1. Introduction
1.1. Baths in the Roman Culture
In the Mediterranean Region, the exploitation of thermo-mineral water springs is a traditional phenomenon [
1]. Since ancient times, water has been associated with positive effects on health, strengthening its relationship to humankind [
2]. Herodotus spoke of the divinity of water and its potential ability to heal all diseases, including blindness, deafness, and dumbness [
3]. In Italy, an ample number of springs that supported bathing establishments and infrastructures those date back to the pre-Roman age left deep and, in some cases, still well visible marks that often represent the basis of modern thermo-mineral resorts.
Campania is one of the richest regions with respect to thermal and mineral water resources [
4]. It may be said that Romans revalued thermalism. In the Roman age, bathing was a social and recreational activity deeply rooted in daily life. There are numerous waters with special chemical and physical properties and declared healthful features and, since Roman times (VIII–V centuries BC), these waters have been used by humans for therapeutic purposes. Thermalism was developed by the Romans by first building waterworks and thermal structures in the cities and then large spas in Baia, Campi Flegrei, Agnano, Ischia [
5], and many other locations that have been highly praised for curing a wide range of pathologies due to the volcanic origin of their waters and their diversified physico–chemical and thermic properties [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19].
In Nitrodi’s spring, already known to the first Greek settlers in southern Italy and dedicated to Apollo and the Nitrodi Nymphs, everyone found a remedy—war wounds healed in a short time, old men became looser, and women emerged from the water more beautiful. Thus, the thermal waters of Nitrodi became a very important cult center between the 1st century BC and the 3rd century AD. The healing power of Nitrodi’s water was considered a gift from the nymphs and the god Apollo. As evidence of that ancient cult, today we can admire the votive reliefs in the Archaeological Museum of Naples, found in Nitrodi in 1757 [
20,
21,
22,
23,
24,
25,
26,
27]. Additional historical information based on the selection and identification of sources and the existing literature is available in this article’s
Supplementary Materials. Copies of votive reliefs are shown in
Figure S1.
1.2. Thermal Water Classifications and Potential Applications
Thermal treatment is an ancient branch of so-called “natural medicine” that is commonly employed, via different methods, for therapeutic purposes [
28]; it is a treatment approach that is used in addition to conventional treatment in several countries with abundant thermal and mineral springs.
The term “thermal water” refers to natural mineral waters whose features are suitable for therapeutic use (Directive 2009/54/EC of the European Parliament and of the
Council of 18 June 2009 on the exploitation and marketing of natural mineral waters). The therapeutic value of mineral waters has largely been studied by European balneologists. Mineral springs with different mineral contents are often recommended for specific therapeutic uses. They are classified according to their main physical and chemical characteristics (temperature, pressure, ionic concentration, radioactivity, and presence of specific ions or active chemical groups). The classification performed by Marotta and Sica dates back to 1933 and, in Italy, represents the current water classification regulations through which licenses to use mineral waters are disseminated [
29]. This classification includes three parameters: temperature, fixed residue, and chemical composition [
30,
31,
32].
While hydrotherapy (HT) generally employs ordinary tap water, in the context of medical treatment, balneotherapy (BT) utilizes thermal mineral water from natural springs, natural gases (CO
2, iodine, sulfur, radon), peloids (mud), and other edaphic remedies (e.g., hay). It is often used for relieving chronic pain, which is a common symptom of several illnesses [
33]. Additionally, BT is usually practiced in spas, where their peculiar atmosphere is part of a complex therapeutic program, which is why the term is often used synonymously with spa therapy [
34].
Thermal water is generally used at a temperature between 34 °C and 36 °C. Water reduces the severity of pain in rheumatic joints, as the hydrostatic force relieves pain and reduces loading. The buoyancy and warmth of water may block nociception by acting on thermal and mechano-receptors. Warm water can also enhance blood flow, thus helping in dissipating algogenic chemicals and facilitating muscle relaxation [
35]. Indeed, it has been described that immersion in thermal mineral water or the application of mudpacks may have beneficial effects on muscle tone, joint mobility, and pain intensity [
36]. Increases in buoyancy and hydrostatic pressure during immersion in thermal water cause many physiological changes. Immersion up to the suprasternal notch in spa water (35 °C) results in a cascade of reactions, including increased diuresis, natriuresis, and cardiac output. Since sulfur spa baths have been successfully used in various skin immune-mediated afflictions, it has been suggested that the absorption of the trace elements present in mineral water and mudpacks through the skin may affect the immune system [
35].
2. Nitrodi’s Spring Water: Advances and Opportunities
In addition to conventional treatment, natural products (thermal water, plants, and minerals) have played a key role in drug discovery, not only in dermatological and rheumatic diseases but also in other therapeutic areas [
37,
38,
39,
40]. Both balneological and hydroponic therapies at ‘the oldest spa in the world’, namely Nitrodi’s spring, have proved effective with respect to several disorders and conditions.
The Nitrodi’s station is located in the village of Buonopane in the south of Ischia Island and is surrounded by verdant hills. The park winds along characteristic terraces that follow the form of the land in accordance with local rural traditions. The Nitrodi’s spa complex has showers and washbasins, which issue pure water directly from the springs without chemical storage or physical treatment of any sort and at its natural temperature of 28 °C. There are comfortable dressing rooms, waterfall showers, washbasins, chairs, and beach umbrellas in sections of the park. Health management is ensured according to the current legislation (Legge Regionale 29 luglio 2008, n. 8. “Disciplina della ricerca ed utilizzazione delle acque minerali e termali, delle risorse geotermiche e delle acque di sorgente, Art. 24 Direzione sanitaria nelle aziende termali, negli stabilimenti termali e nei reparti termali). Nitrodi’s thermal baths host from 25,000 to 30,000 customers per year. Clients can spend short periods of 1–2 days there or, in several cases, they come and stay for longer treatments and therefore spend periods of 10–12–15 days there. Psoriasis, dermatitis, wounds, and ulcers are the most frequent pathologies treated. There are no standard or personalized therapeutic programs; therapy consists of washing via taking showers without any contraindication. Nitrodi also has an aromatherapy itinerary, which gives visitors the chance to come into direct contact with its aromatic plants and medicinal herbs, thus reinforcing the effect of its waters.
