Antioxidant, Pro-Survival and Pro-Regenerative Effects of Conditioned Medium from Wharton’s Jelly Mesenchymal Stem Cells on Developing Zebrafish Embryos
Abstract
:1. Introduction
2. Results
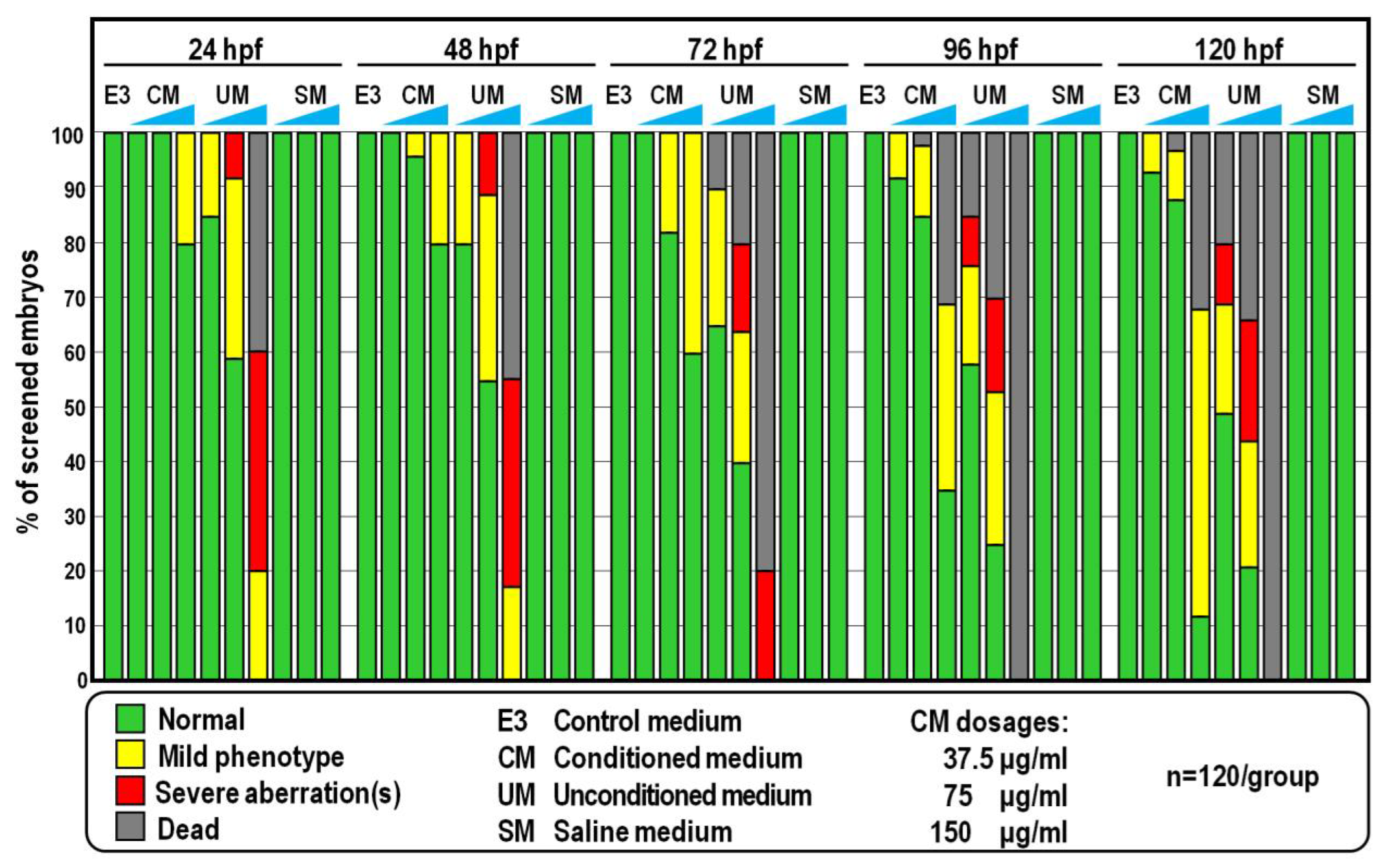
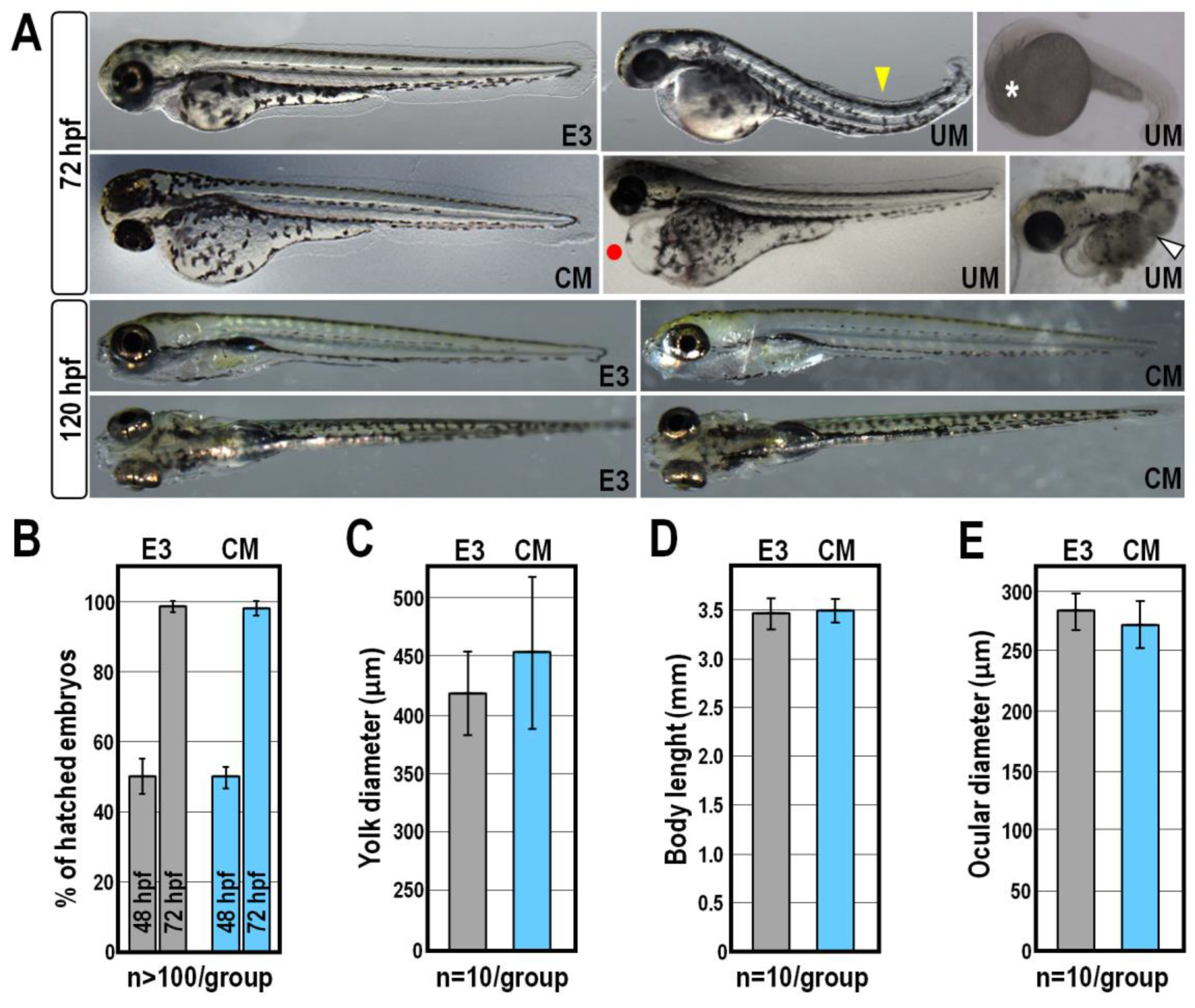
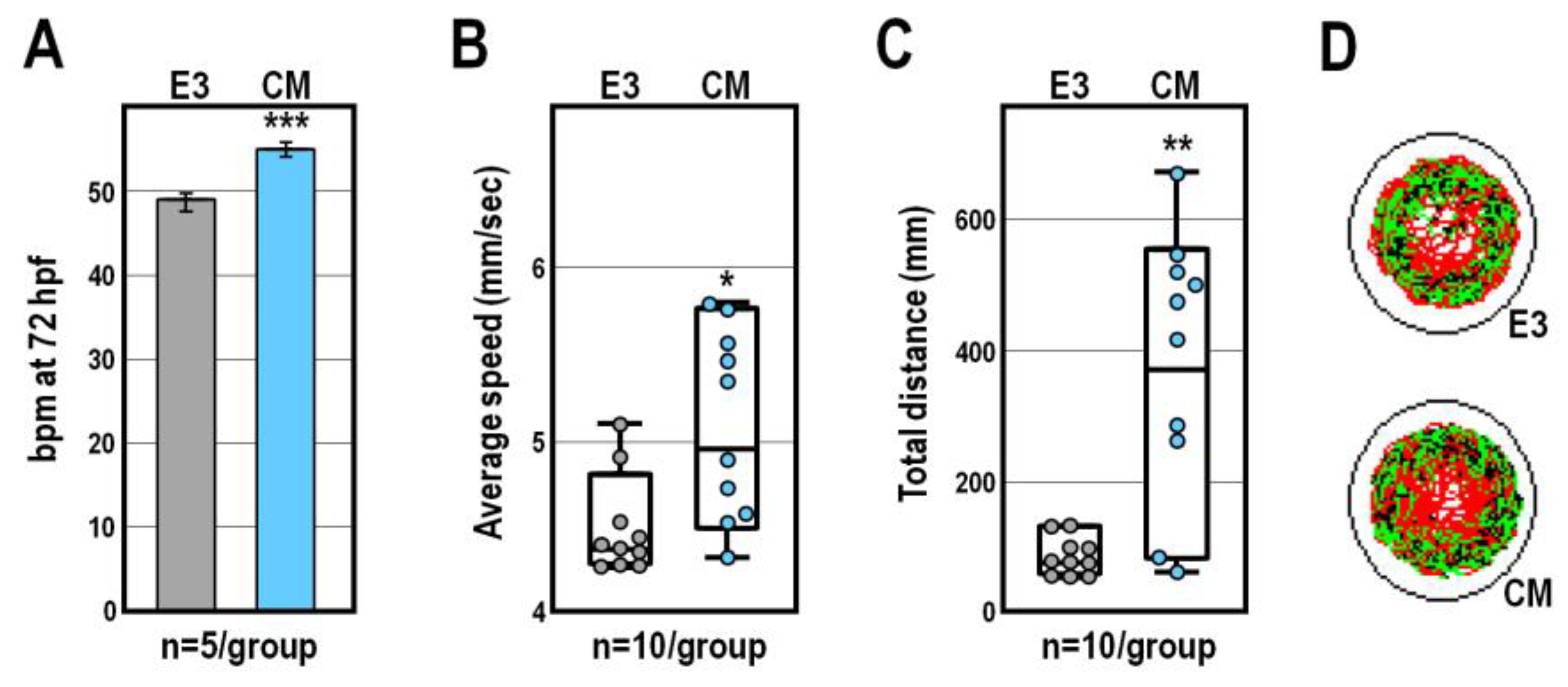
2.1. Morphological and Behavioural Effects of CM Exposure
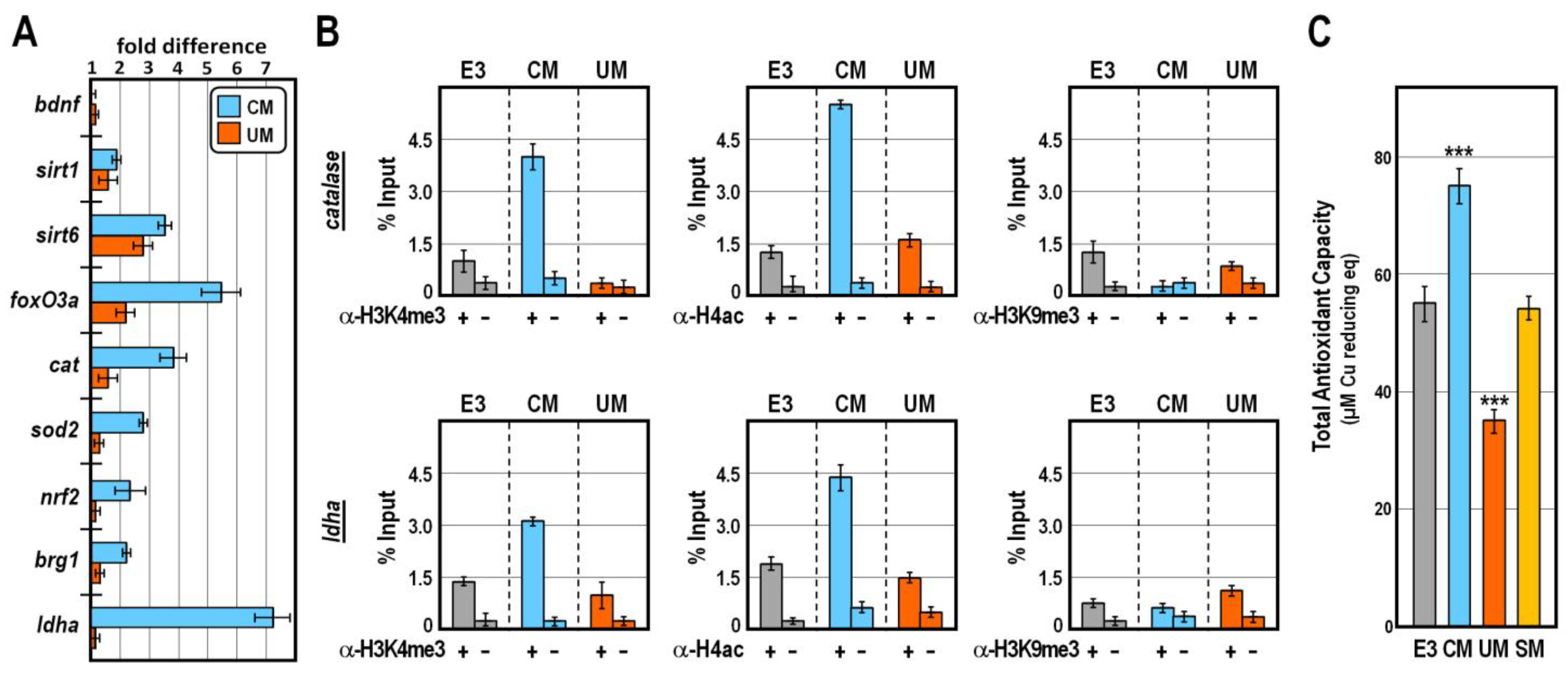
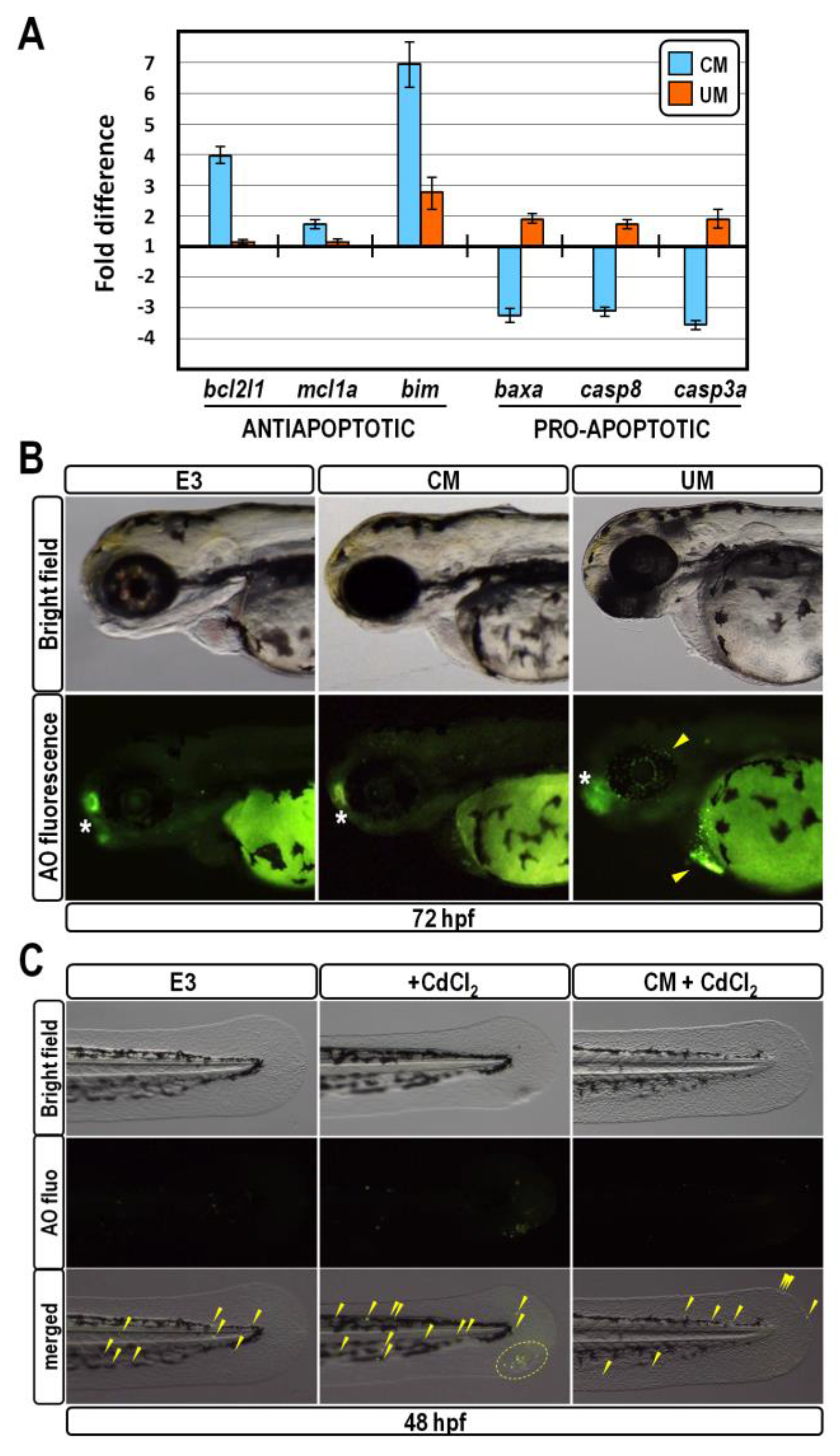
2.2. CM Exposure Impacts on the Antioxidant Gene Network
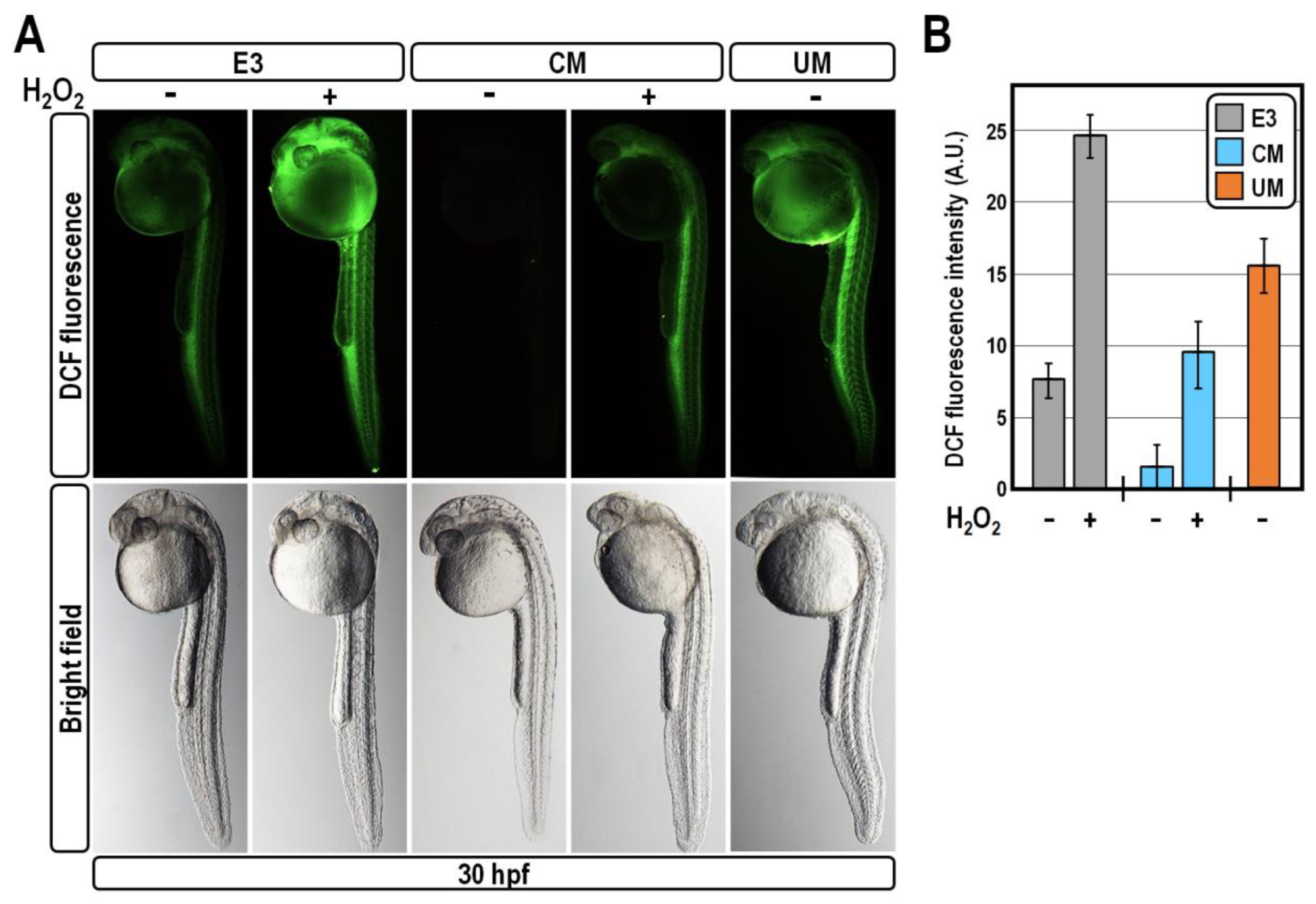
2.3. CM Exposure Establishes Antioxidant Defence by Reducing ROS Level
2.4. CM Exposure Promotes Pro-Survival Effect in Developing Zebrafish
2.5. Stimulatory Effect of CM Exposure on Caudal Fin Regeneration
3. Discussion
4. Materials and Methods
4.1. Preparation of Conditioned Medium
4.2. Zebrafish Maintenance and Breeding
4.3. Zebrafish Embryo Exposure to CM and Evaluation of Phenotype
4.4. Heart Rate Detection and Locomotor Activity Assay
4.5. RNA Extraction, Reverse Transcription and Real-Time Quantitative PCR
4.6. Chromatin Immunoprecipitation (ChIP) Assay
4.7. Determination of Total Antioxidant Capacity and ROS Production
4.8. Acridine Orange Staining
4.9. Caudal Fin Regeneration Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Halabian, R.; Imani Fooladi, A.A. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Atluri, S.; Manchikanti, L.; Hirsch, J.A. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain. Physician 2020, 23, E71–E83. [Google Scholar] [PubMed]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jin, S.Y.; Song, J.S.; Seo, K.K.; Cho, K.H. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann. Dermatol. 2012, 24, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Chudickova, M.; Vackova, I.; Machova Urdzikova, L.; Jancova, P.; Kekulova, K.; Rehorova, M.; Turnovcova, K.; Jendelova, P.; Kubinova, S. The Effect of Wharton Jelly-Derived Mesenchymal Stromal Cells and Their Conditioned Media in the Treatment of a Rat Spinal Cord Injury. Int. J. Mol. Sci. 2019, 20, 4516. [Google Scholar] [CrossRef]
