Improving Cancer Targeting: A Study on the Effect of Dual-Ligand Density on Targeting of Cells Having Differential Expression of Target Biomarkers
Abstract
:1. Introduction
2. Results
2.1. Nanoparticle Synthesis and Characterization
2.2. In Vitro Cellular Studies
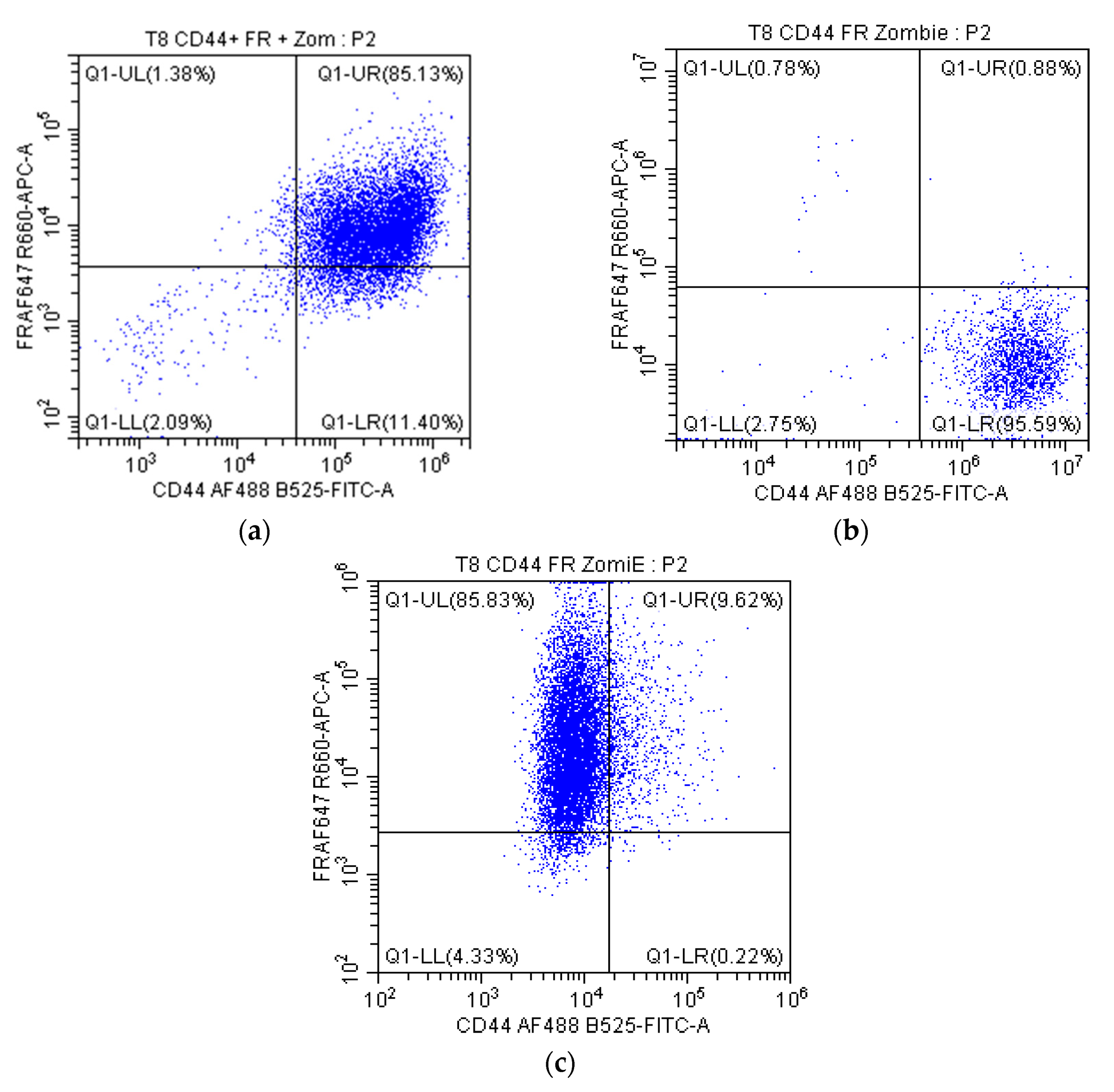
2.2.1. Receptor Expression in Cells
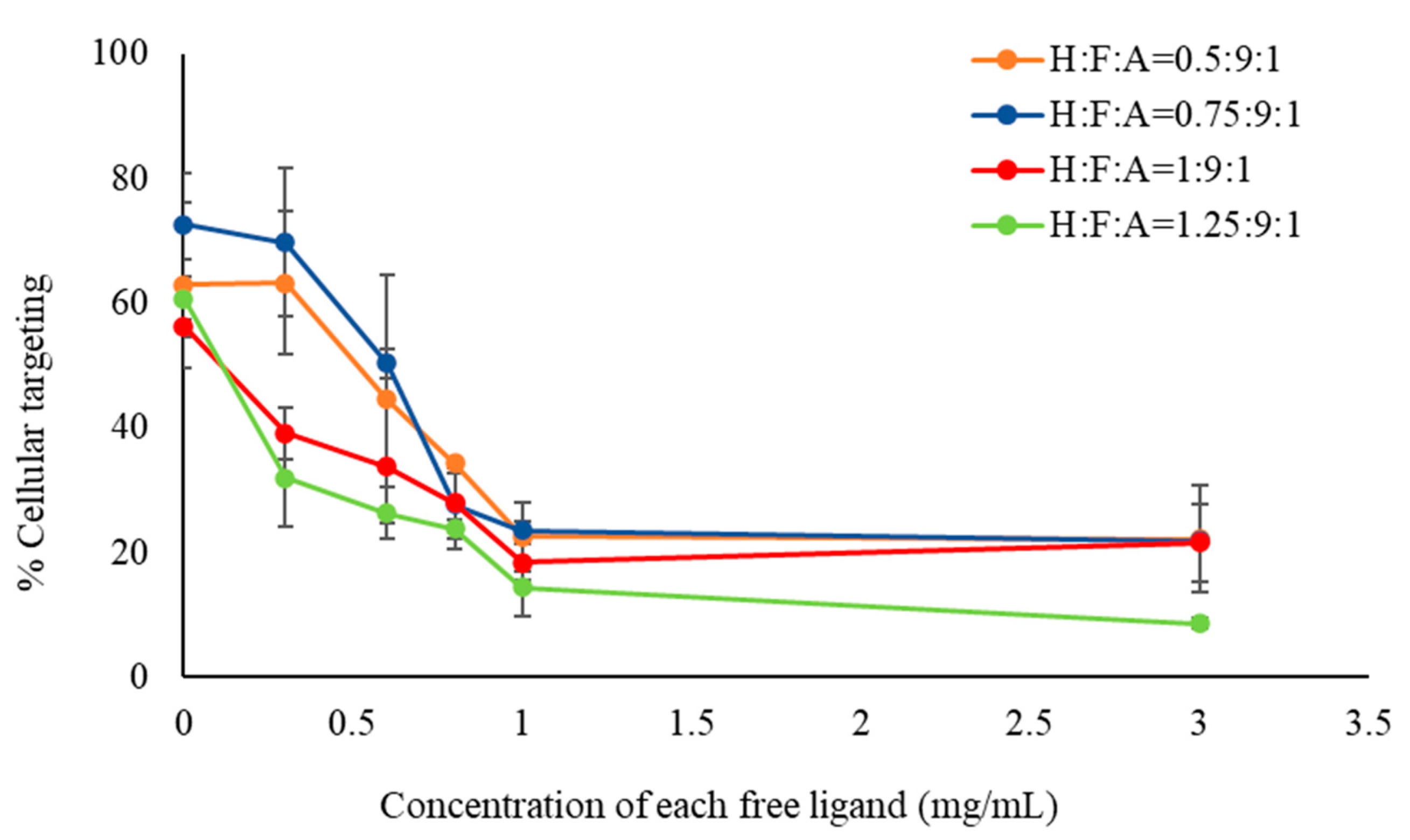
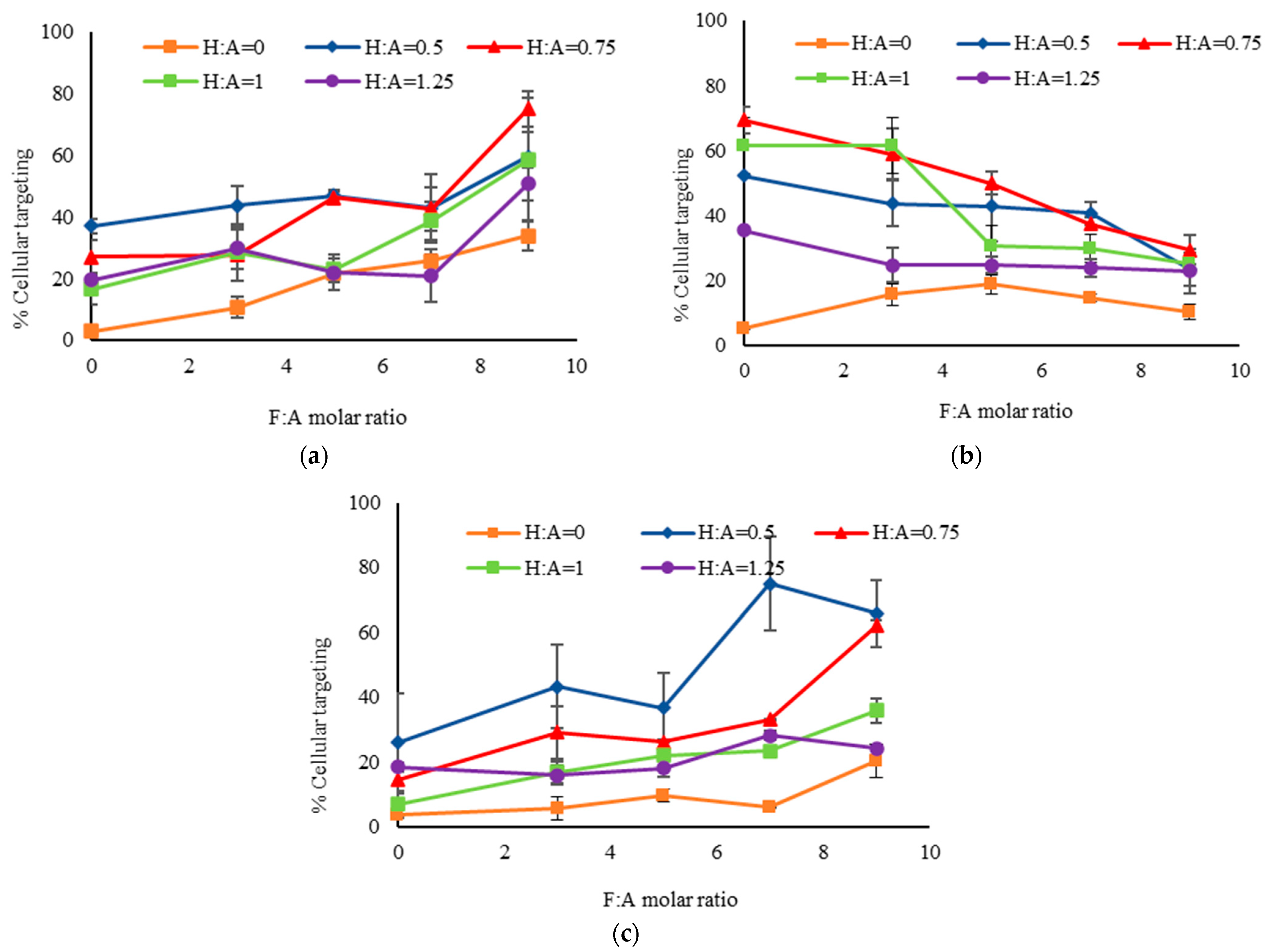
2.2.2. Evaluation of Cellular Targeting
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, M.; Ho, K.; Keating, A.; Shoichet, M.S. Doxorubicin-Conjugated Immuno-Nanoparticles for Intracellular Anticancer Drug Delivery. Adv. Funct. Mater. 2009, 19, 1689–1696. [Google Scholar] [CrossRef]
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Dharanivasan, G.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov. Today 2017, 22, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Cisterna, B.A.; Kamaly, N.; Choi, W.I.; Tavakkoli, A.; Farokhzad, O.C.; Vilos, C. Targeted nanoparticles for colorectal cancer. Nanomedicine 2016, 11, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Gambhir, S.S. Molecular Imaging with theranostic nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Peiris, P.; He, F.; Covarrubias, G.; Raghunathan, S.; Turan, O.; Lorkowski, M.; Gnanasambandam, B.; Wu, C.; Schiemann, W.P.; Karathanasis, E. Precise targeting of cancer metastasis using multi-ligand nanoparticles incorporating four different ligands. Nanoscale 2018, 10, 6861–6871. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Tian, H.; Zhu, Q.; Wang, F.; Fan, Z.; Zhou, S.; Wang, X.; Xie, L.; Hou, Z. Redox-Responsive and Dual-Targeting Hyaluronic Acid− Methotrexate Prodrug Self-Assembling Nanoparticles for Enhancing Intracellular Drug Self-Delivery. Mol. Pharm. 2019, 16, 3133–3144. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Cao, W.; Yang, J.; Lian, H.; Li, X.; Sun, Y.; Wang, Y.; Wang, S.; He, Z. Dual targeting folate-conjugated hyaluronic acid polymeric micelles for paclitaxel delivery. Int. J. Pharm. 2011, 421, 160–169. [Google Scholar] [CrossRef]
