Bridging the Gap between Gut Microbiota and Alzheimer’s Disease: A Metaproteomic Approach for Biomarker Discovery in Transgenic Mice
Abstract
1. Introduction
2. Results
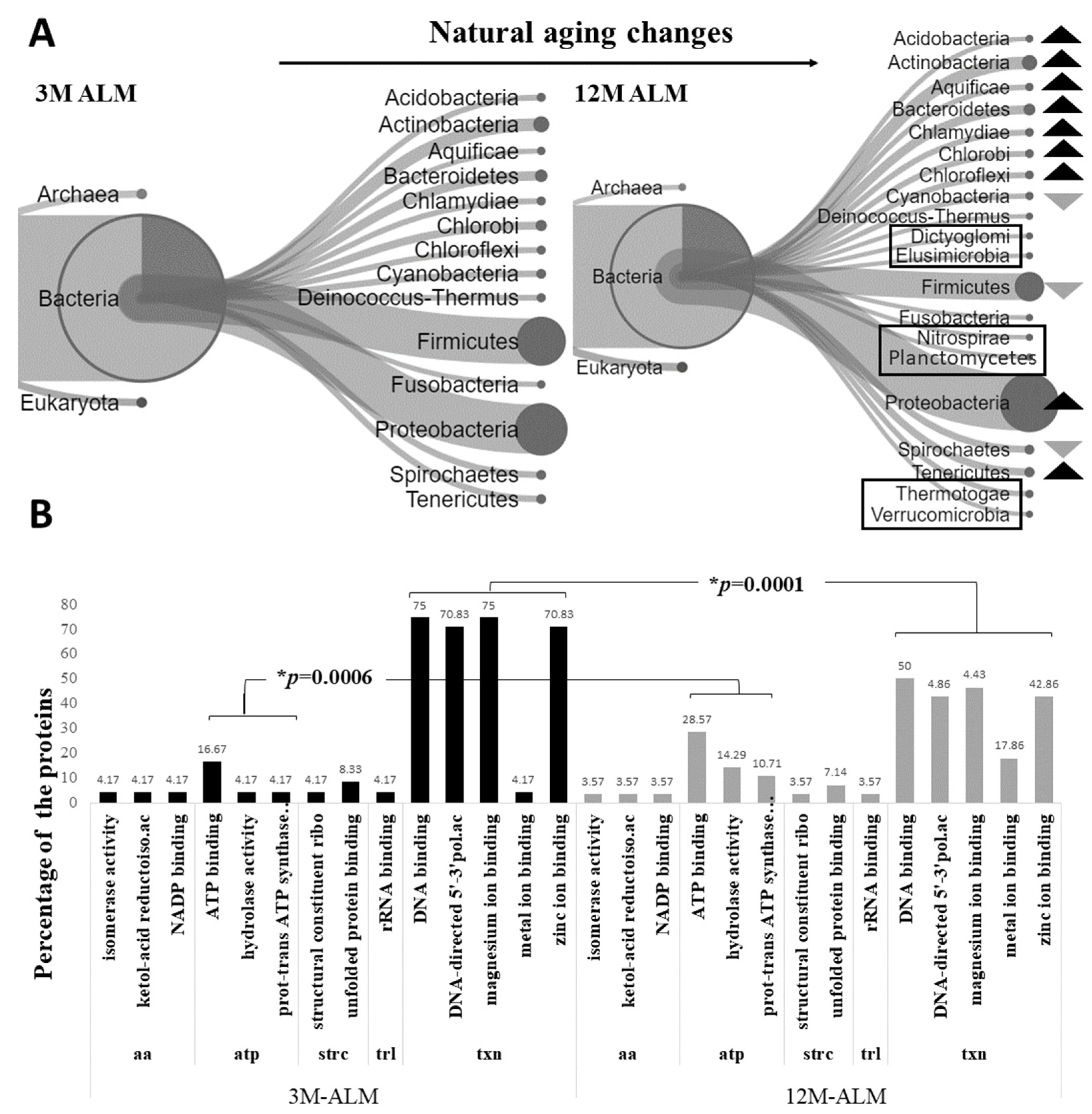
2.1. Metaproteomic Alterations in the Microbiota of Natural Aging
2.2. Metaproteomic Alterations in the Microbiota of Transgenic Aging
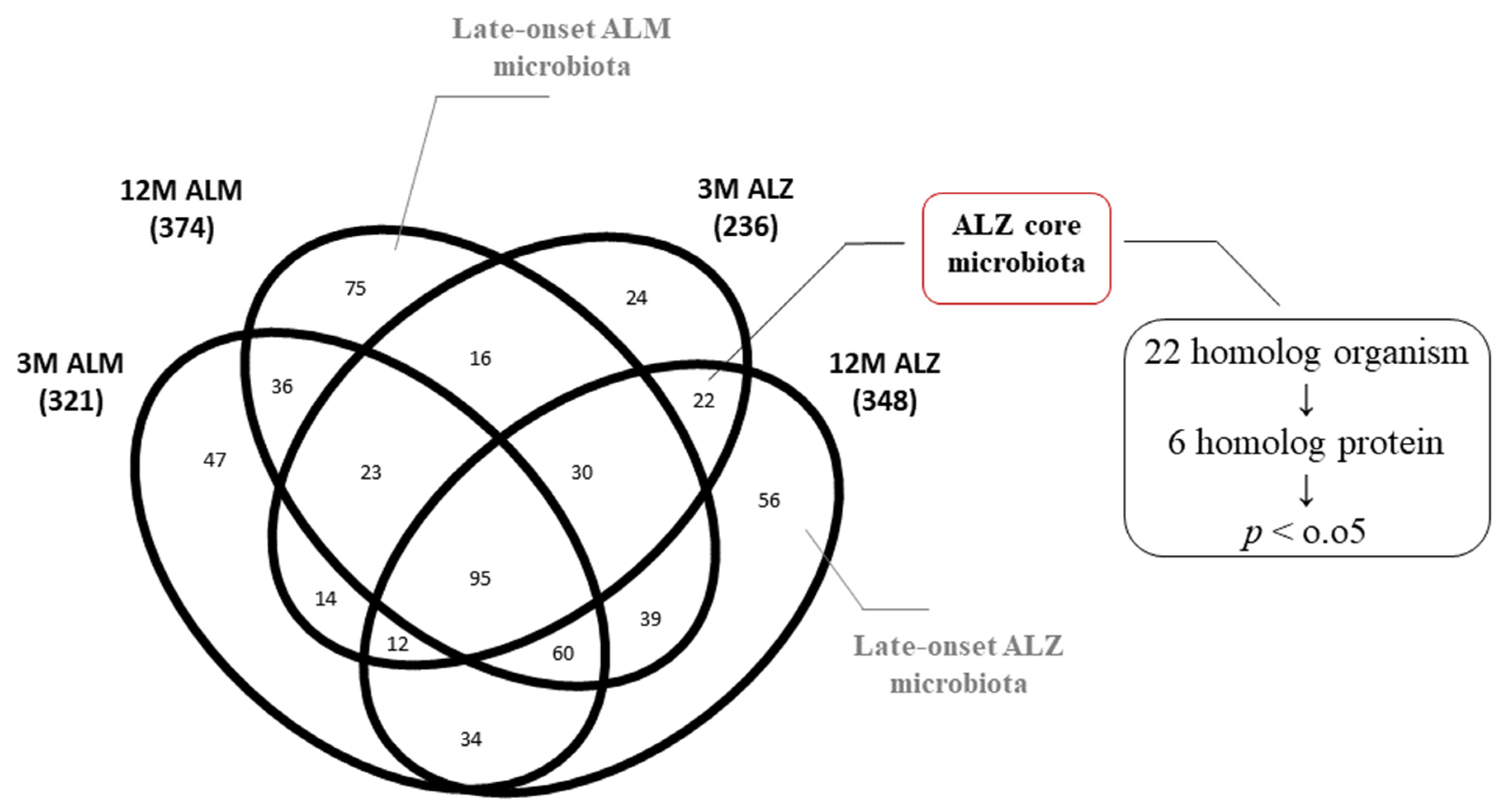
2.3. Homolog Metaproteome Alterations in the Core Microbiota of 3M ALZ and 12M ALZ Cohorts
2.4. Metaproteomic Alterations in the 12M Microbiota of Pure ALZ and ALM Models
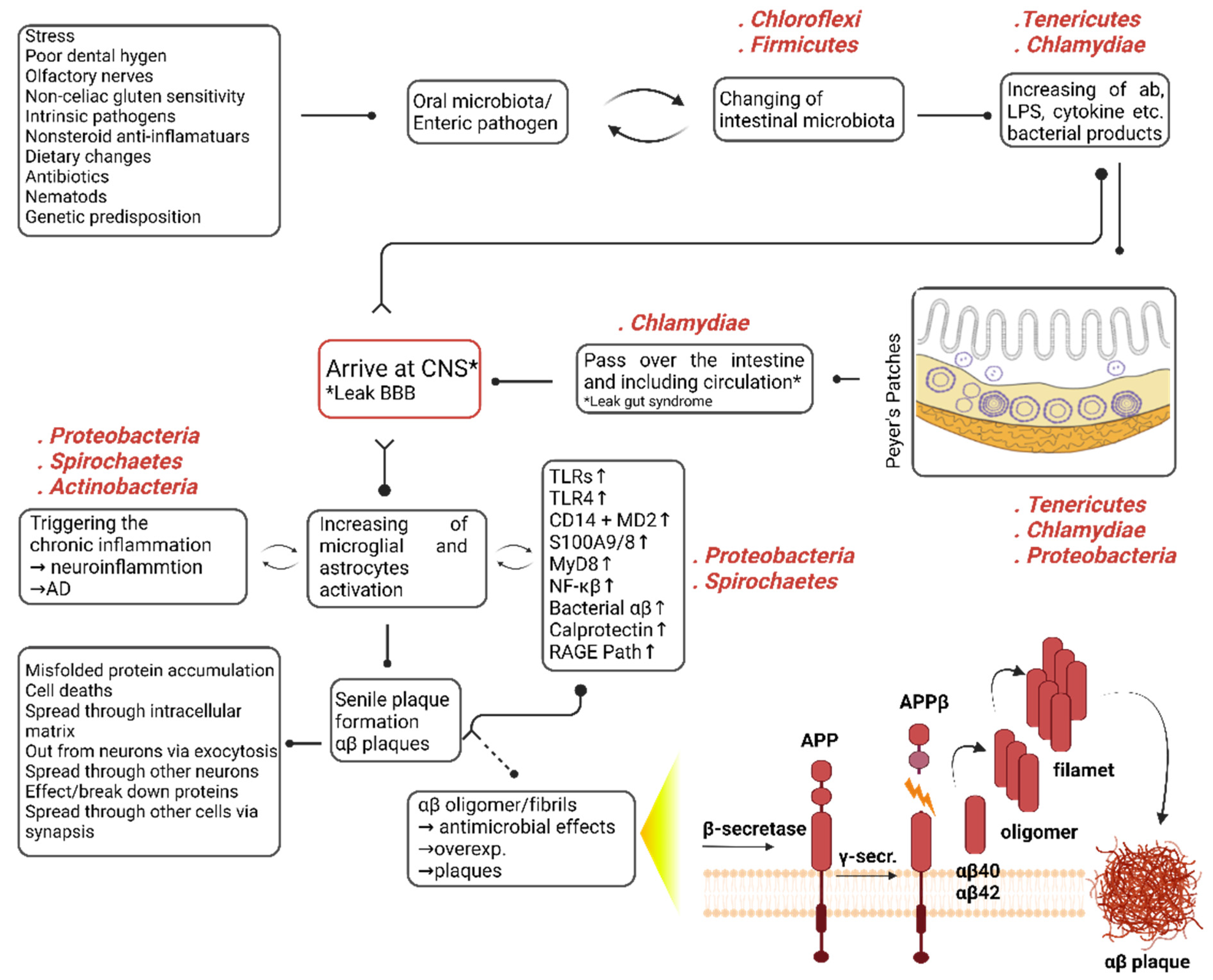
3. Discussion
3.1. Metaproteomic Changes in the Microbiota of Natural Aging
3.2. Metaproteomic Changes in the Microbiota of Transgenic (Tg) Aging
3.3. Metaproteome Changing of Homolog 3M- and 12M-ALZ Core Microbiota
3.4. Metaproteome Changing of Unique 12-Months ALZ and ALM Microbiota
4. Materials and Methods
4.1. Murine Samples
4.2. Microbial Extraction from Murine Fecal Samples
4.3. Protein Extraction
4.4. Microdialysis and Protein Concentration Determination
4.5. FASP (Filter Aided Sample Preparation) Protocol
4.6. Nano LC/MS-MS
4.7. Data Reduction and Analysis
4.8. Data Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alagöz, A.N. Mikrobiyota ve Nörodejenerasyon. J. Biotechnol. Strateg. Health Res. 2017, 1, 115–122. [Google Scholar]
- Xu, Z.; Knight, R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 2014, 113, S1–S5. [Google Scholar] [CrossRef]
- Yılmaz, K.; Altındiş, M. Sindirim sistemi mikrobiyotasi ve fekal transplantasyon. Nobel Med. 2017, 13, 9–15. [Google Scholar]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Microbiology: Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Mandal, R.S.; Saha, S.; Das, S. Metagenomic Surveys of Gut Microbiota. Genom. Proteom. Bioinform. 2015, 13, 148–158. [Google Scholar] [CrossRef]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2016, 595, 489–503. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Catanzaro, R.; Anzalone, M.; Calabrese, F.; Milazzo, M.; Capuana, M.; Italia, A.; Occhipinti, S.; Marotta, F. The gut microbiota and its correlations with the central nervous system disorders. Panminerva Medica 2014, 57, 127–143. [Google Scholar]
- Frasca, D.; Blomberg, B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2015, 17, 7–19. [Google Scholar] [CrossRef]
- Köhler, C.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.L.; Carvalho, A.F. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Bertrand, R.L. Serotonin release and uptake in the gastrointestinal tract. Auton. Neurosci. 2010, 153, 47–57. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Lukiw, W.J. Alzheimer’s disease and the microbiome. Front. Cell. Neurosci. 2013, 7, 153. [Google Scholar] [CrossRef]
- Marizzoni, M.; Provasi, S.; Cattaneo, A.; Frisoni, G.B. Microbiota and neurodegenerative diseases. Curr. Opin. Neurol. 2017, 30, 630–638. [Google Scholar] [CrossRef]