- Joseph, A.; Baiju, I.; Bhat, I.A.; Pandey, S.; Bharti, M.; Verma, M.; Pratap Singh, A.; Ansari, M.M.; Chandra, V.; Saikumar, G.; et al. Mesenchymal stem cell-conditioned media: A novel alternative of stem cell therapy for quality wound healing. J. Cell Physiol. 2020, 235, 5555–5569. [Google Scholar] [CrossRef]
- Gunawardena, T.N.A.; Rahman, M.T.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019, 13, 569–586. [Google Scholar] [CrossRef]
- Zhao, Q.; Han, Z.; Wang, J.; Han, Z. Development and investigational new drug application of mesenchymal stem/stromal cells products in China. Stem Cells Transl. Med. 2021, 10 (Suppl. 2), S18–S30. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, V.; Spinelli, G. Environmental epigenetics in zebrafish. Epigenetics Chromatin 2017, 10, 46. [Google Scholar] [CrossRef]
- Cavalieri, V. Model organisms and their application in environmental epigenetics. In Environmental Epigenetics in Toxicology and Public Health; Fry, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–87. [Google Scholar]
- Gonzales, J.M., Jr. Preliminary evaluation on the effects of feeds on the growth and early reproductive performance of zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 412–417. [Google Scholar] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, A.; Nissen, R.M.; Sun, Z.; Swindell, E.C.; Farrington, S.; Hopkins, N. Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. USA 2004, 101, 12792–12797. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.; Kwon, Y.; Park, K.S.; Jeong, J.H.; Choi, S.J.; Bang, S.I.; Chang, J.W.; Lee, C. Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and Wharton’s jelly. Int. J. Mol. Sci. 2021, 22, 845. [Google Scholar] [CrossRef] [PubMed]
- Bordet, S.; Nguyen, T.M.; Knoppers, B.M.; Isasi, R. Use of umbilical cord blood for stem cell research. J. Obstet. Gynaecol. Can. 2010, 32, 58–61. [Google Scholar] [CrossRef]