- Carlson, C.B.; Mowery, P.; Owen, R.M.; Dykhuizen, E.C.; Kiessling, L.L. Selective Tumor Cell Targeting Using Low-Affinity, Multivalent Interactions. ACS Chem. Biol. 2007, 2, 119–127. [Google Scholar] [CrossRef]
- Montet, X.; Funovics, M.; Montet-Abou, K.; Weissleder, R.; Josephson, L. Multivalent Effects of RGD Peptides Obtained by Nanoparticle Display. J. Med. Chem. 2006, 49, 6087–6093. [Google Scholar] [CrossRef] [PubMed]
- Gantert, M.; Lewrick, F.; Adrian, J.E.; Rössler, J.; Steenpass, T.; Schubert, R.; Peschka-Süss, R. Receptor-Specific Targeting with Liposomes In Vitro Based on Sterol-PEG 1300 Anchors. Pharm. Res. 2009, 26, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Smytha, M.R.; O’Kennedy, R. Oriented Immobilization of Antibodies and Its Applications in Immunoassays and Immunosensors. Analyst 1996, 121, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug Deliv. 2017, 2017, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Anzengruber, M.; Nepustil, L.; Kurtaj, F.; Tahir, A.; Skoll, K.; Sami, H.; Wirth, M.; Gabor, F. A Versatile Brij-Linker for One-Step Preparation of Targeted Nanoparticles. Pharmaceutics 2023, 15, 1403. [Google Scholar] [CrossRef] [PubMed]
- Syed, H.; Qhattal, S.; Liu, X. Characterization of CD44-Mediated Cancer Cell Uptake and Intracellular Distribution of Hyaluronan-Grafted Liposomes. Mol. Pharm. 2011, 8, 1233–1246. [Google Scholar]
- Moradi, E.; Vllasaliu, D.; Garnett, M.; Falcone, F.; Stolnik, S. Ligand density and clustering effects on endocytosis of folate modified nanoparticles. RSC Adv. 2012, 2, 3025–3033. [Google Scholar] [CrossRef]
- Emami, F.; Duwa, R.; Banstola, A.; Woo, S.M.; Kwon, T.K. Dual receptor specific nanoparticles targeting EGFR and PD-L1 for enhanced delivery of docetaxel in cancer therapy. Biomed. Pharmacother. 2023, 165, 115023. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef]
