Starving the Beast: Limiting Coenzyme A Biosynthesis to Prevent Disease and Transmission in Malaria
Abstract
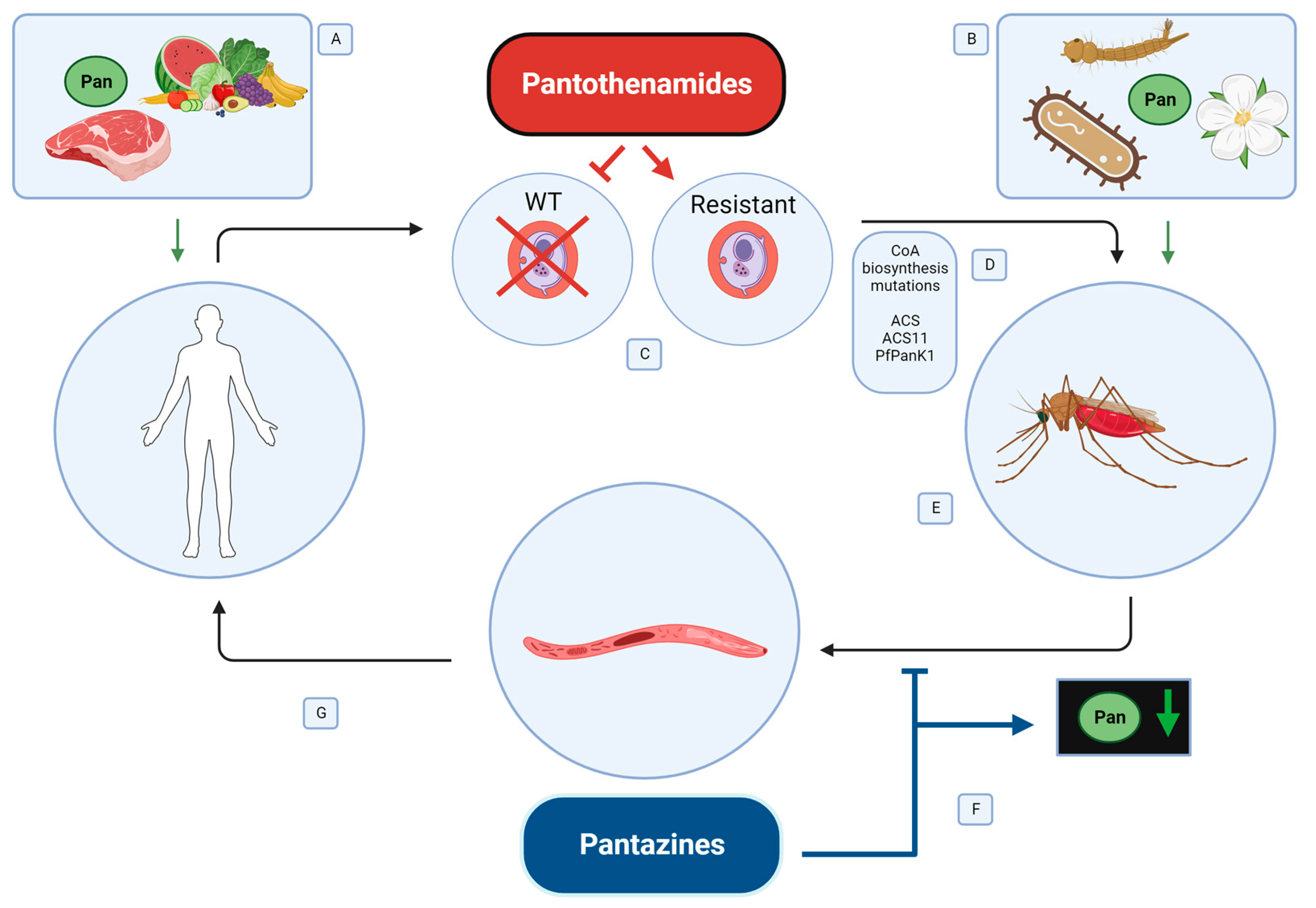
:1. Introduction
2. Coenzyme A
Pantothenate and CoA Biosynthesis
3. Pantothenate
3.1. Pantothenate Synthesis
3.2. Pantothenate Sources in the Malaria Parasite Life Cycle
3.3. The Mosquito Midgut Microbiome as a Source of Pantothenate
3.4. Sources of Pantothenate for Plasmodium spp.
3.5. Pantothenate Transport into Plasmodium Parasites
4. Pantothenate Kinase
4.1. Structure, Function, and Expression of Pantothenate Kinases
4.2. Pantothenate Kinase Disruption
5. Development of Drugs Targeting the CoA Biosynthesis Pathway
5.1. Pantothenamides to Target Malaria Parasites in the Human Host
5.2. Pantazines to Target Malaria Parasites in the Mosquito Host
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Malaria Report 2021; WHO: Geneva, Switzerland, 2021; Volume 13, pp. 15–38. [Google Scholar]
- Hancock, P.A.; Hendriks, C.J.M.; Tangena, J.A.; Gibson, H.; Hemingway, J.; Coleman, M.; Gething, P.W.; Cameron, E.; Bhatt, S.; Moyes, C.L. Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLoS Biol. 2020, 18, e3000633. [Google Scholar] [CrossRef]
- Susanna, D.; Pratiwi, D. Current status of insecticide resistance in malaria vectors in the Asian countries: A systematic review. F1000Research 2022, 10, 200. [Google Scholar] [CrossRef]
- Plowe, C.V. Malaria chemoprevention and drug resistance: A review of the literature and policy implications. Malar. J. 2022, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Abubakr, M.; Ali, Y.; Siddig, E.E.; Mohamed, N.S. Vector control strategy for Anopheles stephensi in Africa. Lancet Microbe 2022, 3, e403. [Google Scholar] [CrossRef]
- Mnzava, A.; Monroe, A.C.; Okumu, F. Anopheles stephensi in Africa requires a more integrated response. Malar. J. 2022, 21, 4–9. [Google Scholar] [CrossRef]
- Sinka, M.E.; Pironon, S.; Massey, N.C.; Longbottom, J.; Hemingway, J.; Moyes, C.L.; Willis, K.J. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. USA 2020, 117, 24900–24908. [Google Scholar] [CrossRef] [PubMed]
- Takken, W.; Lindsay, S. Increased threat of urban malaria from Anopheles stephensi mosquitoes, Africa. Emerg. Infect. Dis. 2019, 25, 1431–1433. [Google Scholar] [CrossRef]