- Tremlett, H.; Bauer, K.C.; Appel-Cresswell, S.; Finlay, B.B.; Waubant, E. The gut microbiome in human neurological disease: A review. Ann. Neurol. 2017, 81, 369–382. [Google Scholar] [CrossRef]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the devel-opment of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Bayer; Bayer, T.A.; Wirths, O. Intracellular accumulation of amyloid-beta—A predictor for synaptic dysfunction and neuron loss in Alzheimer’s disease. Front. Aging Neurosci. 2010, 2, 8. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Girard, S.D.; Jacquet, M.; Baranger, K.; Migliorati, M.; Escoffier, G.; Bernard, A.; Khrestchatisky, M.; Féron, F.; Rivera, S.; Roman, F.S.; et al. Onset of hippocampus-dependent memory impairments in 5XFAD transgenic mouse model of Alz-heimer’s disease. Hippocampus 2014, 24, 762–772. [Google Scholar] [CrossRef]
- Girard, S.D.; Baranger, K.; Gauthier, C.; Jacquet, M.; Bernard, A.; Escoffier, G.; Marchetti, E.; Khrestchatisky, M.; Rivera, S.; Roman, F.S.; et al. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD mouse model of Alz-heimer’s disease. J. Alzheimer’s Dis. 2013, 33, 781–796. [Google Scholar] [CrossRef]
- Eimer, W.A.; Vassar, R. Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Aβ42 accumulation and Caspase-3 activation. Mol. Neurodegener. 2013, 8, 2. [Google Scholar] [CrossRef]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coin-ciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196.e29–196.e40. [Google Scholar] [CrossRef]
- Brandscheid, C.; Schuck, F.; Reinhardt, S.; Schäfer, K.-H.; Pietrzik, C.U.; Grimm, M.; Hartmann, T.; Schwiertz, A.; Endres, K. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer’s Mouse Model. J. Alzheimer’s Dis. 2017, 56, 775–788. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Geng, M.; Xie, Z.; Chu, X.; Yang, J.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino ac-ids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, K.-E.; Kim, J.-K.; Kim, D.-H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019, 9, 11814. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Kodamullil, A.T.; Zekri, F.; Sood, M.; Hengerer, B.; Canard, L.; McHale, D.; Hofmann-Apitius, M. Trial watch: Tracing investment in drug development for Alzheimer disease. Nat. Rev. Drug Discov. 2017, 16, 819. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Lo Monaco, M.R.; Landi, F.; Bernabei, R.; Marzetti, E. Of Microbes and Minds: A Narrative Review on the Second Brain Aging. Front. Med. 2018, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, G.; Wilcock, D.; Henderson, D.; Gordon, M.; Morgan, D. Intrahippocampal LPS injections reduce Aβ load in APP+PS1 transgenic mice. Neurobiol. Aging 2001, 22, 1007–1012. [Google Scholar] [CrossRef]
