A Short Review of the Toxicity of Dentifrices—Zebrafish Model as a Useful Tool in Ecotoxicological Studies
Abstract
:1. Introduction
2. Search Strategy
3. Discussion
3.1. Fluoride
3.2. Abrasive Substances
3.3. Detergents
3.4. Antibacterial Agents
3.5. Whitening and Flavoring Agents
| Agents | Effect | Comment | Reference |
|---|---|---|---|
| Whitening agents | |||
| Antarctic lichen | General toxicity (mechanism: undetermined) | Exposure to extracts of Amandinea sp. and Umbilicaria Antarctica from 6 hpf to 120 hpf significantly reduced the survival rate in zebrafish larvae at the concentration of 200 μg/mL and higher. | [103] |
| Cetraria islandica | Inhibition of melanogenesis (mechanism: tyrosinase inhibition) | 48 h exposure (from 8 to 56 hpf) to subtoxic concentrations of extracts from Cetraria islandica (44 µg/mL) reduced pigmentation in zebrafish. | [104] |
| Letharia vulpine | Inhibition of melanogenesis (mechanism: tyrosinase inhibition) | 48 h exposure (from 8 to 56 hpf) to subtoxic concentrations of extracts from Letharia vulpine (30 µg/mL) reduced pigmentation in zebrafish. | [104] |
| Lichen metabolites | Hepatotoxicity (mechanism: undetermined) | Evernic acid (60.2 μM), vulpic acid (15.5 μM), and psoromic acid (3.6 μM) showed liver toxicity in a transgenic line of zebrafish with liver-specific expression (fabp10a:DsRed2) after 3 days of exposure from 6 dpf. | [105] |
| Flavouring agents | |||
| Menthol | General toxicity (mechanism: undetermined) | 72 h embryo exposure to menthol at 0.01 mg/mL and higher resulted in an increased mortality and malformation rate, and a decreased hatching rate. | [106] |
| Nociception (mechanism: nociceptors stimulation) | Menthol (1.2 mM) induces acute immediate orofacial nociception behavior in adult zebrafish. | [107,108,109] | |
| Haemolysis (mechanism: related to prooxidant properties) | Menthol (180–200 μmol/L) induced brisk hemolysis in zebrafish G6PD deficiency model after 48 h exposure | [110] | |
| Eucalyptus extracts | General toxicity (mechanism: related to Fe3+ presence) | Adult zebrafish exposed for 96 h to tannins (140 mg·L−1) from eucalyptus leaf leachate reached 100% cumulative mortality | [111] |
| Haemolysis (mechanism: nociceptors stimulation) | Eucalyptus oil (1:5000 in fishwater) induces hemolytic phenotype in zebrafish G6PD deficiency model after 72 h exposure | [112] | |
| Hyperlocomotion (mechanism: related to irritant properties) | Biomass smoke condensates from Eucalyptus globulus (30 μg EOM/mL) elevates locomotor activity measured in the dark in 6 dpf zebrafish larvae after 60 min of exposure | [113] | |
| Cinnamon extracts | General toxicity, Morphological abnormalities (mechanism: undetermined) Inhibition of angiogenesis (mechanism: linked with PKC-dependent phosphorylation of MAPK) | Exposure to cinnamon extracts from Cinnamon zeylanicum exhibited LC50 of 0.0508 mg/mL and caused gross morphological deformities (especially of the spine, tail, cartilage, heart, and jaw), abnormal heartbeat, and delayed hatching rate. Moreover, cinnamon extract concentration of 250 μg/mL inhibited angiogenesis after 16 h exposure from 6–8 hpf. | [114,115] |
| Cinnamaldehyde | Morphological abnormalities, Vascular malformations and cardiotoxicity, Decreased hatching rate (mechanism: undetermined) | Pure cinnamaldehyde induced toxicity in 3–4 dpf zebrafish (line Tg(Fli1:EGFP)) after exposure at around 6 hpf with a 50% effect concentration (EC50) of 34–41 µM. | [116] |
| General toxicity, Neurotoxicity, Hypolocomotion (mechanism: neurotoxicity associated with increased oxidative stress) | The LD50 of Cinnamaldehyde was determined to be 8.362 mg/L in larval zebrafish exposed from 0 to 120 hpf. | [117] | |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Cvikl, B.; Lussi, A.; Gruber, R. The in vitro impact of toothpaste extracts on cell viability. Eur. J. Oral Sci. 2015, 123, 179–185. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Mitsui, T. New Cosmetic Science; Elsevier: Amsterdam, The Netherlands; Lausanne, Switzerland; New York, NY, USA; Oxford, UK; Shannon, Ireland; Tokyo, Japan, 1997; pp. 480–490. [Google Scholar]
- Menendez, A.; Li, F.; Michalek, S.M.; Kirk, K.; Makhija, S.K.; Childers, N.K. Comparative analysis of the antibacterial effects of combined mouthrinses on Streptococcus mutans. Oral Microbiol. Immunol. 2005, 20, 31–34. [Google Scholar] [CrossRef]