- Brohn, F.H.; Trager, W. Coenzyme A requirement of malaria parasites: Enzymes of coenzyme A biosynthesis in normal duck erythrocytes and erythrocytes infected with Plasmodium lophurae. Proc. Natl. Acad. Sci. USA 1975, 72, 2456–2458. [Google Scholar] [CrossRef] [PubMed]
- Simão-Gurge, R.M.; Thakre, N.; Strickland, J.; Isoe, J.; Delacruz, L.R.; Torrevillas, B.K.; Rodriguez, A.M.; Riehle, M.A.; Luckhart, S. Activation of Anopheles stephensi pantothenate kinase and coenzyme a biosynthesis reduces infection with diverse Plasmodium species in the mosquito host. Biomolecules 2021, 11, 807. [Google Scholar] [CrossRef]
- Sibon, O.C.M.; Strauss, E. Coenzyme A: To make it or uptake it? Nat. Rev. Mol. Cell Biol. 2016, 17, 605–606. [Google Scholar] [CrossRef]
- Choudhary, C.; Weinert, B.T.; Nishida, Y.; Verdin, E.; Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Sabari, B.R.; Zhang, D.; Allis, C.D.; Zhao, Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 2017, 18, 90–101. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Czumaj, A.; Szrok-Jurga, S.; Hebanowska, A.; Turyn, J.; Swierczynski, J.; Sledzinski, T.; Stelmanska, E. The pathophysiological role of CoA. Int. J. Mol. Sci. 2020, 21, 9057. [Google Scholar] [CrossRef]
- Naquet, P.; Kerr, E.W.; Vickers, S.D.; Leonardi, R. Regulation of coenzyme A levels by degradation: The ‘Ins and Outs’. Prog. Lipid Res. 2020, 78, 101028. [Google Scholar] [CrossRef]
- de Vries, L.E.; Lunghi, M.; Krishnan, A.; Kooij, T.W.A.; Soldati-Favre, D. Pantothenate and CoA biosynthesis in Apicomplexa and their promise as antiparasitic drug targets. PLoS Pathog. 2021, 17, 1010124. [Google Scholar] [CrossRef]
- Nurkanto, A.; Jeelani, G.; Santos, H.J.; Rahmawati, Y.; Mori, M.; Nakamura, Y.; Goto, K.; Saikawa, Y.; Annoura, T.; Tozawa, Y.; et al. Characterization of Plasmodium falciparum pantothenate kinase and identification of its inhibitors from natural products. Front. Cell. Infect. Microbiol. 2021, 11, 639065. [Google Scholar] [CrossRef]
- Seipelo, K.; Mogwera, P.; Strauss, E.; De Villiers, M. Membrane Permeability and Transport studies of Coenzyme A, Its Precursors and Antimetabolites. 2020. Available online: https://scholar.sun.ac.za/items/ddae9589-f9d7-426a-b6b2-91da797df707 (accessed on 24 July 2023).
