Regulation of Grain Chalkiness and Starch Metabolism by FLO2 Interaction Factor 3, a bHLH Transcription Factor in Oryza sativa
Abstract
1. Introduction
2. Results
2.1. Reverse Genetic Analysis Reveals OsFIF3 as a Potential Regulator of Chalkiness Formation
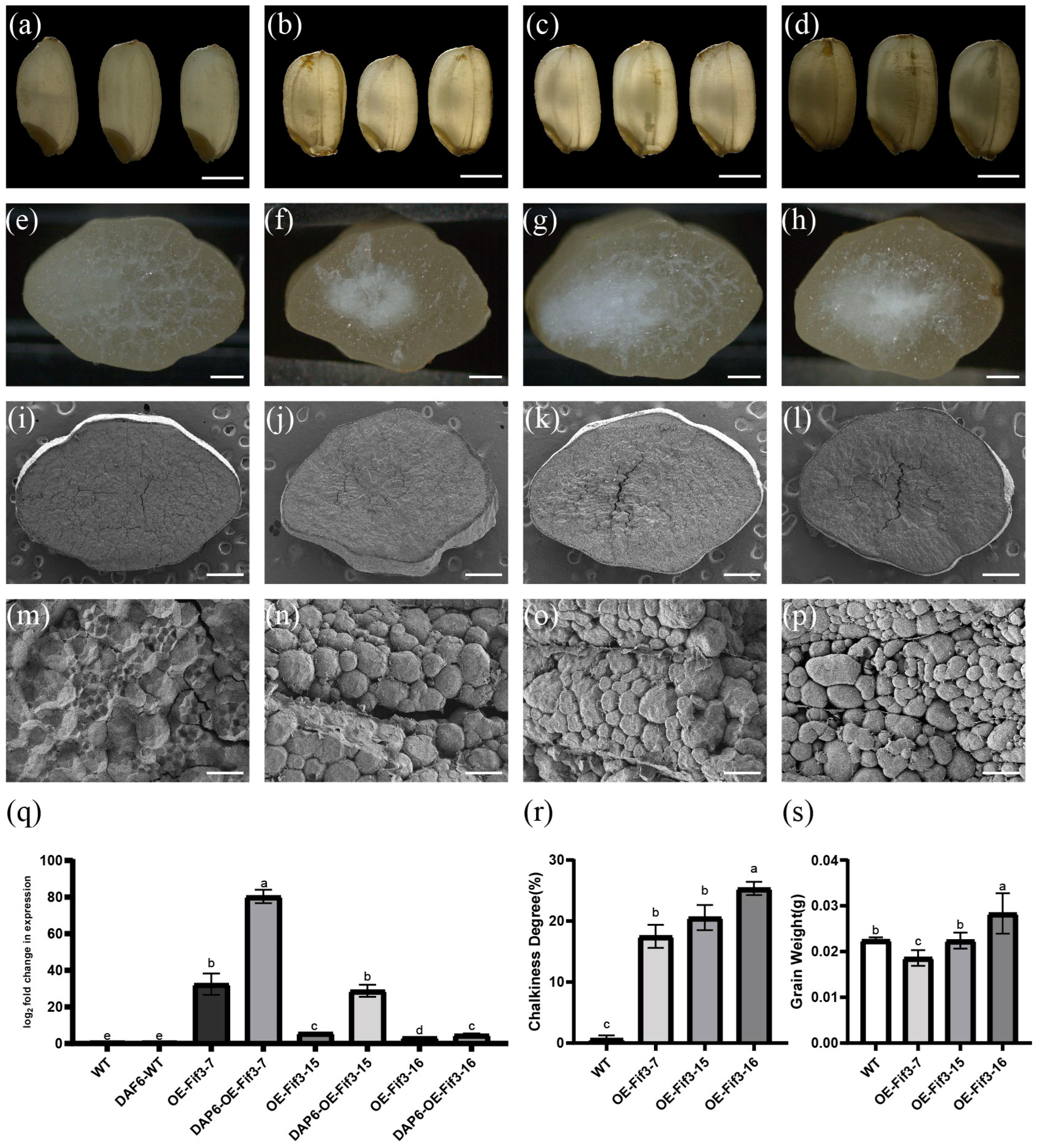
2.2. Overexpression of OsFIF3 Enhances Chalkiness Degrees in Rice Grains
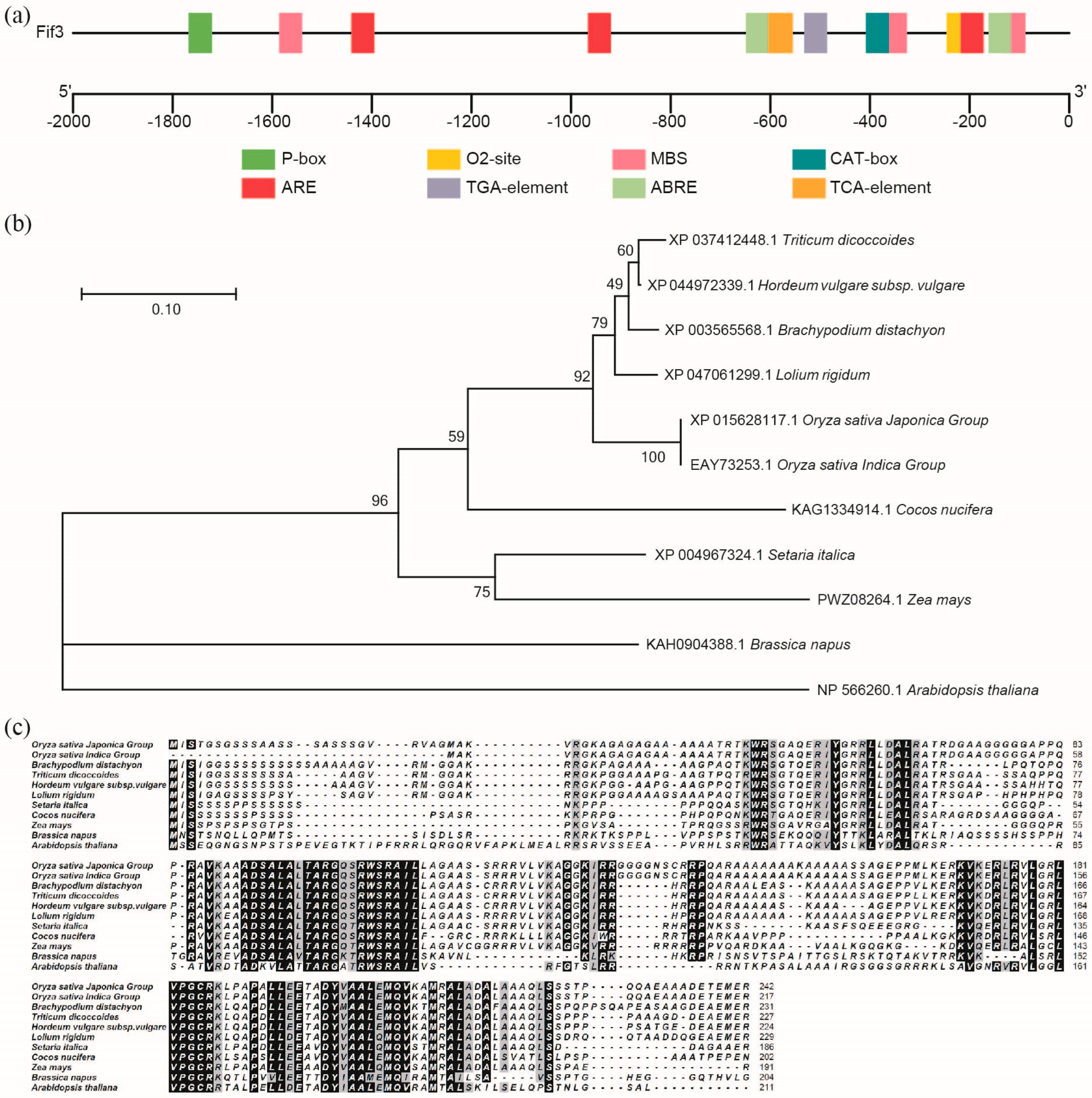
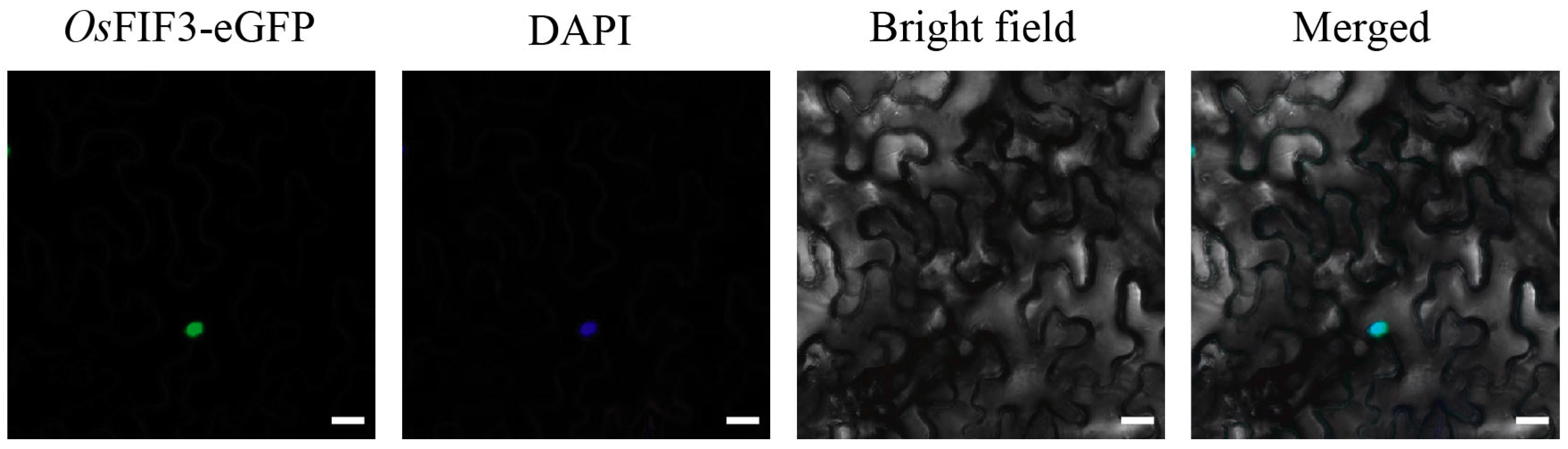
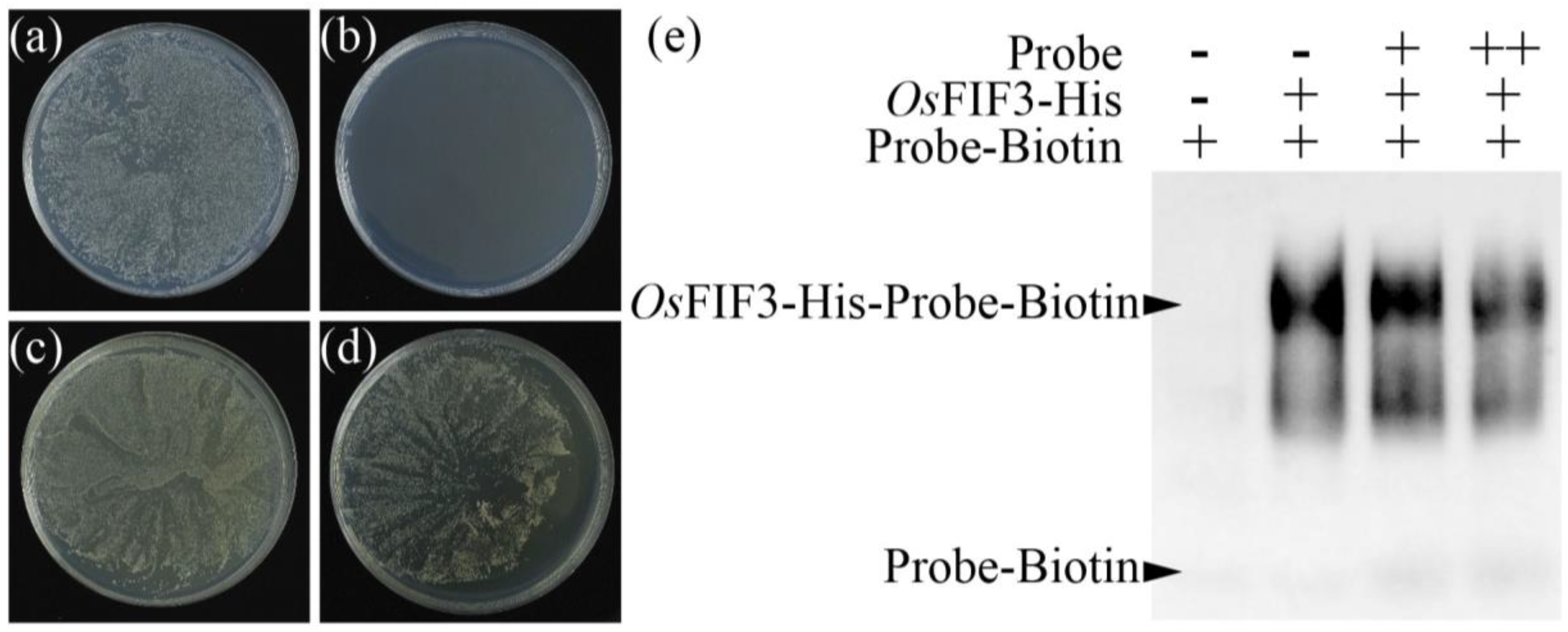
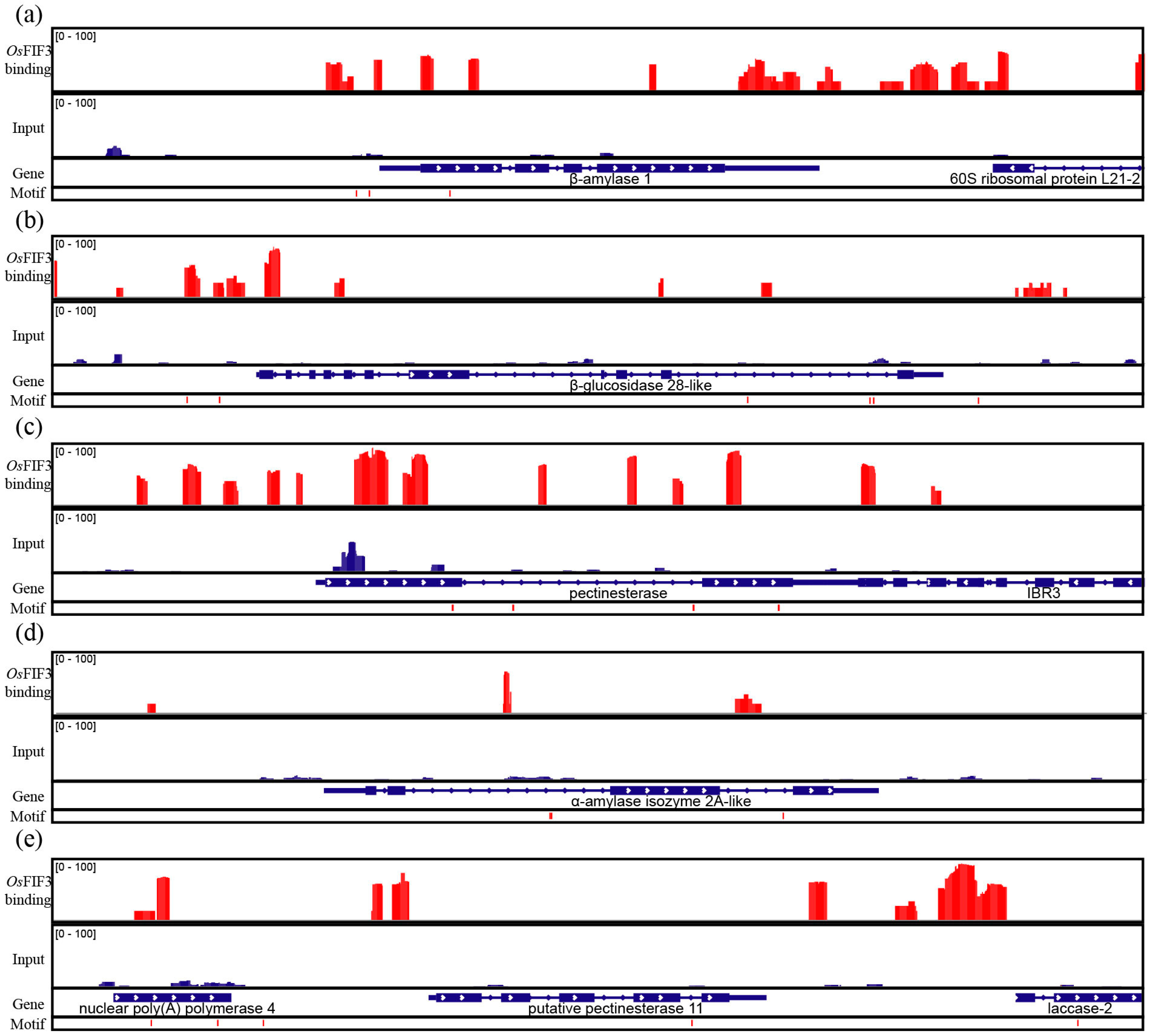
2.3. Subcellular Localization of OsFIF3 and Its Interaction DNA Sequence
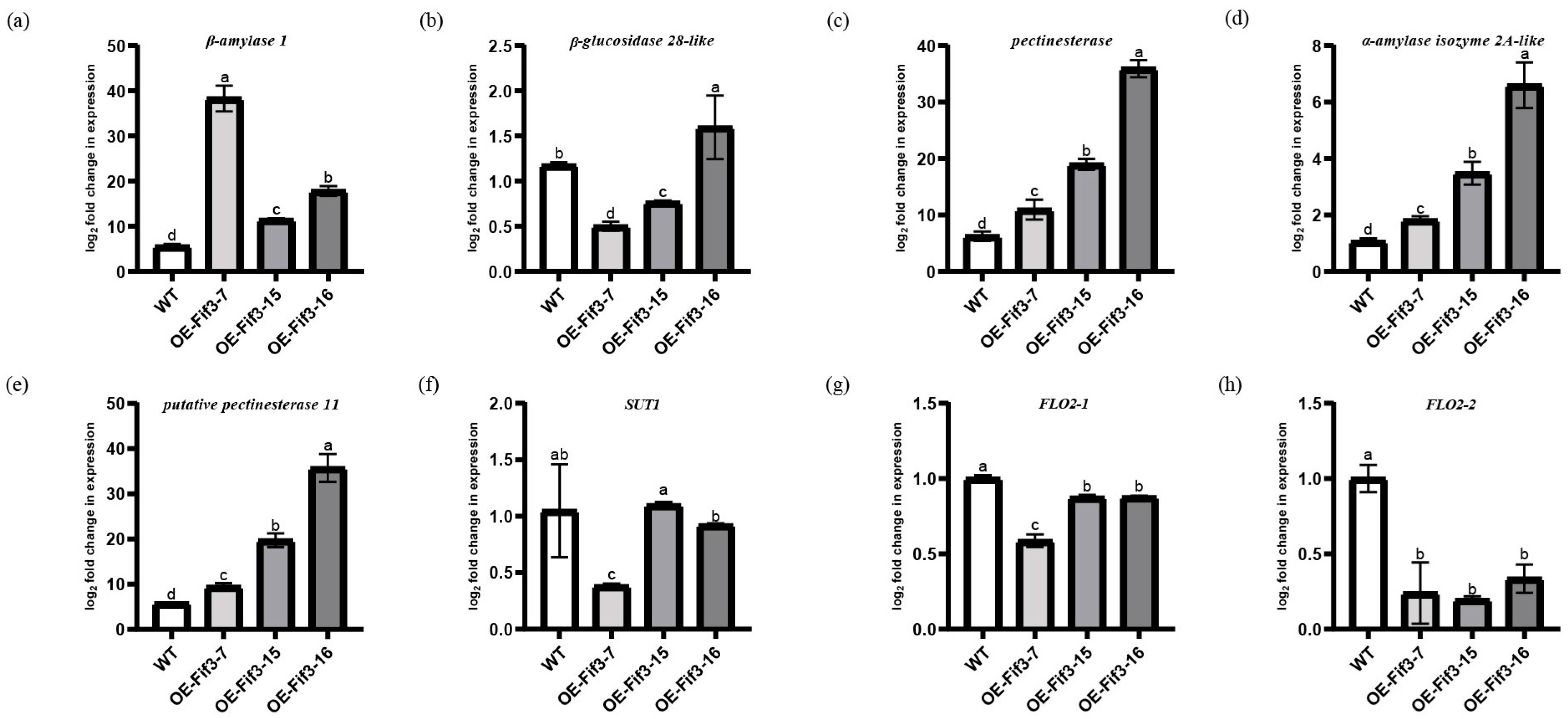
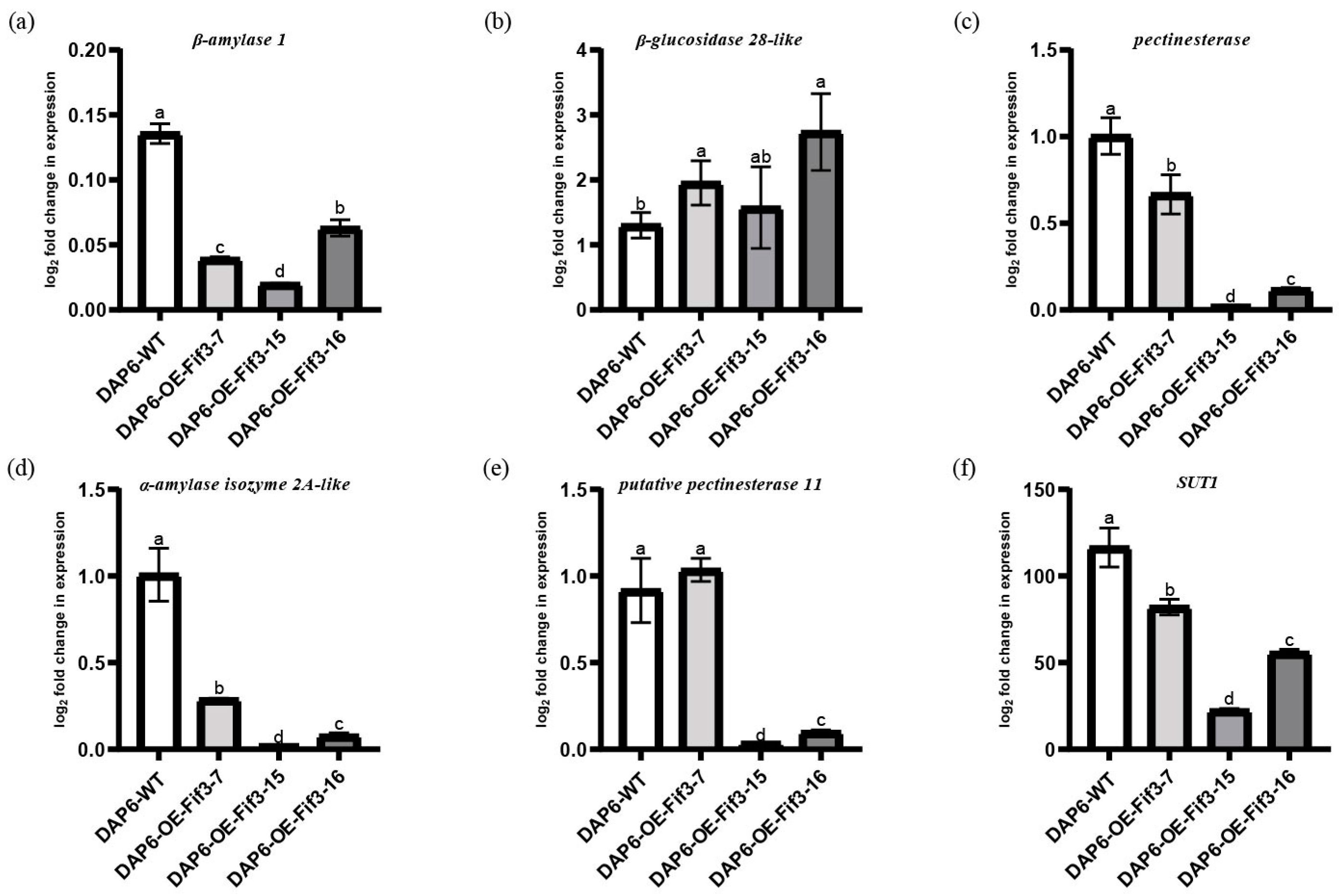
2.4. OsFIF3 Regulates Starch Metabolism and Chalkiness Related Gene SUT1
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Scanning Electron Microscopy
4.3. Vector Construction and Rice Transformation
4.4. RNA Extraction and RT-qPCR Analysis
4.5. Subcellular Localization
4.6. EMSA
4.7. DAP-Seq
4.8. Bioinformatics Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marathi, B.; Guleria, S.; Mohapatra, T.; Parsad, R.; Mariappan, N.; Kurungara, V.K.; Atwal, S.S.; Prabhu, K.V.; Singh, N.K.; Singh, A.K. QTL analysis of novel genomic regions associated with yield and yield related traits in new plant type based recombinant inbred lines of rice (Oryza sativa L.). BMC Plant Biol. 2012, 12, 137. [Google Scholar] [CrossRef]