We aimed to investigate how Nitrodi’s water affects the inflammatory microenvironment and exerts its biological properties. Therefore, in this study, we present the results from our own studies and that of other studies in order to highlight the advances pertaining to Nitrodi’s water and the opportunities arising from its clinical application.
2.1. The Physical, Chemical, and Biological Characteristics of Nitrodi’s Water
Over time, the water from Nitrodi’s spring has been subjected to various official analyses. The first analysis was conducted in 1984, and the findings were confirmed by the University of Naples Federico II in an analysis recognized by the Italian Ministry of Health (Decree no. 3509, 9 October 2003). Our group recently undertook an experimental project on Nitrodi’s water wherein additional chemical and chemical–physical analyses were performed [
41], the main results of which are reported in
Table 1.
In addition to analyses of the water’s chemical composition and physical characteristics, evaluations of its Polycyclic Aromatic Hydrocarbons (PAHs), Organochlorines (OCLs), Organophosphorus Pesticides (OPPs), Polychlorinated Biphenyls (PCBs), and Trihalomethanes (THMs) were conducted through extraction and mass spectrometry analyses. All analyte concentration values were below the Limit of Quantification (LOQ). Future projects will include biological characterization and an analysis of the microbiome of Nitrodi’s water. In fact, Nitrodi’s water was sterilized to perform in vitro experiments with epithelial cells, thus alleviating the risk of microbial contamination. This strategy allowed our group to analyze only the effects of soluble factors (not yet known) and mineral components (reported in
Table 1) on cell activities. Therefore, the results obtained to date reproduce only a part of the beneficial effects of Nitrodi’s water.
Nitrodi’s water was categorized as mildly mineralized and hypothermal (20–30 °C) based on its chemical composition and temperature, as proposed by Nasermoaddeli and Kagamimori’s classification [
33]. Additionally, as proposed by Marotta and Sica in the most well-known classification in Italy [
29,
42], as well as by Mancioli [
43], the results indicated that Nitrodi’s water falls into the category of medium-mineral water, meaning that it essentially consists of sulfate-alkaline. Regarding its clinical use, it is considered a bicarbonated water, although it does not completely meet the European Union’s requisites for such natural mineral water. It is also alkaline-earthy and naturally hypothermal [
29,
33,
41,
42,
43]. Our analysis of its physical and chemical composition allows us to speculate on the beneficial properties of Nitrodi’s water. In particular, the presence of bicarbonate ions and sulphates could be important for hydropinotherapy in patients suffering from pain and other symptoms caused by biliary dyskinesias and biliary sand or following a cholecystectomy [
44]. Bicarbonate mineral water improves skin regeneration by increasing keratinocyte proliferation and migration and inducing collagen and elastic fibers in the dermis [
45]. Bicarbonate ions are also useful for musculoskeletal rehabilitation as they reduce the effects of muscular exertion. Its sodium and silica content could be responsible for the beneficial effects of Nitrodi’s water on skin disorders, as already demonstrated by different research groups [
46].
There is a growing interest in the application of Nitrodi’s water as an active ingredient in cosmetics (unpublished data). The richness of the bicarbonate and the mixture of minerals contained in Nitrodi’s water favor natural skin exfoliation, i.e., peeling, thus removing damaged and aged skin [
47]. Exfoliation induces keratinocyte and fibroblast migration, structural reorganization, and the deposition of new collagen, which are all effects induced by Nitrodi’s water [
41].
It is also conceivable that the therapeutic effects of Nitrodi’s water could be associated with the native microbiome. Interestingly, Nicoletti et al. have demonstrated that Comano spring water-derived bacterial lysates exert regenerative effects on human skin fibroblasts. Similar to Nitrodi’s water, Comano’s water, found in the province of Trento (Northern Italy), is bicarbonate-calcium-magnesium-based and recommended for cutaneous disorders and upper airway diseases [
48]. Nitrodi’s water is certified as microbiologically pure as it contains no pathogenetic microorganisms. However, the anti-inflammatory and regenerative properties of these waters cannot be solely traced back to their mineral composition but the combination of their mineral composition and specific bacterial species. Therefore, studying Nitrodi’s microbiome is necessary to better understand the therapeutic bases of these waters.
2.2. Beneficial Effects of Nitrodi’s Water: Data from the Past
The effects of Nitrodi’s spring water were extensively studied in the late 1960s to evaluate the different modalities of exposure to the water. These studies showed that both internal and external use yielded several benefits in the treatment of certain disorders and in physiological processes such as wound healing [
43].
In 1984, Mancioli published ‘posthumous’, a research publication commissioned by the Center Studies on the Island of Ischia, which was carried out over a three-year period between 1968 and 1970 at the Study Center «P. Malcovati Lacco Ameno Terme» on behalf of the Order of Merit of Labor Angelo Rizzoli. The studies were intended to ascertain, in a modern scientific sense, the therapeutic validity of using Nitrodi’s spring water in view of the realization of a grandiose valorization project.
In the first part of the present manuscript, an extensive analysis of the properties of Nitrodi’s water, based on the studies performed in 1969 by Prof. M. Talenti from the Institute of Hygiene of the University of Rome, was performed.
Water is one of the most important stimulators of diuresis, and in some waters, this property acquires a clearly higher value than that of ordinary waters. With this in mind, Mancioli addressed the initial experiments that evaluated the ability of Nitrodi’s spring water to modify the diuresis of normal subjects, and this was compared to water from the aqueduct, which was considered the control.
Although it was not easy to create a defined limit between common drinking water and diuretic waters, through reviewing the evidence, the author recognized the ability of Nitrodi’s spring water to (i) cause polyuria and (ii) induce polyuria in a more intense and quicker way compared to common drinking water. The results of Mancioli’s analysis showed that the diuresis caused by Nitrodi’s water was more intense and, above all, took place much more rapidly than what occurred after the ingestion of the aqueduct water. The author also demonstrated that the diuretic action of Nitrodi’s mineral water gradually increased with the repeated consumption of the water for several consecutive days compared to the control. The acid uric turnover was likewise evaluated, showing that Nitrodi’s water was able to markedly decrease uricoemia. Changes in the blood and urine levels of uric acid observed during the treatment of Nitrodi’s water suggested the ability of this water to mobilize uric acid from tissue deposits [
43].