- Jung, H.-S.; Moon, D.-S.; Lee, J.-K. Quantitative Analysis and Efficient Surface Modification of Silica Nanoparticles. J. Nanomater. 2012. [CrossRef]
- He, X.; Nie, H.; Wang, K.; Tan, W.; Wu, X.; Zhang, P. In Vivo Study of Biodistribution and Urinary Excretion of Surface-Modified Silica Nanoparticles. Proc. Natl. Acad. Sci. USA 2003, 125, 9597–9603. [Google Scholar] [CrossRef] [PubMed]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Park, J.; Sangyong, J. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2012, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Toporkiewicz, M.; Meissner, J.; Matusewicz, L.; Czogalla, A.; Sikorski, A.F. Toward a magic or imaginary bullet? Ligands for drug targeting to cancer cells: Principles, hopes, and challenges. Int. J. Nanomed. 2015, 10, 1399–1414. [Google Scholar]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H. Drug targeting and tumor heterogeneity. J. Control. Release 2009, 133, 2–3. [Google Scholar] [CrossRef]
- Kirpotin, D.B.; Drummond, D.C.; Shao, Y.; Shalaby, M.R.; Hong, K.; Nielsen, U.B.; Marks, J.D.; Benz, C.C.; Park, J.W. Antibody Targeting of Long-Circulating Lipidic Nanoparticles Does Not Increase Tumor Localization but Does Increase Internalization in Animal Models. Cancer Res. 2006, 66, 6732–6772. [Google Scholar] [CrossRef]
- Pirollo, K.F.; Chang, E.H. Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends Biotechnol. 2008, 26, 552–558. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M.; Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnology 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.N.; Zaro, J.L.; Shen, W.-C. Expert Review Cell Penetrating Peptides: Intracellular Pathways and Pharmaceutical Perspectives. Pharm. Res. 2007, 24, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Schneemilch, M.; Gaisford, S.; Quirke, N. Nanoparticle-membrane interactions. J. Exp. Nanosci. 2018, 13, 62–81. [Google Scholar] [CrossRef]