- Nevame, A.Y.M.; Emon, R.M.; Malek, M.A.; Hasan, M.M.; Alam, M.A.; Muharam, F.M.; Aslani, F.; Rafii, M.Y.; Ismail, M.R. Relationship between High Temperature and Formation of Chalkiness and Their Effects on Quality of Rice. Biomed. Res. Int. 2018, 2018, 1653721. [Google Scholar] [CrossRef] [PubMed]
- Yosuke, Y.; Hiroyoshi, I.; Minako, T.; Seishi, N.; Ryo, O. Chalkiness in rice: Potential for evaluation with image analysis. Crop Sci. 2007, 47, 2113–2120. [Google Scholar]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Alam, M.A.; Abdul Rahim, H.; Malek, M.A.; Latif, M.A. Introgression of blast resistance genes into the elite rice variety MR263 through marker-assisted backcrossing. J. Sci. Food Agric. 2016, 96, 1297–1305. [Google Scholar] [CrossRef]
- Siebenmorgen, T.J.; Grigg, B.C.; Lanning, S.B. Impacts of preharvest factors during kernel development on rice quality and functionality. Annu. Rev. Food Sci. Technol. 2013, 4, 101–115. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Li, D.; Liu, C.M. Rice caryopsis development II: Dynamic changes in the endosperm. J. Integr. Plant Biol. 2016, 58, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Lounifi, I.; Cueff, G.; Collet, B.; Clement, G.; Balzergue, S.; Huguet, S.; Valot, B.; Galland, M.; Rajjou, L. Multi-Omics Approaches Unravel Specific Features of Embryo and Endosperm in Rice Seed Germination. Front. Plant Sci. 2022, 13, 867263. [Google Scholar] [CrossRef] [PubMed]
- Hikaru, S.; Takeshi, O. New Endosperm Mutations Induced by Chemical Mutagens in Rice, Oryza sativa L. Jpn. J. Breed. 1981, 31, 316–326. [Google Scholar]
- She, K.C.; Kusano, H.; Koizumi, K.; Yamakawa, H.; Hakata, M.; Imamura, T.; Fukuda, M.; Naito, N.; Tsurumaki, Y.; Yaeshima, M.; et al. A novel factor FLOURY ENDOSPERM2 is involved in regulation of rice grain size and starch quality. Plant Cell 2010, 22, 3280–3294. [Google Scholar] [CrossRef]
- Murozuka, E.; Massange-Sanchez, J.A.; Nielsen, K.; Gregersen, P.L.; Braumann, I. Genome wide characterization of barley NAC transcription factors enables the identification of grain-specific transcription factors exclusive for the Poaceae family of monocotyledonous plants. PLoS ONE 2018, 13, e0209769. [Google Scholar] [CrossRef]
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef]