- Torres-Hernández, B.A.; Colón, L.R.; Rosa-Falero, C.; Torrado, A.; Miscalichi, N.; Ortíz, J.G.; González-Sepúlveda, L.; Pérez-Ríos, N.; Suárez-Pérez, E.; Bradsher, J.N.; et al. Reversal of pentylenetetrazole-altered swimming and neural activity-regulated gene expression in zebrafish larvae by valproic acid and valerian extract. Psychopharmacology 2016, 233, 2533–2547. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Tomain, M.; D’Aniello, S.; Bertolucci, C. Bdnf loss affects activity, sociability, and anxiety-like behaviour in zebrafish. Behav. Brain Res. 2023, 436, 114115. [Google Scholar] [CrossRef]
- Nabinger, D.D.; Altenhofen, S.; Buatois, A.; Facciol, A.; Peixoto, J.V.; da Silva, J.M.K.; Chatterjee, D.; Rübensam, G.; Gerlai, R.; Bonan, C.D. Acute administration of a dopamine D2/D3 receptor agonist alters behavioral and neural parameters in adult zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 125, 110753. [Google Scholar] [CrossRef]
- Kataba, A.; Botha, T.L.; Nakayama, S.M.M.; Yohannes, Y.B.; Ikenaka, Y.; Wepener, V.; Ishizuka, M. Acute exposure to environmentally relevant lead levels induces oxidative stress and neurobehavioral alterations in larval zebrafish (Danio rerio). Aquat. Toxicol. 2020, 227, 105607. [Google Scholar] [CrossRef] [PubMed]
- Annona, G.; Tarallo, A.; Nittoli, V.; Varricchio, E.; Sordino, P.; D’Aniello, S.; Paolucci, M. Short-term exposure to the simple polyphenolic compound gallic acid induces neuronal hyperactivity in zebrafish larvae. Eur. J. Neurosci. 2021, 53, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhao, Y.; Liu, W.; Li, Z.; Souders, C.L., 2nd; Martyniuk, C.J. Butylated hydroxytoluene induces hyperactivity and alters dopamine-related gene expression in larval zebrafish (Danio rerio). Environ. Pollut. 2020, 257, 113624. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Liu, L.; Xu, W.; Chen, C.; Li, M.; Shi, Y.; Wu, X.; Chen, K.; Wang, C. Zeolitic Imidazolate Framework-8 Nanoparticles Exhibit More Severe Toxicity to the Embryo/Larvae of Zebrafish (Danio rerio) When Co-Exposed with Cetylpyridinium Chloride. Antioxidants 2022, 11, 945. [Google Scholar] [CrossRef]