- Awasthy, D.; Ambady, A.; Bhat, J.; Sheikh, G.; Ravishankar, S.; Subbulakshmi, V.; Mukherjee, K.; Sambandamurthy, V.; Sharma, U. Essentiality and functional analysis of type I and type III pantothenate kinases of Mycobacterium tuberculosis. Microbiology 2010, 156, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Chohnan, S.; Zhang, Y.M.; Virga, K.G.; Lee, R.E.; Rock, C.O.; Jackowski, S. A pantothenate kinase from Staphylococcus aureus refractory to feedback regulation by coenzyme A. J. Biol. Chem. 2005, 280, 3314–3322. [Google Scholar] [CrossRef]
- Leonardi, R.; Jackowski, S. Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus 2007, 2. [Google Scholar] [CrossRef]
- Jiménez-Cortés, J.G.; García-Contreras, R.; Bucio-Torres, M.I.; Cabrera-Bravo, M.; Córdoba-Aguilar, A.; Benelli, G.; Salazar-Schettino, P.M. Bacterial symbionts in human blood-feeding arthropods: Patterns, general mechanisms and effects of global ecological changes. Acta Trop. 2018, 186, 69–101. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, Y.; Chen, J.; Pan, Z.; Pang, L.; Wang, Y.; Zhang, Q.; Strand, M.R.; Chen, X.X.; Huang, J. Parasite reliance on its host gut microbiota for nutrition and survival. ISME J. 2022, 16, 2574–2586. [Google Scholar] [CrossRef]
- Augagneur, Y.; Jaubert, L.; Schiavoni, M.; Pachikara, N.; Garg, A.; Usmani-Brown, S.; Wesolowski, D.; Zeller, S.; Ghosal, A.; Cornillot, E.; et al. Identification and functional analysis of the primary pantothenate transporter, PfPAT, of the human malaria parasite Plasmodium falciparum. J. Biol. Chem. 2013, 288, 20558–20567. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate role of gut microbiota in vitamin B nutrition and its influences on human health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional roles of B-vitamins in the gut and gut microbiome. Mol. Nutr. Food Res. 2020, 64, 2000426. [Google Scholar] [CrossRef]
- Souza, R.S.; Virginio, F.; Riback, T.I.S.; Suesdek, L.; Barufi, J.B.; Genta, F.A. Microorganism-based larval diets affect mosquito development, size and nutritional reserves in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Front. Physiol. 2019, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Barredo, E.; DeGennaro, M. Not just from blood: Mosquito nutrient acquisition from nectar sources. Trends Parasitol. 2020, 36, 473–484. [Google Scholar] [CrossRef]
- Peach, D.A.H.; Gries, G. Mosquito phytophagy—Sources exploited, ecological function, and evolutionary transition to haematophagy. Entomol. Exp. Appl. 2020, 168, 120–136. [Google Scholar] [CrossRef]
- Martin, V.N.; Schaeffer, R.N.; Fukami, T. Potential effects of nectar microbes on pollinator health. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210155. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Pozo, M.I.; Álvarez-Pérez, S.; Lievens, B.; Fukami, T. Yeast–nectar interactions: Metacommunities and effects on pollinators. Curr. Opin. Insect Sci. 2021, 44, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Colda, A.; Bossaert, S.; Verreth, C.; Vanhoutte, B.; Honnay, O.; Keulemans, W.; Lievens, B. Inoculation of pear flowers with Metschnikowia reukaufii and Acinetobacter nectaris enhances attraction of honeybees and hoverflies, but does not increase fruit and seed set. PLoS ONE 2021, 16, e0250203. [Google Scholar] [CrossRef]
- Peach, D.A.H.; Carroll, C.; Meraj, S.; Gomes, S.; Galloway, E.; Balcita, A.; Coatsworth, H.; Young, N.; Uriel, Y.; Gries, R.; et al. Nectar-dwelling microbes of common tansy are attractive to its mosquito pollinator, Culex pipiens L. BMC Ecol. Evol. 2021, 21, 29. [Google Scholar] [CrossRef]
