Instantaneous Inactivation of Herpes Simplex Virus by Silicon Nitride Bioceramics
Abstract
:1. Introduction
2. Results
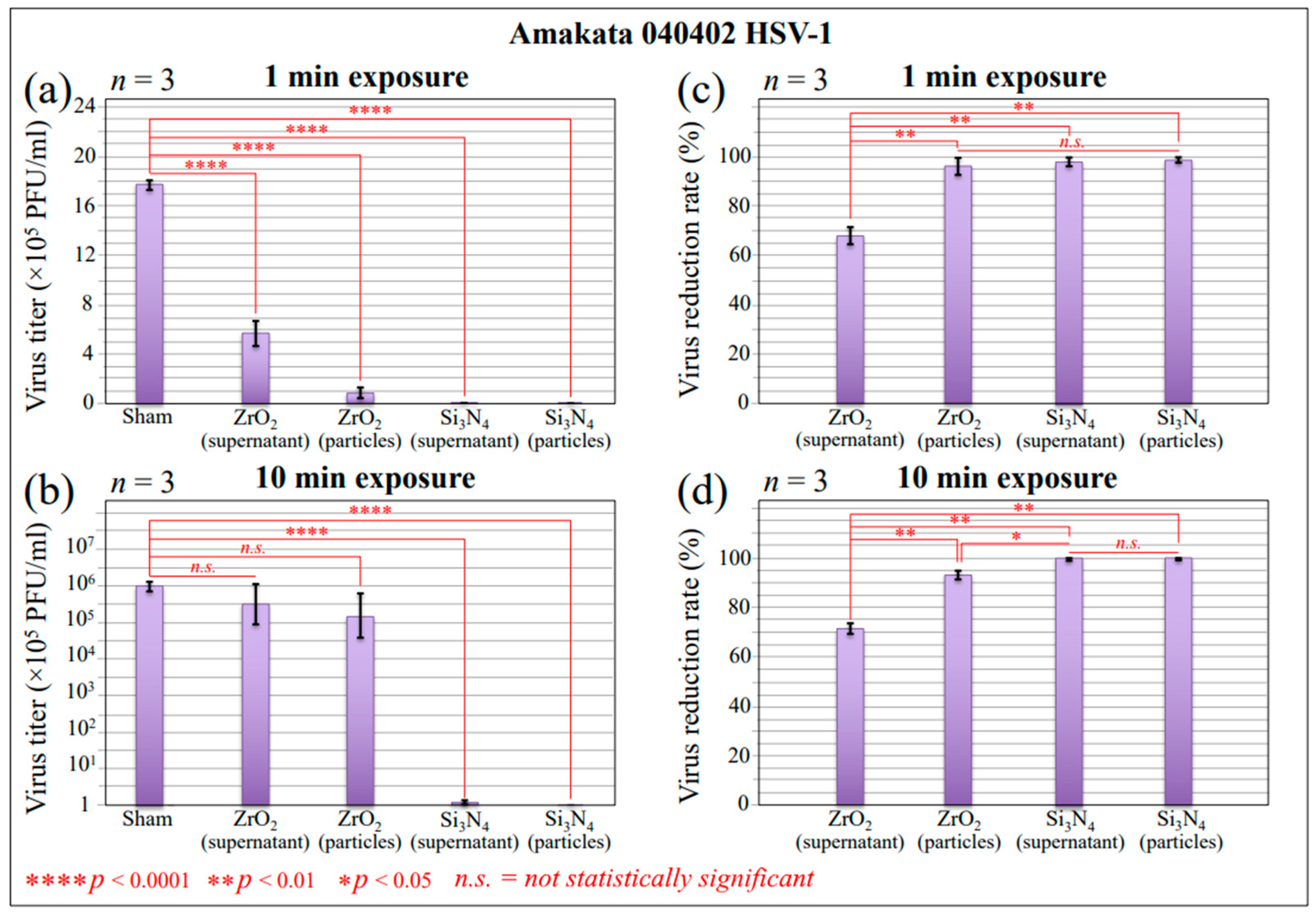
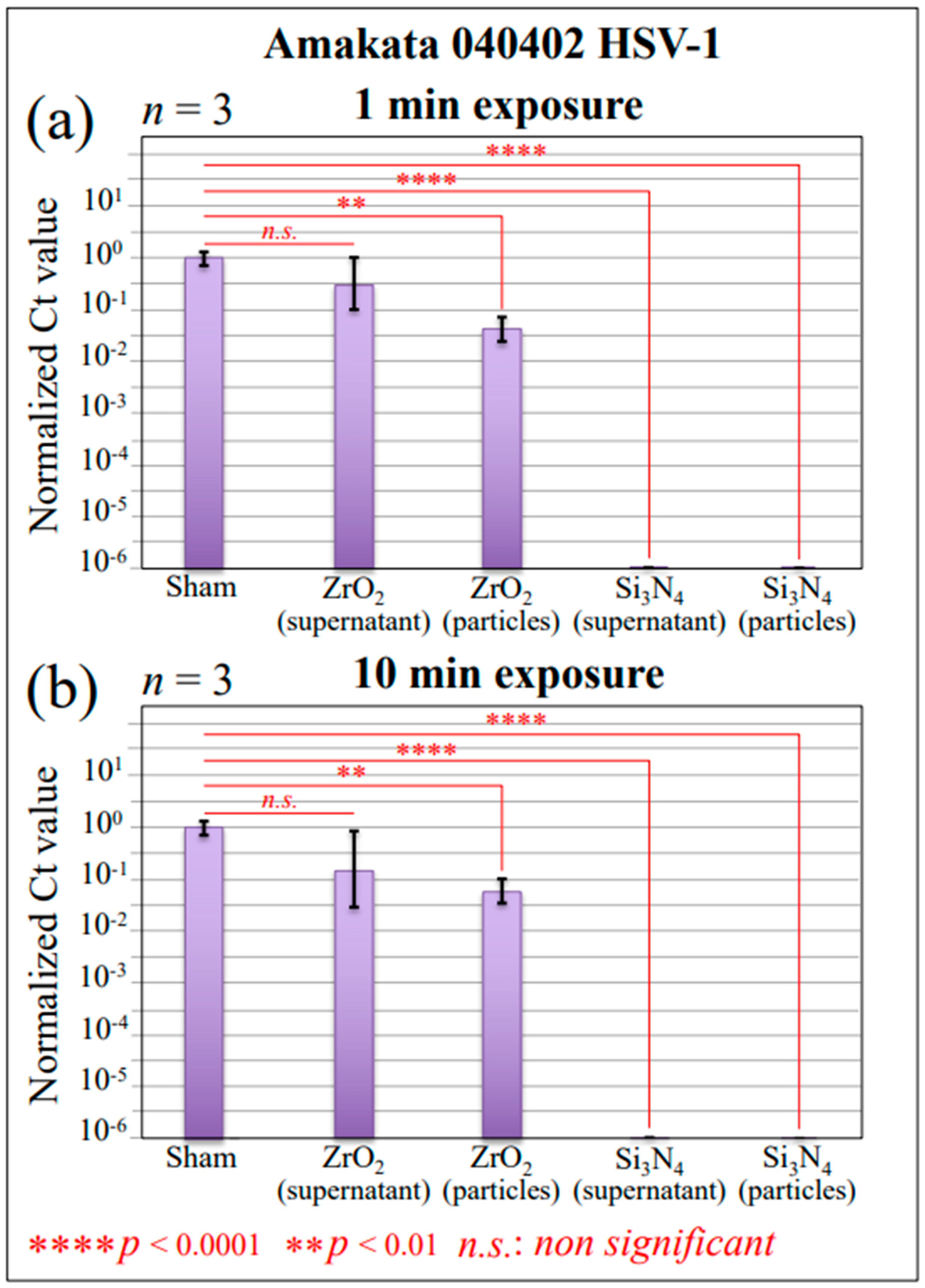
2.1. Infectivity and Polymerase Chain Reaction Results
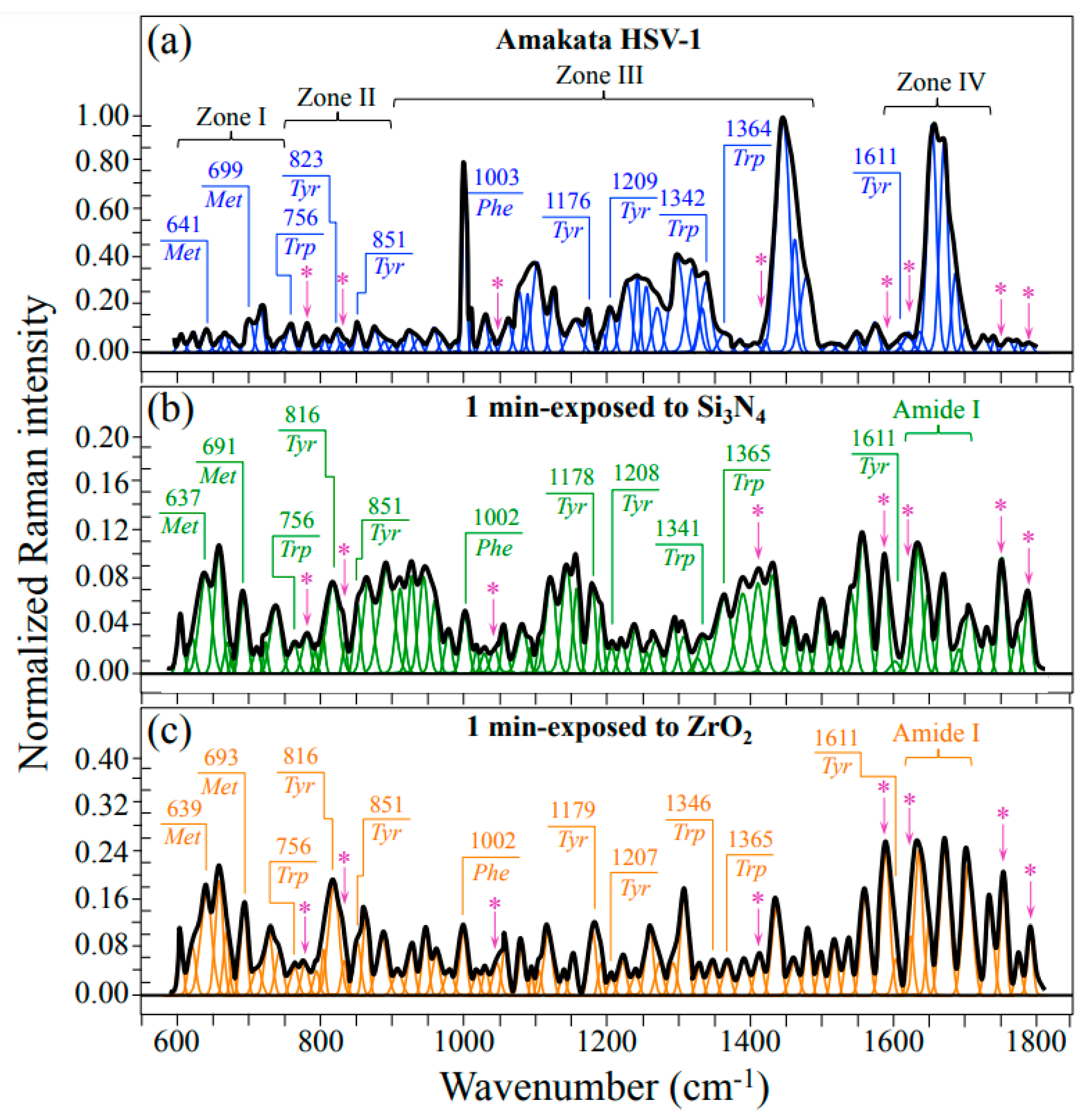
2.2. Raman Spectroscopic Results
3. Discussion
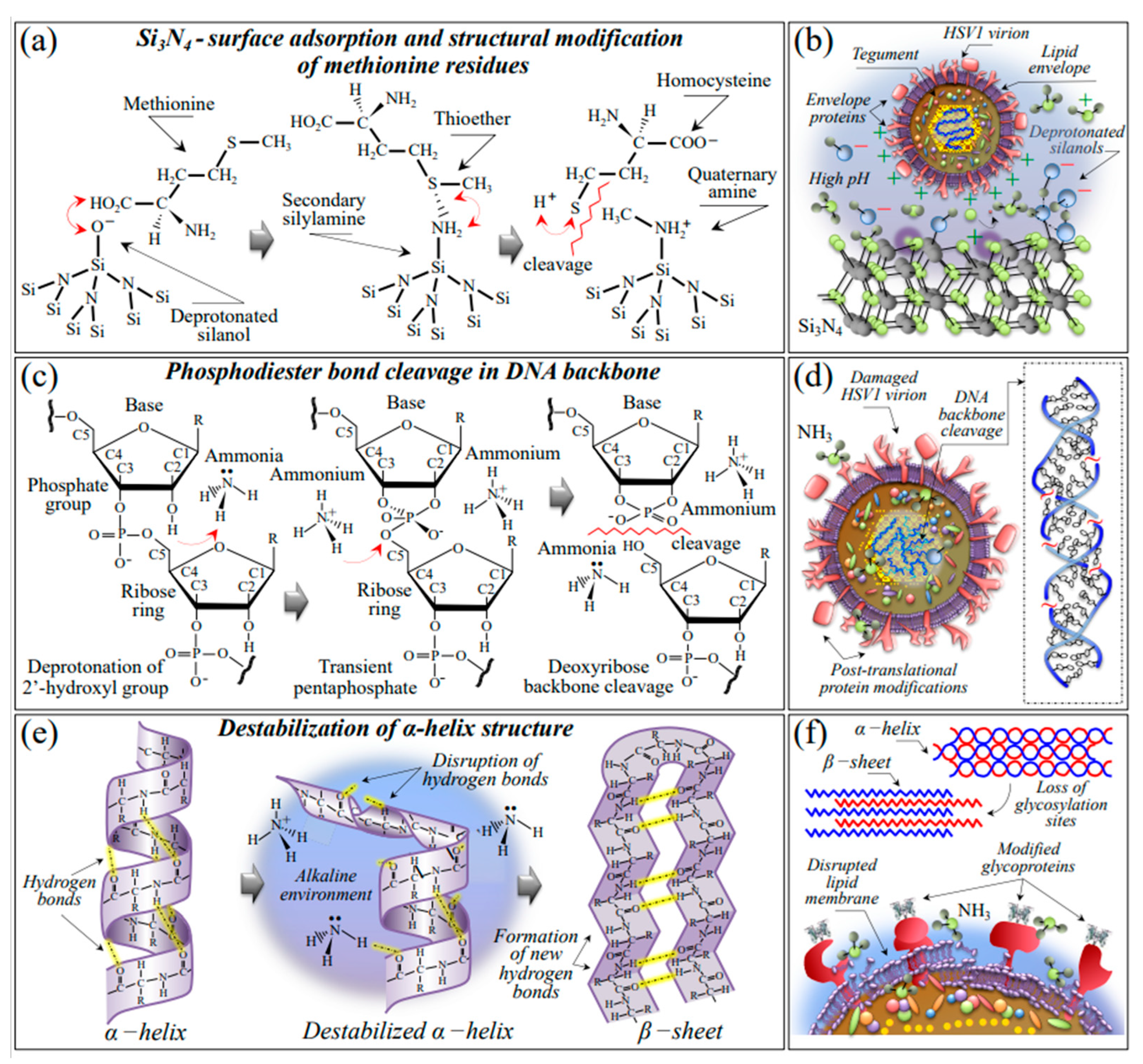
3.1. Hydrolytic Reactions and Kinetics of Si3N4 in an Aqueous Environment
3.2. Mechanisms of Viral Inactivation by Si3N4 Ceramic Powder
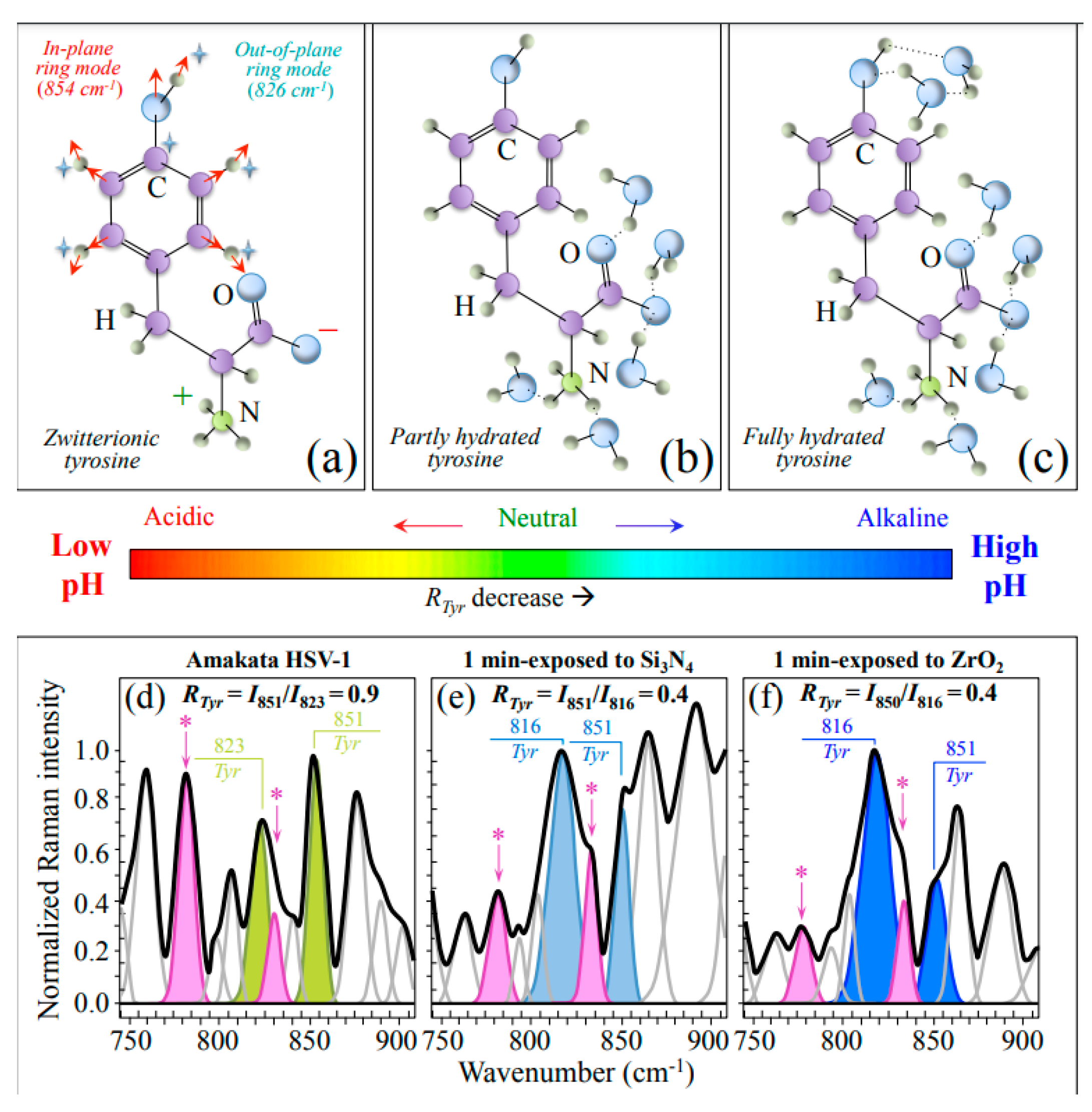
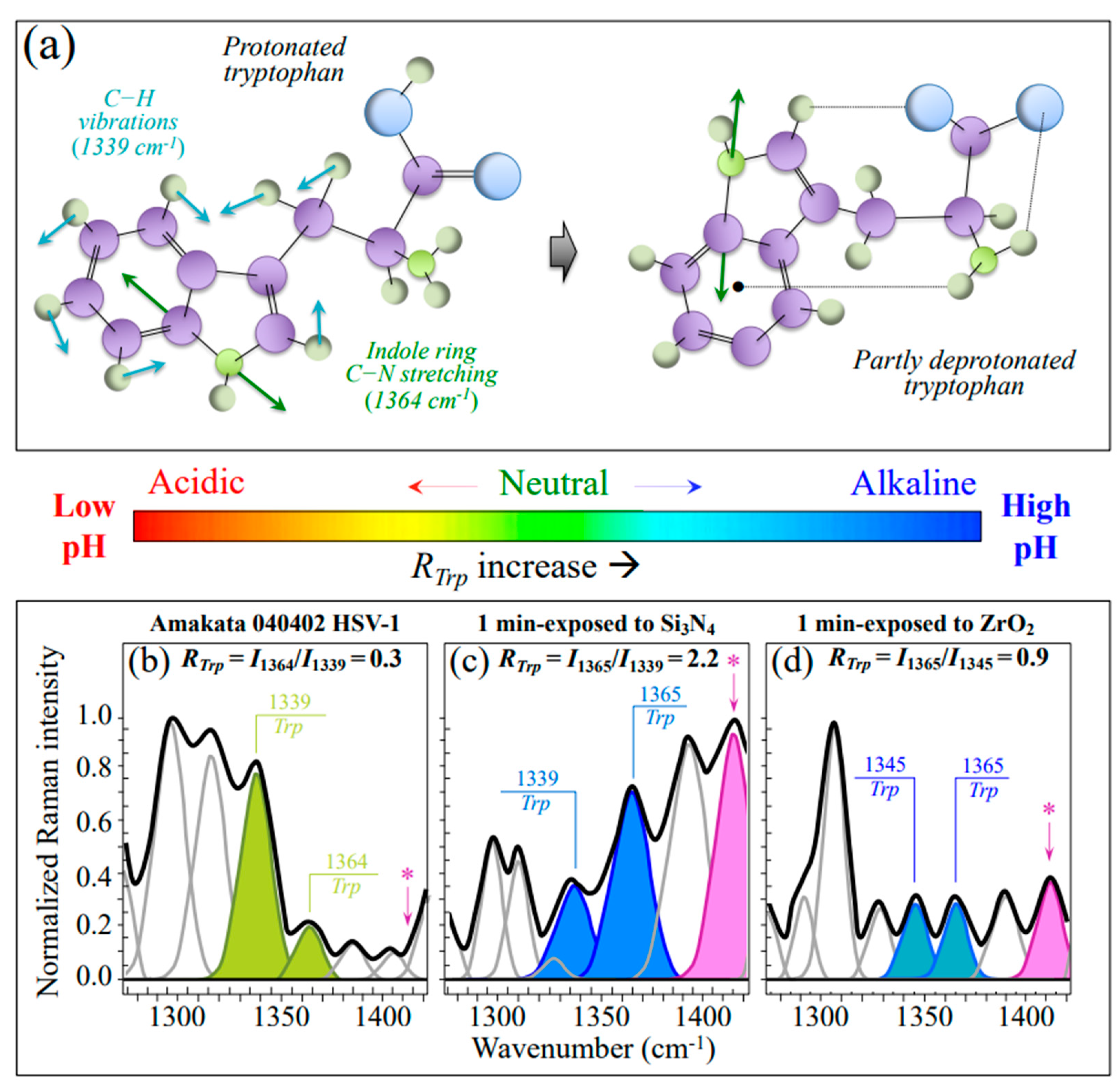
3.2.1. Vibrational Proofs of Enhanced pH at the Virion Surface
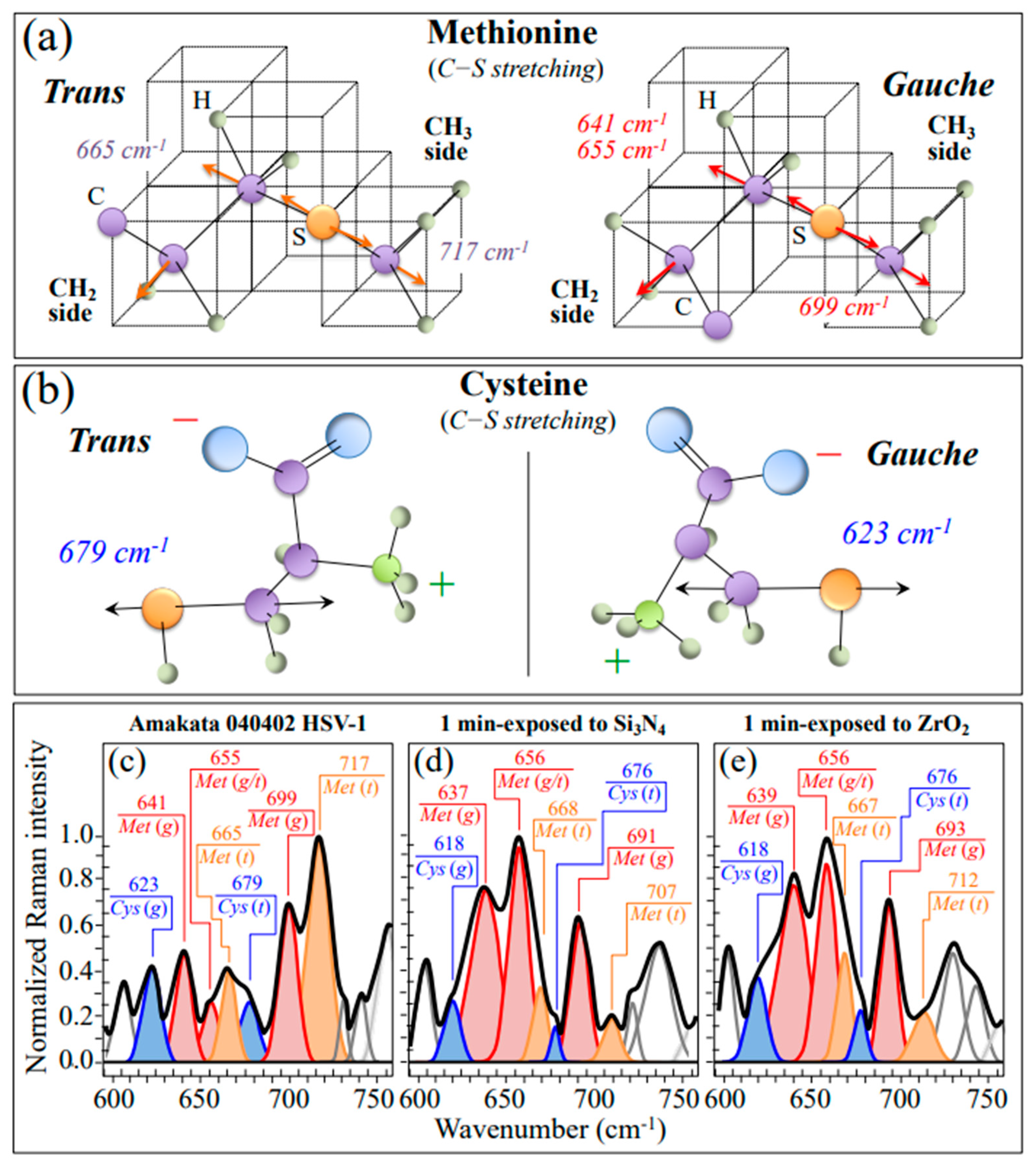
3.2.2. Oxidation of S-Containing Amino Acid Residues
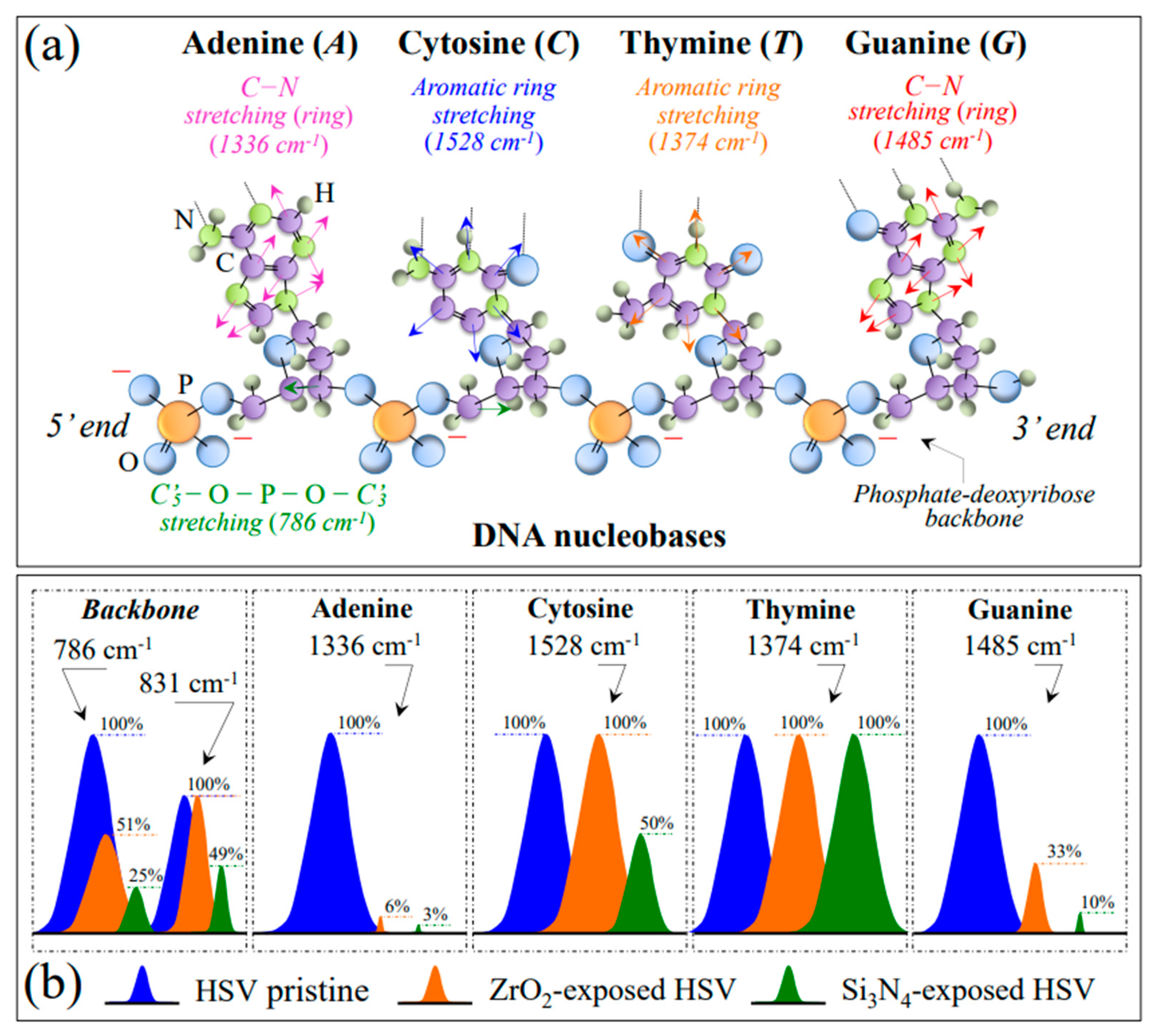
3.2.3. Damage and Fragmentation of the DNA Structure
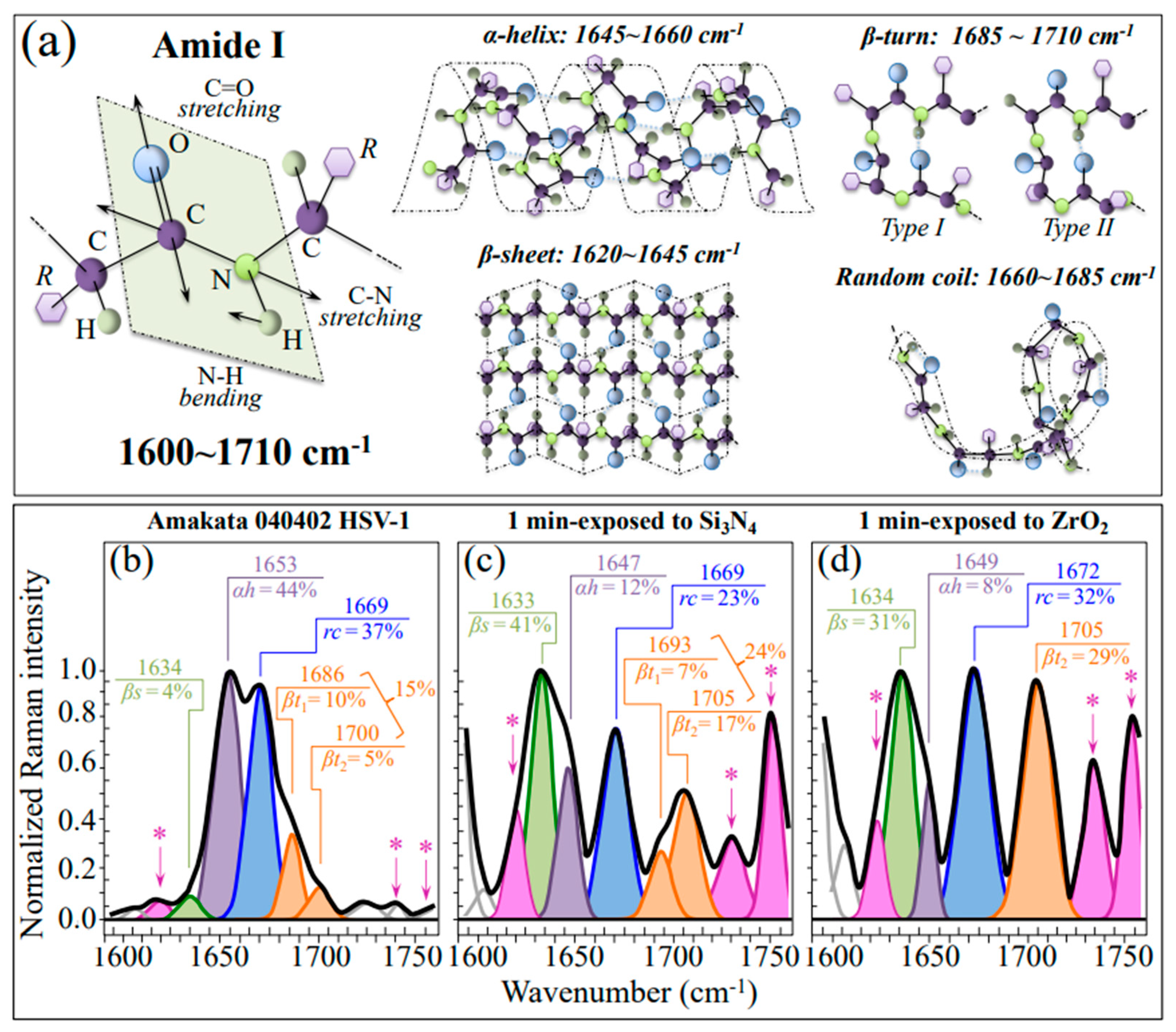
3.2.4. Destabilization of the Protein Secondary Structure
3.3. The Destabilizing Effect of ZrO2 Powder on HSV-1 Virions
3.4. The Direct and Matrix-Based Use of Si3N4 against Herpes Viruses
4. Materials and Methods
4.1. Virus Sample and Cell Infection Procedure
4.2. Ceramic Powders, Inactivation Protocol, and Virus Titration
4.3. Polymerase Chain Reaction Procedures
4.4. Raman Samples and Spectroscopic Procedures
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mettenleiter, T.C.; Klupp, B.G.; Granzow, H. Herpesvirus assembly: An update. Virus Res. 2009, 143, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Baines, J.D. Herpesvirus capsid assembly: Insights from structural analysis. Curr. Opin. Virol. 2015, 14, 142–149. [Google Scholar]
- Roizman, B.; Knipe, D.M.; Whitley, R.J. Herpes simplex viruses. In Fields Virology; Knipe, D.M., Howley, P.M., Cohen, J.I., Griffin, D.E., Lamb, R.A., Martin, M.A., Racaniello, V.R., Roizman, B., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1823–1897. [Google Scholar]
- Atanasiu, D.; Saw, W.T.; Cohen, G.H.; Eisenberg, R.J. Cascade of events governing cell-cell fusion induced by Herpes simplex virus glycoproteins gD, gH/gL, and gB. J. Virol. 2010, 84, 12292–12299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.C.; Wisner, T.W.; Wright, C.C. Herpes simplex virus glycoproteins gB and gD function in a redundant fashion to promote secondary envelopment. J. Virol. 2011, 85, 4910–4926. [Google Scholar] [CrossRef] [Green Version]
- Friedman, H.M.; Wang, L.; Fishman, N.O.; Lambris, J.D.; Eisenberg, R.J.; Cohen, G.H.; Lubinski, J. Immune evasion properties of Herpes simplex virus Type 1 glycoprotein gC. J. Virol. 1996, 70, 4253–4260. [Google Scholar] [CrossRef] [PubMed]
- Madavaraju, K.; Koganti, R.; Volety, I.; Yadavalli, T.; Shukla, D. Herpes simplex virus cell entry mechanisms: An update. Front. Cell. Infect. Microbiol. 2020, 10, 17578. [Google Scholar] [CrossRef] [PubMed]
- Croughan, W.S.; Behbehani, A.M. Comparative study of inactivation of Herpes simplex virus types 1 and 2 by commonly used antiseptic agents. J. Clin. Microbiol. 1988, 26, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Monma, Y.; Kawana, T.; Shimizu, F. In vitro inactivation of Herpes simplex virus by a biological response modifier, PSK. Antiviral Res. 1997, 35, 131–138. [Google Scholar] [CrossRef]
- Asculai, S.S.; Weis, M.T.; Rancourt, M.W.; Kupferberg, A. Inactivation of Herpes simplex viruses by nonionic surfactants. Antimicrob. Agents Chemother. 1978, 13, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Docherty, J.J.; Pollock, J.J. Inactivation of Herpes simplex virus types 1 and 2 by synthetic histidine peptides. Antimicrob. Agents Chemother. 1987, 31, 1562–1566. [Google Scholar] [CrossRef] [Green Version]
- Lewin, A.A.; Schnipper, L.E.; Crumpacker, C.S. Photodynamic inactivation of Herpes simplex virus by hematoporphyrin derivative and light. Exp. Biol. Med. 1980, 163, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.H.; Uchida, T. The effect of ammonium chloride on the multiplication of Herpes simplex virus type 1 in Vero cells. Virus Res. 1989, 13, 271–281. [Google Scholar] [CrossRef]
