Sex Differences in Neurovascular Control: Implications for Obstructive Sleep Apnea
Abstract
:1. Introduction
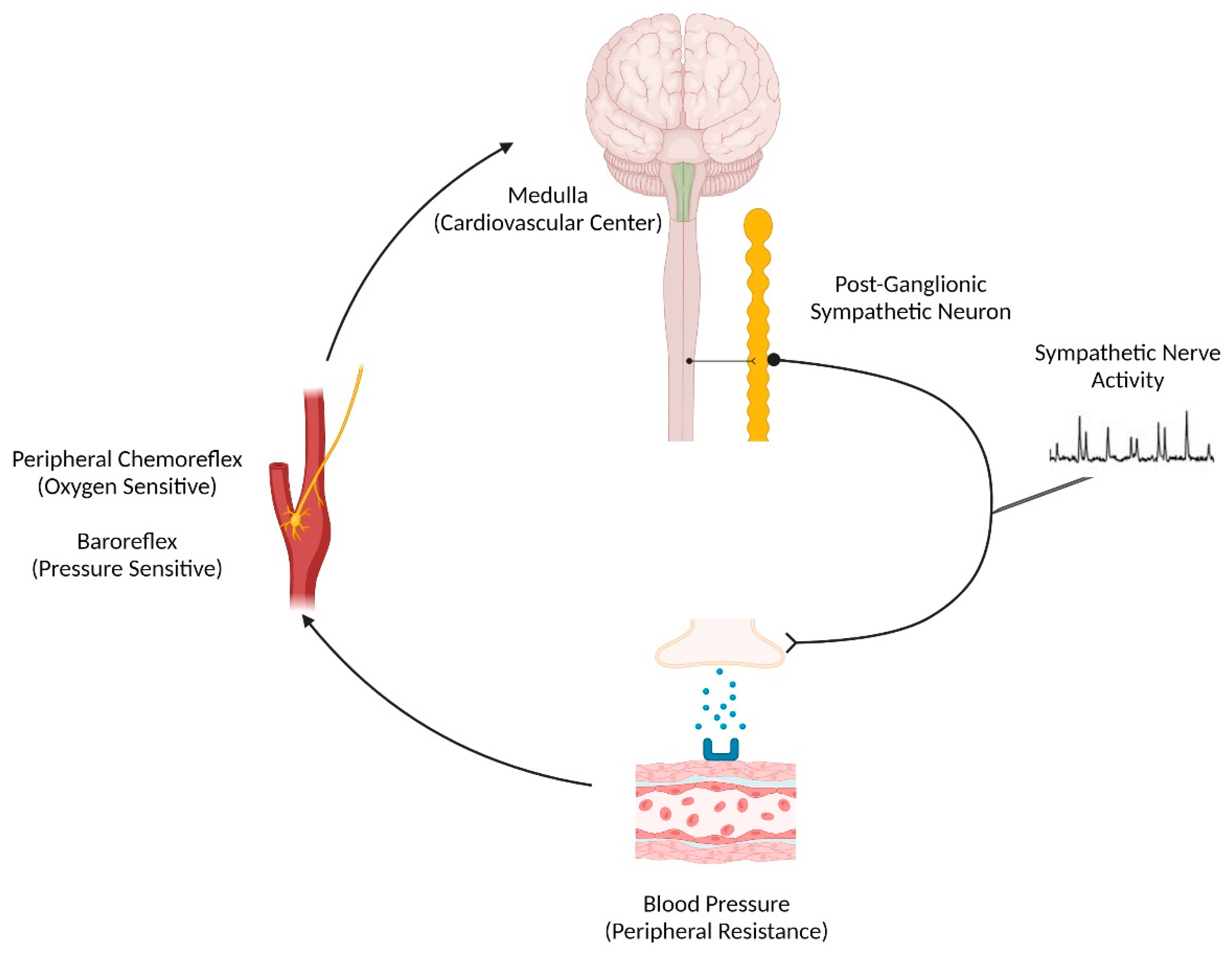
2. Sex Differences in Neurovascular Control of Blood Pressure
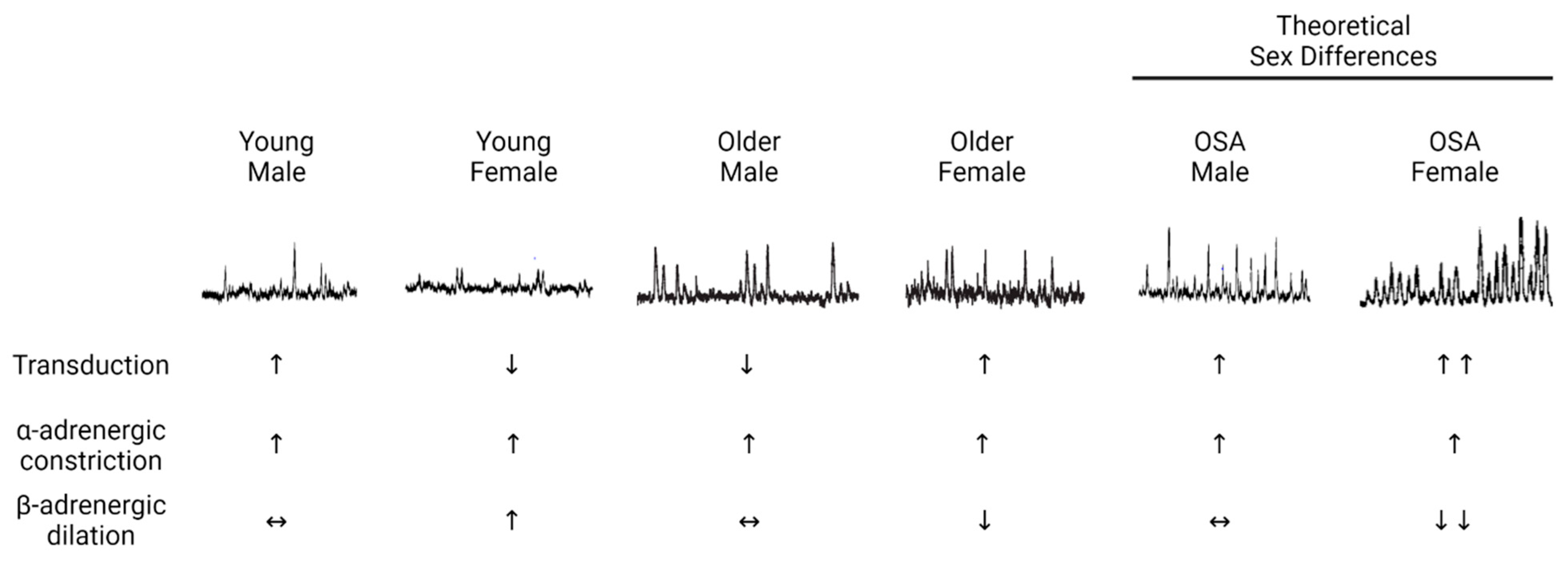
2.1. α-Adrenergic Signaling
2.2. β-Adrenergic Signaling
2.3. Changes with Aging
3. Sleep Disruption and Cardiovascular Risk: Sex Differences
3.1. Epidemiological Data
3.2. Experimental Studies on Sleep Disruption
4. Obstructive Sleep Apnea
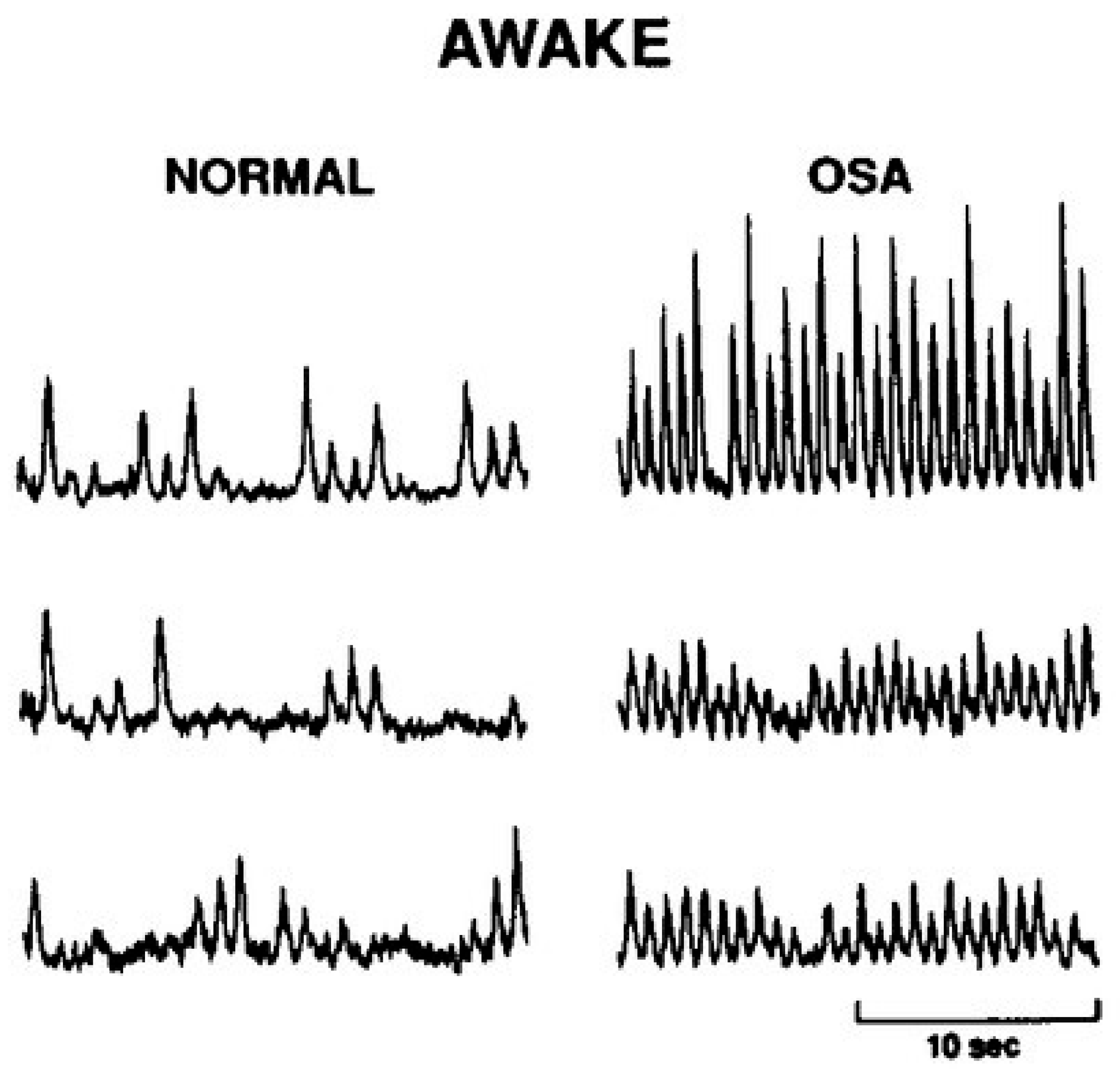
4.1. Sympathetic Regulation of Blood Pressure in OSA
4.2. Intermittent Hypoxia Studies
5. Future Directions
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef]
- Hasbani, N.R.; Ligthart, S.; Brown, M.R.; Heath, A.S.; Bebo, A.; Ashley, K.E.; Boerwinkle, E.; Morrison, A.C.; Folsom, A.R.; Aguilar, D.; et al. American Heart Association’s Life’s Simple 7: Lifestyle Recommendations, Polygenic Risk, and Lifetime Risk of Coronary Heart Disease. Circulation 2022, 145, 808–818. [Google Scholar] [CrossRef]
- Bock, J.M.; Vungarala, S.; Covassin, N.; Somers, V.K. Sleep Duration and Hypertension: Epidemiological Evidence and Underlying Mechanisms. Am. J. Hypertens. 2022, 35, 3–11. [Google Scholar] [CrossRef]
- Covassin, N.; Bukartyk, J.; Singh, P.; Calvin, A.D.; St Louis, E.K.; Somers, V.K. Effects of Experimental Sleep Restriction on Ambulatory and Sleep Blood Pressure in Healthy Young Adults: A Randomized Crossover Study. Hypertension 2021, 78, 859–870. [Google Scholar] [CrossRef]
- Carter, J.R.; Fonkoue, I.T.; Greenlund, I.M.; Schwartz, C.E.; Mokhlesi, B.; Smoot, C.A. Sympathetic neural responsiveness to sleep deprivation in older adults: Sex differences. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H315–H322. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Stranges, S.; Kandala, N.B.; Miller, M.A.; Taggart, F.M.; Kumari, M.; Ferrie, J.E.; Shipley, M.J.; Brunner, E.J.; Marmot, M.G. Gender-specific associations of short sleep duration with prevalent and incident hypertension: The Whitehall II Study. Hypertension 2007, 50, 693–700. [Google Scholar] [CrossRef]
- Grandner, M.; Mullington, J.M.; Hashmi, S.D.; Redeker, N.S.; Watson, N.F.; Morgenthaler, T.I. Sleep Duration and Hypertension: Analysis of >700,000 Adults by Age and Sex. J. Clin. Sleep Med. 2018, 14, 1031–1039. [Google Scholar] [CrossRef]