- Qin, P.; Zhang, G.; Hu, B.; Wu, J.; Chen, W.; Ren, Z.; Liu, Y.; Xie, J.; Yuan, H.; Tu, B.; et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci. Adv. 2021, 7, eabc8873. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.R.; Deng, X.J.; Gao, X.L.; Chen, J.; Wan, J.; Jiang, H.; Xu, Z.J. Effect of drought on rice quality. Chin. Agric. Sci. 2004, 20, 282. [Google Scholar]
- O’Malley, R.C.; Huang, S.C.; Song, L.; Lewsey, M.G.; Bartlett, A.; Nery, J.R.; Galli, M.; Gallavotti, A.; Ecker, J.R. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell 2016, 165, 1280–1292. [Google Scholar] [CrossRef]
- Bartlett, A.; O’Malley, R.C.; Huang, S.C.; Galli, M.; Nery, J.R.; Gallavotti, A.; Ecker, J.R. Mapping genome-wide transcription-factor binding sites using DAP-seq. Nat. Protoc. 2017, 12, 1659–1672. [Google Scholar] [CrossRef]
- Wu, M.; Ren, Y.; Cai, M.; Wang, Y.; Zhu, S.; Zhu, J.; Hao, Y.; Teng, X.; Zhu, X.; Jing, R.; et al. Rice FLOURY ENDOSPERM10 encodes a pentatricopeptide repeat protein that is essential for the trans-splicing of mitochondrial nad1 intron 1 and endosperm development. N. Phytol. 2019, 223, 736–750. [Google Scholar] [CrossRef]
- Zhong, M.S.; Liu, X.; Liu, F.; Ren, Y.L.; Wan, J.M. FLOURY ENDOSPERM12 encoding alanine aminotransferase 1 regulates carbon and nitrogen metabolism in rice. J. Plant Biol. 2019, 62, 61–73. [Google Scholar] [CrossRef]
