Synthesis, Characterization, and Bioactivity of Mesoporous Bioactive Glass Codoped with Zinc and Silver
Abstract
:1. Introduction
2. Results
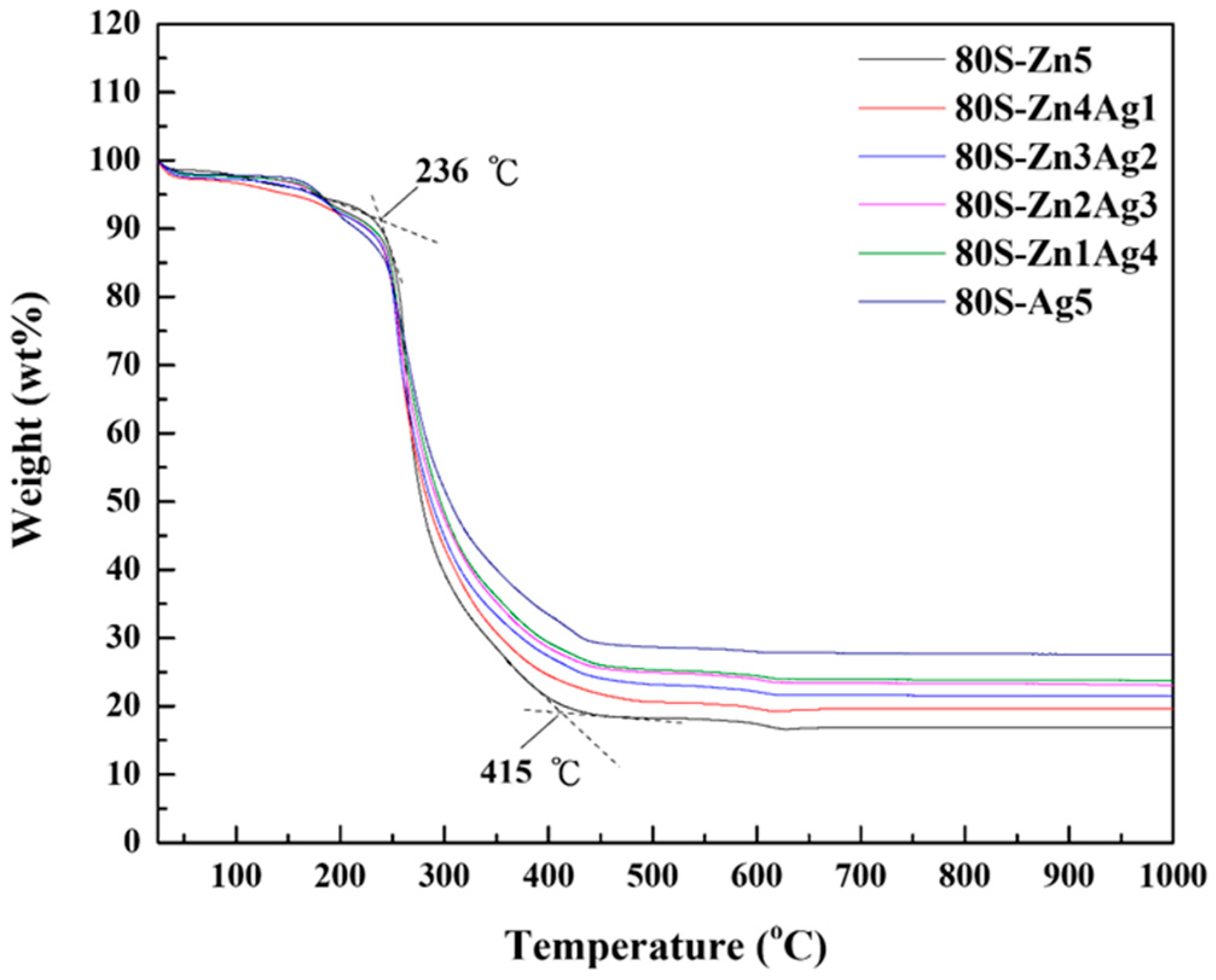
