates the activity of peroxiredoxin 5 via covalent modification. Mol. Cell. Biochem. 2019, 461, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Lashley, T.; Tossounian, M.-A.; Costello Heaven, N.; Wallworth, S.; Peak-Chew, S.; Bradshaw, A.; Cooper, J.M.; de Silva, R.; Srai, S.K.; Malanchuk, O.; et al. Extensive Anti-CoA Immunostaining in Alzheimer’s Disease and Covalent Modification of Tau by a Key Cellular Metabolite Coenzyme A. Front. Cell. Neurosci. 2021, 15, 739425. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for Examination and Processing of Human Semen, 6th ed.; University Press Cambridge: Cambridge, UK, 2021.
- MacLeod, J. The role of oxygen in the metabolism and motility of human spermatozoa. Am. J. Physiol. Leg. Content 1943, 138, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Tosic, J.; Walton, A. Formation of hydrogen peroxide by spermatozoa and its inhibitory effect of respiration. Nature 1946, 158, 485. [Google Scholar] [CrossRef] [PubMed]
- Matsushita-Fournier, D.; O’Flaherty, C.S. Oxidative Stress Induces Redox-Dependent Modifications of Human Sperm and Seminal Plasma Proteins and Damages the Paternal Genome. Ph.D. Thesis, McGill University, Montreal, QC, Canada, April 2015. [Google Scholar]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic Aldehydes Generated by Sperm Metabolism Activate Mitochondrial Reactive Oxygen Species Generation and Apoptosis by Targeting Succinate Dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvolini, E.; Buldreghini, E.; Lucarini, G.; Vignini, A.; Di Primio, R.; Balercia, G. Nitric oxide synthase and tyrosine nitration in idiopathic asthenozoospermia: An immunohistochemical study. Fertil. Steril. 2012, 97, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Peeker, R.; Abramsson, L.; Marklund, S.L. Superoxide dismutase isoenzymes in human seminal plasma and spermatozoa. Mol. Hum. Reprod. 1997, 3, 1061–1066. [Google Scholar] [CrossRef]
- Woo, H.A.; Kang, S.W.; Kim, H.K.; Yang, K.S.; Chae, H.Z.; Rhee, S.G. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J. Biol. Chem. 2003, 278, 47361–47364. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, C. The Enzymatic Antioxidant System of Human Spermatozoa. Adv. Androl. 2014, 2014, 626374. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.; Yu, A.; Moawad, A.; O’Flaherty, C. Peroxiredoxin 6 regulates the phosphoinositide 3-kinase/AKT pathway to maintain human sperm viability. Mol. Hum. Reprod. 2019, 25, 787–796. [Google Scholar] [CrossRef]
- Moawad, A.; Fernandez, M.; Scarlata, E.; Dodia, C.; Feinstein, S.; Fisher, A.; O’Flaherty, C. Deficiency of peroxiredoxin 6 or inhibition of its phospholipase A2 activity impair the in vitro sperm fertilizing competence in mice. Sci. Rep. 2017, 7, 12994. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Feinstein, S.I.; Fisher, A.B. Peroxiredoxin 6 as an antioxidant enzyme: Protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. J. Cell. Biochem. 2008, 104, 1274–1285. [Google Scholar] [CrossRef] [Green Version]
- Bumanlag, E.; Scarlata, E.; O’Flaherty, C. Peroxiredoxin 6 Peroxidase and Ca2+-Independent Phospholipase A2 Activities Are Essential to Support Male-Mouse Fertility. Antioxidants 2022, 11, 226. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic. Biol. Med. 2006, 40, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Kuribayashi, Y.; Gagnon, C. Effect of sperm lipid peroxidation on fertilization. J. Androl. 1996, 17, 151–157. [Google Scholar] [PubMed]
- De Lamirande, E.; Leclerc, P.; Gagnon, C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol. Hum. Reprod. 1997, 3, 175–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafini, S.; O’Flaherty, C. Redox Regulation to Modulate Phosphorylation Events in Human Spermatozoa. Antioxid. Redox Signal. 2022, 37, 437–450. [Google Scholar] [CrossRef]
- Lefievre, L.; Jha, K.N.; De Lamirande, E.; Visconti, P.E.; Gagnon, C. Activation of protein kinase A during human sperm capacitation and acrosome reaction. J. Androl. 2002, 23, 709–716. [Google Scholar]
- Visconti, P.E.; Johnson, L.R.; Oyaski, M.; Fornes, M.; Moss, S.B.; Gerton, G.L.; Kopf, G.S. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev. Biol. 1997, 192, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Biggers, J.D.; Whitten, W.K.; Whittngham, D.G. The culture of mouse embryos in vitro. In Methods in Mammalian Embryology; Daniel, J.C., Ed.; Freeman: San Francisco, CA, USA, 1971; pp. 86–116. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO Press: Geneva, Switzerland, 2010; Volume 5.