- Hu, T.; Tian, Y.; Zhu, J.; Wang, Y.; Jing, R.; Lei, J.; Sun, Y.; Yu, Y.; Li, J.; Chen, X.; et al. OsNDUFA9 encoding a mitochondrial complex I subunit is essential for embryo development and starch synthesis in rice. Plant Cell Rep. 2018, 37, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Ichiho, M.; Munetoshi, A.; Hiro, Y.H.; Yoshio, S. Altered tissue-specific expression at the Wx gene of the opaque mutants in rice. Euphytica 1999, 105, 91–97. [Google Scholar]
- Ryoo, N.; Yu, C.; Park, C.S.; Baik, M.Y.; Park, I.M.; Cho, M.H.; Bhoo, S.H.; An, G.; Hahn, T.R.; Jeon, J.S. Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.). Plant Cell Rep. 2007, 26, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Okuno, K.; Kawakami, J.; Satoh, H.; Omura, T. High amylose mutants of rice, Oryza sativa L. Theor. Appl. Genet. 1985, 69, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, Y.; Liu, F.; Ren, Y.; Zhou, K.; Lv, J.; Zheng, M.; Zhao, S.; Zhang, L.; Wang, C.; et al. FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J. 2014, 77, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, C.; Xing, Y.; Yun, P.; Luo, L.; Yan, B.; Peng, B.; Xie, W.; Wang, G.; Li, X.; et al. Chalk5 encodes a vacuolar H(+)-translocating pyrophosphatase influencing grain chalkiness in rice. Nat. Genet. 2014, 46, 398–404. [Google Scholar] [CrossRef]