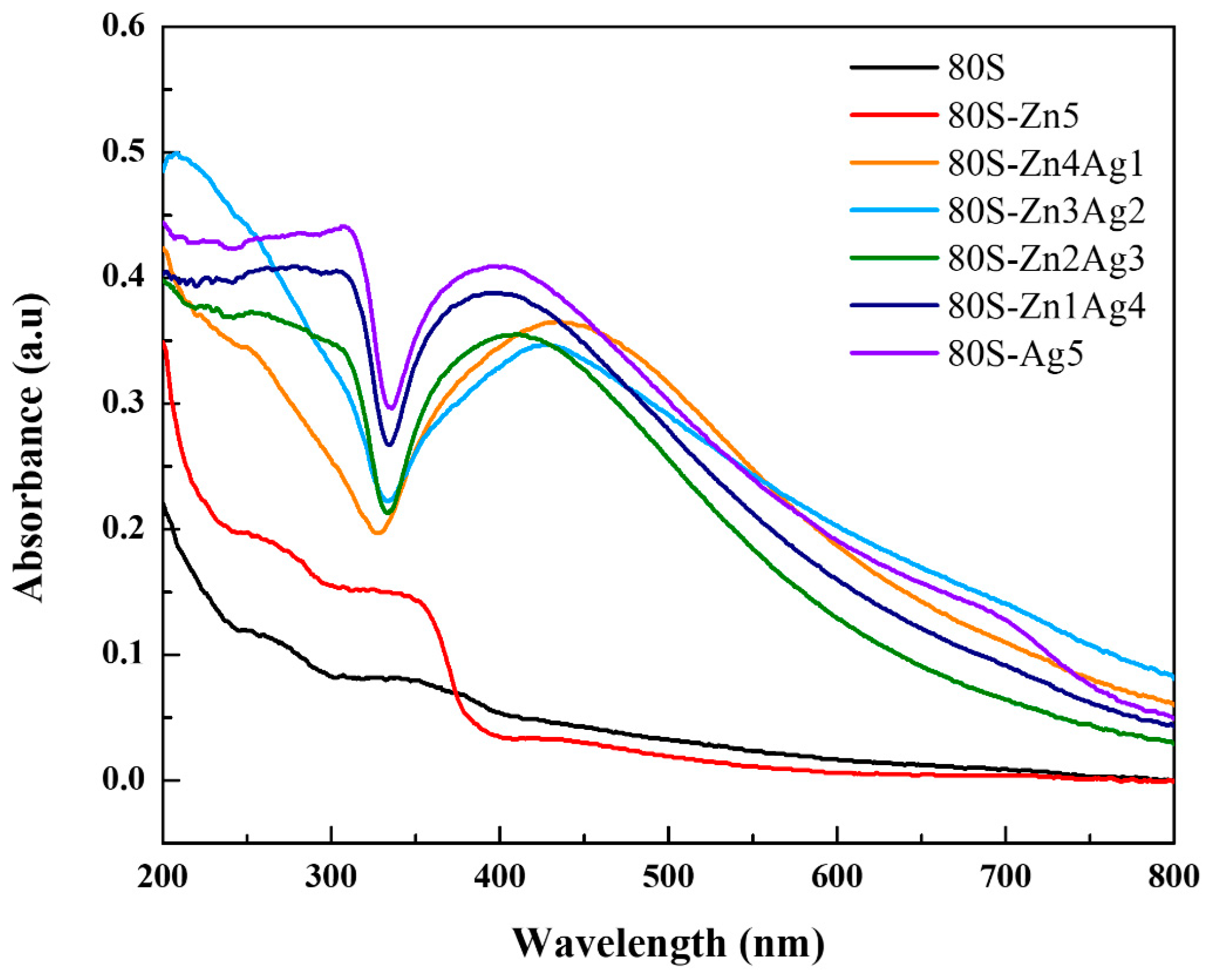
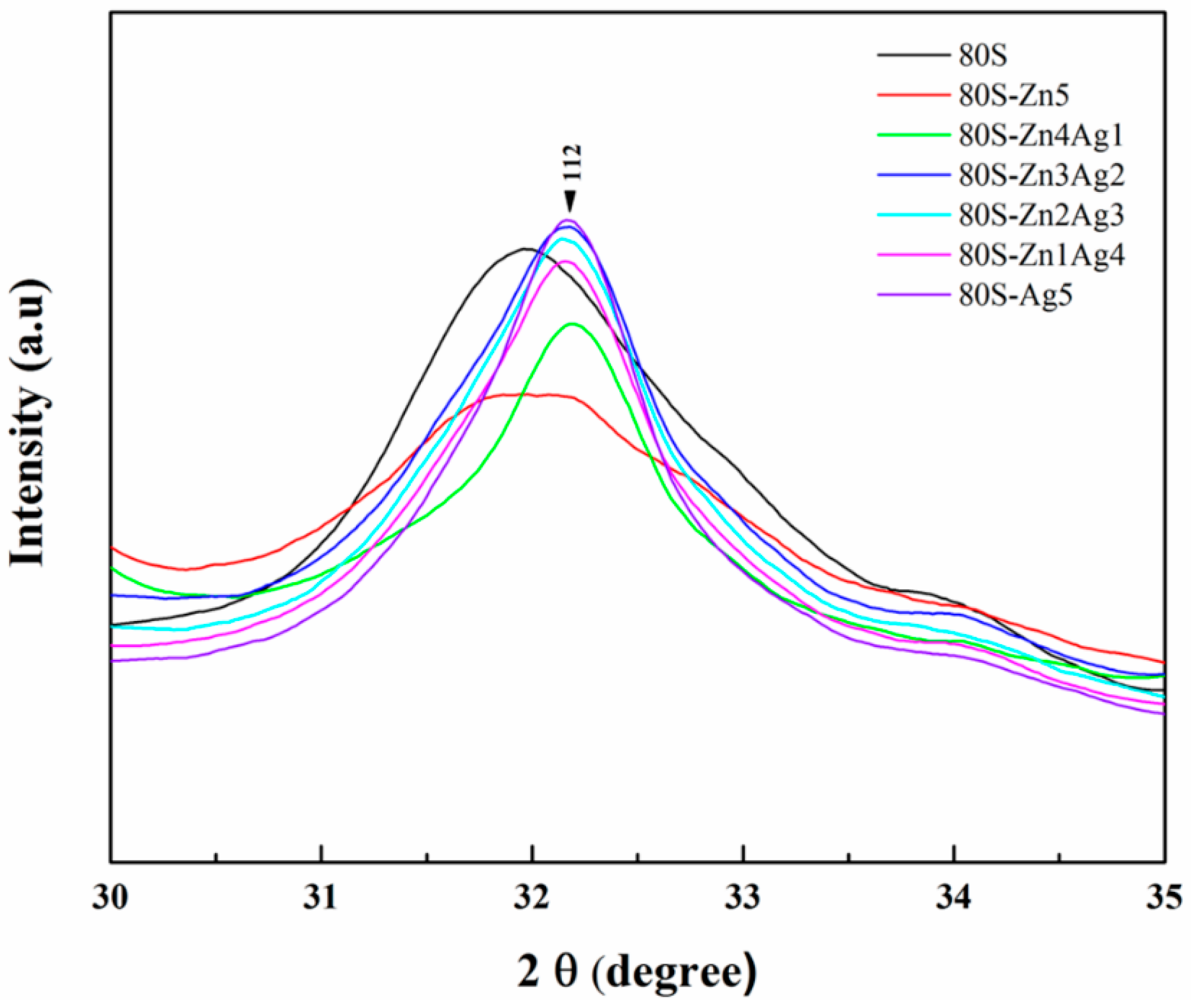