- Rojas-Chapana, J.A.; Correa-Duarte, M.A.; Ren, Z.; Kempa, K.; Giersig, M. Enhanced Introduction of Gold Nanoparticles into Vital Acidothiobacillus ferrooxidans by Carbon Nanotube-based Microwave Electroporation. Nano Lett. 2023, 19, 40. [Google Scholar] [CrossRef]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Jiang, X.; Rö, C.; Hafner, M.; Brandholt, S.; Dö, R.M.; Nienhaus, G.U. Endo- and Exocytosis of Zwitterionic Quantum Dot Nanoparticles by Live HeLa Cells. ACS Nano 2010, 4, 6787–6797. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials 1988, 9, 356–362. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Aubert, T.; Grasset, F.; Mornet, S.; Duguet, E.; Cador, O.; Cordier, S.; Molard, Y.; Demange, V.; Mortier, M.; Haneda, H. Functional silica nanoparticles synthesized by water-in-oil microemulsion processes. J. Colloid Interface Sci. 2010, 341, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, C.; Qian, Q.; Ma, J.; Huang, P.; Zhang, X.; Pan, L.; Gao, G.; Fu, H.; Fu, S.; et al. Folic acid-conjugated silica capped gold nanoclusters for targeted fluorescence/X-ray computed tomography imaging. J. Nanobiotechnol. 2013, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Berríos, M.P.; Vivero-Escoto, J.L. In vitro evaluation of folic acid-conjugated redox-responsive mesoporous silica nanoparticles for the delivery of cisplatin. Int. J. Nanomed. 2016, 11, 6251–6265. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Li, Z.; Wang, W.; Sun, H.; Liu, M. Synthesis and Characterization of Hyaluronic Acid Modified Colloidal Mesoporous Silica Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 275, 012009. [Google Scholar] [CrossRef]
- Zhao, Q.; Geng, H.; Wang, Y.; Gao, Y.; Huang, J.; Wang, Y.; Zhang, J.; Wang, S. Hyaluronic Acid Oligosaccharide Modified Redox-Responsive Mesoporous Silica Nanoparticles for Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 20290–20299. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, H.; Xu, S.; Zhi, C.; Nakanishi, H.; Gao, X.D. Folate-conjugated, mesoporous silica functionalized boron nitride nanospheres for targeted delivery of doxorubicin. Mater. Sci. Eng. C 2019, 96, 552–560. [Google Scholar] [CrossRef]