Mancioli also evaluated the effects of the water in the context of gastritis and subacute and chronic gastroduodenitis. Patients suffering from gastric and duodenal ulcers visited Nitrodi’s spring spontaneously and reported an undeniable benefit from drinking the water. Furthermore, Nitrodi’s water was found to be singularly effective in the topical treatment of varicose ulcers, numb sores, sinus tracts, etc., confirming an ancient local empirical tradition [
43].
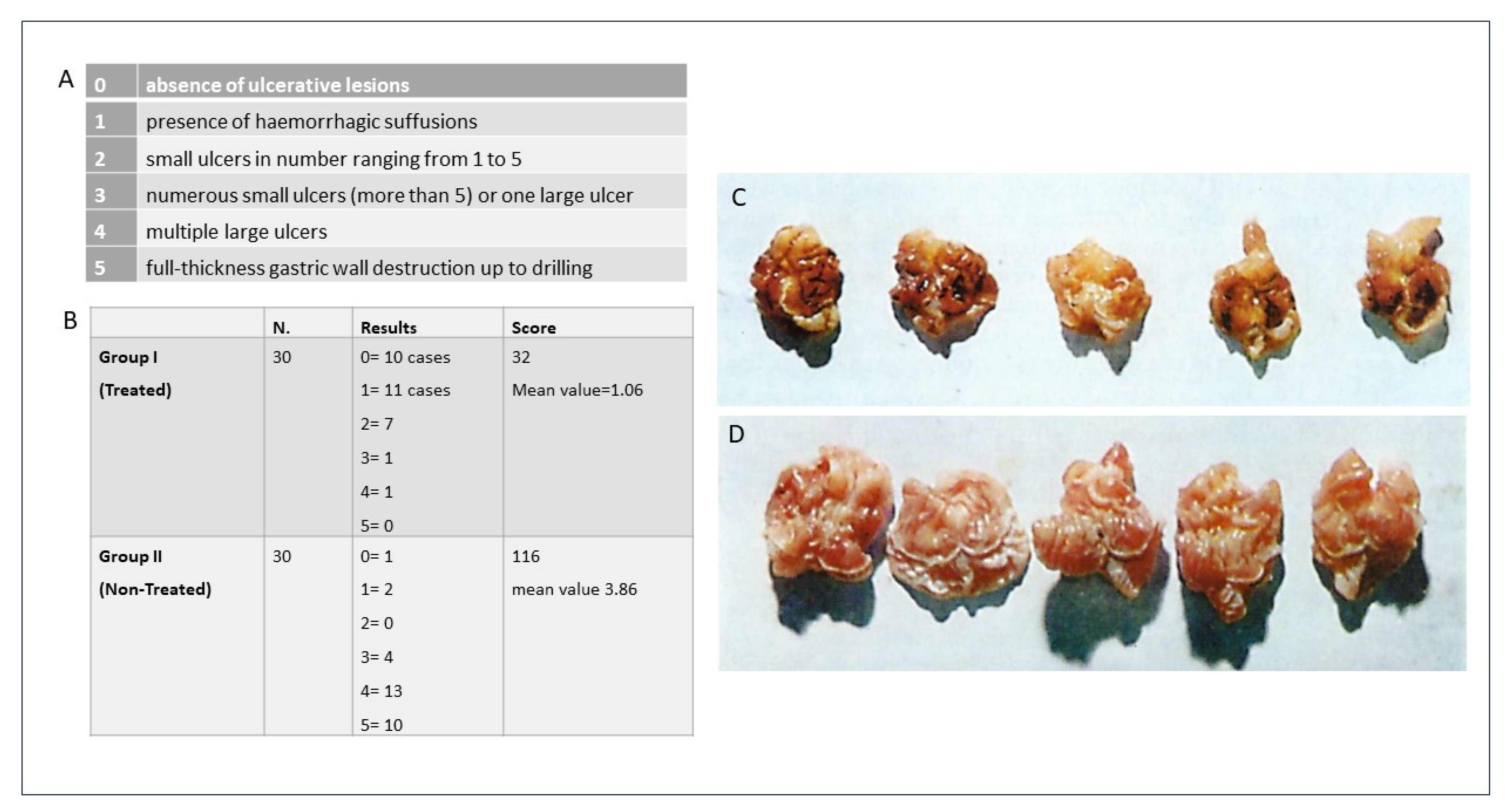
To this end, in the final series of experiments conducted by the author, 60 albino female Spreague Dawley rats were treated in an in vivo model to evaluate the efficacy of the Nitrodi’s spring water. The animals were divided into two groups: those treated for 15 consecutive days with Nitrodi’s water twice a day (30 cc/kg of weight) and those treated using equal amounts of water from the aqueduct. At the end of the treatment stage, an experimental ulcer was induced via the intraperitoneal injection of reserpine at a dose of 0.5 mgr/kg. Approximately 16 h after the intraperitoneal injection of reserpine, the animals were sacrificed, and their stomachs were removed via laparotomy. To evaluate the extent of the lesions found and to establish a criterion of comparison between the various findings, the author followed a classification according to an arbitrary scale. The histological exam demonstrated that, in the animals previously treated with Nitrodi’s water, compared to the control group, the ulcers affected only the most superficial layers of the mucosa and rarely involved the muscularis mucosae. Furthermore, a qualitative analysis showed the scarcity of the lesions, as they were mostly expressed as hemorrhagic suffusions or superficial ulcerations in the animals treated with Nitrodi’s water (
Figure 1).
3. Beneficial Effects of Nitrodi’s Water: Ongoing Data
Recently, among scientists and clinicians, interest in the properties of Nitrodi’s water has grown. Although many clinical and experimental data are still unpublished, we believe that the knowledge of this water will soon develop and that more potential applications will arise. In 2020, we decided to confirm some of the evidence described by Mancioli, expand the knowledge of the effects of this water, and elucidate some of the biological mechanisms sustaining the therapeutic efficacy of Nitrodi’s water, providing a robust scientific basis that could confirm its usage in hydroponic and balneological therapy.