- Greenlund, I.M.; Carter, J.R. Sympathetic neural responses to sleep disorders and insufficiencies. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H337–H349. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Bairey Merz, C.N.; Cheng, S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 19–26. [Google Scholar] [CrossRef]
- Hart, E.C.; Charkoudian, N.; Wallin, B.G.; Curry, T.B.; Eisenach, J.H.; Joyner, M.J. Sex differences in sympathetic neural-hemodynamic balance: Implications for human blood pressure regulation. Hypertension 2009, 53, 571–576. [Google Scholar] [CrossRef]
- Hart, E.C.; Charkoudian, N.; Wallin, B.G.; Curry, T.B.; Eisenach, J.; Joyner, M.J. Sex and ageing differences in resting arterial pressure regulation: The role of the β-adrenergic receptors. J. Physiol. 2011, 589 Pt 21, 5285–5297. [Google Scholar] [CrossRef]
- Freedman, R.R.; Sabharwal, S.C.; Desai, N. Sex differences in peripheral vascular adrenergic receptors. Circ. Res. 1987, 61, 581–585. [Google Scholar] [CrossRef]
- Christou, D.D.; Jones, P.P.; Jordan, J.; Diedrich, A.; Robertson, D.; Seals, D.R. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 2005, 111, 494–498. [Google Scholar] [CrossRef]
- Jones, P.P.; Christou, D.D.; Jordan, J.; Seals, D.R. Baroreflex buffering is reduced with age in healthy men. Circulation 2003, 107, 1770–1774. [Google Scholar] [CrossRef]
- Briant, L.J.; Burchell, A.E.; Ratcliffe, L.E.; Charkoudian, N.; Nightingale, A.K.; Paton, J.F.; Joyner, M.J.; Hart, E.C. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: Insights into sex, ageing and blood pressure control. J. Physiol. 2016, 594, 4753–4768. [Google Scholar] [CrossRef]
- Keir, D.A.; Badrov, M.B.; Tomlinson, G.; Notarius, C.F.; Kimmerly, D.S.; Millar, P.J.; Shoemaker, J.K.; Floras, J.S. Influence of Sex and Age on Muscle Sympathetic Nerve Activity of Healthy Normotensive Adults. Hypertension 2020, 76, 997–1005. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Phillips, B.G.; Kato, M.; Hering, D.; Bieniaszewski, L.; Somers, V.K. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 2005, 45, 522–525. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.R.; Durocher, J.J.; Larson, R.A.; DellaValla, J.P.; Yang, H. Sympathetic neural responses to 24-hour sleep deprivation in humans: Sex differences. Am. J. Physiol. Heart. Circ. Physiol. 2012, 302, H1991–H1997. [Google Scholar] [CrossRef]
- O’Connor, C.; Thornley, K.S.; Hanly, P.J. Gender differences in the polysomnographic features of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2000, 161, 1465–1472. [Google Scholar] [CrossRef]