- Stein, G.E. Pharmacology of new antiherpes agents: Famciclovir and valacyclovir. J. Am. Pharm. Assoc. 1997, 37, 157–163. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ur Rehman, M.I.M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef] [PubMed]
- Schnipper, L.E.; Lewin, A.A.; Swartz, M.; Crumpacker, C.S. Mechanisms of photodynamic inactivation of Herpes simplex viruses. Comparison between methylene blue, light plus electricity, and hematoporphyrin plus light. J. Clin. Investig. 1980, 65, 432–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Yew, P.Y.M.; Owh, C.; Chee, P.L.; Loh, X.J. Sanitizing agents for virus inactivation and disinfection. View 2020, 1, e16. [Google Scholar] [CrossRef]
- Elveborg, S.; Monteil, V.M.; Mirazimi, A. Methods of inactivation of highly pathogenic viruses for molecular, serology or vaccine development purposes. Pathogens 2022, 11, 271. [Google Scholar] [CrossRef]
- Grinde, B. Herpesviruses: Latency and reactivation—Viral strategies and host response. J. Oral Microbiol. 2013, 5, 22766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzotti, G.; Ohgitani, E.; Shin-Ya, M.; Adachi, T.; Marin, E.; Boschetto, F.; Zhu, W.; Mazda, O. Instantaneous “catch-and-kill” inactivation of SARS-CoV-2 by nitride ceramics. Clin. Translat. Med. 2020, 10, e212. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Boschetto, F.; Ohgitani, E.; Fujita, Y.; Shin-Ya, M.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Marin, E.; Zhu, W.; et al. Mechanisms of instantaneous inactivation of SARS-CoV-2 by silicon nitride bioceramic. Mater. Today Bio 2021, 12, 100144. [Google Scholar] [CrossRef]
- Pezzotti, G.; Boschetto, F.; Ohgitani, E.; Fujita, Y.; Zhu, W.; Marin, E.; McEntire, B.J.; Bal, B.S.; Mazda, O. Silicon nitride: A potent solid-state bioceramic inactivator of ssRNA viruses. Sci. Rep. 2021, 11, 2977. [Google Scholar] [CrossRef]
- Preuss, T.; Kamstrup, S.; Kyvsgaard, N.C.; Nansen, P.; Miller, A.; Lei, J.C. Comparison of two different methods for inactivation of viruses in serum. Clin. Diagn. Lab. Immunol. 1997, 4, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G. Silicon nitride as a biomaterial. J. Ceram. Soc. Jpn. 2023, 131, 398–428. [Google Scholar] [CrossRef]
- Paione, C.M.; Baino, F. Non-oxide ceramics for bone implant application: State-of-the-art overview with an emphasis on the acetabular cup of hip joint prosthesis. Ceramics 2023, 6, 994–1016. [Google Scholar] [CrossRef]
- Minami, M.; Kita, M.; Yan, X.-Q.; Yamamoto, T.; Iida, T.; Sekigawa, K.; Iwakura, Y.; Imanishi, J. Role of IFN-γ and tumor necrosis Factor-α in Herpes simplex virus Type 1 infection. J. Interferon Cytokine Res. 2002, 22, 671–676. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ichimura, H.; Iida, T.; Minami, M.; Kobayashi, K.; Kita, M.; Sotozono, C.; Tagawa, Y.-I.; Iwakura, Y.; Imanishi, J. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-a infection. J. Interferon Cytokine Res. 1999, 19, 609–615. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, W.; Liu, L.; Huang, Y.; He, J.; Xie, W.; Wu, P.; Du, C. Baseline correction for Raman spectra using an improved asym-metric least squares method. Anal. Methods 2014, 6, 4402–4407. [Google Scholar] [CrossRef]
- Pezzotti, G.; Boschetto, F.; Ohgitani, E.; Fujita, Y.; Shin-Ya, M.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Marin, E.; Zhu, W.; et al. Raman molecular fingerprints of SARS-CoV-2 British variant and the concept of Raman barcode. Adv. Sci. 2021, 2021, 2103287. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Kobara, M.; Nakaya, T.; Imamura, H.; Miyamoto, N.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Ohgitani, E.; Marin, E.; et al. Raman spectroscopy of oral Candida species: Molecular-scale analyses, chemometrics, and barcode identification. Int. J. Mol. Sci. 2022, 23, 5359. [Google Scholar] [CrossRef]