- Herber, D.L.; Mercer, M.; Roth, L.M.; Symmonds, K.; Maloney, J.; Wilson, N.; Freeman, M.J.; Morgan, D.; Gordon, M.N. Microglial Activation is Required for Aβ Clearance After Intracranial Injection of Lipopolysaccharide in APP Transgenic Mice. J. Neuroimmune Pharmacol. 2007, 2, 222–231. [Google Scholar] [CrossRef]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human gut microbiota: The links with dementia development. Protein Cell 2016, 8, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Main, B.S.; Minter, M.R. Microbial Immuno-Communication in Neurodegenerative Diseases. Front. Neurosci. 2017, 11, 151. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, Z.; Xie, G.; Liu, M.; Yuan, B.; Cai, H.; Wang, W.; Cheng, P. Implications of gut microbiota in neuro-degenerative diseases. Front. Immunol. 2022, 13, 325. [Google Scholar]
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ho, C.-T.; Zhang, X. Neuroprotection of Food Bioactives in Neurodegenerative Diseases: Role of the Gut Microbiota and Innate Immune Receptors. J. Agric. Food Chem. 2023, 71, 2718–2733. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Dandekar, M.P. Implication of Paraprobiotics in Age-Associated Gut Dysbiosis and Neurodegenerative Diseases. NeuroMolecular Med. 2022, 25, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Borsom, E.M.; Conn, K.; Keefe, C.R.; Herman, C.; Orsini, G.M.; Hirsch, A.H.; Avila, M.P.; Testo, G.; Jaramillo, S.A.; Cope, E.K. Predicting neu-rodegenerative disease using Prepathology gut microbiota composition: A longitudinal study in mice modeling Alzheimer’s disease pathologies. Microbiol. Spectr. 2023, 11, e03458-22. [Google Scholar] [CrossRef]
- Beker, M.C.; Caglayan, B.; Yalcin, E.; Caglayan, A.B.; Turkseven, S.; Gurel, B.; Kelestemur, T.; Sertel, E.; Sahin, Z.; Kutlu, S.; et al. Time-of-Day Dependent Neuronal Injury After Ischemic Stroke: Implication of Circadian Clock Transcriptional Factor Bmal1 and Survival Kinase AKT. Mol. Neurobiol. 2017, 55, 2565–2576. [Google Scholar] [CrossRef]
- Demircan, T.; Keskin, I.; Dumlu, S.N.; Aytürk, N.; Avşaroğlu, M.E.; Akgün, E.; Öztürk, G.; Baykal, A.T. Detailed tail proteomic analysis of axolotl (Ambystoma mexicanum) using an mRNA-seq reference database. Proteomics 2017, 17, 1600338. [Google Scholar] [CrossRef]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef]
- Emery, D.C.; Shoemark, D.K.; Batstone, T.E.; Waterfall, C.M.; Coghill, J.A.; Cerajewska, T.L.; Davies, M.; West, N.X.; Allen-Birt, S.J. 16S rRNA Next Generation Sequencing Analysis Shows Bacteria in Alzheimer’s Post-Mortem Brain. Front. Aging Neurosci. 2017, 9, 195. [Google Scholar] [CrossRef]
- Bradley, W.G.; Mash, D.C. Beyond Guam: The cyanobacteria/BMAA hypothesis of the cause of ALS and other neuro-degenerative diseases. Amyotroph. Lateral Scler. 2009, 10, 7–20. [Google Scholar] [CrossRef]
- Hu, X.; Wang, T.; Jin, F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 2016, 59, 1006–1023. [Google Scholar] [CrossRef]