- Phasomkusolsil, S.; Pantuwatana, K.; Tawong, J.; Khongtak, W.; Kertmanee, Y.; Monkanna, N.; Khaosanorh, S.; Wanja, E.W.; Davidson, S.A. Sugar and multivitamin diet effects on the longevity and mating capacity of laboratory-reared male Anopheline mosquitoes. J. Am. Mosq. Control. Assoc. 2017, 33, 175–183. [Google Scholar] [CrossRef]
- Tan, S.B.; Nazni, W.A.; Misni, S.; Zuraini, Z.; Lee, H.L. Effects of vitamin B fortified sucrose solution on the longevity and reproductive potentials of laboratory-bred Culex quinquefasciatus say adult. Trop. Biomed. 2016, 33, 141–148. [Google Scholar]
- Serrato-Salas, J.; Gendrin, M. Involvement of Microbiota in Insect Physiology: Focus on B vitamins. mBio 2023, 14, e0222522. [Google Scholar] [CrossRef]
- Thakre, N.; Simao Gurge, R.M.; Isoe, J.; Kivi, H.; Strickland, J.; Delacruz, L.R.; Rodriguez, A.M.; Haney, R.; Sadeghi, R.; Joy, T.; et al. Manipulation of pantothenate kinase in Anopheles stephensi suppresses pantothenate levels with minimal impacts on mosquito fitness. Insect Biochem. Mol. Biol. 2022, 149, 103834. [Google Scholar] [CrossRef]
- Alto, B.W.; Muturi, E.J.; Lampman, R.L. Effects of nutrition and density in Culex pipiens. Medical and Veterinary Entomology 2012, 26, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Levi, T.; Ben-Dov, E.; Shahi, P.; Borovsky, D.; Zaritsky, A. Growth and development of Aedes aegypti larvae at limiting food concentrations. Acta Trop. 2014, 133, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Price, D.P.; Schilkey, F.D.; Ulanov, A.; Hansen, I.A. Small mosquitoes, large implications: Crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasites Vectors 2015, 8, 252. [Google Scholar] [CrossRef]
- Rivera-Pérez, C.; Clifton, M.E.; Noriega, F.G. How micronutrients influence the physiology of mosquitoes. Curr. Opin. Insect Sci. 2017, 23, 112–117. [Google Scholar] [CrossRef]
- Price, D.R.G.; Wilson, A.C.C. A substrate ambiguous enzyme facilitates genome reduction in an intracellular symbiont. BMC Biol. 2014, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Shigenobu, S.; Watanabe, H.; Hattori, M.; Sakaki, Y.; Ishikawa, H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 2000, 407, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.R.; Sun, X.; Wang, T.Y.; Yan, J.Y.; Yao, Y.L.; Li, C.Q.; Luan, J.B. Pantothenate mediates the coordination of whitefly and symbiont fitness. ISME J. 2021, 15, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hasegawa, D.K.; Kaur, N.; Kliot, A.; Pinheiro, P.V.; Luan, J.; Stensmyr, M.C.; Zheng, Y.; Liu, W.; Sun, H.; et al. The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biol. 2016, 14, 110. [Google Scholar] [CrossRef]
- Sun, X.; Liu, B.Q.; Li, C.Q.; Chen, Z.B.; Xu, X.R.; Luan, J.B. A novel microRNA regulates cooperation between symbiont and a laterally acquired gene in the regulation of pantothenate biosynthesis within Bemisia tabaci whiteflies. Mol. Ecol. 2022, 31, 2611–2624. [Google Scholar] [CrossRef] [PubMed]
- Ricci, I.; Damiani, C.; Capone, A.; DeFreece, C.; Rossi, P.; Favia, G. Mosquito/microbiota interactions: From complex relationships to biotechnological perspectives. Curr. Opin. Microbiol. 2012, 15, 278–284. [Google Scholar] [CrossRef]
- Villegas, L.M.; Pimenta, P.F.P. Metagenomics, paratransgenesis and the Anopheles microbiome: A portrait of the geographical distribution of the anopheline microbiota based on a meta-analysis of reported taxa. Mem. Inst. Oswaldo Cruz 2014, 109, 672–684. [Google Scholar] [CrossRef]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasites Vectors 2013, 6, 146. [Google Scholar] [CrossRef]