- Cheng, W.; Nie, J.; Xu, L.; Liang, C.; Peng, Y.; Liu, G.; Wang, T.; Mei, L.; Huang, L.; Zeng, X. pH-Sensitive Delivery Vehicle Based on Folic Acid-Conjugated Polydopamine-Modified Mesoporous Silica Nanoparticles for Targeted Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 18462–18473. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Xu, M.; Wu, C.; Miyoshi, H.; Liu, Y. Investigation of folate-conjugated fluorescent silica nanoparticles for targeting delivery to folate receptor-positive tumors and their internalization mechanism. Int. J. Nanomed. 2011, 6, 2023–2032. [Google Scholar]
- Cao, J.; Zhang, Y.; Wu, Y.; Wu, J.; Wang, W.; Wu, Q.; Yuan, Z. The effects of ligand valency and density on the targeting ability of multivalent nanoparticles based on negatively charged chitosan nanoparticles. Colloids Surf. B Biointerfaces 2018, 161, 508–518. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Zhu, L.; Weller, H.; Mews, A.; Parak, W.J.; Barz, M.; Feliu, N. Ligand density on nanoparticles: A parameter with critical impact on nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 22–36. [Google Scholar] [CrossRef]
- Elias, D.R.; Poloukhtine, A.; Popik, V.; Tsourkas, A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine 2013, 9, 194–201. [Google Scholar] [CrossRef]
- Saneja, A.; Nayak, D.; Srinivas, M.; Kumar, A.; Khare, V.; Katoch, A.; Goswami, A.; Vishwakarma, R.A.; Sawant, S.D.; Gupta, P.N. Development and mechanistic insight into enhanced cytotoxic potential of hyaluronic acid conjugated nanoparticles in CD44 overexpressing cancer cells. Eur. J. Pharm. Sci. 2017, 97, 79–91. [Google Scholar] [CrossRef]
- Edelman, R.; Assaraf, Y.G.; Levitzky, I.; Shahar, T.; Livney, Y.D. Hyaluronic acid-serum albumin conjugate-based nanoparticles for targeted cancer therapy. Oncotarget 2017, 8, 24337–24353. [Google Scholar] [CrossRef]




| Molar Ratios | Hydrodynamic Diameter (nm) | PDI | |
|---|---|---|---|