3.1. Anti-Inflammatory and Anti-Oxidant Effects of Nitrodi’s Water
Inflammation represents the immune system’s response to infection with foreign organisms and injury [
49] and underlies a wide variety of physiological and pathological processes such as arthritis, cancer, atherosclerosis, and neurodegenerative diseases [
49].
After wounding, the first phase begins with vascular constriction and fibrin clot formation. Once bleeding is controlled, inflammatory cells migrate to the wound (chemotaxis) and initiate the inflammatory phase. The first responders are neutrophils, activated by pro-inflammatory mediators released by damaged tissue and by Damage-Associated Molecular Pattern molecules (DAMPs). Neutrophils remove foreign material from the wounds. During this process, substances such as reactive oxygen species (ROS) and proteases are generated by neutrophils, causing some additional damage [
50]. Next, immune cells arrive at the scene, including monocytes that differentiate into macrophages and coordinate the wound healing process. As the inflammation subsides and the number of leukocytes diminishes, the wound undergoes a lengthy period of remodeling and resolution, which is mediated by fibroblasts and keratinocytes. Certain medical conditions, mainly diabetes or immunosuppression, can have negative impacts on the wound healing response. There remains an urgent need to develop effective alternative therapeutics that can be used in combination with conventional treatments in order to improve wound healing and resolve impaired wounds.
Based on our long-standing experience in the study of allergic and autoimmune inflammatory processes [
51,
52,
53,
54,
55,
56,
57,
58,
59,
60], an experimental strategy using different in vitro techniques was set up to assess the effects of Nitrodi’s spring water in many aspects associated with low-grade inflammation and stress-related conditions. The results from these experiments are illustrated in
Figure 2.
3.2. Cell Treatment with Filtered Nitrodi’s Water
Firstly, the phenomenon of S-nitrosylation, understood as the addition of nitric oxide (NO) to the thiol side chain of cysteine residues within proteins, has been the subject of a lot of academic interest. The aberrant S-nitrosylation of proteins is associated with many diseases [
61]. NO is also a key messenger in the pathogenesis of inflammation and exerts this effect by activating innate and adaptive immunity. Cyclooxygenase-2 (COX-2) expression has pathological significance; COX-2 could be activated by S-nitrosylation inducing MMP-2, which is responsible for the degradation of the ECM. To analyze the effects of Nitrodi’s water on protein S-nitrosylation, the expression of MMP-2 was investigated in colorectal adenocarcinoma cells (RKO cell line). The results obtained from these experiments showed that MMP-2 protein expression and protein S-nitrosylation were markedly downregulated in RKO cells treated with Nitrodi’s water. Overall, the results revealed that the molecular mechanisms through which Nitrodi’s spring water exerts its anti-inflammatory effects might involve the downregulation of protein S-nitrosylation [
61].
Recent evidence has shown that oxidative stress plays a pivotal role in the development and perpetuation of inflammation [
62]. Oxidative stress implies increased intracellular ROS levels, which are involved in both chronological aging and photo-aging [
41]. Promising results have been obtained from using natural antioxidant compounds as pharmaceutical tools against several inflammatory diseases in in vitro systems, in vivo models, and in clinical trials. Napolitano et al. showed that Nitrodi’s water is a promising source of natural antioxidants that could potentially be useful in chronic inflammation and anti-aging strategies. Treatment with these waters reduced the basal production of ROS in a model of dermal fibroblasts, namely BJ cells. These data were also confirmed in other models of epithelial cells, or rather keratinocytes. In response to stress-associated stimuli, such as H
2O
2, the levels of ROS in the treated cells were lower compared to the untreated cells, suggesting that Nitrodi’s water is able to contain the damage caused by oxidative stress in dermal fibroblasts. Instead, Nitrodi’s water was not able to reduce ROS release in response to H
2O
2 in keratinocytes. This behavior can be explained by the fact that ROS are used by keratinocytes to communicate with the underlying cells in order to promote wound repair. Since ROS also operate as intracellular signaling molecules in response to external stimuli, it has been demonstrated that Nitrodi’s water does not affect ROS generation in response to bacterial-derived peptides [
41].
3.3. Regenerative Properties of Nitrodi’s Water
Topical treatment with thermal waters exerts regenerative action on skin probably due to the favorable combination of a local wet environment and an anti-inflammatory effect. In vitro studies have demonstrated that Nitrodi’s water exerts a positive effect on dermal fibroblast and keratinocyte activities.
In particular, the expression of extracellular signal-regulated protein kinases 1 and 2 (ERK 1/2), which are involved in growth and cell-cycle progression, was induced in dermal fibroblasts via treatment with Nitrodi’s water. Nitrodi’s water can sustain dermal fibroblast survival both in starving and growing conditions [
41].
Dermal fibroblast migration is a pivotal step in the healing process. Nitrodi’s water induced both chemotaxis and wound-scratch closure in dermal fibroblasts.
Upon injury, dermal fibroblasts migrate into wound granulation tissue and differentiate into myofibroblasts, which play a pivotal role in the wound contraction and deposition of ECM proteins. Alpha smooth muscle actin (alpha-SMA) expression is used as a marker of myofibroblast differentiation [
63]. Nitrodi’s water can induce alpha-SMA expression in dermal fibroblasts, thus promoting fibroblast–myofibroblast transition.
The ECM is crucial for structural support and cellular attachment, regulating biochemical pathways and directly modulating cell adhesion, proliferation, and migration. The healing process occurs through the formation of the synthesis of the ECM, much of which is due to fibroblasts. In the newly formed ECM, all of the ECM proteins are created, with the exception of elastin [
62]. Treating dermal fibroblasts with Nitrodi’s water promotes the deposition of collagen type I and fibronectin, which are major ECM proteins that are essential for both cell adhesion and structural support [
64].
Keratinocytes play a key role in ensuring that the wound healing process is properly carried out through coordinated action with fibroblasts and immune cells. Cells can migrate, proliferate, and differentiate, contributing to the re-epithelialization of epidermal tissue [
65]. These processes are impaired in all types of chronic wounds. Treatment with Nitrodi’s water induces keratinocyte proliferation and chemotaxis.