- Shi, H.; Yu, X.; Cheng, G. Impact of the microbiome on mosquito-borne diseases. Protein Cell 2023. online ahead of print. [Google Scholar] [CrossRef]
- Huang, W.; Wang, S.; Jacobs-Lorena, M. Use of microbiota to fight mosquito-borne disease. Front. Genet. 2020, 11, 196. [Google Scholar] [CrossRef]
- Dacey, D.P.; Chain, F.J.J. The challenges of microbial control of mosquito-borne diseases due to the gut microbiome. Front. Genet. 2020, 11, 504354. [Google Scholar] [CrossRef] [PubMed]
- Newton, I.L.G.; Rice, D.W. The Jekyll and Hyde symbiont: Could Wolbachia be a nutritional mutualist? J. Bacteriol. 2020, 202, 8. [Google Scholar] [CrossRef] [PubMed]
- Nikoh, N.; Hosokawa, T.; Moriyama, M.; Oshima, K.; Hattori, M.; Fukatsu, T. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc. Natl. Acad. Sci. USA 2014, 111, 10257–10262. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. Camb. Philos. Soc. 2015, 90, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Shrivastava, N.K.; Nayak, A.; Singh, H. Wolbachia: A prospective solution to mosquito borne diseases. Int. J. Mosq. Res. 2018, 5, 1–8. [Google Scholar]
- Crotti, E.; Damiani, C.; Pajoro, M.; Gonella, E.; Rizzi, A.; Ricci, I.; Negri, I.; Scuppa, P.; Rossi, P.; Ballarini, P.; et al. Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders. Environ. Microbiol. 2009, 11, 3252–3264. [Google Scholar] [CrossRef]
- Crotti, E.; Rizzi, A.; Chouaia, B.; Ricci, I.; Favia, G.; Alma, A.; Sacchi, L.; Bourtzis, K.; Mandrioli, M.; Cherif, A.; et al. Acetic acid bacteria, newly emerging symbionts of insects. Appl. Environ. Microbiol. 2010, 76, 6963–6970. [Google Scholar] [CrossRef]
- Wilson, A.C.C.; Duncan, R.P. Signatures of host/symbiont genome coevolution in insect nutritional endosymbioses. Proc. Natl. Acad. Sci. USA 2015, 112, 10255–10261. [Google Scholar] [CrossRef]
- Divo, A.A.; Geary, T.G.; Davis, N.L.; Jensen, J.B. Nutritional Requirements of Plasmodium falciparum in Culture. I. Exogenously Supplied Dialyzable Components Necessary for Continuous Growth. J. Protozool. 1985, 32, 59–64. [Google Scholar] [CrossRef]
- Hart, R.J.; Abraham, A.; Aly, A.S.I. Genetic characterization of coenzyme A biosynthesis reveals essential distinctive functions during malaria parasite development in blood and mosquito. Front. Cell. Infect. Microbiol. 2017, 7, 260. [Google Scholar] [CrossRef]
- Martin, R.E. The transportome of the malaria parasite. Biol. Rev. 2020, 95, 305–332. [Google Scholar] [CrossRef]
- Kenthirapalan, S.; Waters, A.P.; Matuschewski, K.; Kooij, T.W.A. Functional profiles of orphan membrane transporters in the life cycle of the malaria parasite. Nat. Commun. 2016, 7, 10519. [Google Scholar] [CrossRef]
- Srinivasan, B.; Baratashvili, M.; Van Der Zwaag, M.; Kanon, B.; Colombelli, C.; Lambrechts, R.A.; Schaap, O.; Nollen, E.A.; Podgoršek, A.; Kosec, G.; et al. Extracellular 4′-phosphopantetheine is a source for intracellular coenzyme A synthesis. Nat. Chem. Biol. 2015, 11, 784–792. [Google Scholar] [CrossRef]