- Santos, J.; Barreto, Â.; Almeida, C.; Azevedo, C.; Domingues, I.; Amorim, M.J.B.; Maria, V.L. Toxicity of boron and vanadium nanoparticles on Danio rerio embryos—Phenotypical, biochemical, and behavioral alterations. Aquat. Toxicol. 2021, 238, 105930. [Google Scholar] [CrossRef] [PubMed]
- Haridevamuthu, B.; Guru, A.; Murugan, R.; Sudhakaran, G.; Pachaiappan, R.; Almutairi, M.H.; Almutairi, B.O.; Juliet, A.; Arockiaraj, J. Neuroprotective effect of Biochanin a against Bisphenol A-induced prenatal neurotoxicity in zebrafish by modulating oxidative stress and locomotory defects. Neurosci. Lett. 2022, 790, 136889. [Google Scholar] [CrossRef]
- Cavalieri, V. Histones, Their Variants and Post-translational Modifications in Zebrafish Development. Front. Cell Dev. Biol. 2020, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Raghunath, A.; Perumal, E. Role of epigenetics in zebrafish development. Gene 2019, 718, 144049. [Google Scholar] [CrossRef]
- Cavalieri, V. The Expanding Constellation of Histone Post-Translational Modifications in the Epigenetic Landscape. Genes 2021, 12, 1596. [Google Scholar] [CrossRef]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef]
- Zhang, J.; Ohta, T.; Maruyama, A.; Hosoya, T.; Nishikawa, K.; Maher, J.M.; Shibahara, S.; Itoh, K.; Yamamoto, M. BRG1 interacts with Nrf2 to selectively mediate HO-1 induction in response to oxidative stress. Mol. Cell Biol. 2006, 26, 7942–7952. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hai, C. Novel insights into redox system and the mechanism of redox regulation. Mol. Biol. Rep. 2016, 43, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Puri, D.; Gala, H.; Mishra, R.; Dhawan, J. High-wire act: The poised genome and cellular memory. FEBS J. 2015, 282, 1675–1691. [Google Scholar] [CrossRef] [PubMed]
- Di Caro, V.; Cavalieri, V.; Melfi, R.; Spinelli, G. Constitutive promoter occupancy by the MBF-1 activator and chromatin modification of the developmental regulated sea urchin alpha-H2A histone gene. J. Mol. Biol. 2007, 365, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Kahl, R.; Kampkötter, A.; Wätjen, W.; Chovolou, Y. Antioxidant enzymes and apoptosis. Drug Metab. Rev. 2004, 36, 747–762. [Google Scholar] [CrossRef]