- Narkiewicz, K.; van de Borne, P.J.; Montano, N.; Dyken, M.E.; Phillips, B.G.; Somers, V.K. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation 1998, 97, 943–945. [Google Scholar] [CrossRef]
- Harvey, R.E.; Ranadive, S.M.; Limberg, J.K.; Baker, S.E.; Nicholson, W.T.; Curry, T.B.; Barnes, J.N.; Joyner, M.J. Forearm vasodilatation to a β(2) -adrenergic receptor agonist in premenopausal and postmenopausal women. Exp. Physiol. 2020, 105, 886–892. [Google Scholar] [CrossRef]
- Harvey, R.E.; Barnes, J.N.; Charkoudian, N.; Curry, T.B.; Eisenach, J.H.; Hart, E.C.; Joyner, M.J. Forearm vasodilator responses to a β-adrenergic receptor agonist in premenopausal and postmenopausal women. Physiol. Rep. 2014, 2, e12032. [Google Scholar] [CrossRef] [PubMed]
- Kneale, B.J.; Chowienczyk, P.J.; Brett, S.E.; Coltart, D.J.; Ritter, J.M. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J. Am. Coll. Cardiol. 2000, 36, 1233–1238. [Google Scholar] [CrossRef]
- Kenny, R.A.; Bhangu, J.; King-Kallimanis, B.L. Epidemiology of syncope/collapse in younger and older Western patient populations. Prog. Cardiovasc. Dis. 2013, 55, 357–363. [Google Scholar] [CrossRef]
- Jones, P.P.; Shapiro, L.F.; Keisling, G.A.; Jordan, J.; Shannon, J.R.; Quaife, R.A.; Seals, D.R. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation 2001, 104, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.E.; Limberg, J.K.; Dillon, G.A.; Curry, T.B.; Joyner, M.J.; Nicholson, W.T. Aging Alters the Relative Contributions of the Sympathetic and Parasympathetic Nervous System to Blood Pressure Control in Women. Hypertension 2018, 72, 1236–1242. [Google Scholar] [CrossRef]
- Barnes, J.N.; Hart, E.C.; Curry, T.B.; Nicholson, W.T.; Eisenach, J.H.; Wallin, B.G.; Charkoudian, N.; Joyner, M.J. Aging enhances autonomic support of blood pressure in women. Hypertension 2014, 63, 303–308. [Google Scholar] [CrossRef]
- Breskovic, T.; Steinback, C.D.; Salmanpour, A.; Shoemaker, J.K.; Dujic, Z. Recruitment pattern of sympathetic neurons during breath-holding at different lung volumes in apnea divers and controls. Auton. Neurosci. 2011, 164, 74–81. [Google Scholar] [CrossRef]
- Klassen, S.A.; Shoemaker, J.K. Action potential subpopulations within human muscle sympathetic nerve activity: Discharge properties and governing mechanisms. Auton. Neurosci. 2021, 230, 102743. [Google Scholar] [CrossRef]
- Badrov, M.B.; Lalande, S.; Olver, T.D.; Suskin, N.; Shoemaker, J.K. Effects of aging and coronary artery disease on sympathetic neural recruitment strategies during end-inspiratory and end-expiratory apnea. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1040–H1050. [Google Scholar] [CrossRef]