- Healy, C.M.; Cruchley, A.T.; Thornhill, M.H.; Williams, D.M. The effect of sodium lauryl sulphate, triclosan and zinc on the permeability of normal oral mucosa. Oral Dis. 2000, 6, 118–123. [Google Scholar] [CrossRef]
- Khamverdi, Z.; Kasraie Sh Rezaei-Soufi, L.; Jebeli, S. Comparison of the effects of two whitening toothpastes on microhardness of the enamel and a microhybride composite resin: An in vitro study. J. Dent. 2010, 7, 139–145. [Google Scholar]
- Duffin, S.; Duffin, M.; Grootveld, M. Revisiting Fluoride in the Twenty-First Century: Safety and Efficacy Considerations. Front. Oral Health 2022, 3, 873157. [Google Scholar] [CrossRef]
- Yueh, M.F.; Tukey, R.H. Triclosan: A Widespread Environmental Toxicant with Many Biological Effects. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 251–272. [Google Scholar] [CrossRef]
- Sarmah, S.; Marrs, J.A. Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Functio. Int. J. Mol. Sci. 2016, 17, 2123. [Google Scholar] [CrossRef]
- Van der Heyden, C.; Huysseune, A. Dynamics of tooth formation and replacement in the zebrafish (Danio rerio) (Teleostei, Cyprinidae). Dev. Dyn. 2000, 219, 486–496. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Vita, N.A.; Brohem, C.A.; Canavez, A.D.P.M.; Oliveira, C.F.S.; Kruger, O.; Lorencini, M.; Carvalho, C.M. Parameters for assessing the aquatic environmental impact of cosmetic products. Toxicol. Lett. 2018, 287, 70–82. [Google Scholar] [CrossRef]
- Toumba, K.J.; Twetman, S.; Splieth, C.; Parnell, C.; van Loveren, C.; Lygidakis, N.A. Guidelines on the use of fluoride for caries prevention in children: An updated EAPD policy document. Eur. Arch. Paediatr. Dent. 2019, 20, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Sánchez, M.; Llama-Palacios, A.; Sanz, M.; Herrera, D. Antibacterial Effects of Toothpastes Evaluated in an In vitro Biofilm Model. Oral Health Prev. Dent. 2017, 15, 251–257. [Google Scholar] [CrossRef]
- Mason, S.; Young, S.; Qaqish, J.; Frappin, G.; Goyal, C. Stain control with two modified stannous fluoride/sodium tripolyphosphate toothpastes: A randomised controlled proof of concept study. J. Dent. 2019, 91, 100009. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cao, J.; Zhao, Y.; Wu, P.; Li, X.; Khodaei, F.; Han, Y.; Wang, J. Fluoride impairs ovary development by affecting oogenesis and inducing oxidative stress and apoptosis in female zebrafish (Danio rerio). Chemosphere 2020, 256, 127105. [Google Scholar] [CrossRef]
- Li, M.; Cao, J.; Chen, J.; Song, J.; Zhou, B.; Feng, C.; Wang, J. Waterborne fluoride exposure changed the structure and the expressions of steroidogenic-related genes in gonads of adult zebrafish (Danio rerio). Chemosphere 2016, 145, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xue, W.; Cao, J.; Song, J.; Jia, R.; Li, M. Fluoride caused thyroid endocrine disruption in male zebrafish (Danio rerio). Aquat. Toxicol. 2016, 171, 48–58. [Google Scholar] [CrossRef]
- Mondal, P.; Shaw, P.; Bandyopadhyay, A.; Dey Bhowmik, A.; Chakraborty, A.; Sudarshan, M.; Chattopadhyay, A. Mixture effect of arsenic and fluoride at environmentally relevant concentrations in zebrafish (Danio rerio) liver: Expression pattern of Nrf2 and related xenobiotic metabolizing enzymes. Aquat. Toxicol. 2019, 213, 105219. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Chattopadhyay, A. Induction of oxidative stress and related transcriptional effects of sodium fluoride in female zebrafish liver. Bull. Environ. Contam. Toxicol. 2014, 93, 64–70. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Priya, P.; Chattopadhyay, A. Sodium fluoride affects zebrafish behaviour and alters mRNA expressions of biomarker genes in the brain: Role of Nrf2/Keap1. Environ. Toxicol. Pharmacol. 2015, 40, 352–359. [Google Scholar] [CrossRef]
- Wolf, H.F.; Hassell, T.M. Color Atlas of Dental Hygiene—Periodontology; Thieme Publishing Group: Stuttgart, Germany, 2006. [Google Scholar]
- Kasiak, M.; Kasiak, M. Toothpastes–composition and effects. Farm. Pol. 2009, 65, 665–672. [Google Scholar]
- Matthews-Brzozowska, T.; Surdacka, A.; Jóźwiak, K. Ocena mikroskopowa drobin surowców ściernych niektórych past do zębów. Czas Stomatol. 1991, 6, 416–418. [Google Scholar]
- ISO 11609; 2017 Dentistry—Dentifrices—Requirements, Test Methods and Marking. 2017-06, 3, 22. ISO: Geneva, Switzerland, 2017.