- Gillim-Ross, L.; Taylor, J.; Scholl, D.R.; Ridenour, J.; Masters, P.S.; Wentworth, D.E. Discovery of novel human and animal cells infected by the severe acute respiratory syndrome coronavirus by replication-specific multiplex reverse transcription-PCR. J. Clin. Microbiol. 2004, 42, 3196–3206. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E.; Stewart, W.E.; De Somer, P. Studies on the mechanism of the priming effect of interferon on interferon production by cell cultures exposed to Poly(rI) · Poly(rC). Infect. Immun. 1973, 8, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Emeny, J.M.; Morgan, M.J. Regulation of the interferon system: Evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 1979, 43, 247–252. [Google Scholar] [CrossRef]
- Podstawka, E.; Ozaki, Y.; Proniewicz, L.M. Part II: Surface-enhanced Raman spectroscopy investigation of methionine containing heterodipeptides adsorbed on colloidal silver. Appl. Spectrosc. 2004, 58, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Fischer, G. Conformational and infrared spectral studies of L-methionine and its N-deuterated isotopomer as isolated zwitterions. J. Phys. Chem. 2002, 106, 41–50. [Google Scholar] [CrossRef]
- Hernandez, B.; Pfluger, F.; Adenier, A.; Kruglik, S.K.; Ghomi, M. Side chain flexibility and protonation states of sulfur atom containing amino acids. Phys. Chem. Chem. Phys. 2011, 13, 17284–17294. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.C.; Yu, N.-T. Laser-excited Raman spectroscopy of bio-molecules. I. Native lysozyme and its constituent amino acids. J. Mol. Biol. 1970, 50, 509–524. [Google Scholar] [CrossRef]
- Torreggiani, A.; Barata-Vallejo, S.; Chatgilialoglu, C. Combined Raman and IR spectroscopic study on the radical-based modifications of methionine. Anal. Bioanal. Chem. 2011, 401, 1231–1239. [Google Scholar] [CrossRef]
- Davies, M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta—Proteins Proteom. 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Schoneich, C. Methionine oxidation by reactive oxygen species: Reaction mechanisms and relevance to Alzheimer’s disease. Biochim. Biophys. Acta—Protein Proteom. 2005, 1703, 111–119. [Google Scholar] [CrossRef]
- Hildebrandt, P.G.; Copeland, R.A.; Spiro, T.G.; Otlewski, J.; Laskowski, M., Jr.; Prendergast, F.G. Tyrosine hydrogen-bonding and environmental effects in proteins probed by ultraviolet resonance Raman spectroscopy. Biochemistry 1988, 27, 5426–5433. [Google Scholar] [CrossRef]
- Hernandez, B.; Coic, Y.-M.; Pluger, F.; Kruglik, S.G.; Ghomi, M. All characteristic Raman markers of tyrosine and tyrosinate originate from phenol ring fundamental vibrations. J. Raman Spectrosc. 2016, 47, 210–220. [Google Scholar] [CrossRef]
- Harada, I.; Miura, T.; Takeuchi, H. Origin of the doublet at 1360 and 1340 cm-1 in the Raman spectra of tryptophan and related compounds. Spectrochim. Acta 1986, 42, 307–312. [Google Scholar] [CrossRef]
- Takeuchi, H. Raman structural markers of tryptophan and histidine side chains in proteins. Biopolym. Biospectrosc. 2003, 72, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cortes, S.; Garcia-Ramos, J.V. SERS of cytosine and its methylated derivatives on metal colloids. J. Raman Spectrosc. 1992, 23, 61–66. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Seuvre, A.M.; Koenig, J.L. FT-IR and laser-Raman spectra of cytosine and cytidine. Carbohydr. Res. 1986, 146, 1–13. [Google Scholar] [CrossRef]
- Madzharova, F.; Heiner, Z.; Guehlke, M.; Kneipp, J. Surface-enhanced hyper-Raman spectra of adenine, guanine, cytosine, thy-mine, and uracil. J. Phys. Chem. C 2016, 120, 15415–15423. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.P.; Marques, M.P.M.; Valero, R.; Tomkinson, J.; de Carvalho, L.A.E.B. Guanine: A combined study using vibrational spectroscopy and theoretical methods. Spectrosc. Int. J. 2012, 27, 273–292. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.P.; Valero, R.; Tomkinson, J.; Marques, M.P.M.; de Carvalho, L.A.E.B. Applying vibrational spectroscopy to the study of nucleobases—Adenine as a case study. New J. Chem. 2013, 37, 2691–2699. [Google Scholar] [CrossRef] [Green Version]