- Coovadia, Y.; Shoemaker, J.K.; Usselman, C.W. The effects of sex and menstrual cycle phase on sympathetic action potential recruitment patterns during hypercapnic-hypoxic apnea. Auton. Neurosci. 2023, 247, 103093. [Google Scholar] [CrossRef]
- Knutson, K.L.; Van Cauter, E.; Rathouz, P.J.; DeLeire, T.; Lauderdale, D.S. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep 2010, 33, 37–45. [Google Scholar] [CrossRef]
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [CrossRef]
- Ong, J.C.; Crawford, M.R. Insomnia and Obstructive Sleep Apnea. Sleep Med. Clin. 2013, 8, 389–398. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Redline, S.; Nieto, F.J.; Baldwin, C.M.; Newman, A.B.; Resnick, H.E.; Punjabi, N.M. Association of usual sleep duration with hypertension: The Sleep Heart Health Study. Sleep 2006, 29, 1009–1014. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Casini, A.; Macchi, C.; Abbate, R.; Gensini, G.F. Insomnia and risk of cardiovascular disease: A meta-analysis. Eur. J. Prev. Cardiol. 2014, 21, 57–64. [Google Scholar] [CrossRef]
- Javaheri, S.; Redline, S. Insomnia and Risk of Cardiovascular Disease. Chest 2017, 152, 435–444. [Google Scholar] [CrossRef]
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep Disordered Breathing and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef]
- Grunstein, R.R.; Stenlöf, K.; Hedner, J.; Sjöström, L. Impact of obstructive sleep apnea and sleepiness on metabolic and cardiovascular risk factors in the Swedish Obese Subjects (SOS) Study. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 410–418. [Google Scholar]
- Ogawa, Y.; Kanbayashi, T.; Saito, Y.; Takahashi, Y.; Kitajima, T.; Takahashi, K.; Hishikawa, Y.; Shimizu, T. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: A study with microneurographic technique. Sleep 2003, 26, 986–989. [Google Scholar] [CrossRef]
- Kato, M.; Phillips, B.G.; Sigurdsson, G.; Narkiewicz, K.; Pesek, C.A.; Somers, V.K. Effects of sleep deprivation on neural circulatory control. Hypertension 2000, 35, 1173–1175. [Google Scholar] [CrossRef]
- Sayk, F.; Teckentrup, C.; Becker, C.; Heutling, D.; Wellhöner, P.; Lehnert, H.; Dodt, C. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R191–R197. [Google Scholar] [CrossRef]
- Carlson, J.T.; Hedner, J.; Elam, M.; Ejnell, H.; Sellgren, J.; Wallin, B.G. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 1993, 103, 1763–1768. [Google Scholar] [CrossRef]