Co-culture experiments are being set up in order to study keratinocyte–fibroblast interactions in the wound healing process (unpublished data). In fact, there is a large body of evidence to suggest that keratinocytes stimulate fibroblasts to synthesize growth factors, which in turn stimulate keratinocyte proliferation in a double-paracrine manner. Importantly, fibroblasts can acquire a myofibroblast phenotype under the control of keratinocytes [
65]. The results from these experiments will have a major impact on both clinical practices and the cosmetics industry.
Overall, these in vitro data are scientific proof of the beneficial effects Nitrodi’s water has on the skin. The anti-inflammatory, anti-oxidant, and regenerative effects of Nitrodi’s water that have been observed in in vitro studies are shown in
Figure 2.
3.4. Future Perspectives and Potential Applications in Clinical Practice
Long regarded as an alternative treatment in European countries, balneotherapy (BT) was one of the first non-pharmacological treatments evaluated in different acute and chronic diseases.
The mechanisms by which BT acts in the treatment of autoimmune diseases are not fully understood. While this therapy does not replace but rather supplements traditional drug therapy, it is certainly beneficial in appropriate cases [
66,
67].
BT is defined as the use of mineral water, gases, or peloids for health promotion and the prevention or treatment of diseases. In a Hungarian double-blind, controlled pilot study, Tiszasüly and Kolop mud packs were shown to have a favorable effect in the treatment of knee osteoarthritis. Indeed, Tiszasüly and Kolop peloids are inorganic because of their notable mineral content and considerable lack of organic components [
68]. A systematic review on the beneficial effects of different BT modalities, including radon–carbon dioxide baths, mud packs, hot sulfur baths, and Dead or Red Sea baths for patients with RA, has been published [
69]. In 2018, Fioravanti et al. demonstrated the efficacy of BT in patients with fibromyalgia by using the thermal water of Vetriolo (Trento, Italy), which descend from the Vetriolo spring in the thick of the fir woods at an altitude of 1500 m. Indeed, Vetriolo’s water is a highly mineralized (fixed residue at 180 °C 1702 mg/L) strongly acidic (pH 5.7) sulfate (1100 mg/L) water that is rich in calcium (111.0 mg/L), magnesium (65.5 mg/L), and iron (315 mg/L) [
70]. Several studies have indicated the efficacy of BT with natural mineral water for psoriasis and atopic dermatitis [
71].
BT plays a role in the complex, multimodal therapy of rheumatic diseases; therefore, the American College of Rheumatology has positioned it as an essential complementary therapy for rheumatic diseases [
72].
Important features of BT include the temperature and composition of the mineral water used, which depends on the respective country. Mudpacks or peloids can be applied wet or dry (in the form of sand), and their properties depend on their origin. Waters can be enriched with gases, mainly CO
2 or radon. All these types of BT usually take the form of baths for the whole body or the affected portion of the body [
73]. The benefits of BT include improved motion and reduced stiffness and pain. These benefits may have a positive impact on RA symptoms, which could improve the quality of life of these patients [
73,
74,
75].
Although the experimental evidence described in the present review collectively suggests that Nitrodi’s water exerts in vitro regenerative action by promoting wound healing, in vitro wound healing assays cannot mimic the complexity of the conditions that occur during the in vivo wound healing process. Hence, data obtained from in vitro assays should be corroborated with in vivo models. Therefore, we are planning a large observational prospective study to evaluate the effects of crenotherapy on wound repair and regeneration using Nitrodi’s thermal water in a model digital ulcer (DUs) from patients affected by SSc over a 12-month follow-up period. We will perform tests such as the Scleroderma Health Assessment Questionnaire (SHAQ), pain and fatigue VAS, SF36 (Quality of Life–QoL), HAQ (General Disability). In addition, we will assess the overall DU number and reduction in new DUs, as well as some qualitative parameters, such as the presence of infections, presence of devitalized tissue (necrosis/fibrin), amount of exudates, presence of granulation tissue, and epithelization of the wound. Finally, we will evaluate morphology microcirculation using nailfold capillaroscopy [
60,
76]. These findings could contribute to developing novel strategies that aim to complement conventional treatments by using Nitrodi’s water as a potential treatment for skin diseases and age-related skin deterioration.
4. Conclusions
Studies on the cellular biology of the properties of thermal waters in the world are limited. Italy represents a unique case in that it is particularly rich in springs that share similar therapeutic effects and have similar salt and thermal features to that of Nitrodi’s spring water, probably because they have the same hydro-geological origin.
The present review presents the results of all of the in vitro studies performed to confirm the therapeutic role of Nitrodi’s water. The elucidation of the biological mechanisms underlying the benefits of Nitrodi’s water will contribute to the development of potential therapies for skin diseases. Nitrodi’s water could be a promising anti-inflammatory agent for the skin and a potential wound-healing therapeutic agent. In addition, the antioxidant properties of Nitrodi’s water could be exploited to prevent symptoms related to photo-induced skin aging.
These results concur with all the previously reported therapeutic properties of Nitrodi’s spring water and thus reinforce the concept that the natural resource is an important complementary therapy to traditional medicine.
Author Contributions
I.M., F.T., F.N. and F.W.R. conceived and designed the study, acquired literature data, interpreted literature data, and drafted the manuscript. F.D.C. and V.D. acquired literature data, interpreted literature data, and drafted the manuscript. A.d.P., N.M. and M.D.R. supervised the project, interpreted literature data, drafted the manuscript, and performed critical revisions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from Miur, Campania Bioscience PON03PE_00060_8. I. Mormile is funded by from MIUR PON R&I 2014-2020-PON_INN_RTDA_2021_87. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated or analyzed for this review article.