- Friedland, R.P. Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Boles, B.R. Microbial amyloids—Functions and interactions within the host. Curr. Opin. Microbiol. 2013, 16, 93–99. [Google Scholar] [CrossRef]
- Branton, W.G.; Ellestad, K.K.; Maingat, F.; Wheatley, B.M.; Rud, E.; Warren, R.L.; Holt, R.A.; Surette, M.G.; Power, C. Brain Microbial Populations in HIV/AIDS: α-Proteobacteria Predominate Independent of Host Immune Status. PLoS ONE 2013, 8, e54673. [Google Scholar] [CrossRef]
- Païssé, S.; Valle, C.; Servant, F.; Courtney, M.; Burcelin, R.; Amar, J.; Lelouvier, B. Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 2016, 56, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.J.; Kim, K.A.; Jang, S.E.; Woo, J.Y.; Han, M.J.; Kim, D.H. Correction: Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS ONE 2015, 10, e0142521. [Google Scholar]
- Hufnagel, D.A.; Tükel, Ç.; Chapman, M.R. Disease to Dirt: The Biology of Microbial Amyloids. PLoS Pathog. 2013, 9, e1003740. [Google Scholar] [CrossRef]
- Fransen, F.; Van Beek, A.A.; Borghuis, T.; El Aidy, S.; Hugenholtz, F.; van der Gaast–de Jongh, C.; Savelkoul, H.F.J.; De Jonge, M.I.; Boekschoten, M.V.; Smidt, H.; et al. Aged Gut Microbiota Contributes to Systemical Inflammaging after Transfer to Germ-Free Mice. Front. Immunol. 2017, 8, 1385. [Google Scholar] [CrossRef]
- Bäuerl, C.; Collado, M.C.; Diaz Cuevas, A.; Viña, J.; Pérez Martínez, G. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol. 2018, 66, 464–471. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. Ebiomedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Miklossy, J. Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 46. [Google Scholar] [CrossRef]
- Miklossy, J. Alzheimer’s disease—A neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J. Neuroinflammation 2011, 8, 90. [Google Scholar] [CrossRef]
- Miklossy, J. Alzheimer’s Disease, Spirochetes—A Causal Relationship. Innov. Aging 2017, 1, 274. [Google Scholar] [CrossRef][Green Version]
- Lee, H.-J.; Hwang, Y.-H.; Kim, D.-H. Lactobacillus plantarum C29-Fermented Soybean (DW2009) Alleviates Memory Impairment in 5XFAD Transgenic Mice by Regulating Microglia Activation and Gut Microbiota Composition. Mol. Nutr. Food Res. 2018, 62, e1800359. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, L. Dr. Oskar Fischer’s Curious Little Alzheimer’s Germ. Curr. Opin. Neurol. Sci. 2017, 1, 160–178. [Google Scholar]
- La Rosa, F.; Clerici, M.; Ratto, D.; Occhinegro, A.; Licito, A.; Romeo, M.; Di Iorio, C.; Rossi, P. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients 2018, 10, 1267. [Google Scholar] [CrossRef]
- Askarova, S.; Umbayev, B.; Masoud, A.R.; Kaiyrlykyzy, A.; Safarova, Y.; Tsoy, A.; Kushugulova, A.; Olzhayev, F. The Links Between the Gut Microbiome, Aging, Modern Lifestyle and Alzheimer’s Disease. Front. Cell. Infect. Microbiol. 2020, 10, 104. [Google Scholar] [CrossRef]