- Cole, L.K.; Ross, L.S. Apoptosis in the developing zebrafish embryo. Dev. Biol. 2001, 240, 123–142. [Google Scholar] [CrossRef]
- Chan, P.K.; Cheng, S.H. Cadmium-induced ectopic apoptosis in zebrafish embryos. Arch. Toxicol. 2003, 77, 69–79. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Zhao, J.; Zhang, Z.; Yang, R.; Xie, J.; Liu, X.; Qi, S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE 2014, 9, e96161. [Google Scholar] [CrossRef] [PubMed]
- Kusindarta, D.L.; Wihadmadyatami, H.; Fibrianto, Y.H.; Nugroho, W.S.; Susetya, H.; Musana, D.K.; Wijayanto, H.; Prihatna, S.A.; Wahyuni, A.E. Human umbilical mesenchymal stem cells conditioned medium promote primary wound healing regeneration. Vet. World 2016, 9, 605–610. [Google Scholar] [CrossRef]
- Im, G.B.; Kim, Y.H.; Kim, Y.J.; Kim, S.W.; Jung, E.; Jeong, G.J.; Wang, K.; Kim, J.; Kim, D.I.; Kim, T.H.; et al. Enhancing the Wound Healing Effect of Conditioned Medium Collected from Mesenchymal Stem Cells with High Passage Number Using Bioreducible Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4835. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Sano, K.; Inohaya, K.; Kawaguchi, M.; Yoshizaki, N.; Iuchi, I.; Yasumasu, S. Purification and characterization of zebrafish hatching enzyme—An evolutionary aspect of the mechanism of egg envelope digestion. FEBS J. 2008, 275, 5934–5946. [Google Scholar] [CrossRef] [PubMed]
- Trikić, M.Z.; Monk, P.; Roehl, H.; Partridge, L.J. Regulation of zebrafish hatching by tetraspanin cd63. PLoS ONE 2011, 6, e19683. [Google Scholar] [CrossRef]
- Johnson, A.; Carew, E.; Sloman, K.A. The effects of copper on the morphological and functional development of zebrafish embryos. Aquat. Toxicol. 2007, 84, 431–438. [Google Scholar] [CrossRef]