- Wülknitz, P. Cleaning power and abrasivity of European toothpastes. Adv. Dent. Res. 1997, 11, 576–579. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, M.; Liendo, F.; Deorsola, F.A.; Bensaid, S.; Giordani, S. Toxicological profile of calcium carbonate nanoparticles for industrial applications. Colloids Surf. B Biointerfaces 2020, 190, 110947. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.T.; Bufalino, M.R.; Hartlen, K.D.; Kitaev, V.; Lee, L.E. Cytotoxicity evaluation of silica nanoparticles using fish cell lines. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Kotil, T.; Akbulut, C.; Yön, N.D. The effects of titanium dioxide nanoparticles on ultrastructure of zebrafish testis (Danio rerio). Micron 2017, 100, 38–44. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Shi, H.; Tian, L.; Guo, C.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 2013, 8, e74606. [Google Scholar] [CrossRef]
- Duan, J.; Hu, H.; Li, Q.; Jiang, L.; Zou, Y.; Wang, Y.; Sun, Z. Combined toxicity of silica nanoparticles and methylmercury on cardiovascular system in zebrafish (Danio rerio) embryos. Environ. Toxicol. Pharmacol. 2016, 44, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liang, S.; Yu, Y.; Li, Y.; Wang, L.; Wu, Z.; Chen, Y.; Miller, M.R.; Sun, Z. Inflammation-coagulation response and thrombotic effects induced by silica nanoparticles in zebrafish embryos. Nanotoxicology 2018, 12, 470–484. [Google Scholar] [CrossRef]
- Dumitrescu, E.; Karunaratne, D.P.; Prochaska, M.K.; Liu, X.; Wallace, K.N.; Andreescu, S. Developmental toxicity of glycine-coated silica nanoparticles in embryonic zebrafish. Environ. Pollut. 2017, 229, 439–447. [Google Scholar] [CrossRef]
- Zhu, B.; He, W.; Hu, S.; Kong, R.; Yang, L. The fate and oxidative stress of different sized SiO2 nanoparticles in zebrafish (Danio rerio) larvae. Chemosphere 2019, 225, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.; Verma, S.K.; Panda, P.K.; Jha, E.; Das, B.; Mukherjee, K.; Suar, M. In Vivo Molecular Toxicity Profile of Dental Bioceramics in Embryonic Zebrafish (Danio rerio). Chem. Res. Toxicol. 2018, 31, 914–923. [Google Scholar] [CrossRef]
- Pham, D.H.; De Roo, B.; Nguyen, X.B.; Vervaele, M.; Kecskés, A.; Ny, A.; Copmans, D.; Vriens, H.; Locquet, J.P.; Hoet, P.; et al. Use of Zebrafish Larvae as a Multi-Endpoint Platform to Characterize the Toxicity Profile of Silica Nanoparticles. Sci. Rep. 2016, 6, 37145. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, X.; Sun, M.; Wang, Y.; Wu, M.; Zhang, C.; Wang, Y.; Liu, B.; Zhang, Y.; Zhao, X.; et al. An assessment of the impact of SiO2 nanoparticles of different sizes on the rest/wake behavior and the developmental profile of zebrafish larvae. Small 2013, 9, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Addy, M.; Moran, J. Toothpaste detergents: A potential source of oral soft tissue damage? Int. J. Dent. Hyg. 2008, 6, 193–198. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, X.; Sun, M.; Wei, Z.; Wang, Y.; Gao, A.; Chen, D.; Zhao, X.; Feng, X. Exploring the Effects of Different Types of Surfactants on Zebrafish Embryos and Larvae. Sci. Rep. 2015, 5, 10107. [Google Scholar] [CrossRef]
- Kharaeva, Z.F.; Mustafaev, M.S.; Khazhmetov, A.V.; Gazaev, I.H.; Blieva, L.Z.; Steiner, L.; Mayer, W.; Luca, C.; Korkina, L.G. Anti-Bacterial and Anti-Inflammatory Effects of Toothpaste with Swiss Medicinal Herbs towards Patients Suffering from Gingivitis and Initial Stage of Periodontitis: From Clinical Efficacy to Mechanisms. Dent. J. 2020, 8, 10. [Google Scholar] [CrossRef]
- Nonnenmacher, C.; Dalpke, A.; Mutters, R.; Heeg, K. Quantitative detection of periodontopathogens by real-time PCR. J. Microbiol. Methods 2004, 59, 117–125. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.K.; Cho, J.Y.; Lee, J.M.; Hong, S.H. Quantification of subgingival bacterial pathogens at different stages of periodontal diseases. Curr. Microbiol. 2012, 65, 22–27. [Google Scholar] [CrossRef]