2.1. Characterization of 80S-ZnAg
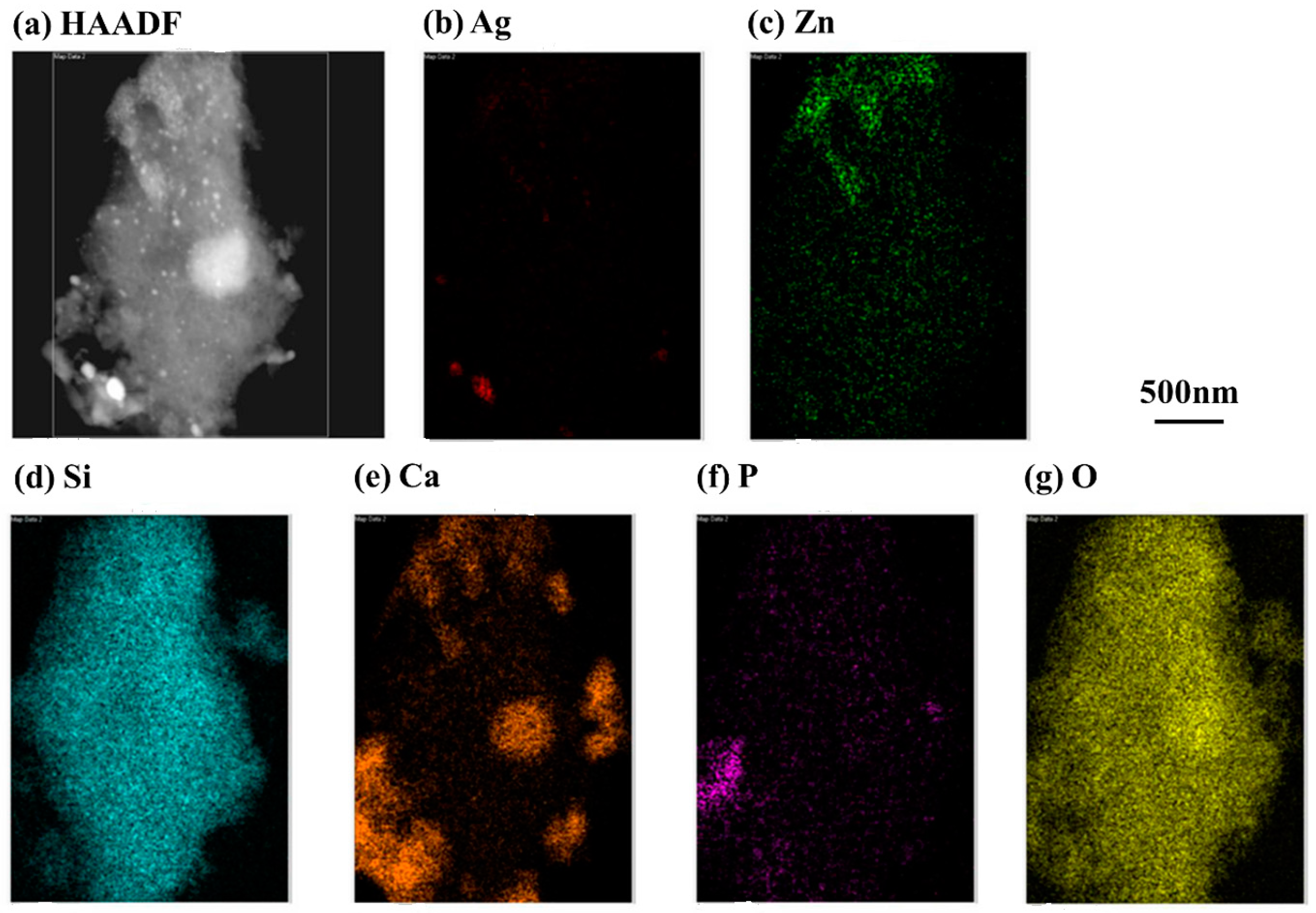
2.2. Chemical Characterization