- Notingher, I.; Green, C.; Dyer, C. Discrimination between ricin and sulphur mustard toxicity in vitro using Raman spectroscopy. J. R. Soc. Interface 2004, 1, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, H. Mutagenic potentials of damaged nucleic acids produced by reactive oxygen/nitrogen species: Approaches using synthetic oligonucleotides and nucleotides. Nucleic Acid Res. 2003, 31, 517–531. [Google Scholar] [CrossRef] [Green Version]
- Douki, T.; Cadet, J. Peroxynitrite mediated oxidation of purine bases in nucleosides and DNA. Free Radic. Res. Commun. 1996, 24, 369–380. [Google Scholar] [CrossRef]
- Boorstein, R.J.; Hilbert, T.P.; Cadet, J.; Cunningham, R.P.; Teebor, G.W. UV-induced pyrimidine hydrates in DNA are repaired by bacterial and mammalian DNA glycosylase activities. Biochemistry 1989, 28, 6164–6170. [Google Scholar] [CrossRef]
- Shugar, D.; Fox, J.J. Spectrophotometric studies of nucleic acid derivatives and related compounds as a function of pH. I. Pyrimidines. Biochim. Biophys. Acta 1952, 9, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Vigny, P. The photochemistry of nucleic acids. In Bioorganic Photochemistry: Photochemistry and the Nucleic Acids; Morrison, H., Ed.; Wiley and Sons: New York, NY, USA, 1990; Volume 1, pp. 1–272. [Google Scholar]
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef]
- Nonoyama, N.; Oshima, H.; Shoda, C.; Suzuki, H. The reaction of peroxynitrite with organic molecules bearing a biologically important functionality. The multiplicity of reaction modes as exemplified by hydroxylation, nitration, nitrosation, dealkylation, oxygenation, and oxidative dimerization and cleavage. Bull. Chem. Soc. Jpn. 2001, 74, 2385–2395. [Google Scholar]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- McAvan, B.S.; Bowsher, L.A.; Powell, T.; O’Hara, J.F.; Spitali, M.; Goodacre, R.; Doig, A.J. Raman spectroscopy to monitor post-translational modifications and degradation in monoclonal anti-body therapeutics. Anal. Chem. 2020, 92, 10381–10389. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Post-translational modifications of coronavirus proteins: Roles and function. Future Virol. 2018, 13, 405–430. [Google Scholar] [CrossRef] [Green Version]
- Pace, C.N.; Scholtz, J.M. A helix propensity scale based on experimental studies of peptides and proteins. Biophys. J. 1998, 75, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Colombo, G.; Meli, M.; Morra, G.; Gabizon, R.; Gasset, M. Methionine sulfoxides on prion protein helix-3 switch on the α-fold destabilization required for conversion. PLoS ONE 2009, 4, e4296. [Google Scholar] [CrossRef] [Green Version]
- Pezzotti, G. Silicon nitride: A bioceramic with a gift. ACS Appl. Mater. Interfaces 2019, 11, 26619–26636. [Google Scholar] [CrossRef]
- Laarz, E.; Zhmud, B.V.; Bergstroem, L. Dissolution and de-agglomeration of silicon nitride in aqueous medium. J. Am. Ceram. Soc. 2000, 83, 2394–2400. [Google Scholar] [CrossRef]
- Sawyer, C.N.; McCarty, P.L. Chemistry for Environmental Engineering, 3rd ed.; McGraw-Hill Book Co.: New York, NY, USA, 1978; p. 532. [Google Scholar]
- Parker, D.R.; Norvell, W.A.; Chaney, R.L. GEOCHEM-PC—A chemical speciation program for IBM and compatible personal computers. In Chemical Equilibria and Reaction Models; Loeppert, R.H., Schwab, A.D., Goldberg, S., Eds.; Soil Science Society of America: Madison, WI, USA, 1995; pp. 253–269. [Google Scholar]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.; Marison, I.W.; von Stockar, U. The importance of ammonia in mammalian cell culture. J. Biotechnol. 1996, 46, 161–185. [Google Scholar] [CrossRef]