| H:A | F:A | ||
| 0 | 0 | 271 ± 4 | 0.23 ± 0.02 |
| 3 | 264 ± 3 | 0.21 ± 0.01 | |
| 5 | 261 ± 3 | 0.23 ± 0.01 | |
| 7 | 247 ± 2 | 0.21 ± 0.01 | |
| 9 | 234 ± 2 | 0.20 ± 0.01 | |
| 0.5 | 0 | 269 ± 5 | 0.15 ± 0.02 |
| 3 | 156 ± 3 | 0.07 ± 0.04 | |
| 5 | 155 ± 2 | 0.12 ± 0.02 | |
| 7 | 207 ± 2 | 0.22 ± 0.01 | |
| 9 | 225 ± 1 | 0.22 ± 0.02 | |
| 0.75 | 0 | 155 ± 4 | 0.16 ± 0.01 |
| 3 | 219 ± 7 | 0.20 ± 0.01 | |
| 5 | 194 ± 3 | 0.16 ± 0.01 | |
| 7 | 193 ± 3 | 0.19 ± 0.02 | |
| 9 | 252 ± 6 | 0.22 ± 0.01 | |
| 1 | 0 | 198 ± 5 | 0.13 ± 0.01 |
| 3 | 147 ± 2 | 0.28 ± 0.02 | |
| 5 | 239 ± 1 | 0.24 ± 0.01 | |
| 7 | 195 ± 1 | 0.17 ± 0.04 | |
| 9 | 250 ± 2 | 0.23 ± 0.03 | |
| 1.25 | 0 | 211 ± 4 | 0.12 ± 0.02 |
| 3 | 231 ± 3 | 0.24 ± 0.02 | |
| 5 | 164 ± 1 | 0.21 ± 0.02 | |
| 7 | 181 ± 2 | 0.16 ± 0.03 | |
| 9 | 193 ±2 | 0.19 ± 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, N.; David, A.E. Improving Cancer Targeting: A Study on the Effect of Dual-Ligand Density on Targeting of Cells Having Differential Expression of Target Biomarkers. Int. J. Mol. Sci. 2023, 24, 13048. https://doi.org/10.3390/ijms241713048
Sultana N, David AE. Improving Cancer Targeting: A Study on the Effect of Dual-Ligand Density on Targeting of Cells Having Differential Expression of Target Biomarkers. International Journal of Molecular Sciences. 2023; 24(17):13048. https://doi.org/10.3390/ijms241713048
Chicago/Turabian StyleSultana, Nayer, and Allan E. David. 2023. "Improving Cancer Targeting: A Study on the Effect of Dual-Ligand Density on Targeting of Cells Having Differential Expression of Target Biomarkers" International Journal of Molecular Sciences 24, no. 17: 13048. https://doi.org/10.3390/ijms241713048
APA StyleSultana, N., & David, A. E. (2023). Improving Cancer Targeting: A Study on the Effect of Dual-Ligand Density on Targeting of Cells Having Differential Expression of Target Biomarkers. International Journal of Molecular Sciences, 24(17), 13048. https://doi.org/10.3390/ijms241713048