of 80S-Zn3Ag2
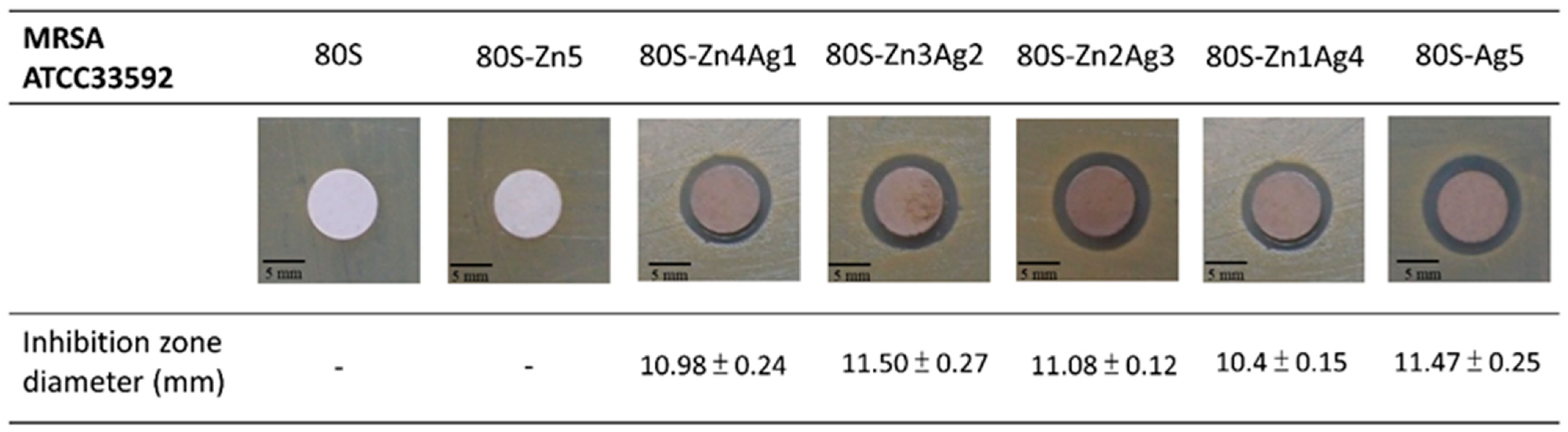
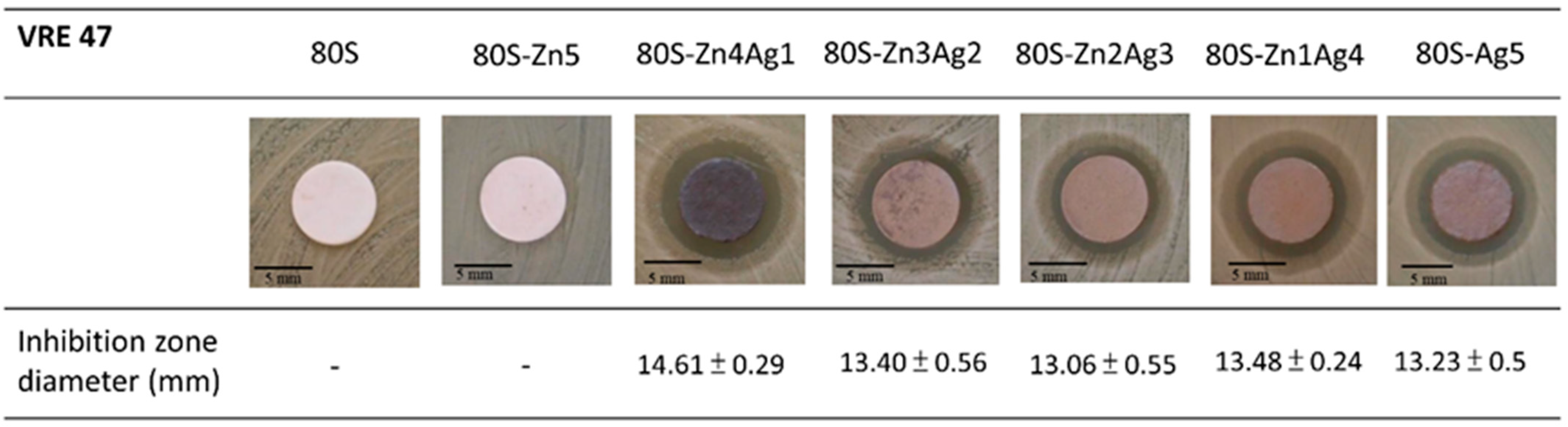