- Harach, T.; Marungruang, N.; Dutilleul, N.; Cheatham, V.; Mc Coy, K.D.; Neher, J.J.; Jucker, M.; Fåk, F.; Lasser; Bolmont, T. Reduction of Alzheimer’s disease beta-amyloid pathology in the absence of gut microbiota. arXiv 2015, arXiv:1509.02273. [Google Scholar]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lang, T.; Shen, J.; Dai, J.; Tian, L.; Wang, X. Core Gut Bacteria Analysis of Healthy Mice. Front. Microbiol. 2019, 10, 887. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta)genomic Data. mBio 2014, 5, e00889-14. [Google Scholar] [CrossRef]
- Lukiw, W.J. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer’s Disease. Front. Microbiol. 2016, 7, 1544. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef]
- Sergeant, N.; Wattez, A.; Galvn-Valencia, M.; Ghestem, A.; David, J.-P.; Lemoine, J.; Sautire, P.-E.; Dachary, J.; Mazat, J.-P.; Michalski, J.-C.; et al. Association of atp synthase α-chain with neurofibrillary degeneration in alzheimer’s disease. Neuroscience 2003, 117, 293–303. [Google Scholar] [CrossRef]
- Terni, B.; Boada, J.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Mitochondrial ATP-Synthase in the Entorhinal Cortex Is a Target of Oxidative Stress at Stages I/II of Alzheimer’s Disease Pathology. Brain Pathol. 2010, 20, 222–233. [Google Scholar] [CrossRef]
- Beck, S.J.; Guo, L.; Phensy, A.; Tian, J.; Wang, L.; Tandon, N.; Gauba, E.; Lu, L.; Pascual, J.M.; Kroener, S.; et al. Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer’s disease. Nat. Commun. 2016, 7, 11483. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Brinton, R.D.; Yin, F. Mitochondria-Targeted Therapeutics for Alzheimer’s Disease: The Good, the Bad, the Potential. Antioxid. Redox Signal. 2021, 34, 611–630. [Google Scholar] [CrossRef]
- Mangione, M.R.; Vilasi, S.; Marino, C.; Librizzi, F.; Canale, C.; Spigolon, D.; Bucchieri, F.; Fucarino, A.; Passantino, R.; Cappello, F.; et al. Hsp60, amateur chaperone in amyloid-beta fibrillogenesis. Biochim. Biophys. Acta 2016, 1860, 2474–2483. [Google Scholar] [CrossRef]
- Singh, N.K.; Rao, P.; Asea, A. Heat Shock Proteins and the Brain: Implications for Neurodegenerative Diseases and Neuroprotection. In Heat Shock Proteins and the Brain: Implications for Neurodegenerative Diseases and Neuroprotection; Springer: Dordrecht, The Netherlands, 2008; p. 371. [Google Scholar] [CrossRef]
- Veereshwarayya, V.; Kumar, P.; Rosen, K.M.; Mestril, R.; Querfurth, H.W. Differential Effects of Mitochondrial Heat Shock Protein 60 and Related Molecular Chaperones to Prevent Intracellular β-Amyloid-induced Inhibition of Complex IV and Limit Apoptosis. J. Biol. Chem. 2006, 281, 29468–29478. [Google Scholar] [CrossRef]