- Hart, R.J.; Cornillot, E.; Abraham, A.; Molina, E.; Nation, C.S.; Ben Mamoun, C.; Aly, A.S.I. Genetic characterization of Plasmodium putative pantothenate kinase genes reveals their essential role in malaria parasite transmission to the mosquito. Sci. Rep. 2016, 6, 33518. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J.; Lawres, L.; Fritzen, E.; Mamoun, C.B.; Aly, A.S.I. Plasmodium yoelii vitamin B5 pantothenate transporter candidate is essential for parasite transmission to the mosquito. Sci. Rep. 2014, 4, srep05665. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.; Singer, M.; Lemgruber, L.; Silva, P.A.G.C.; Frischknecht, F.; Mair, G.R. A putative small solute transporter is responsible for the secretion of G377 and TRAP-containing secretory vesicles during Plasmodium gamete egress and sporozoite motility. PLoS Pathog. 2016, 12, 1005734. [Google Scholar] [CrossRef]
- Spry, C.; Kirk, K.; Saliba, K.J. Coenzyme A biosynthesis: An antimicrobial drug target. FEMS Microbiol. Rev. 2008, 32, 56–106. [Google Scholar] [CrossRef]
- Hauck, E.S.; Antonova-Koch, Y.; Drexler, A.; Pietri, J.; Pakpour, N.; Liu, D.; Blacutt, J.; Riehle, M.A.; Luckhart, S. Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi. Microbes Infect. 2013, 15, 775–787. [Google Scholar] [CrossRef]
- Souvannaseng, L.; Hun, L.V.; Baker, H.; Klyver, J.M.; Wang, B.; Pakpour, N.; Bridgewater, J.M.; Napoli, E.; Giulivi, C.; Riehle, M.A.; et al. Inhibition of JNK signaling in the Asian malaria vector Anopheles stephensi extends mosquito longevity and improves resistance to Plasmodium falciparum infection. PLoS Pathog. 2018, 14, e1007418. [Google Scholar] [CrossRef] [PubMed]
- Bennink, S.; Kiesow, M.J.; Pradel, G. The development of malaria parasites in the mosquito midgut. Cell. Microbiol. 2016, 18, 905–918. [Google Scholar] [CrossRef]
- Smith, R.C.; Jacobs-Lorena, M. Plasmodium-mosquito interactions: A tale of roadblocks and detours. Adv. Insect Physiol. 2010, 39, 119–149. [Google Scholar] [CrossRef]
- Srivastava, A.; Philip, N.; Hughes, K.R.; Georgiou, K.; MacRae, J.I.; Barrett, M.P.; Creek, D.J.; McConville, M.J.; Waters, A.P. Stage-specific changes in Plasmodium metabolism required for differentiation and adaptation to different host and vector environments. PLoS Pathog. 2016, 12, 1006094. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.S.; Yun, M.K.; Zhang, Y.M.; Chohnan, S.; Rock, C.O.; White, S.W.; Jackowski, S.; Park, H.W.; Leonardi, R. Prokaryotic type II and type III pantothenate kinases: The same monomer fold creates dimers with distinct catalytic properties. Structure 2006, 14, 1251–1261. [Google Scholar] [CrossRef]
- Bum, S.H.; Senisterra, G.; Rabeh, W.M.; Vedadi, M.; Leonardi, R.; Zhang, Y.M.; Rock, C.O.; Jackowski, S.; Park, H.W. Crystal structures of human pantothenate kinases: Insights into allosteric regulation and mutations linked to a neurodegeneration disorder. J. Biol. Chem. 2007, 282, 27984–27993. [Google Scholar] [CrossRef]
- Alfonso-Pecchio, A.; Garcia, M.; Leonardi, R.; Jackowski, S. Compartmentalization of mammalian pantothenate kinases. PLoS ONE 2012, 7, 0049509. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, C.; Lv, S.; Zhou, B. Pantothenate kinase-associated neurodegeneration: Insights from a Drosophila model. Hum. Mol. Genet. 2009, 18, 3659–3672. [Google Scholar] [CrossRef]
- Jackowski, S. Proposed therapies for pantothenate-kinase-associated neurodegeneration. J. Exp. Neurosci. 2019, 13, 4–6. [Google Scholar] [CrossRef]
- Afshar, K.; Gönczy, P.; DiNardo, S.; Wasserman, S.A. fumble encodes a pantothenate kinase homolog required for proper mitosis and meiosis in Drosophila melanogaster. Genetics 2001, 157, 1267–1276. [Google Scholar] [CrossRef]
- Tjhin, E.T.; Spry, C.; Sewell, A.L.; Hoegl, A.; Barnard, L.; Sexton, A.E.; Siddiqui, G.; Howieson, V.M.; Maier, A.G.; Creek, D.J.; et al. Mutations in the pantothenate kinase of Plasmodium falciparum confer diverse sensitivity profiles to antiplasmodial pantothenate analogues. PLoS Pathog. 2018, 14, 1006918. [Google Scholar] [CrossRef]