- Budick, S.A.; O’Malley, D.M. Locomotor repertoire of the larval zebrafish: Swimming, turning and prey capture. J. Exp. Biol. 2000, 203 Pt 17, 2565–2579. [Google Scholar] [CrossRef]
- Ellis, L.D.; Seibert, J.; Soanes, K.H. Distinct models of induced hyperactivity in zebrafish larvae. Brain Res. 2012, 1449, 46–59. [Google Scholar] [CrossRef]
- Cadenas, E. Basic mechanisms of antioxidant activity. Biofactors 1997, 6, 391–397. [Google Scholar] [CrossRef]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2016, 26, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Brand, K.A.; Hermfisse, U. Aerobic glycolysis by proliferating cells: A protective strategy against reactive oxygen species. FASEB J. 1997, 11, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Ying, M.; Jin, C.; Li, J.; Hu, X. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct. Target. Ther. 2021, 6, 242. [Google Scholar] [CrossRef] [PubMed]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Cho, I.T.; Kim, T.S.; Bae, G.W.; Kim, I.H.; Kim, I.Y. Effects of lactate dehydrogenase suppression and glycerol-3-phosphate dehydrogenase overexpression on cellular metabolism. Mol. Cell Biochem. 2006, 284, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Huang, L.; Liu, H.; He, W.; Lei, X.; Li, M.; Li, S.; Liang, H.; Chen, G.; Tang, J.; et al. Resveratrol Improves Follicular Development of PCOS Rats by Regulating the Glycolytic Pathway. Mol. Nutr. Food Res. 2021, 65, e2100457. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Sehring, I.M.; Weidinger, G. Recent advancements in understanding fin regeneration in zebrafish. Wiley Interdiscip. Rev. Dev. Biol. 2020, 9, e367. [Google Scholar] [CrossRef]
- He, J.; Liang, Y.; Shi, M.; Guo, B. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem. Eng. J. 2020, 385, 123464. [Google Scholar] [CrossRef]
- Hou, T.; Wang, Z.; Tang, K.; Zhang, S.; Liu, S.; Liu, J.; Fan, X.; Liu, X. The Fin-Improving Effects of Fucosylated Chondroitin Sulfate from Green and Purple Apostichopus japonicus on Caudal Fin Regeneration of Zebrafish Larvae. Aquac. Res. 2023, 2023, 9258423. [Google Scholar] [CrossRef]