- Abnosi, M.H.; Pari, S. Exogenous nitric oxide induced early mineralization in rat bone marrow mesenchymal stem cells via activation of alkaline phosphatase. Iran. Biomed. J. 2019, 23, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Bock, R.M.; Adachi, T.; Rondinella, A.; Boschetto, F.; Zhu, W.; Marin, E.; McEntire, B.; Bal, B.S.; Mazda, O. Silicon nitride surface chemistry: A potent regulator of mesenchymal progenitor cell activity in bone formation. Appl. Mater. Today 2017, 9, 82–95. [Google Scholar] [CrossRef]
- Pezzotti, G.; McEntire, B.J.; Bock, R.; Boffelli, M.; Zhu, W.; Vi-tale, E.; Puppulin, L.; Adachi, T.; Yamamoto, T.; Kanamura, N.; et al. Silicon nitride: A synthetic mineral for vertebrate biology. Sci. Rep. 2016, 6, 31717. [Google Scholar] [CrossRef] [Green Version]
- Pezzotti, G.; Marin, E.; Adachi, T.; Rondinella, A.; Boschetto, F.; Zhu, W.-L.; Sugano, N.; Bock, R.M.; McEntire, B.J.; Bal, B.S. Bioactive silicon nitride: A new therapeutic material for osteoarthropathy. Sci. Rep. 2017, 7, 44848. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, J.B.; Yoon, H.; Ringel, A.E.; Jeanfavre, S.; Clish, C.B.; Haigis, M.C. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 2017, 359, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Burrows, C.J.; Muller, J.G. Oxidative nucleobase modifications leading to strand scission. Chem. Rev. 1998, 98, 1109–1151. [Google Scholar] [CrossRef]
- Olofsson, J.; Grehk, T.M.; Berlind, T.; Persson, C.; Jacobson, S.; Engqvist, H. Evaluation of silicon nitride as a wear resistant and resorbable alternative for total hip joint replacement. Biomatter 2012, 2, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersson, M.; Skjöldebrand, C.; Filho, L.; Engqvist, H.; Persson, C. Morphology and dissolution rate of wear debris from silicon nitride coatings. ACS Biomater. Sci. Eng. 2016, 2, 998–1004. [Google Scholar]
- Kumar, A.; Welch, N.; Mishra, S.; Bellar, A.; Silva, R.N.; Li, L.; Singh, S.S.; Sharkoff, M.; Kerr, A.; Chelluboyina, A.K.; et al. Metabolic reprogramming during hyperammonemia targets mitochondrial function and postmitotic senescence. JCI Insight 2021, 6, e154089. [Google Scholar] [CrossRef]
- Dasarathy, S.; Mookerjee, R.P.; Rackayova, V.; Thrane, V.R.; Vairappan, B.; Ott, P.; Rose, C.F. Ammonia toxicity: From head to toe? Metab. Brain Dis. 2017, 32, 529–538. [Google Scholar] [CrossRef]
- Visek, W.J. Some aspects of ammonia toxicity in animal cells. J. Dairy Sci. 1968, 51, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Siamwiza, M.N.; Lord, R.C.; Chen, M.C.; Takamatsu, T.; Harada, I.; Matsuura, H.; Shimanouchi, T.T. Interpretation of the doublet at 850 and 830 cm-1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry 1975, 14, 4870–4876. [Google Scholar] [CrossRef] [PubMed]
- Lancz, G.J. Effect of pH on the kinetics of Herpes simplex virus inactivation at 36°. Virology 1976, 75, 488–491. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochimica et Biophysica Acta (BBA)-Bioenergetics 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Austin, L.A.; Kang, B.; El-Sayed, M.A.A. New nanotechnology technique for determining drug efficacy using targeted plasmonically enhanced single cell imaging spectroscopy. J. Am. Chem. Soc. 2013, 135, 4688–4691. [Google Scholar] [CrossRef]
- Rodríguez-Zamora, P.; Cordero-Silis, C.A.; Fabila, J.; Luque-Ceballos, J.C.; Buendiá, F.; Heredia-Barbero, A.; Garzón, I.L. Interaction mechanisms and interface configuration of cysteine adsorbed on gold, silver, and copper nanoparticles. Langmuir 2022, 38, 5418–5427. [Google Scholar] [CrossRef]
- López-Tobar, E.; Hernández, B.; Ghomi, M.; Sanchez-Cortes, S. Stability of the disulfide bond in cystine adsorbed on silver and gold nanoparticles as evidenced by SERS data. J. Phys. Chem. C 2013, 117, 1531–1537. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry; biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- Folzer, E.; Diepold, K.; Bomans, K.; Finkler, C.; Schmidt, R.; Bulau, P.; Huwyler, J.; Mahler, H.C.; Koulov, A.V. Selective oxidation of methionine and tryptophan residues in a therapeutic IgG1 molecule. J. Pharm. Sci. 2015, 104, 2824–2831. [Google Scholar] [CrossRef] [PubMed]