Acknowledgments
The authors would like to acknowledge Giuseppe Di Meglio (Fonti delle Ninfe Nitrodi, via Pendio Nitrodi, 80070 Barano d’Ischia) for supplying Nitrodi’s water.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zanetti, C. Acque e siti termali nell’Italia romana le testimonianze degli autori antichi. In Proceedings of the Acque e siti termali nell’Italia romana le testimonianze degli autori antichi, in Aquae Patavinae, Montegrotto, Italy, 6–8 September 2012; Aggiornamenti e nuove prospettive di valorizzazione. Padova University Press: Padova, Italy, 2012; p. 365. [Google Scholar]
- Sorcinelli, P. Storia Sociale dell’acqua; Riti e culture: Milan, Italy, 1998. [Google Scholar]
- Sunseri, G.B. Herod. 2.13.3. Sul Controllo e l’uso Delle Acque in Erodoto. Spunti di Riflessione. ὀρμος. Ric. Di Stor. Antica 2018, 10, 203. Available online: https://www.perseus.tufts.edu/hopper/text?doc=Perseus%3Atext%3A1999.01.0125%3Abook%3D2%3Achapter%3D13%3Asection%3D3 (accessed on 27 August 2023).
- Fagan, G.G. The Genesis of the Roman Public Bath: Recent Approaches and Future Directions. Am. J. Archeol. 2001, 105, 421. [Google Scholar] [CrossRef]
- Plin. Naturalis historia. 31.5.9. Available online: https://la.wikisource.org/w/index.php?title=Naturalis_Historia/Liber_XXXI&action=edit§ion=5 (accessed on 27 August 2023).
- Brundrett, N.G.R.; Simpson, C.J. Innovation and the Baths of Agrippa. Athenaeum 1997, 87, 220. [Google Scholar]
- Di Capua, F. L’idroterapia ai Tempi Dell’impero Romano; Istituto di studi Romani: Rome, Italy, 1940. [Google Scholar]
- Malissard, A. Les Romains et L’eau. Fontaines, Salles de Bains, Thermes, Egouts, Aqueductus; Les Belles Lettres: Paris, France, 1994. [Google Scholar]
- Brödner, E. Die Römischen Thermen und Das Antike Badewesen: Eine kulturhistorische Betrachtung Darmstadt; Wissenschaftliche Buchgesellschaft: Darmstadt, Germany, 1983. [Google Scholar]
- Pasquinucci, M. Terme Romane e Vita Quotidiana; Franco Cosimo Panini: Modena, Italy, 1987. [Google Scholar]
- Nielsen, I. Thermae et Balnea. The Architecture and Cultural History of Roman Public Baths; Aarhus University Press: Aarhus, Denmark, 1990. [Google Scholar]
- Plin. Naturalis historia. 31.2.5. Available online: https://la.wikisource.org/w/index.php?title=Naturalis_Historia/Liber_XXXI&action=edit§ion=2 (accessed on 27 August 2023).
- Vitr. 8.3.5. Available online: https://www.thelatinlibrary.com/vitruvius/vitruvius8.html#3.5 (accessed on 27 August 2023).
- Sen. Naturales Quaestiones. 3.2.1. Available online: https://www.thelatinlibrary.com/sen/sen.qn3.shtml (accessed on 27 August 2023).
- Jackson, R. Waters and Spas in the Classical World. The Medicinal History of Waters and Spas. Porter Edition (London) 1990, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Staccioli, A. Le terme di Roma Antica; Newton Compton Editori: Rome, Italy, 1995. [Google Scholar]
- Fagan, G.G. Bathing in Public in the Roman World Ann Arbor; University of Michigan Press: Ann Arbor, MI, USA, 2002. [Google Scholar]
- Pettenò, E. Acque termali e uso terapeutico del bagno nel mondo romano. In Proceedings of the Termalismo antiguo I Congreso Peninsular, Actas, Arnedillo, Spain, 3–5 October 1996; UNED: Madrid, Spain, 1997. [Google Scholar]
- Perex Agorreta, M.J. Termalismo antiguo I Congreso Peninsular; Casa de Velazquez: Madrid, Italy, 1997; p. 217. [Google Scholar]
- Muscettola, S.A. Gli ex voto alle ninfe di ischia: La parabola di una cultura marginale. RIA 57 2002, 42 nt. 24. [Google Scholar]
- Jasolino, G. De’ Rimedj Naturali che sono nell’Isola di Pithecusa Naples; Bartolomeo Roselli: Napoli, Italy, 1763; Available online: https://books.google.it/books?id=R3JAAAAAcAAJ&printsec=frontcover&hl=it&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false (accessed on 27 August 2023).
- Angeletti, R. Usi terapeutici delle acque nella trattatistica medica della tarda antichità (secoli IV-VII d.C.). In Proceedings of the L’acqua nei secoli altomedievali, Atti delle settimane di studio della Fondazione Centro Italiano di Studi sull’Altomedioevo, Spoleto, Italy, 12–17 April 2008; Fondazione CISAM: Spoleto, Italy, 2008; p. 821. [Google Scholar]
- Mantovanelli, L. Acque Termali e cure Mediche, in Cura, Preghiera e Benessere. Le stazioni Curative Termominerali nell’Italia Romana; Padova University Press: Padova, Italy, 2014. [Google Scholar]
- Forti, L. Rilievi dedicati alle Ninfe Nitrodi. RAAN 36 1951, Table VI–XI, 161. [Google Scholar]
- Iapino, S. I rilievi votivi con dedica ad Apollo e alle Ninfe Nitrodi. La Rass. D’ischia 2003, 3–4, 26. [Google Scholar]
- Chioffi, L. Ischia in età romana: Cosa dicono le iscrizioni. In Proceedings of the II Mediterraneo e la Storia II. Naviganti, Popoli e Culture ad Ischia e in Altri Luoghi Della Costa Tirrenica; Institutum Romanum Finlandiae: Rome, Italy, 2017. [Google Scholar]
- CIL X 6790. Available online: https://arachne.uni-koeln.de/Tei-Viewer/cgi-bin/teiviewer.php?manifest=BOOK-ZID1323145,pg.679; (accessed on 27 August 2023).