- Zainol Abidin, I.Z.; Fazry, S.; Jamar, N.H.; Ediwar Dyari, H.R.; Zainal Ariffin, Z.; Johari, A.N.; Ashaari, N.S.; Johari, N.A.; Megat Abdul Wahab, R.; Zainal Ariffin, S.H. The effects of Piper sarmentosum aqueous extracts on zebrafish (Danio rerio) embryos and caudal fin tissue regeneration. Sci. Rep. 2020, 10, 14165. [Google Scholar] [CrossRef] [PubMed]
- Poleo, G.; Brown, C.W.; Laforest, L.; Akimenko, M.A. Cell proliferation and movement during early fin regeneration in zebrafish. Dev. Dyn. 2001, 221, 380–390. [Google Scholar] [CrossRef]
- Cavalieri, V.; Geraci, F.; Spinelli, G. Diversification of spatiotemporal expression and copy number variation of the echinoid hbox12/pmar1/micro1 multigene family. PLoS ONE 2017, 12, e0174404. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, V.; Guarcello, R.; Spinelli, G. Specific expression of a TRIM-containing factor in ectoderm cells affects the skeletal morphogenetic program of the sea urchin embryo. Development 2011, 138, 4279–4290. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, V.; Melfi, R.; Spinelli, G. Promoter activity of the sea urchin (Paracentrotus lividus) nucleosomal H3 and H2A and linker H1 {alpha}-histone genes is modulated by enhancer and chromatin insulator. Nucleic Acids Res. 2009, 37, 7407–7415. [Google Scholar] [CrossRef]
- Cavalieri, V.; Melfi, R.; Spinelli, G. The Compass-like locus, exclusive to the Ambulacrarians, encodes a chromatin insulator binding protein in the sea urchin embryo. PLoS Genet. 2013, 9, e1003847. [Google Scholar] [CrossRef]
- Cavalieri, V.; Spinelli, G. Ectopic hbox12 Expression Evoked by Histone Deacetylase Inhibition Disrupts Axial Specification of the Sea Urchin Embryo. PLoS ONE 2015, 10, e0143860. [Google Scholar] [CrossRef]
- Cavalieri, V.; Di Bernardo, M.; Anello, L.; Spinelli, G. cis-Regulatory sequences driving the expression of the Hbox12 homeobox-containing gene in the presumptive aboral ectoderm territory of the Paracentrotus lividus sea urchin embryo. Dev. Biol. 2008, 321, 455–469. [Google Scholar] [CrossRef]