- Wakasa, Y.; Yasuda, H.; Oono, Y.; Kawakatsu, T.; Hirose, S.; Takahashi, H.; Hayashi, S.; Yang, L.; Takaiwa, F. Expression of ER quality control-related genes in response to changes in BiP1 levels in developing rice endosperm. Plant J. 2011, 65, 675–689. [Google Scholar] [CrossRef]
- Yasuda, H.; Hirose, S.; Kawakatsu, T.; Wakasa, Y.; Takaiwa, F. Overexpression of BiP has inhibitory effects on the accumulation of seed storage proteins in endosperm cells of rice. Plant Cell Physiol. 2009, 50, 1532–1543. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yeu, S.Y.; Park, B.S.; Koh, H.J.; Song, J.T.; Seo, H.S. Protein disulfide isomerase-like protein 1-1 controls endosperm development through regulation of the amount and composition of seed proteins in rice. PLoS ONE 2012, 7, e44493. [Google Scholar] [CrossRef]
- Kabir, M.H.; Liu, Q.; Yi, S.; Huang, Z.; Xiao, L. Dynamics of phytohormones and their relationship with chalkness of early indica rice under different post-anthesis temperature regimes. Bangladesh J. Agric. Res. 2017, 42, 2408–8293. [Google Scholar]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef]
- Zhang, X.F.; Tong, J.H.; Bai, A.N.; Liu, C.M.; Xiao, L.T.; Xue, H.W. Phytohormone dynamics in developing endosperm influence rice grain shape and quality. J. Integr. Plant Biol. 2020, 62, 1625–1637. [Google Scholar] [CrossRef]