- Tjhin, E.T.; Howieson, V.M.; Spry, C.; van Dooren, G.G.; Saliba, K.J. A novel heteromeric pantothenate kinase complex in apicomplexan parasites. PLoS Pathog. 2021, 17, 1009797. [Google Scholar] [CrossRef]
- Saliba, K.J.; Ferru, I.; Kirk, K. Provitamin B5 (Pantothenol) inhibits growth of the intraerythrocytic malaria parasite. Antimicrob. Agents Chemother. 2005, 49, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.A.M.; Hermkens, P.H.H.; Zeeuwen, P.L.J.M.; Botman, P.N.M.; Blaauw, R.H.; Burghout, P.; Van Galen, P.M.; Mouton, J.W.; Rutjes, F.P.J.T.; Schalkwijk, J. Combination of pantothenamides with vanin inhibitors as a novel antibiotic strategy against gram-positive bacteria. Antimicrob. Agents Chemother. 2013, 57, 4794–4800. [Google Scholar] [CrossRef] [PubMed]
- Spry, C.; Macuamule, C.; Lin, Z.; Virga, K.G.; Lee, R.E.; Strauss, E.; Saliba, K.J. Pantothenamides are potent, on-target inhibitors of Plasmodium falciparum growth when serum pantetheinase is inactivated. PLoS ONE 2013, 8, 0054974. [Google Scholar] [CrossRef]
- Macuamule, C.J.; Tjhin, E.T.; Jana, C.E.; Barnard, L.; Koekemoer, L.; De Villiers, M.; Saliba, K.J.; Strauss, E. A pantetheinase-resistant pantothenamide with potent, on-target, and selective antiplasmodial activity. Antimicrob. Agents Chemother. 2015, 59, 3666–3668. [Google Scholar] [CrossRef] [PubMed]
- Spry, C.; Chai, C.L.L.; Kirk, K.; Saliba, K.J. A class of pantothenic acid analogs inhibits Plasmodium falciparum pantothenate kinase and represses the proliferation of malaria parasites. Antimicrob. Agents Chemother. 2005, 49, 4649–4657. [Google Scholar] [CrossRef]
- De Villiers, M.; Macuamule, C.; Spry, C.; Hyun, Y.M.; Strauss, E.; Saliba, K.J. Structural modification of pantothenamides counteracts degradation by pantetheinase and improves antiplasmodial activity. ACS Med. Chem. Lett. 2013, 4, 784–789. [Google Scholar] [CrossRef]
- de Vries, L.E.; Jansen, P.A.M.; Barcelo, C.; Munro, J.; Verhoef, J.M.J.; Pasaje, C.F.A.; Rubiano, K.; Striepen, J.; Abla, N.; Berning, L.; et al. Preclinical characterization and target validation of the antimalarial pantothenamide MMV693183. Nat. Commun. 2022, 13, 2158. [Google Scholar] [CrossRef]
- Jansen, P.A.M.; van der Krieken, D.A.; Botman, P.N.M.; Blaauw, R.H.; Cavina, L.; Raaijmakers, E.M.; de Heuvel, E.; Sandrock, J.; Pennings, L.J.; Hermkens, P.H.H.; et al. Stable pantothenamide bioisosteres: Novel antibiotics for Gram-positive bacteria. J. Antibiot. 2019, 72, 682–692. [Google Scholar] [CrossRef]
- Howieson, V.M.; Tran, E.; Hoegl, A.; Fam, H.L.; Fu, J.; Sivonen, K.; Li, X.X.; Auclair, K.; Saliba, K.J. Triazole substitution of a labile amide bond stabilizes pantothenamides and improves their antiplasmodial potency. Antimicrob. Agents Chemother. 2016, 60, 7146–7152. [Google Scholar] [CrossRef]
- Spry, C.; Barnard, L.; Kok, M.; Powell, A.K.; Mahesh, D.; Tjhin, E.T.; Saliba, K.J.; Strauss, E.; De Villiers, M. Toward a stable and potent coenzyme-A targeting antiplasmodial agent: Structure-activity relationship studies of N-phenethyl-α-methyl-pantothenamide. ACS Infect. Dis. 2020, 6, 1844–1854. [Google Scholar] [CrossRef]
- Leonardi, R.; Zhang, Y.-M.; Yun, M.-K.; Zhou, R.; Zeng, F.-Y.; Lin, W.; Cui, J.; Chen, T.; Rock, C.O.; White, S.W.; et al. Modulation of pantothenate kinase 3 activity by small molecules that interact with the substrate/allosteric regulatory domain. Chem. Biol. 2010, 17, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, J.; Allman, E.L.; Jansen, P.A.M.; De Vries, L.E.; Verhoef, J.M.J.; Jackowski, S.; Botman, P.N.M.; Beuckens-Schortinghuis, C.A.; Koolen, K.M.J.; Bolscher, J.M.; et al. Antimalarial pantothenamide metabolites target acetyl-coenzyme A biosynthesis in Plasmodium falciparum. Sci. Transl. Med. 2019, 11, eaas9917. [Google Scholar] [CrossRef]