- Dosseva-Panova, V.T.; Popova, C.L.; Panov, V.E. Subgingival microbial profile and production of pro inflammatory cytokines in chronic periodontitis. Folia Med. 2014, 56, 152–160. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Kharaeva, Z.; Korkina, L. Is there a role for antioxidants in the prevention of infection-associated carcinogenesis and in the treatment of infection-driven tumours? Curr. Top. Med. Chem. 2015, 15, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Painter, K.L.; Strange, E.; Parkhill, J.; Bamford, K.B.; Armstrong-James, D.; Edwards, A.M. Staphylococcus aureus adapts to oxidative stress by producing H2O2-resistant small -colony variants via the SOS response. Infect. Immun. 2015, 83, 1830–1844. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, L.M.; Cury, J.A. Fluoride: Its role in dentistry. Braz. Oral Res. 2010, 24, 9–17. [Google Scholar] [CrossRef]
- Randall, J.P.; Seow, W.K.; Walsh, L.J. Antibacterial activity of fluoride compounds and herbal toothpastes on Streptococcus mutants: An in vitro study. Aust. Dent. J. 2015, 60, 368–374. [Google Scholar] [CrossRef]
- American Academy of Pediatric. Dentistry Guideline on xylitol use in caries prevention. Pediatr. Dent. 2011, 33, 157–160. [Google Scholar]
- Chi, D.L.; Tut, O.; Milgrom, P. Cluster-randomized xylitol toothpaste trial for early childhood caries prevention. J. Dent. Child. 2014, 81, 27–32. [Google Scholar]
- Maden, E.A.; Allun, C.; Ozmen, B.; Bazak, P. Antimicrobial effect of toothpaste containing fluoride, xylitol, or xylitol-probiotic on salivary Streptococcus metans and Lactobacillus in children. Niger. J. Clin. Pract. 2018, 21, 134–138. [Google Scholar]
- Adolfsson-Erici, M.; Petterson, M.; Parkkonen, J.; Sturve, J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 2002, 46, 1485–1489. [Google Scholar] [CrossRef]
- Glaser, A. The ubiquitous triclosan, a common antibacterial agent exposed. Pestic. You 2004, 24, 12–17. [Google Scholar]
- Aranami, K.; Readman, J.W. Photolytic degradation of triclosan in freshwater and seawater. Chemosphere 2007, 66, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Dirtu, A.C.; Roosens, L.; Geens, T.; Gheorge, A.; Neels, H.; Covaci, A. Simultaneous determination of bisphenol A, triclosan, and tetrabromobisphenol A in human serum using solid-phase extraction and gas chromatography-electron capture negative-ionization mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Roosens, L.; Neels, H.; Covaci, A. Assessment of human exposure to bisphenol-A, triclosan and tetrabromobisphenol-A through indoor dust intake in Belgium. Chemosphere 2009, 76, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Falisse, E.; Voisin, A.S.; Silvestre, F. Impacts of triclosan exposure on zebrafish early-life stage: Toxicity and acclimation mechanisms. Aquat. Toxicol. 2017, 189, 97–107. [Google Scholar] [CrossRef]
- Ho, J.C.H.; Hsiao, C.D.; Kawakami, K.; Tse, W.K.F. Triclosan (TCS) exposure impairs lipid metabolism in zebrafish embryos. Aquat. Toxicol. 2016, 173, 29–35. [Google Scholar] [CrossRef]
- Oliveira, R.; Domingues, I.; Koppe Grisolia, C.; Soares, A.M. Effects of triclosan on zebrafish early-life stages and adults. Environ. Sci. Pollut. Res. Int. 2009, 16, 679–688. [Google Scholar] [CrossRef]
- Wirt, H.; Botka, R.; Perez, K.E.; King-Heiden, T. Embryonic exposure to environmentally relevant concentrations of triclosan impairs foraging efficiency in zebrafish larvae. Environ. Toxicol. Chem. 2018, 37, 3124–3133. [Google Scholar] [CrossRef]
- Tang, N.; Fan, P.; Chen, L.; Yu, X.; Wang, W.; Wang, W.; Ouyang, F. The Effect of Early Life Exposure to Triclosan on Thyroid Follicles and Hormone Levels in Zebrafish. Front. Endocrinol. 2022, 13, 850231. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Wang, X.; Qian, Q.; Wang, H. Study on the toxic-mechanism of triclosan chronic exposure to zebrafish (Danio rerio) based on gut-brain axis. Sci. Total Environ. 2022, 844, 156936. [Google Scholar] [CrossRef]
- Pullaguri, N.; Nema, S.; Bhargava, Y.; Bhargava, A. Triclosan alters adult zebrafish behavior and targets acetylcholinesterase activity and expression. Environ. Toxicol. Pharmacol. 2020, 75, 103311. [Google Scholar] [CrossRef]
- Pullaguri, N.; Grover, P.; Abhishek, S.; Rajakumara, E.; Bhargava, Y.; Bhargava, A. Triclosan affects motor function in zebrafish larva by inhibiting ache and syn2a genes. Chemosphere 2021, 266, 128930. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mou, L.; Qu, J.; Wu, L.; Liu, C. Adverse effects of triclosan exposure on health and potential molecular mechanisms. Sci. Total Environ. 2023, 879, 163068. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Lee, M.H. Triclosan: An Update on Biochemical and Molecular Mechanisms. Oxidative Med. Cell. Longev. 2019, 2019, 1607304. [Google Scholar] [CrossRef]