- Zhiponova, M.K.; Morohashi, K.; Vanhoutte, I.; Machemer-Noonan, K.; Revalska, M.; Van Montagu, M.; Grotewold, E.; Russinova, E. Helix-loop-helix/basic helix-loop-helix transcription factor network represses cell elongation in Arabidopsis through an apparent incoherent feed-forward loop. Proc. Natl. Acad. Sci. USA 2014, 111, 2824–2829. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef]
- Phan, T.; Ishibashi, Y.; Miyazaki, M.; Tran, H.T.; Okamura, K.; Tanaka, S.; Nakamura, J.; Yuasa, T.; Iwaya, I.M. High temperature-induced repression of the rice Sucrose Transporter (OsSUT1) and starch synthesis-Related genes in sink and source organs at milky ripening stage causes chalky grains. J. Agron. Crop Sci. 2013, 199, 178–188. [Google Scholar]
- Xie, X.; Ma, X.; Zhu, Q.; Zeng, D.; Li, G.; Liu, Y.G. CRISPR-GE: A convenient software toolkit for CRISPR-based genome editing. Mol. Plant 2017, 10, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdottir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Machanick, P. Inferring direct DNA binding from ChIP-seq. Nucleic Acids Res. 2012, 40, e128. [Google Scholar] [CrossRef]