- Alcock, L.J.; Perkins, M.V.; Chalker, J.M. Chemical methods for mapping cysteine oxidation. Chem. Soc. Rev. 2018, 7, 231–268. [Google Scholar] [CrossRef] [Green Version]
- Penta, N.K.; Peethala, B.C.; Amanapu, H.P.; Melman, A.; Babu, S.V. Role of hydrogen bonding on the adsorption of several amino acids on SiO2 and Si3N4 and selective polishing of these materials using ceria dispersions. Coll. Surf. A Physicochem. Eng. Aspects 2013, 429, 67–73. [Google Scholar] [CrossRef]
- Kristensen, H.H.; Wathes, C.M. Ammonia and poultry welfare: A review. World’s Poult. Sci. J. 2000, 56, 235–245. [Google Scholar] [CrossRef]
- Meyer, C.; Meyer, D.; Bickle, T.A.; Giese, B. Chemical restriction: Strand cleavage by ammonia treatment at 8-oxoguanine yields biologically active DNA. ChemBioChem 2003, 4, 610–614. [Google Scholar] [CrossRef]
- Lönnberg, H. Cleavage of RNA phosphodiester bonds by small molecular entities: A mechanistic insight. Org. Biomol. Chem. 2011, 9, 1687–1703. [Google Scholar] [CrossRef]
- Pezzotti, G. A spontaneous solid-state NO donor to fight antibiotic resistant bacteria. Mater. Today Chem. 2018, 9, 80–90. [Google Scholar] [CrossRef]
- Eker, F.; Cao, X.; Nafie, L.; Huang, Q.; Schweitzer-Stenner, R. The structure of alanine based tripeptides in water and dimethyl sulfoxide probed by vibrational spectroscopy. J. Phys. Chem. B 2003, 107, 358–365. [Google Scholar] [CrossRef]
- Koch, O.; Bocola, M.; Klebe, G. Cooperative effects in hydro-gen-bonding of protein secondary structure elements: A systematic analysis of crystal data using Secbase. Prot. Struct. Funct. Bioinform. 2005, 61, 310–317. [Google Scholar] [CrossRef]
- Nathan, C.; Shiloh, M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 2000, 97, 8841–8848. [Google Scholar] [CrossRef] [PubMed]
- Tantipanjaporn, A.; Wong, M.-K. Development and recent advances in lysine and N-terminal bioconjugation for peptides and proteins. Molecules 2023, 28, 1083. [Google Scholar] [CrossRef]
- Turner, A.; Bruun, B.; Minson, T.; Browne, H. Glycoproteins gB, and gHgL of Herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 1998, 72, 873–875. [Google Scholar] [CrossRef]
- Chaopradith, D.T.; Scanlon, D.O.; Catlow, C.R.A. Adsorption of water on yttria-stabilized zirconia. J. Phys. Chem. C 2015, 119, 22526–22533. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Priyadarshini, S.; Al-Maleki, A.R.; Alagiri, M.; Yahya, R.; Saravanan, S.; Vadivelu, J. In vitro toxicity, apoptosis and antimicrobial effects of phytomediated copper oxide nanoparticles. RSC Adv. 2016, 6, 110986–110995. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Lee, T.-S.; Lui, T.-S. Enhanced osteoblastic cell response on zirconia by bio-inspired surface modification. Colloids Surf. B 2013, 106, 37–45. [Google Scholar] [CrossRef]
- Kumaresan, M.; Anand, K.V.; Govindaraju, K.; Tamilselvan, S.; Kumar, V.G. Seaweed Sargassum wightii mediated preparation of zirconia (ZrO2) nanoparticles and their antibacterial activity against gram positive and gram negative bacteria. Microb. Pathog. 2018, 124, 311–315. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, J.; Yang, Y.; Zheng, M.; Yang, J.; Tan, J. Surface modification of zirconia with polydopamine to enhance fibroblast response and decrease bacterial activity in vitro: A potential technique for soft tissue engineering applications. Colloids Surf. B 2015, 136, 74–83. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H.; Zordok, W.A.; El-Didamony, A.M. Spectroscopic, structure and antimicrobial activity of new Y(III) and Zr(IV) ciprofloxacin. Spectrochim. Acta Part A 2011, 78, 854–867. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Khan, S.T.; Adil, S.F.; Kuniyil, M.; Khan, M.; Al-Warthan, A.A.; Siddiqui, M.R.H.; Tahir, M.N. Enhanced antimicrobial activity of biofunctionalized zirconia nanoparticles. ACS Omega 2020, 5, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and role of reactive oxygen and nitrogen species induced by plasma, lasers, chemical agents, and other systems in dentistry. Oxid. Med. Cell. Longev. 2017, 2017, 7542540. [Google Scholar] [CrossRef] [PubMed]