- Iannetta, A.; Caioni, G.; Di Vito, V.; Benedetti, E.; Perugini, M.; Merola, C. Developmental toxicity induced by triclosan exposure in zebrafish embryos. Birth Defects Res. 2022, 114, 175–183. [Google Scholar] [CrossRef]
- Kim, J.; Oh, H.; Ryu, B.; Kim, U.; Lee, J.M.; Jung, C.R.; Kim, C.Y.; Park, J.H. Triclosan affects axon formation in the neural development stages of zebrafish embryos (Danio rerio). Environ. Pollut. 2018, 236, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Li, J.; Dahlgren, R.A.; Wang, X.; Huang, H.; Wang, H. Risk assessment of cardiotoxicity to zebrafish (Danio rerio) by environmental exposure to triclosan and its derivatives. Environ. Pollut. 2020, 265 Pt A, 114995. [Google Scholar] [CrossRef]
- Dar, O.I.; Aslam, R.; Sharma, S.; Jia, A.Q.; Kaur, A.; Faggio, C. Biomolecular alterations in the early life stages of four food fish following acute exposure of Triclosan. Environ. Toxicol. Pharmacol. 2022, 91, 103820. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, F.; Liu, F. Effects of triclosan on antioxidant- and apoptosis-related genes expression in the gill and ovary of zebrafish. Exp. Anim. 2020, 69, 199–206. [Google Scholar] [CrossRef]
- Stenzel, A.; Wirt, H.; Patten, A.; Theodore, B.; King-Heiden, T. Larval exposure to environmentally relevant concentrations of triclosan impairs metamorphosis and reproductive fitness in zebrafish. Reprod. Toxicol. 2019, 87, 79–86. [Google Scholar] [CrossRef]
- Wang, F.; Liu, F.; Chen, W. Exposure to triclosan changes the expression of microRNA in male juvenile zebrafish (Danio rerio). Chemosphere. 2019, 214, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, B.; Han, X.; Mao, Z.; Chen, M.; Du, G.; Talbot, P.; Wang, X.; Xia, Y. The effects of triclosan on pluripotency factors and development of mouse embryonic stem cells and zebrafish. Arch. Toxicol. 2015, 89, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, R.; Huang, H.; Lu, C.; Xia, Y.; Wang, X.; Du, G. Exploration of the developmental toxicity of TCS and PFOS to zebrafish embryos by whole-genome gene expression analyses. Environ. Sci. Pollut. Res. Int. 2021, 28, 56032–56042. [Google Scholar] [CrossRef] [PubMed]
- Saley, A.; Hess, M.; Miller, K.; Howard, D.; King-Heiden, T.C. Cardiac toxicity of triclosan in developing zebrafish. Zebrafish 2016, 13, 399–404. [Google Scholar] [CrossRef]
- Haggard, D.E.; Noyes, P.D.; Waters, K.M.; Tanguay, R.L. Phenotypically anchored transcriptome profiling of developmental exposure to the antimicrobial agent, triclosan, reveals hepatotoxicity in embryonic zebrafish. Toxicol. Appl. Pharmacol. 2016, 308, 32–45. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, H.; Yu, H. Triclosan affects Ca2+ regulatory module and musculature development in skeletal myocyte during early life stages of Zebrafish (Danio rerio). Environ. Sci. Technol. 2019, 53, 11988–11998. [Google Scholar] [CrossRef]
- Fu, J.; Tan, Y.X.R.; Gong, Z.; Bae, S. The toxic effect of triclosan and methyl-triclosan on biological pathways revealed by metabolomics and gene expression in zebrafish embryos. Ecotoxicol. Environ. Saf. 2020, 189, 110039. [Google Scholar] [CrossRef]
- Gyimah, E.; Dong, X.; Qiu, W.; Zhang, Z.; Xu, H. Sublethal concentrations of triclosan elicited oxidative stress, DNA damage, and histological alterations in the liver and brain of adult zebrafish. Environ. Sci. Pollut. Res. Int. 2020, 27, 17329–17338. [Google Scholar] [CrossRef]
- Parenti, C.C.; Ghilardi, A.; Della Torre, C.; Mandelli, M.; Magni, S.; Del Giacco, L.; Binelli, A. Environmental concentrations of triclosan activate cellular defence mechanism and generate cytotoxicity on zebrafish (Danio rerio) embryos. Sci. Total Environ. 2019, 650, 1752–1758. [Google Scholar] [CrossRef]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef]