- Parati, G.; Lombardi, C.; Narkiewicz, K. Sleep apnea: Epidemiology, pathophysiology, and relation to cardiovascular risk. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1671–R1683. [Google Scholar] [CrossRef]
- Carter, J.R.; Grimaldi, D.; Fonkoue, I.T.; Medalie, L.; Mokhlesi, B.; Cauter, E.V. Assessment of sympathetic neural activity in chronic insomnia: Evidence for elevated cardiovascular risk. Sleep 2018, 41, zsy048. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Salman, L.A.; Shulman, R.; Cohen, J.B. Obstructive Sleep Apnea, Hypertension, and Cardiovascular Risk: Epidemiology, Pathophysiology, and Management. Curr. Cardiol. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Baguet, J.P.; Hammer, L.; Lévy, P.; Pierre, H.; Rossini, E.; Mouret, S.; Ormezzano, O.; Mallion, J.M.; Pépin, J.L. Night-time and diastolic hypertension are common and underestimated conditions in newly diagnosed apnoeic patients. J. Hypertens. 2005, 23, 521–527. [Google Scholar] [CrossRef]
- Suzuki, M.; Guilleminault, C.; Otsuka, K.; Shiomi, T. Blood pressure “dipping” and “non-dipping” in obstructive sleep apnea syndrome patients. Sleep 1996, 19, 382–387. [Google Scholar] [CrossRef]
- Fan, H.Q.; Li, Y.; Thijs, L.; Hansen, T.W.; Boggia, J.; Kikuya, M.; Björklund-Bodegård, K.; Richart, T.; Ohkubo, T.; Jeppesen, J.; et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J. Hypertens. 2010, 28, 2036–2045. [Google Scholar] [CrossRef]
- Hansen, T.W.; Li, Y.; Boggia, J.; Thijs, L.; Richart, T.; Staessen, J.A. Predictive role of the nighttime blood pressure. Hypertension 2011, 57, 3–10. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Saaresranta, T.; Riha, R.L. Sex differences in obstructive sleep apnoea. Eur. Respir. Rev. 2019, 28, 190030. [Google Scholar] [CrossRef]
- Lindberg, E.; Benediktsdottir, B.; Franklin, K.A.; Holm, M.; Johannessen, A.; Jögi, R.; Gislason, T.; Real, F.G.; Schlünssen, V.; Janson, C. Women with symptoms of sleep-disordered breathing are less likely to be diagnosed and treated for sleep apnea than men. Sleep Med. 2017, 35, 17–22. [Google Scholar] [CrossRef]
- Quintana-Gallego, E.; Carmona-Bernal, C.; Capote, F.; Sánchez-Armengol, A.; Botebol-Benhamou, G.; Polo-Padillo, J.; Castillo-Gómez, J. Gender differences in obstructive sleep apnea syndrome: A clinical study of 1166 patients. Respir. Med. 2004, 98, 984–989. [Google Scholar] [CrossRef]
- Basoglu, O.K.; Tasbakan, M.S. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: A clinical study of 2827 patients. Sleep Breath. 2018, 22, 241–249. [Google Scholar] [CrossRef]