- De Villiers, M.; Spry, C.; Macuamule, C.J.; Barnard, L.; Wells, G.; Saliba, K.J.; Strauss, E. Antiplasmodial mode of action of pantothenamides: Pantothenate kinase serves as a metabolic activator not as a target. ACS Infect. Dis. 2017, 3, 527–541. [Google Scholar] [CrossRef]
- Glennon, E.K.K.; Dankwa, S.; Smith, J.D.; Kaushansky, A. Opportunities for host-targeted therapies for malaria. Trends Parasitol. 2018, 34, 843–860. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Adderley, J.; Leroy, D.; Drewry, D.H.; Wilson, D.W.; Kaushansky, A.; Doerig, C. Host-directed therapy, an untapped opportunity for antimalarial intervention. Cell Rep. Med. 2021, 2, 100423. [Google Scholar] [CrossRef]
- Sharma, L.K.; Leonardi, R.; Lin, W.; Boyd, V.A.; Goktug, A.; Shelat, A.A.; Chen, T.; Jackowski, S.; Rock, C.O. A high-throughput screen reveals new small-molecule activators and inhibitors of pantothenate kinases. J. Med. Chem. 2015, 58, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, C.; Frank, M.W.; Tangallapally, R.; Yun, M.K.; Edwards, A.; White, S.W.; Lee, R.E.; Rock, C.O.; Jackowski, S. Pantothenate kinase activation relieves coenzyme A sequestration and improves mitochondrial function in mice with propionic acidemia. Sci. Transl. Med. 2021, 13, eabf5965. [Google Scholar] [CrossRef]
- Subramanian, C.; Frank, M.W.; Tangallapally, R.; Yun, M.K.; White, S.W.; Lee, R.E.; Rock, C.O.; Jackowski, S. Relief of CoA sequestration and restoration of mitochondrial function in a mouse model of propionic acidemia. J. Inherit. Metab. Dis. 2023, 46, 28–42. [Google Scholar] [CrossRef]
- Sharma, L.K.; Subramanian, C.; Yun, M.K.; Frank, M.W.; White, S.W.; Rock, C.O.; Lee, R.E.; Jackowski, S. A therapeutic approach to pantothenate kinase associated neurodegeneration. Nat. Commun. 2018, 9, 4399. [Google Scholar] [CrossRef]
- Diarra, R.A.; Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; et al. Testing configurations of attractive toxic sugar bait (ATSB) stations in Mali, West Africa, for improving the control of malaria parasite transmission by vector mosquitoes and minimizing their effect on non-target insects. Malar. J. 2021, 20, 184. [Google Scholar] [CrossRef]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riske, B.F.; Luckhart, S.; Riehle, M.A. Starving the Beast: Limiting Coenzyme A Biosynthesis to Prevent Disease and Transmission in Malaria. Int. J. Mol. Sci. 2023, 24, 13915. https://doi.org/10.3390/ijms241813915
Riske BF, Luckhart S, Riehle MA. Starving the Beast: Limiting Coenzyme A Biosynthesis to Prevent Disease and Transmission in Malaria. International Journal of Molecular Sciences. 2023; 24(18):13915. https://doi.org/10.3390/ijms241813915
Chicago/Turabian StyleRiske, Brendan F., Shirley Luckhart, and Michael A. Riehle. 2023. "Starving the Beast: Limiting Coenzyme A Biosynthesis to Prevent Disease and Transmission in Malaria" International Journal of Molecular Sciences 24, no. 18: 13915. https://doi.org/10.3390/ijms241813915
APA StyleRiske, B. F., Luckhart, S., & Riehle, M. A. (2023). Starving the Beast: Limiting Coenzyme A Biosynthesis to Prevent Disease and Transmission in Malaria. International Journal of Molecular Sciences, 24(18), 13915. https://doi.org/10.3390/ijms241813915