- Asadpour, E.; Sadeghnia, H.R.; Ghorbani, A.; Boroushaki, M.T. Effect of zirconium dioxide nanoparticles on glutathione peroxidase enzyme in PC12 and N2a cell lines. Iran. J. Pharm. Res. IJPR 2014, 13, 1141–1148. [Google Scholar] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Perez-Leben, E.A. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxy-gen species: Oxidative damage and pathogenesis. Curr. Sci. 1999, 77, 658–666. [Google Scholar]
- Valyi-Nagy, T.; Dermody, T.S. Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histol. Histopathol. 2005, 20, 957–967. [Google Scholar]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef]
- Fu, X.; Jiang, X.; Chen, X.; Zhu, L.; Zhang, G. The differential expression of mitochondrial function-associated proteins and antioxidant enzymes during bovine Herpesvirus 1 infection: A potential mechanism for virus infection-induced oxidative mitochondrial dysfunction. Mediat. Inflamm. 2019, 2019, 7072917. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, S. Isoelectric focusing of Herpes simplex virus. Arch. Virol. 1975, 49, 93–98. [Google Scholar] [CrossRef]
- Wei, C.J.; Wang, S.-C.; Ho, F.-Y. Electrokinetic properties of colloidal zirconia powders in aqueous suspension. J. Am. Ceram. Soc. 1999, 82, 3385–3392. [Google Scholar] [CrossRef]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Lafferty, W.E.; Coombs, R.W.; Benedetti, J.; Critchlow, C.; Corey, L. Recurrences after oral and genital Herpes simplex virus infection. Influence of site of infection and viral type. N. Engl. J. Med. 1987, 316, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Sarta, C. Infection prophylaxis and antiretroviral therapy in patients with HIV infection and malignancy. Curr. Opin. Oncol. 1996, 8, 392–399. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, P.; Blum, M.R. Pharmacokinetics of acyclovir after intravenous and oral administration. J. Antimicrob. Chemother. 2012, 12 (Suppl. SB), 29–37. [Google Scholar] [CrossRef] [PubMed]
- Celum, C.; Wald, A.; Lingappa, J.R.; Magaret, A.S.; Wang, R.S.; Mugo, N.; Mujugira, A.; Baeten, J.M.; Mullins, J.I.; Hughes, J.P.; et al. Acyclovir and trans-mission of HIV-1 from persons infected with HIV-1 and HSV-2. N. Engl. J. Med. 2010, 316, 1444–1449. [Google Scholar]
- Laskin, O.L.; Longstreth, J.A.; Saral, R.; de Miranda, P.; Keeney, R.; Lietman, P.S. Pharmacokinetics and tolerance of acyclovir, a new anti-herpesvirus agent, in humans. Antimicrob. Agents Chemother. 1982, 21, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, E.P.; Flexner, C. Antiviral Agents (Nonretroviral). In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2011; pp. 1593–1622. [Google Scholar]
- Hodge, R.A.V.; Field, H.J. Antiviral Agents for Herpes simplex Virus. Adv. Pharmacol. 2013, 67, 1–38. [Google Scholar]
- Van Vloten, W.A.; Swart, R.N.; Pot, F. Topical acyclovir therapy in patients with recurrent orofacial Herpes simplex infections. J. Antimicrob. Chemother. 1983, 12 (Suppl. SB), 89–93. [Google Scholar] [CrossRef]
- Spruance, S.L.; Nett, R.; Marbury, T.; Wolff, R.; Johnson, J.; Spaulding, T. Acyclovir cream for treatment of Herpes simplex labialis: Results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob. Agents Chemother. 2002, 46, 2238–2243. [Google Scholar] [CrossRef] [Green Version]
- Spruance, S.L.; Schnipper, L.E.; Overall, J.C., Jr.; Kern, E.R.; Wester, B.; Modlin, J.; Wenerstrom, G.; Burton, C.; Arndt, K.A.; Chiu, G.L.; et al. Treatment of Herpes simplex labialis with topical acyclovir in polyethylene glycol. J. Infect. Dis. 1982, 146, 85–90. [Google Scholar] [CrossRef]