- Ye, L.; Pien, G.W.; Weaver, T.E. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med. 2009, 10, 1075–1084. [Google Scholar] [CrossRef]
- Won, C.; Guilleminault, C. Gender differences in sleep disordered breathing: Implications for therapy. Expert Rev. Respir. Med. 2015, 9, 221–231. [Google Scholar] [CrossRef]
- Hedner, J.; Bengtsson-Boström, K.; Peker, Y.; Grote, L.; Råstam, L.; Lindblad, U. Hypertension prevalence in obstructive sleep apnoea and sex: A population-based case-control study. Eur. Respir. J. 2006, 27, 564–570. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Mark, A.L.; Abboud, F.M. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993, 328, 303–307. [Google Scholar] [CrossRef]
- Harrison, D.G.; Coffman, T.M.; Wilcox, C.S. Pathophysiology of Hypertension: The Mosaic Theory and Beyond. Circ. Res. 2021, 128, 847–863. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Pesek, C.A.; Kato, M.; Phillips, B.G.; Davison, D.E.; Somers, V.K. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension 1998, 32, 1039–1043. [Google Scholar] [CrossRef]
- Sequeira, V.C.C.; Bandeira, P.M.; Azevedo, J.C.M. Heart rate variability in adults with obstructive sleep apnea: A systematic review. Sleep Sci. 2019, 12, 214–221. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Montano, N.; Cogliati, C.; van de Borne, P.J.; Dyken, M.E.; Somers, V.K. Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998, 98, 1071–1077. [Google Scholar] [CrossRef]
- Colombari, E.; Sato, M.A.; Cravo, S.L.; Bergamaschi, C.T.; Campos, R.R., Jr.; Lopes, O.U. Role of the medulla oblongata in hypertension. Hypertension 2001, 38, 549–554. [Google Scholar] [CrossRef]
- Guyenet, P.G. Regulation of breathing and autonomic outflows by chemoreceptors. Compr. Physiol. 2014, 4, 1511–1562. [Google Scholar]
- Lugliani, R.; Whipp, B.J.; Seard, C.; Wasserman, K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N. Engl. J. Med. 1971, 285, 1105–1111. [Google Scholar] [CrossRef]
- Holton, P.; Wood, J. The effects of bilateral removal of the carotid bodies and denervation of the carotid sinuses in two human subjects. J. Physiol. 1965, 181, 365–378. [Google Scholar] [CrossRef]
- Narkiewicz, K.; van de Borne, P.J.; Pesek, C.A.; Dyken, M.E.; Montano, N.; Somers, V.K. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 1999, 99, 1183–1189. [Google Scholar] [CrossRef]
- Bernardi, L.; Passino, C.; Serebrovskaya, Z.; Serebrovskaya, T.; Appenzeller, O. Respiratory and cardiovascular adaptations to progressive hypoxia; effect of interval hypoxic training. Eur. Heart J. 2001, 22, 879–886. [Google Scholar] [CrossRef]