- Ramu, S.; Jeyendran, R.S. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. Methods Mol. Biol. 2013, 927, 21–25. [Google Scholar]
- De Lamirande, E.; Gagnon, C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic. Biol. Med. 1995, 18, 487–495. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: Modulation and protein kinase A dependency. Mol. Hum. Reprod. 2004, 10, 355–363. [Google Scholar] [CrossRef] [Green Version]
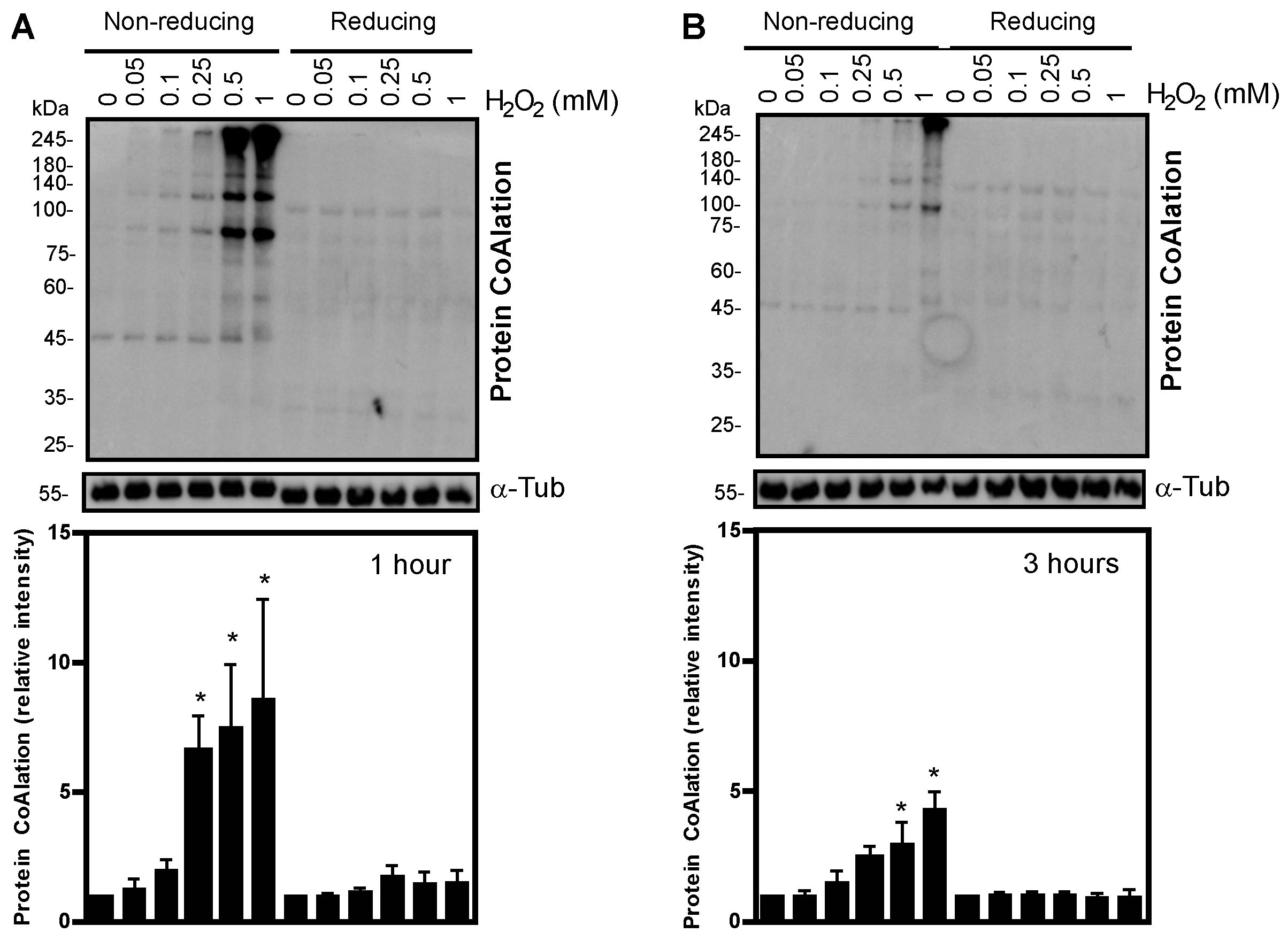
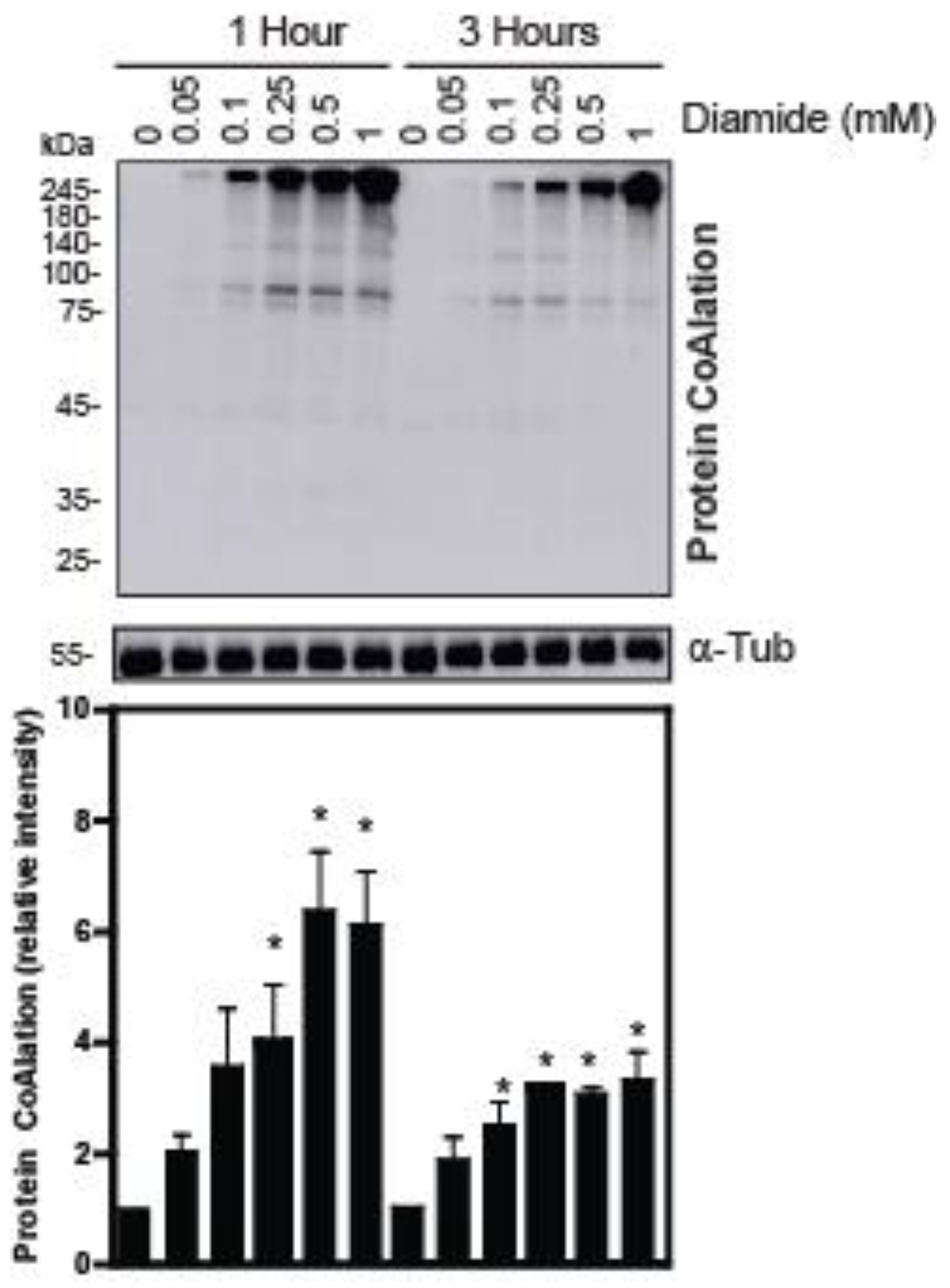
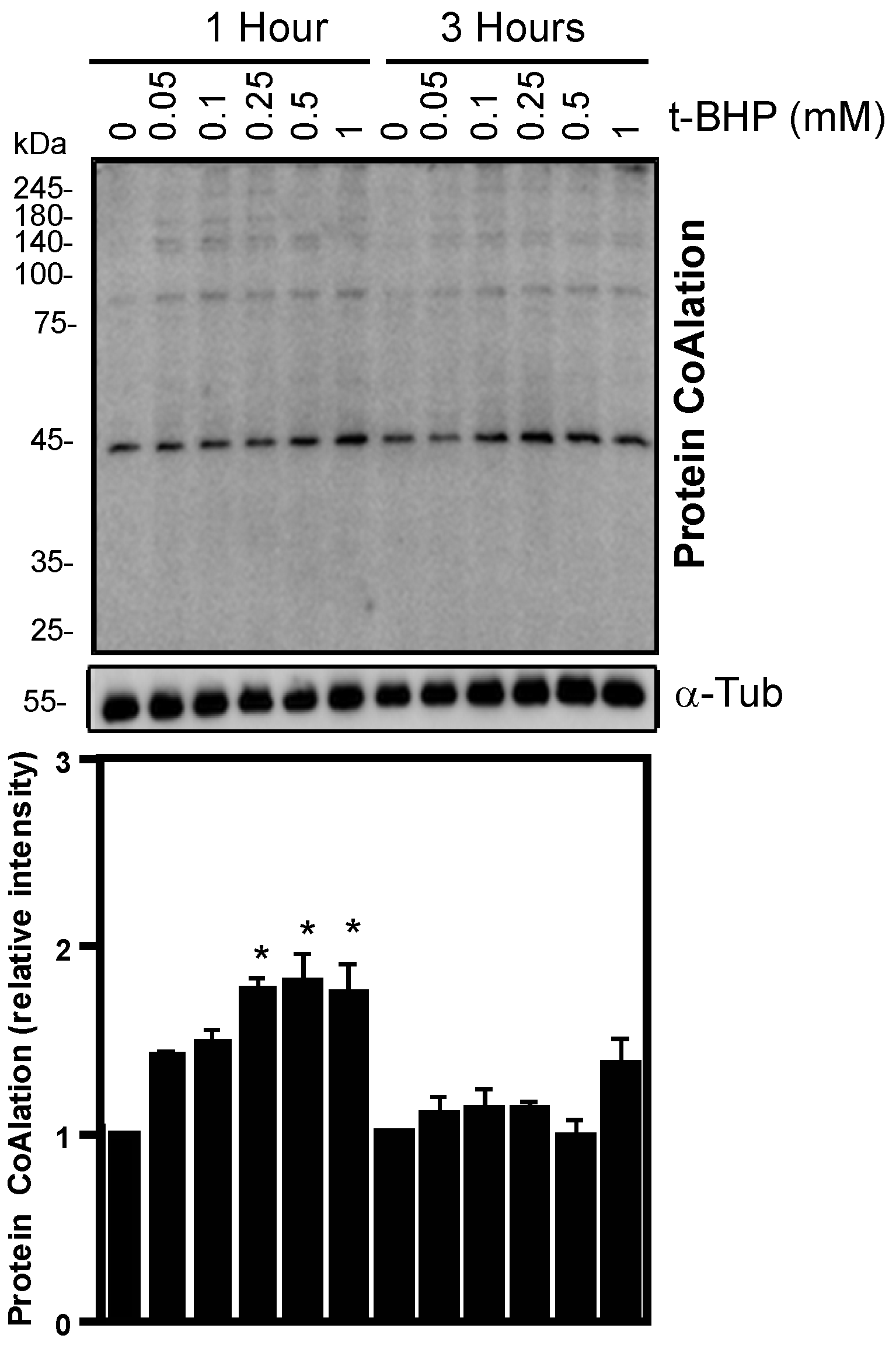
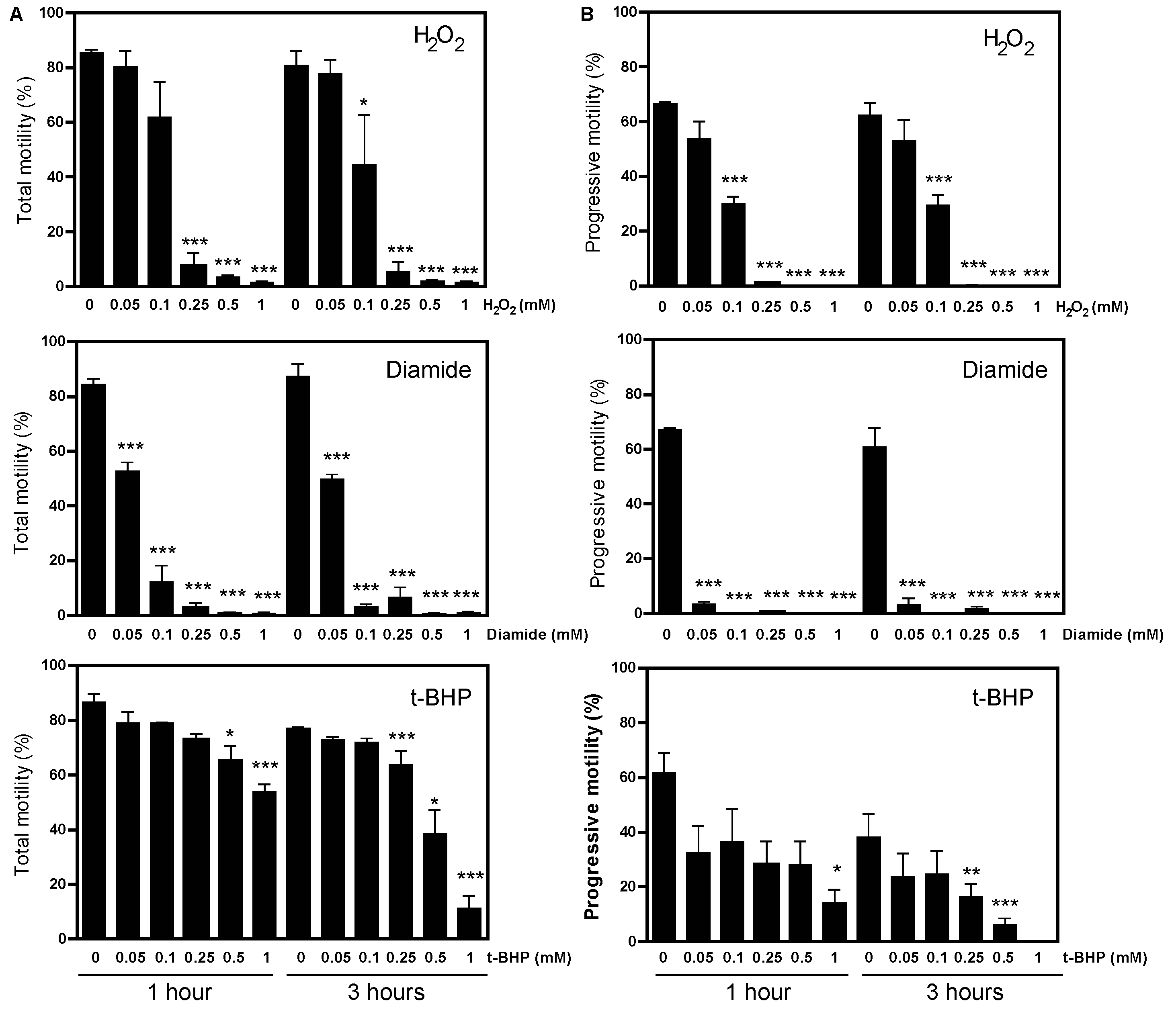
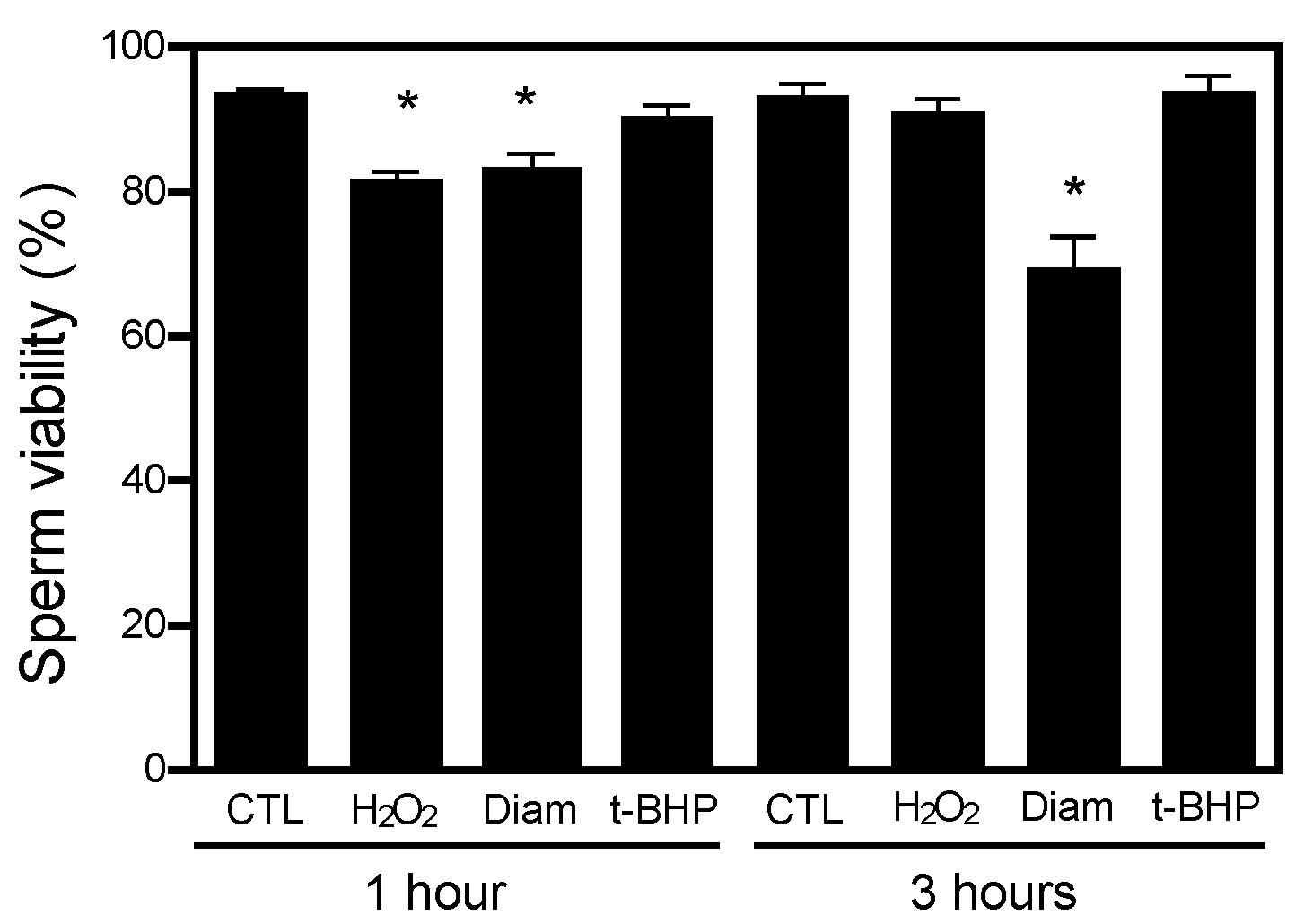
| Source of Variation | Protein CoAlation Levels | Sperm Viability | Total Motility | Progressive Motility |
|---|---|---|---|---|
| H2O2 treatment | p < 0.001 | p = 0.002 | p < 0.0001 | p < 0.0001 |
| time | p = 0.0007 | p = 0.03 | p = 0.20 | p = 0.601 |