- Fiddian, A.P.; Yeo, J.M.; Stubbings, R.; Dean, D. Successful treatment of herpes labialis with topical acyclovir. Br. Med. J. Clin. Res. Ed. 1983, 286, 1699–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiddian, A.P.; Ivanyi, L. Topical acyclovir in the management of recurrent herpes labialis. Br. J. Dermatol. 1983, 109, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; King, M.; Best, J.M.; Banatvala, J.E.; Gibson, J.R.; Klaber, M.R. Failure of acyclovir cream in treatment of recurrent herpes labialis. Br. Med. J. (Clin. Res. Ed.) 1985, 291, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Raborn, G.W.; McGaw, W.T.; Grace, M.; Percy, J.; Samuels, S. Herpes labialis treatment with acyclovir 5% modified aqueous cream: A double-blind randomized trial. Oral Surg. Oral Med. Oral Pathol. 1989, 67, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Raborn, G.W.; McGaw, W.T.; Grace, M.; Houle, L. Herpes labialis treatment with acyclovir 5 per cent ointment. J. Can. Dent. Assoc. 1989, 55, 135–137. [Google Scholar] [PubMed]
- Evans, T.G.; Bernstein, D.I.; Raborn, G.W.; Harmenberg, J.; Kowalski, J.; Spruance, S.L. Double-blind; randomized, placebo-controlled study of topical 5% acyclovir-1% hydrocortisone cream (ME-609) for treatment of UV radiation-induced herpes labialis. Antimicrob. Agents Chemother. 2002, 46, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, E.; Pisanty, S.; Czerninski, R.; Helser, M.; Eliav, E.; Touitou, E. A clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 87, 700–705. [Google Scholar] [CrossRef]
- Opstelten, W.; Neven, A.K.; Eekhof, J. Treatment and prevention of herpes labialis. Can. Farm. Physician 2008, 54, 1683–1687. [Google Scholar]
- Godfrey, H.R.; Godfrey, N.J.; Godfrey, J.C.; Riley, D. A randomized clinical trial on the treatment of oral herpes with topical zinc oxide/glycine. Altern. Ther. Health Med. 2001, 7, 49–56. [Google Scholar]
- Kneist, W.; Hempel, B.; Borelli, S. Clinical double-blind trial of topical zinc sulfate for herpes labialis recidivans. Arzneimittelforschung 1995, 45, 624–626. (In German) [Google Scholar] [PubMed]
- Giannasca, N.J.; Suon, J.S.; Evans, A.C.; Margulies, B.J. Matrix-based controlled release delivery of acyclovir from poly-(ethylene co-vinyl acetate) rings. J. Drug Deliv. Sci. Technol. 2019, 55, 101391. [Google Scholar] [CrossRef]
- Pezzotti, G.; Asai, T.; Adachi, T.; Ohgitani, E.; Yamamoto, T.; Kanamura, N.; Boschetto, F.; Zhu, W.; Zanocco, M.; Marin, E.; et al. Antifungal activity of polymethyl methacrylate/Si3N4 composites against Candida albicans. Acta Biomater. 2021, 126, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Marin, E.; Adachi, T.; Lerussi, F.; Rondinella, A.; Boschetto, F.; Zhu, W.; Kitajima, T.; Inada, K.; McEntire, B.J.; et al. Incorporating Si3N4 into PEEK to produce anti-bacterial, osteoconductive, and radiolucent spinal implants. Macromol. Biosci. 2018, 18, 1800033. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzotti, G.; Ohgitani, E.; Ikegami, S.; Shin-Ya, M.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Marin, E.; Zhu, W.; Okuma, K.; et al. Instantaneous Inactivation of Herpes Simplex Virus by Silicon Nitride Bioceramics. Int. J. Mol. Sci. 2023, 24, 12657. https://doi.org/10.3390/ijms241612657
Pezzotti G, Ohgitani E, Ikegami S, Shin-Ya M, Adachi T, Yamamoto T, Kanamura N, Marin E, Zhu W, Okuma K, et al. Instantaneous Inactivation of Herpes Simplex Virus by Silicon Nitride Bioceramics. International Journal of Molecular Sciences. 2023; 24(16):12657. https://doi.org/10.3390/ijms241612657
Chicago/Turabian StylePezzotti, Giuseppe, Eriko Ohgitani, Saki Ikegami, Masaharu Shin-Ya, Tetsuya Adachi, Toshiro Yamamoto, Narisato Kanamura, Elia Marin, Wenliang Zhu, Kazu Okuma, and et al. 2023. "Instantaneous Inactivation of Herpes Simplex Virus by Silicon Nitride Bioceramics" International Journal of Molecular Sciences 24, no. 16: 12657. https://doi.org/10.3390/ijms241612657