- Mateika, J.H. A reminder that experimentally induced intermittent hypoxia is an incomplete model of obstructive sleep apnea and its outcome measures. J. Appl. Physiol. 2019, 127, 1620–1621. [Google Scholar] [CrossRef]
- Puri, S.; Panza, G.; Mateika, J.H. A comprehensive review of respiratory, autonomic and cardiovascular responses to intermittent hypoxia in humans. Exp. Neurol. 2021, 341, 113709. [Google Scholar] [CrossRef]
- Keir, D.A.; Duffin, J.; Millar, P.J.; Floras, J.S. Simultaneous assessment of central and peripheral chemoreflex regulation of muscle sympathetic nerve activity and ventilation in healthy young men. J. Physiol. 2019, 597, 3281–3296. [Google Scholar] [CrossRef]
- Jouett, N.P.; Moralez, G.; Raven, P.B.; Smith, M.L. Losartan reduces the immediate and sustained increases in muscle sympathetic nerve activity after hyperacute intermittent hypoxia. J. Appl. Physiol. 2017, 122, 884–892. [Google Scholar] [CrossRef]
- Ott, E.P.; Jacob, D.W.; Baker, S.E.; Holbein, W.W.; Scruggs, Z.M.; Shoemaker, J.K.; Limberg, J.K. Sympathetic neural recruitment strategies following acute intermittent hypoxia in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R961–R971. [Google Scholar] [CrossRef]
- Jacob, D.W.; Ott, E.P.; Baker, S.E.; Scruggs, Z.M.; Ivie, C.L.; Harper, J.L.; Manrique-Acevedo, C.M.; Limberg, J.K. Sex differences in integrated neurocardiovascular control of blood pressure following acute intermittent hypercapnic hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R626–R636. [Google Scholar] [CrossRef]
- Hinojosa-Laborde, C.; Mifflin, S.W. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension 2005, 46, 1016–1021. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yuan, Z.F.; Yang, C.H.; Shen, Y.J.; Lin, J.Y.; Lai, C.J. Estrogen Modulates the Sensitivity of Lung Vagal C Fibers in Female Rats Exposed to Intermittent Hypoxia. Front. Physiol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Iwamoto, E.; Hanson, B.E.; Bock, J.M.; Casey, D.P. Intermittent hypoxia enhances shear-mediated dilation of the internal carotid artery in young adults. J. Appl. Physiol. 2020, 129, 603–611. [Google Scholar] [CrossRef]
- Hanson, B.E.; Iwamoto, E.; Mouser, B.L.; Miller, K.A.; Casey, D.P. Hypoxia offsets the decline in brachial artery flow-mediated dilation after acute inactivity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R787–R796. [Google Scholar] [CrossRef]