| H2O2 treatment × time | p = 0.077 | p = 0.014 | p = 0.83 | p = 0.99 |
| Diamide treatment | p < 0.0001 | p = 0.003 | p < 0.0001 | p < 0.0001 |
| time | p < 0.0001 | p = 0.04 | p = 0.60 | p = 0.47 |
| Diamide treatment × time | p = 0.0151 | p = 0.06 | p = 0.45 | p = 0.57 |
| t-BHP treatment | p < 0.0001 | p = 0.49 | p < 0.0001 | p < 0.0001 |
| time | p < 0.0001 | p = 0.45 | p = 0.61 | p < 0.001 |
| t-BHP treatment × time | p = 0.0006 | p = 0.29 | p = 0.24 | p = 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrone, O.; Serafini, S.; Yu, B.Y.K.; Filonenko, V.; Gout, I.; O’Flaherty, C. Changes of the Protein CoAlation Pattern in Response to Oxidative Stress and Capacitation in Human Spermatozoa. Int. J. Mol. Sci. 2023, 24, 12526. https://doi.org/10.3390/ijms241512526
Petrone O, Serafini S, Yu BYK, Filonenko V, Gout I, O’Flaherty C. Changes of the Protein CoAlation Pattern in Response to Oxidative Stress and Capacitation in Human Spermatozoa. International Journal of Molecular Sciences. 2023; 24(15):12526. https://doi.org/10.3390/ijms241512526
Chicago/Turabian StylePetrone, Olivia, Steven Serafini, Bess Yi Kun Yu, Valeriy Filonenko, Ivan Gout, and Cristian O’Flaherty. 2023. "Changes of the Protein CoAlation Pattern in Response to Oxidative Stress and Capacitation in Human Spermatozoa" International Journal of Molecular Sciences 24, no. 15: 12526. https://doi.org/10.3390/ijms241512526
APA StylePetrone, O., Serafini, S., Yu, B. Y. K., Filonenko, V., Gout, I., & O’Flaherty, C. (2023). Changes of the Protein CoAlation Pattern in Response to Oxidative Stress and Capacitation in Human Spermatozoa. International Journal of Molecular Sciences, 24(15), 12526. https://doi.org/10.3390/ijms241512526