- Melillo, L. Thermalism in ancient world. Med. Secoli 1995, 7, 461–483. [Google Scholar]
- Sica, C.; Marotta, D. Composizione e Classificazione Delle Acque Minerali Italiane; Annali di Chimica Applicata (Italia): Roma, Italy, 1933; Volume 23. [Google Scholar]
- Seite, S. Thermal waters as cosmeceuticals: La Roche-Posay thermal spring water example. Clin. Cosmet. Investig. Dermatol. 2013, 6, 23–28. [Google Scholar] [CrossRef]
- Matz, H.; Orion, E.; Wolf, R. Balneotherapy in dermatology. Dermatol. Ther. 2003, 16, 132–140. [Google Scholar] [CrossRef]
- Petraccia, L.; Liberati, G.; Masciullo, S.G.; Grassi, M.; Fraioli, A. Water, mineral waters and health. Clin. Nutr. 2006, 25, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Nasermoaddeli, A.; Kagamimori, S. Balneotherapy in medicine: A review. Environ. Health Prev. Med. 2005, 10, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.; Kutschan, S.; Freuding, M.; Schmidt, T.; Josfeld, L.; Huebner, J. Water therapies (hydrotherapy, balneotherapy or aqua therapy) for patients with cancer: A systematic review. J. Cancer Res. Clin. Oncol. 2022, 148, 1277–1297. [Google Scholar] [CrossRef] [PubMed]
- van Tubergen, A.; van der Linden, S. A brief history of spa therapy. Ann. Rheum. Dis. 2002, 61, 273–275. [Google Scholar] [CrossRef]
- Verhagen, A.P.; Bierma-Zeinstra, S.M.; Boers, M.; Cardoso, J.R.; Lambeck, J.; de Bie, R.; de Vet, H.C. Balneotherapy (or spa therapy) for rheumatoid arthritis. Cochrane Database Syst. Rev. 2015, 2015, CD000518. [Google Scholar] [CrossRef]
- Davis, S.C.; Perez, R. Cosmeceuticals and natural products: Wound healing. Clin. Dermatol. 2009, 27, 502–506. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Liang, J.; Kang, D.; Wang, Y.; Yu, Y.; Fan, J.; Takashi, E. Carbonate ion-enriched hot spring water promotes skin wound healing in nude rats. PLoS ONE 2015, 10, e0117106. [Google Scholar] [CrossRef]
- Rossi, F.W.; Rivellese, F.; Napolitano, F.; Mosella, F.; Selleri, C.; Montuori, N.; de Paulis, A. Effects of Polyurethane Foam Dressings as an Add-on Therapy in the Management of Digital Ulcers in Scleroderma Patients. Transl. Med. UniSa 2020, 22, 10–14. [Google Scholar]
- Napolitano, F.; Postiglione, L.; Mormile, I.; Barrella, V.; de Paulis, A.; Montuori, N.; Rossi, F.W. Water from Nitrodi’s Spring Induces Dermal Fibroblast and Keratinocyte Activation, Thus Promoting Wound Repair in the Skin: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 5357. [Google Scholar] [CrossRef]
- Sica, C.; Marotta, D. Composizione e Classificazione Delle Acque Minerali Italiane; Annali di Chimica Applicata (Italia): Roma, Italy, 1929; Volume 19. [Google Scholar]
- Mancioli, M. Le Proprietà Terapeutiche Delle Acque di Nitrodi e Olmitello; Edizioni Analisi: Napoli, Italy, 1984. [Google Scholar]
- Mennuni, G.; Petraccia, L.; Fontana, M.; Nocchi, S.; Stortini, E.; Romoli, M.; Esposito, E.; Priori, F.; Grassi, M.; Geraci, A.; et al. The therapeutic activity of sulphate-bicarbonate-calcium-magnesiac mineral water in the functional disorders of the biliary tract. Clin. Ter. 2014, 165, e346–e352. [Google Scholar] [CrossRef] [PubMed]
- Faga, A.; Nicoletti, G.; Gregotti, C.; Finotti, V.; Nitto, A.; Gioglio, L. Effects of thermal water on skin regeneration. Int. J. Mol. Med. 2012, 29, 732–740. [Google Scholar] [CrossRef]
- Araujo, A.; Sarraguca, M.C.; Ribeiro, M.P.; Coutinho, P. Physicochemical fingerprinting of thermal waters of Beira Interior region of Portugal. Environ. Geochem. Health 2017, 39, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Trevisol, T.C.; Henriques, R.O.; Souza, A.J.A.; Furigo, A., Jr. An overview of the use of proteolytic enzymes as exfoliating agents. J. Cosmet. Dermatol. 2022, 21, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Saler, M.; Tresoldi, M.M.; Faga, A.; Benedet, M.; Cristofolini, M. Regenerative effects of spring water-derived bacterial lysates on human skin fibroblast in in vitro culture: Preliminary results. J. Int. Med. Res. 2019, 47, 5777–5786. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Napolitano, F.; Rossi, F.W.; Pesapane, A.; Varricchio, S.; Ilardi, G.; Mascolo, M.; Staibano, S.; Lavecchia, A.; Ragno, P.; Selleri, C.; et al. N-Formyl Peptide Receptors Induce Radical Oxygen Production in Fibroblasts Derived from Systemic Sclerosis by Interacting with a Cleaved Form of Urokinase Receptor. Front. Immunol. 2018, 9, 574. [Google Scholar] [CrossRef]
- Mormile, I.; Rossi, F.W.; Prevete, N.; Granata, F.; Pucino, V.; de Paulis, A. The N-Formyl Peptide Receptors and Rheumatoid Arthritis: A Dangerous Liaison or Confusing Relationship? Front. Immunol. 2021, 12, 685214. [Google Scholar] [CrossRef]
- Montuori, N.; Pesapane, A.; Giudice, V.; Serio, B.; Rossi, F.W.; De Paulis, A.; Selleri, C. 67 kDa laminin receptor (67LR) in normal and neoplastic hematopoietic cells: Is its targeting a feasible approach? Transl. Med. UniSa 2016, 15, 8–14. [Google Scholar]