- Jesus, F.T.; Oliveira, R.; Silva, A.; Catarino, A.L.; Soares, A.M.; Nogueira, A.J.; Domingues, I. Lethal and sub lethal effects of the biocide chlorhexidine on aquatic organisms. Ecotoxicology 2013, 22, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Kalliath, C.; Mukunda, A.; Pynadath, M.; Venugopal, V.; Prethweeraj, J. Comparison between the effect of commercially available chemical teeth whitening paste and teeth whitening paste containing ingredients of herbal origin on human enamel. Ayu 2018, 39, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Vranić, E.; Lacević, A.; Mehmedagić, A.; Uzunović, A. Formulation ingredients for toothpastes and mouthwashes. Bosn. J. Basic. Med. Sci. 2004, 4, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kroona, L.; Warfvinge, G.; Isaksson, M.; Ahlgren, C.; Dahlin, J.; Sörensen, Ö.; Bruze, M. Quantification of l-carvone in toothpastes available on the Swedish market. Contact Dermat. 2017, 77, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Carmichael, C.; Varga, Z.M.; Tiersch, T.R. Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio. Theriogenology 2007, 68, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Kouya, T.; Tsuchiya, R.; Valdez DMJr Jin, B.; Koshimoto, C.; Kasai, M.; Edashige, K. Cryobiological properties of immature zebrafish oocytes assessed by their ability to be fertilized and develop into hatching embryos. Cryobiology 2011, 62, 8–14. [Google Scholar] [CrossRef]
- Lahnsteiner, F. The effect of internal and external cryoprotectants on zebrafish (Danio rerio) embryos. Theriogenology 2008, 69, 384–396. [Google Scholar] [CrossRef]
- Maes, J.; Verlooy, L.; Buenafe, O.E.; de Witte, P.A.; Esguerra, C.V.; Crawford, A.D. Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae. PLoS ONE 2012, 7, e43850. [Google Scholar] [CrossRef]
- Audira, G.; Siregar, P.; Chen, J.R.; Lai, Y.H.; Huang, J.C.; Hsiao, C.D. Systematical exploration of the common solvent toxicity at whole organism level by behavioral phenomics in adult zebrafish. Environ. Pollut. 2020, 266 Pt 1, 115239. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, H.C.; Kim, J.; You, K.; Cho, Y.; Kim, S. Toxicity impact of hydrogen peroxide on the fate of zebrafish and antibiotic resistant bacteria. J. Environ. Manag. 2022, 302 Pt B, 114072. [Google Scholar] [CrossRef]
- Dumitrescu, E.; Karunaratne, D.P.; Babu, S.V.; Wallace, K.N.; Andreescu, S. Interaction, transformation and toxicity assessment of particles and additives used in the semiconducting industry. Chemosphere 2018, 192, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, S.; Xiong, Z. Protective effect of polysaccharide from Ligusticum chuanxiong hort against H2O2-induced toxicity in zebrafish embryo. Carbohydr. Polym. 2019, 221, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Lite, C.; Freddy, A.J.; Issac, P.K.; Pasupuleti, M.; Saraswathi, N.T.; Arasu, M.V.; Al-Dhabi, N.A.; Arshad, A.; Arockiaraj, J. Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Dev. Comp. Immunol. 2021, 114, 103863. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Muraki, K.; Fuse, Y.; Kobayashi, M. Evaluation of Antioxidant Activity of Spice-Derived Phytochemicals Using Zebrafish. Int. J. Mol. Sci. 2020, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Fiskus, W.; Coothankandaswamy, V.; Chen, J.; Ma, H.; Ha, K.; Saenz, D.T.; Krieger, S.S.; Mill, C.P.; Sun, B.; Huang, P.; et al. SIRT2 Deacetylates and Inhibits the Peroxidase Activity of Peroxiredoxin-1 to Sensitize Breast Cancer Cells to Oxidant Stress-Inducing Agents. Cancer Res. 2016, 76, 5467–5478. [Google Scholar] [CrossRef]
- Han, E.J.; Um, J.H.; Kim, E.A.; Lee, W.; Kang, N.; Oh, J.Y.; Park, S.Y.; Jeon, Y.J.; Ahn, C.B.; Lee, S.H.; et al. Protective Effects of An Water Extracts Prepared from Loliolus beka Gray Meat Against H2O2-Induced Oxidative Stress in Chang Liver Cells and Zebrafish Embryo Model. Adv. Exp. Med. Biol. 2017, 975 Pt 1, 585–601. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, E.A.; Kim, Y.S.; Yu, S.K.; Choi, C.; Lee, J.S.; Kim, Y.T.; Nah, J.W.; Jeon, Y.J. Protective effects of polysaccharides from Psidium guajava leaves against oxidative stresses. Int. J. Biol. Macromol. 2016, 91, 804–811. [Google Scholar] [CrossRef]
- Paravani, E.V.; Simoniello, M.F.; Poletta, G.L.; Zolessi, F.R.; Casco, V.H. Cypermethrin: Oxidative stress and genotoxicity in retinal cells of the adult zebrafish. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 826, 25–32. [Google Scholar] [CrossRef]
- Paravani, E.V.; Simoniello, M.F.; Poletta, G.L.; Casco, V.H. Cypermethrin induction of DNA damage and oxidative stress in zebrafish gill cells. Ecotoxicol. Environ. Saf. 2019, 173, 1–7. [Google Scholar] [CrossRef]
- Kim, J.E.; Min, S.K.; Hong, J.M.; Kim, K.H.; Han, S.J.; Yim, J.H.; Park, H.; Kim, I.C. Anti-inflammatory effects of methanol extracts from the Antarctic lichen, Amandinea sp. in LPS-stimulated raw 264.7 macrophages and zebrafish. Fish. Shellfish. Immunol. 2020, 107 Pt A, 301–308. [Google Scholar] [CrossRef]