- Lindeman, L.C.; Vogt-Kielland, L.T.; Aleström, P.; Collas, P. Fish’n ChIPs: Chromatin immunoprecipitation in the zebrafish embryo. Methods Mol. Biol. 2009, 567, 75–86. [Google Scholar] [CrossRef]
- Link, V.; Shevchenko, A.; Heisenberg, C.P. Proteomics of early zebrafish embryos. BMC Dev. Biol. 2006, 6, 1. [Google Scholar] [CrossRef]
- Covassin, L.; Amigo, J.D.; Suzuki, K.; Teplyuk, V.; Straubhaar, J.; Lawson, N.D. Global analysis of hematopoietic and vascular endothelial gene expression by tissue specific microarray profiling in zebrafish. Dev. Biol. 2006, 299, 551–562. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reina, C.; Cardella, C.; Lo Pinto, M.; Pucci, G.; Acuto, S.; Maggio, A.; Cavalieri, V. Antioxidant, Pro-Survival and Pro-Regenerative Effects of Conditioned Medium from Wharton’s Jelly Mesenchymal Stem Cells on Developing Zebrafish Embryos. Int. J. Mol. Sci. 2023, 24, 13191. https://doi.org/10.3390/ijms241713191
Reina C, Cardella C, Lo Pinto M, Pucci G, Acuto S, Maggio A, Cavalieri V. Antioxidant, Pro-Survival and Pro-Regenerative Effects of Conditioned Medium from Wharton’s Jelly Mesenchymal Stem Cells on Developing Zebrafish Embryos. International Journal of Molecular Sciences. 2023; 24(17):13191. https://doi.org/10.3390/ijms241713191
Chicago/Turabian StyleReina, Chiara, Clara Cardella, Margot Lo Pinto, Gaia Pucci, Santina Acuto, Aurelio Maggio, and Vincenzo Cavalieri. 2023. "Antioxidant, Pro-Survival and Pro-Regenerative Effects of Conditioned Medium from Wharton’s Jelly Mesenchymal Stem Cells on Developing Zebrafish Embryos" International Journal of Molecular Sciences 24, no. 17: 13191. https://doi.org/10.3390/ijms241713191
APA StyleReina, C., Cardella, C., Lo Pinto, M., Pucci, G., Acuto, S., Maggio, A., & Cavalieri, V. (2023). Antioxidant, Pro-Survival and Pro-Regenerative Effects of Conditioned Medium from Wharton’s Jelly Mesenchymal Stem Cells on Developing Zebrafish Embryos. International Journal of Molecular Sciences, 24(17), 13191. https://doi.org/10.3390/ijms241713191