- Montuori, N.; Pesapane, A.; Rossi, F.W.; Giudice, V.; De Paulis, A.; Selleri, C.; Ragno, P. Urokinase type plasminogen activator receptor (uPAR) as a new therapeutic target in cancer. Transl. Med. UniSa 2016, 15, 15–21. [Google Scholar]
- Rossi, F.W.; Prevete, N.; Rivellese, F.; Napolitano, F.; Montuori, N.; Postiglione, L.; Selleri, C.; de Paulis, A. The Urokinase/Urokinase Receptor System in Mast Cells: Effects of its Functional Interaction with fMLF Receptors. Transl. Med. UniSa 2016, 15, 34–41. [Google Scholar] [PubMed]
- Lembo, C.; Raimondo, A.; de Paulis, A.; Mormile, I.; Rossi, F.W.; Lembo, S.; Balato, A. Clinical predictors of psoriatic arthritis and osteoclast differentiation. Exp. Dermatol. 2021, 30, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Salzano, F.A.; Rossi, F.W.; Rivellese, F.; Dellepiane, M.; Guastini, L.; Mora, R.; Marone, G.; Salami, A.; De Paulis, A. Role(s) of formyl-peptide receptors expressed in nasal epithelial cells. J. Biol. Regul. Homeost. Agents. 2011, 25, 553–564. [Google Scholar] [PubMed]
- Di Spigna, G.; Rossi, F.W.; Mormile, I.; Ladogana, P.; Buonavolonta, L.; Covelli, B.; Salzano, S.; Napolitano, F.; Giannini, A.; Postiglione, L. Serum Metalloprotease 3 (MMP-3) biomarker of therapeutic efficacy during treatment of rheumatoid arthritis. J. Biol. Regul. Homeost. Agents. 2021, 35, 1041–1045. [Google Scholar] [CrossRef]
- Di Spigna, G.; Ladogana, P.; Covelli, B.; Ricciardone, M.; Salzano, S.; Spalletti Cernia, D.; Mormile, I.; Varriale, G.; Catapano, O.; Spadaro, G.; et al. Component resolved diagnosis by recombinant allergens in patients with allergies to inhalants. J. Biol. Regul. Homeost. Agents. 2020, 34, 1729–1737. [Google Scholar] [CrossRef]
- Cesoni Marcelli, A.; Loffredo, S.; Petraroli, A.; Carucci, L.; Mormile, I.; Ferrara, A.L.; Spadaro, G.; Genovese, A.; Bova, M. Nailfold Videocapillaroscopy Findings in Bradykinin-Mediated Angioedema. J. Investig. Allergol. Clin. Immunol. 2021, 31, 404–416. [Google Scholar] [CrossRef]
- Aversano, A.; Rossi, F.W.; Cammarota, F.; De Paulis, A.; Izzo, P.; De Rosa, M. Nitrodi thermal water downregulates protein S-nitrosylation in RKO cells. Int. J. Mol. Med. 2020, 46, 1359–1366. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Rossi, F.W.; Napolitano, F.; Pesapane, A.; Mascolo, M.; Staibano, S.; Matucci-Cerinic, M.; Guiducci, S.; Ragno, P.; di Spigna, G.; Postiglione, L.; et al. Upregulation of the N-formyl Peptide receptors in scleroderma fibroblasts fosters the switch to myofibroblasts. J. Immunol. 2015, 194, 5161–5173. [Google Scholar] [CrossRef]
- Poljsak, B.; Dahmane, R.G.; Godic, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat 2012, 21, 33–36. [Google Scholar] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, S.; Abu-Shakra, M.; Flusser, D. Balneotherapy in autoimmune disease. Isr. J. Med. Sci. 1997, 33, 258–261. [Google Scholar] [PubMed]
- Morer, C.; Roques, C.F.; Francon, A.; Forestier, R.; Maraver, F. The role of mineral elements and other chemical compounds used in balneology: Data from double-blind randomized clinical trials. Int. J. Biometeorol. 2017, 61, 2159–2173. [Google Scholar] [CrossRef]
- Kiraly, M.; Kovari, E.; Hodosi, K.; Balint, P.V.; Bender, T. The effects of Tiszasuly and Kolop mud pack therapy on knee osteoarthritis: A double-blind, randomised, non-inferiority controlled study. Int. J. Biometeorol. 2020, 64, 943–950. [Google Scholar] [CrossRef]
- Brosseau, L.; MacLeay, L.; Robinson, V.; Casimiro, L.; Pelland, L.; Welles, G.; Tugwell, P.; McGowan, J. Efficacy of Balneotherapy for Osteoarthritis of the Knee: A Systematic Review. Phys. Ther. Rev. 2002, 7, 209–222. [Google Scholar] [CrossRef]
- Fioravanti, A.; Manica, P.; Bortolotti, R.; Cevenini, G.; Tenti, S.; Paolazzi, G. Is balneotherapy effective for fibromyalgia? Results from a 6-month double-blind randomized clinical trial. Clin. Rheumatol. 2018, 37, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Moini Jazani, A.; Ayati, M.H.; Nadiri, A.A.; Nasimi Doost Azgomi, R. Efficacy of hydrotherapy, spa therapy, and balneotherapy for psoriasis and atopic dermatitis: A systematic review. Int. J. Dermatol. 2023, 62, 177–189. [Google Scholar] [CrossRef]
- Panush, R.S. American College of Rheumatology Position Statement: Complementary and Alternative Therapies for Rheumatic Disease. Rheum. Dis. Clin. N. Am. 2000, 26, 189–192. [Google Scholar] [CrossRef]
- Gutenbrunner, C.; Bender, T.; Cantista, P.; Karagulle, Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int. J. Biometeorol. 2010, 54, 495–507. [Google Scholar] [CrossRef]
- Verhagen, A.P.; Bierma-Zeinstra, S.M.; Boers, M.; Cardoso, J.R.; Lambeck, J.; De Bie, R.; De Vet, H.C. Balneotherapy (or spa therapy) for rheumatoid arthritis. An abridged version of Cochrane Systematic Review. Eur. J. Phys. Rehabil. Med. 2015, 51, 833–847. [Google Scholar] [PubMed]
- Fioravanti, A.; Cantarini, L.; Guidelli, G.M.; Galeazzi, M. Mechanisms of action of spa therapies in rheumatic diseases: What scientific evidence is there? Rheumatol. Int. 2011, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).