- Malaspina, P.; Catellani, E.; Burlando, B.; Brignole, D.; Cornara, L.; Bazzicalupo, M.; Candiani, S.; Obino, V.; De Feo, V.; Caputo, L.; et al. Depigmenting potential of lichen extracts evaluated by in vitro and in vivo tests. PeerJ 2020, 8, e9150. [Google Scholar] [CrossRef] [PubMed]
- Lauinger, I.L.; Vivas, L.; Perozzo, R.; Stairiker, C.; Tarun, A.; Zloh, M.; Zhang, X.; Xu, H.; Tonge, P.J.; Franzblau, S.G.; et al. Potential of lichen secondary metabolites against Plasmodium liver stage parasites with FAS-II as the potential target. J. Nat. Prod. 2013, 76, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.Y.; Hu, Y.; Zhang, B.J. Safety evaluation of the temporary consolidant based on a zebrafish embryo model. Toxicol. Vitr. 2018, 51, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Rocha Barreto, R.; Lima Veras, P.J.; de Oliveira Leite, G.; Vieira-Neto, A.E.; Sessle, B.J.; Villaça Zogheib, L.; Rolim Campos, A. Botulinum toxin promotes orofacial antinociception by modulating TRPV1 and NMDA receptors in adult zebrafish. Toxicon Off. J. Int. Soc. Toxinology 2022, 210, 158–166. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Leite, G.; Santos, S.A.A.R.; Bezerra, F.M.D.H.; Sena ESilva, F.E.; de Castro Ribeiro, A.D.; Roma, R.R.; Silva, R.R.S.; Santos, M.H.C.; Santos, A.L.E.; Teixeira, C.S.; et al. Is the orofacial antinociceptive effect of lectins intrinsically related to their specificity to monosaccharides? Int. J. Biol. Macromol. 2020, 161, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.C.R.; Santos, S.A.A.R.; Coelho, R.F.; Alves, Y.A.; Vieira-Neto, A.E.; Tavares, K.C.S.; Magalhaes, F.E.A.; Campos, A.R. Oleanolic acid promotes orofacial antinociception in adult zebrafish (Danio rerio) through TRPV1 receptors. Chem. Biol. Interact. 2019, 299, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Patrinostro, X.; Carter, M.L.; Kramer, A.C.; Lund, T.C. A model of glucose-6-phosphate dehydrogenase deficiency in the zebrafish. Exp. Hematol. 2013, 41, 697–710.e2. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, M.; Deng, Y.; Li, J.; Li, J.; Pang, W.; Xie, L.; Jiang, D.; Huang, Z.; He, T.; et al. Acute toxicity of eucalyptus leachate tannins to zebrafish and the mitigation effect of Fe3+ on tannin toxicity. Ecotoxicol. Environ. Saf. 2022, 229, 113077. [Google Scholar] [CrossRef]
- Arogbokun, O.; Shevik, M.; Slusher, T.; Farouk, Z.; Elfstrum, A.; Weber, J.; Cusick, S.E.; Lund, T. Traditional African remedies induce hemolysis in a glucose-6-phopshate dehydrogenase deficient zebrafish model. Sci. Rep. 2020, 10, 19172. [Google Scholar] [CrossRef]
- Martin, W.K.; Padilla, S.; Kim, Y.H.; Hunter, D.L.; Hays, M.D.; DeMarini, D.M.; Hazari, M.S.; Gilmour, M.I.; Farraj, A.K. Zebrafish irritant responses to wildland fire-related biomass smoke are influenced by fuel type, combustion phase, and byproduct chemistry. J. Toxicol. Environ. Health A 2021, 84, 674–688. [Google Scholar] [CrossRef]
- Bansode, R.R.; Leung, T.; Randolph, P.; Williams, L.L.; Ahmedna, M. Cinnamon extract inhibits angiogenesis in zebrafish and human endothelial cells by suppressing VEGFR1, VEGFR2, and PKC-mediated MAP kinase. Food Sci. Nutr. 2013, 1, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.F.; Hashim, Z.; Soon, W.T.; Rahman, N.S.A.; Zainudin, A.N.; Majid, F.A.A. Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J. Tradit. Complement. Med. 2017, 7, 452–465. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Narain, V.; Bondesson, M. E-cigarette vaping liquids and the flavoring chemical cinnamaldehyde perturb bone, cartilage and vascular development in zebrafish embryos. Aquat. Toxicol. 2021, 240, 105995. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Zeng, N.; Ding, Y.; Zhao, X.; Gao, C.; Li, Y.; Wang, H.; Liu, X.; Niu, Y.; Sun, Y.; et al. Cinnamaldehyde causes developmental neurotoxicity in zebrafish via the oxidative stress pathway that is rescued by astaxanthin. Food Funct. 2022, 13, 13028–13039. [Google Scholar] [CrossRef] [PubMed]
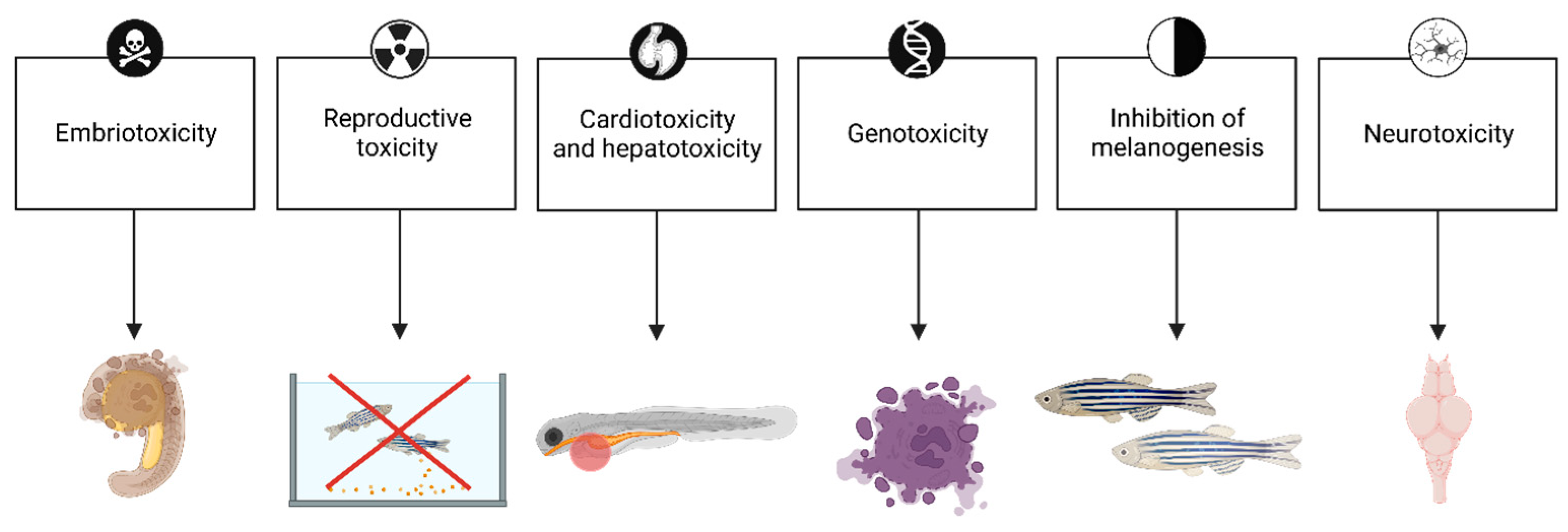

| Effect | Comment | Stage | Treatment (Concentration; Exposure Time) | Reference |
|---|---|---|---|---|