- Rombauts, S.; Dehais, P.; Van Montagu, M.; Rouze, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Zhong, W.; Wang, K.; Gong, X.; Xia, Y.; Nong, J.; Xiao, L.; Xia, S. Regulation of Grain Chalkiness and Starch Metabolism by FLO2 Interaction Factor 3, a bHLH Transcription Factor in Oryza sativa. Int. J. Mol. Sci. 2023, 24, 12778. https://doi.org/10.3390/ijms241612778
Tang X, Zhong W, Wang K, Gong X, Xia Y, Nong J, Xiao L, Xia S. Regulation of Grain Chalkiness and Starch Metabolism by FLO2 Interaction Factor 3, a bHLH Transcription Factor in Oryza sativa. International Journal of Molecular Sciences. 2023; 24(16):12778. https://doi.org/10.3390/ijms241612778
Chicago/Turabian StyleTang, Xianyu, Weiping Zhong, Kunmei Wang, Xin Gong, Yunong Xia, Jieying Nong, Langtao Xiao, and Shitou Xia. 2023. "Regulation of Grain Chalkiness and Starch Metabolism by FLO2 Interaction Factor 3, a bHLH Transcription Factor in Oryza sativa" International Journal of Molecular Sciences 24, no. 16: 12778. https://doi.org/10.3390/ijms241612778
APA StyleTang, X., Zhong, W., Wang, K., Gong, X., Xia, Y., Nong, J., Xiao, L., & Xia, S. (2023). Regulation of Grain Chalkiness and Starch Metabolism by FLO2 Interaction Factor 3, a bHLH Transcription Factor in Oryza sativa. International Journal of Molecular Sciences, 24(16), 12778. https://doi.org/10.3390/ijms241612778