- Walls, K.C.; Coskun, P.; Gallegos-Perez, J.-L.; Zadourian, N.; Freude, K.; Rasool, S.; Blurton-Jones, M.; Green, K.N.; LaFerla, F.M. Swedish Alzheimer Mutation Induces Mitochondrial Dysfunction Mediated by HSP60 Mislocalization of Amyloid Precursor Protein (APP) and Beta-Amyloid. J. Biol. Chem. 2012, 287, 30317–30327. [Google Scholar] [CrossRef]
- Mamelak, M. Alzheimer’ s disease, oxidative stress and gammahydroxybutyrate. Neurobiol. Aging 2007, 28, 1340–1360. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Hong, X.; Li, S.; Wang, Y. Quantitative Proteomics Reveals the Mechanism of Oxygen Treatment on Lenses of Alzheimer’s Disease Model Mice. J. Alzheimer’s Dis. 2016, 54, 275–286. [Google Scholar] [CrossRef]
- Lee, T.-R.; Lee, H.-Y.; Huang, S.-H.; Chan, H.-T.; Lyu, P.-C.; Chan, H.-L. Comparative proteomics analysis of normal and memory-deficient Drosophila melanogaster heads. Zoöl. Stud. 2013, 52, 10. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Cho, H.J.; Kaufmann, J.C.; McLachlan, D.R.C. The molecular mechanisms of scrapie encephalopathy and relevance to human neurodegenerative disease. Can. J. Veter. Res. Rev. Can. Rech. Veter. 1990, 54, 49–57. [Google Scholar]
- Lukiw, W.; Kruck, T.; McLachlan, D. Linker histone-DNA complexes: Enhanced stability in the presence of aluminum lactate and implications for Alzheimer’s disease. FEBS Lett. 1989, 253, 59–62. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Kumar, D.K.V.; Choi, S.H.; Washicosky, K.J.; Eimer, W.A.; Tucker, S.; Ghofrani, J.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 340ra72. [Google Scholar] [CrossRef]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef]
- Gorevic, P. Genetic Factors in the Amyloid Diseases; Up To Date: Waltham, MA, USA, 2014; pp. 1–31. [Google Scholar]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef]
- Shi, D.Y.; Bierhaus, A.; Nawroth, P.P.; Stern, D.M. RAGE and Alzheimer’s disease: A progression factor for amyloid-β- induced cellular perturbation? J. Alzheimer’s Dis. 2009, 16, 833–843. [Google Scholar]
- Allen, H.B. Alzheimer’s Disease: Assessing the Role of Spirochetes, Biofilms, the Immune System, and Amyloid-β with Regard to Potential Treatment and Prevention. J. Alzheimer’s Dis. 2016, 53, 1271–1276. [Google Scholar] [CrossRef]
- Lim, J.-E.; Kou, J.; Song, M.; Pattanayak, A.; Jin, J.; Lalonde, R.; Fukuchi, K.-I. MyD88 Deficiency Ameliorates β-Amyloidosis in an Animal Model of Alzheimer’s Disease. Am. J. Pathol. 2011, 179, 1095–1103. [Google Scholar] [CrossRef]
- Chen, S.G.; Stribinskis, V.; Rane, M.J.; Demuth, D.R.; Gozal, E.; Roberts, A.M.; Jagadapillai, R.; Liu, R.; Choe, K.; Shivakumar, B.; et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci. Rep. 2016, 6, 34477. [Google Scholar] [CrossRef]
- Frost, B.; Diamond, M.I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010, 11, 155–159. [Google Scholar] [CrossRef]