| Reproductive toxicity | Larval exposure to triclosan caused adverse effects in adults including delays in metamorphosis, as well as impairment of fecundity and fertility. Meanwhile, offspring were characterized by decreased survival and delayed maturation, without effect on reproductive capacity. | 21–35 dpf larvae | 40 μg/L; 15 days | [73] |
| General toxicity | Triclosan changes the expression of miRNAs involved in translation, transcription, and DNA-templated, protein transport, and motor neuron axon guidance. | 2-month-old male zebrafish | 68 μg/L for 42 days | [74] |
| Embryotoxicity Malformations | Recorded mortality and morphological changes in zebrafish embryos at 10 and 24 hpf. | 2–24 hpf embryos | 300 μg/L, 8 and 22 h | [75] |
| Triclosan decreased the hatching rate in 72 hpf larvae, as well as caused a significant decrease in body length in 120 hpf larvae. | 4–120 hpf larvae | 300 μg/L, 4 to 120 hpf | [76] | |
| Triclosan induced craniofacial morphosis in zebrafish and decreased the body length, head size, and eye size in a concentration-dependent manner. | 96 hpf larvae | 0.2, 0.4, 0.6, and 0.8 mg/L; from 4 to 96 hpf | [69] | |
| Cardiotoxicity | Incidence of pericardial edema, and impacts on heart structure and heart function. | 8–120 hpf larvae | 40, and 400 μg/L | [77] |
| Hepatotoxicity | TCS may be hepatotoxic in zebrafish; gene enrichment analysis further supported the role of the liver as a target organ for TCS toxicity. | 6–48 hpf embryo | 1–10 µM; from 8 to 120 hpf | [78] |
| Muscles | Trunk skeletal muscle abnormalities, presumably by the Ca2+ regulatory module between the dihydropyridine receptor and Ryanodine receptor 1. | 96 hpf larvae | 0.52, 1.04, and 1.73 μM; from 24 to 120 hpf | [79] |
| Decreased acetylcholinesterase (AChE) activity in skeletal muscles, and the AChE gene was significantly downregulated only in the skeletal muscle, with observed downregulation of the myelin basic protein (MBP) gene. | Adult zebrafish (nine months old) | 0.3 and 0.6 mg/L; for 48 h | [64] | |
| Behavior | Triclosan reduced locomotion concomitant with increased freezing duration and induced anxiety-like behavior. | Adult zebrafish (nine months old) | 0.3 and 0.6 mg/L; for 48 h | [65] |
| Changes in biomarkers | Significant dysregulations in the expression of the urea transporter (UT), glucose-6-phosphate dehydrogenase (G6PD), alanine transaminase (ALT), glutamate dehydrogenase (GDH), phosphoglucomutase (PGM), and fatty acid synthase (FASN), together with changes in alanine, urea, glucose, 6-phosphogluconalactone, and palmitic acid. | 96 hpf larvae | 30 μg/L and 300 μg/L; for 96 hpf | [80] |
| Decrease in superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) in the brain and liver of adult zebrafish. Also, the contents of the glutathione system (GSH and GSSH), as well as the activity of the glutathione reductase (GR), assayed in the liver, were reduced while the contents of malondialdehyde (MDA) were elevated in the liver. | Adult zebrafish (five months old) | 50, 100, and 150 μg/L for 30 days | [81] | |
| Decrease in activity of glutathione-S-transferase (GST), P-glycoprotein efflux, and ethoxyresorufin-o-deethylase (EROD), and increase in oxidative stress parameters. | 0–120 hpf larvae | 0.1 μg/L and 1 μg/L; from 0 to 120 hpf; from 96 to 120 hpf | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachurski, P.; Świątkowski, W.; Ciszewski, A.; Sarna-Boś, K.; Michalak, A. A Short Review of the Toxicity of Dentifrices—Zebrafish Model as a Useful Tool in Ecotoxicological Studies. Int. J. Mol. Sci. 2023, 24, 14339. https://doi.org/10.3390/ijms241814339
Stachurski P, Świątkowski W, Ciszewski A, Sarna-Boś K, Michalak A. A Short Review of the Toxicity of Dentifrices—Zebrafish Model as a Useful Tool in Ecotoxicological Studies. International Journal of Molecular Sciences. 2023; 24(18):14339. https://doi.org/10.3390/ijms241814339
Chicago/Turabian StyleStachurski, Piotr, Wojciech Świątkowski, Andrzej Ciszewski, Katarzyna Sarna-Boś, and Agnieszka Michalak. 2023. "A Short Review of the Toxicity of Dentifrices—Zebrafish Model as a Useful Tool in Ecotoxicological Studies" International Journal of Molecular Sciences 24, no. 18: 14339. https://doi.org/10.3390/ijms241814339
APA StyleStachurski, P., Świątkowski, W., Ciszewski, A., Sarna-Boś, K., & Michalak, A. (2023). A Short Review of the Toxicity of Dentifrices—Zebrafish Model as a Useful Tool in Ecotoxicological Studies. International Journal of Molecular Sciences, 24(18), 14339. https://doi.org/10.3390/ijms241814339