- Panza, G.S.; Puri, S.; Lin, H.S.; Badr, M.S.; Mateika, J.H. Daily Exposure to Mild Intermittent Hypoxia Reduces Blood Pressure in Male Patients with Obstructive Sleep Apnea and Hypertension. Am. J. Respir. Crit. Care Med. 2022, 205, 949–958. [Google Scholar] [CrossRef]
- Cetin-Atalay, R.; Meliton, A.Y.; Sun, K.A.; Glass, M.E.; Woods, P.S.; Peng, Y.J.; Fang, Y.; Hamanaka, R.B.; Prabhakar, N.R.; Mutlu, G.M. Intermittent hypoxia inhibits epinephrine-induced transcriptional changes in human aortic endothelial cells. Sci. Rep. 2022, 12, 17167. [Google Scholar] [CrossRef]
- Guo, H.; Ding, H.; Yan, Y.; Chen, Q.; Zhang, J.; Chen, B.; Cao, J. Intermittent hypoxia-induced autophagy via AMPK/mTOR signaling pathway attenuates endothelial apoptosis and dysfunction in vitro. Sleep Breath. 2021, 25, 1859–1865. [Google Scholar] [CrossRef]
- Casey, D.P.; Ueda, K.; Wegman-Points, L.; Pierce, G.L. Muscle contraction induced arterial shear stress increases endothelial nitric oxide synthase phosphorylation in humans. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H854–H859. [Google Scholar] [CrossRef]
- Bock, J.M.; Hanson, B.E.; Asama, T.F.; Feider, A.J.; Hanada, S.; Aldrich, A.W.; Dyken, M.E.; Casey, D.P. Acute inorganic nitrate supplementation and the hypoxic ventilatory response in patients with obstructive sleep apnea. J. Appl. Physiol. 2021, 130, 87–95. [Google Scholar] [CrossRef]
- Dinenno, F.A. Skeletal muscle vasodilation during systemic hypoxia in humans. J. Appl. Physiol. 2016, 120, 216–225. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef]
- Sayegh, A.L.C.; Fan, J.L.; Vianna, L.C.; Dawes, M.; Paton, J.F.R.; Fisher, J.P. Sex differences in the sympathetic neurocirculatory responses to chemoreflex activation. J. Physiol. 2022, 600, 2669–2689. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bock, J.M.; Greenlund, I.M.; Somers, V.K.; Baker, S.E. Sex Differences in Neurovascular Control: Implications for Obstructive Sleep Apnea. Int. J. Mol. Sci. 2023, 24, 13094. https://doi.org/10.3390/ijms241713094
Bock JM, Greenlund IM, Somers VK, Baker SE. Sex Differences in Neurovascular Control: Implications for Obstructive Sleep Apnea. International Journal of Molecular Sciences. 2023; 24(17):13094. https://doi.org/10.3390/ijms241713094
Chicago/Turabian StyleBock, Joshua M., Ian M. Greenlund, Virend K. Somers, and Sarah E. Baker. 2023. "Sex Differences in Neurovascular Control: Implications for Obstructive Sleep Apnea" International Journal of Molecular Sciences 24, no. 17: 13094. https://doi.org/10.3390/ijms241713094