- Apajalahti, J.H.A.; Särkilahti, L.K.; Mäki, B.R.; Heikkinen, J.P.; Nurminen, P.H.; Holben, W.E. Effective Recovery of Bacterial DNA and Percent-Guanine-Plus-Cytosine-Based Analysis of Community Structure in the Gastrointestinal Tract of Broiler Chickens. Appl. Environ. Microbiol. 1998, 64, 4084–4088. [Google Scholar] [CrossRef]
- Tanca, A.; Palomba, A.; Pisanu, S.; Deligios, M.; Fraumene, C.; Manghina, V.; Pagnozzi, D.; Addis, M.F.; Uzzau, S. A straightforward and efficient analytical pipeline for metaproteome characterization. Microbiome 2014, 2, 49. [Google Scholar] [CrossRef]
- Ngo, S.; Guo, Z. Key residues for the oligomerization of Aβ42 protein in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2011, 414, 512–516. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Singh, R.G.; Tanca, A.; Palomba, A.; Van Der Jeugt, F.; Verschaffelt, P.; Uzzau, S.; Martens, L.; Dawyndt, P.; Mesuere, B. Unipept 4.0: Functional Analysis of Metaproteome Data. J. Proteome Res. 2018, 18, 606–615. [Google Scholar] [CrossRef] [PubMed]


| 3M-ALM | 12M-ALM | 3M-ALZ | 12MALZ | |
|---|---|---|---|---|
| Peptide | 502 | 636 | 338 | 584 |
| Protein | 484 | 636 | 338 | 584 |
| Organism | 321 | 374 | 236 | 348 |
| Unique phyla | 1 * | 5 * | 1 * | 1 * |
| Phyla | 15 | 19 | 16 | 16 |
| Acidobacteria Actinobacteria Aquificae Bacteroidetes Chlamydiae Chlorobi Chloroflexi Cyanobacteria Deinococcus-thermus * Elusimicrobia Firmicutes Fusobacteria Proteobacteria Spirochaetes Tenericutes | Acidobacteria Actinobacteria Aquificae Bacteroidetes Chlamydiae Chlorobi Chloroflexi Cyanobacteria Deinococcus-thermus * Dictiyoglomi Firmicutes Fusobacteria * Nitrospirae * Planctomycetes Proteobacteria Spirochaetes Tenericutes * Thermotogae * Verrumicrobia | Actinobacteria Aquificae Bacteroidetes Chlamydiae Chlorobi Chloroflexi Cyanobacteria Deinococcus-thermus * Dictiyoglomi Firmicutes Fusobacteria Proteobacteria Spirochaetes Tenericutes Thermotogae Verrumicrobia | Actinobacteria Aquificae Bacteroidetes Chlamydiae Chlorobi Chloroflexi * Coprothermobacterota Cyanobacteria Deinococcus-thermus Firmicutes Fusobacteria Proteobacteria Spirochaetes Tenericutes Thermotogae Verrumicrobia |
| UniProt ID | Protein Name | t-Test | F-Chance | Organism | Phylum |
|---|---|---|---|---|---|
| P13357 | ATP synthase subunit beta | 0.01 | −0.88 | Cellulophaga lytica | Bacteroidetes |
| Q9XXK1 | ATP synthase subunit alpha, mitochondrial | 0.01 | −0.08 | Caenorhabditis elegans | Metazoa |
| P37282 | 60 kDa chaperonin | 0.08 | 0.49 | Lactococcus lactis subsp. lactis (strain IL1403) | Firmicutes |
| C0QVC8 | Probable transaldolase | 0.01 | 1.91 | Brachyspira hyodysenteriae (strain ATCC 49526/WA1) | Spirochaetes |
| A0K2Y1 | ATP synthase subunit alpha | 0.01 | 9.31 | Burkholderia cenocepacia (strain HI2424) | Proteobacteria |
| A8GV24 | DNA-directed RNA polymerase subunit beta | 0 | 11.62 | Rickettsia bellii (strain OSU 85-389) | Proteobacteria |
| Pathways | Molecular Function | %Peptide |
|---|---|---|
| Energy metabolism | ATP binding | 66.67 |
| Proton-transporting ATP synthase activity, rotational mechanism | 50 | |
| hydrolase activity | 16.67 | |
| Pentose-phosphate | Sedoheptulose-7-phosphate: D-glyceraldehyde-3-phosphate glyceronetransferase activity | 16.67 |
| Aldehyde-lyase activity | 16.67 | |
| Protein structure | Unfolded protein binding | 16.67 |
| Transcription | DNA binding | 16.67 |
| DNA-directed 5′-3′ RNA polymerase activity | 16.67 | |
| Ribonucleoside binding | 16.67 |
| Phyla | 3-12M ALM | 3-12M ALZ | U 12M ALZ | Notes |
|---|---|---|---|---|
| Acidobacter | + | 0 | 0 | Prospective (beneficial) |
| Actinobacteria | + | + | + | Prospective (aging marker) |
| Aquificae | + | + | 0 | In progress |
| Bacteroidetes | + | + | 0 | In progress |
| Chlamydiae | + | − | − | Prospective (beneficial) |
| Chlorobi | + | + | 0 | In progress |
| Chloroflexi | + | + | u | Prospective (aging marker) |
| Cyanobacteria | − | + | u | Prospective (late-onset marker) |
| Deinococcus-Thermus | 0 | + | 0 | In progress |
| Dictyoglomi | u | 0 | 0 | For the first time |
| Firmicutes | − | + | + | Prospective (late-onset marker) |
| Fusobacteria | 0 | − | 0 | In progress |
| Nitrospirae | u | 0 | 0 | For the first time |
| Planctomycetes | u | 0 | 0 | For the first time |
| Proteobacteria | + | + | − | In progress |
| Spirochaetes | − | + | 0 | Prospective (harmful) |
| Tenericutes | + | − | − | Prospective (beneficial) |
| Thermotogae | u | − | 0 | Prospective (beneficial) |
| Verrucomicrobia | u | + | 0 | In progress |
| Coprothermobacterota | 0 | u | 0 | Prospective (harmful) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayan, E.; DeMirci, H.; Serdar, M.A.; Palermo, F.; Baykal, A.T. Bridging the Gap between Gut Microbiota and Alzheimer’s Disease: A Metaproteomic Approach for Biomarker Discovery in Transgenic Mice. Int. J. Mol. Sci. 2023, 24, 12819. https://doi.org/10.3390/ijms241612819
Ayan E, DeMirci H, Serdar MA, Palermo F, Baykal AT. Bridging the Gap between Gut Microbiota and Alzheimer’s Disease: A Metaproteomic Approach for Biomarker Discovery in Transgenic Mice. International Journal of Molecular Sciences. 2023; 24(16):12819. https://doi.org/10.3390/ijms241612819
Chicago/Turabian StyleAyan, Esra, Hasan DeMirci, Muhittin Abdulkadir Serdar, Francesca Palermo, and Ahmet Tarık Baykal. 2023. "Bridging the Gap between Gut Microbiota and Alzheimer’s Disease: A Metaproteomic Approach for Biomarker Discovery in Transgenic Mice" International Journal of Molecular Sciences 24, no. 16: 12819. https://doi.org/10.3390/ijms241612819
APA StyleAyan, E., DeMirci, H., Serdar, M. A., Palermo, F., & Baykal, A. T. (2023). Bridging the Gap between Gut Microbiota and Alzheimer’s Disease: A Metaproteomic Approach for Biomarker Discovery in Transgenic Mice. International Journal of Molecular Sciences, 24(16), 12819. https://doi.org/10.3390/ijms241612819