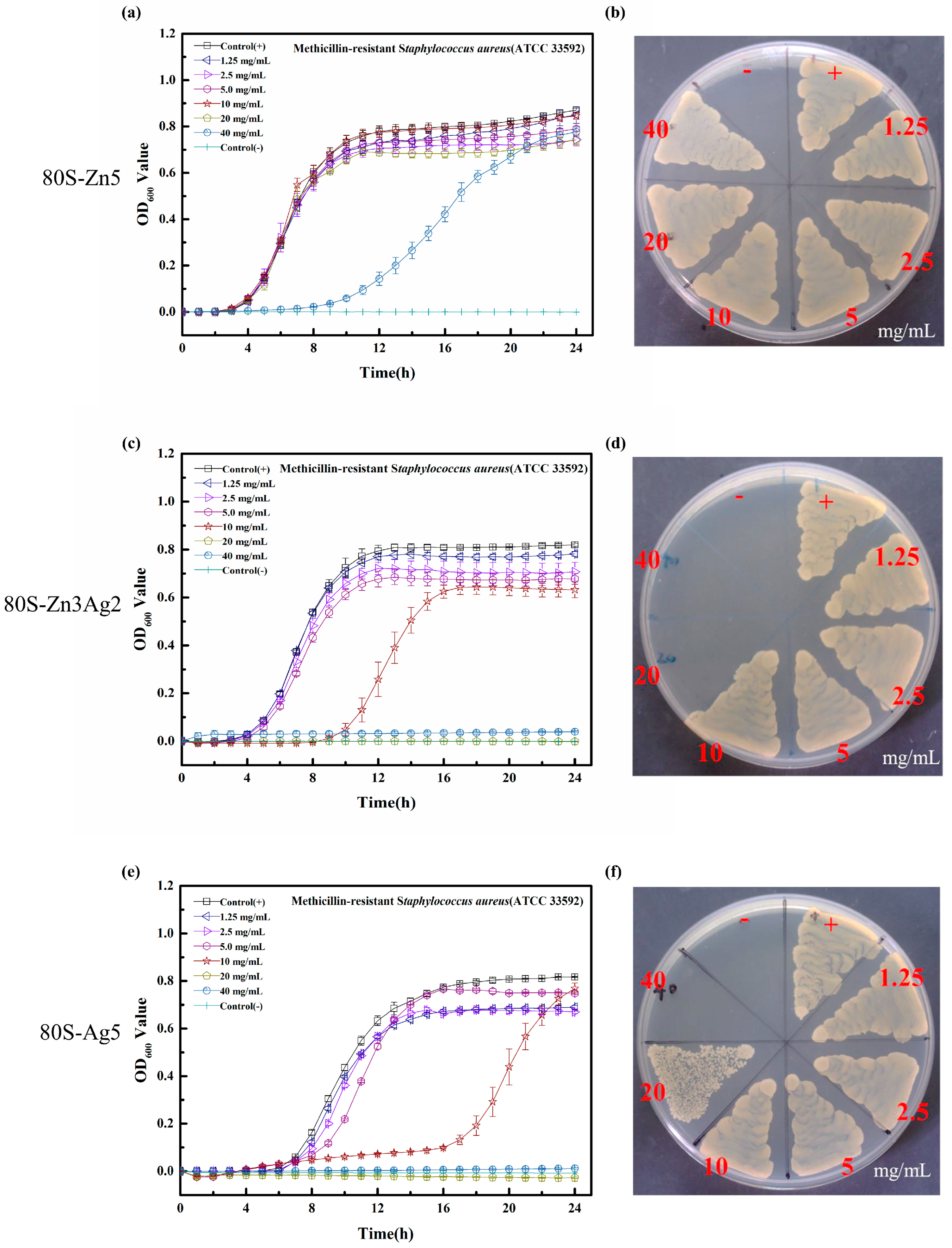
2.3. Antibacterial Activity of 80S-ZnAg
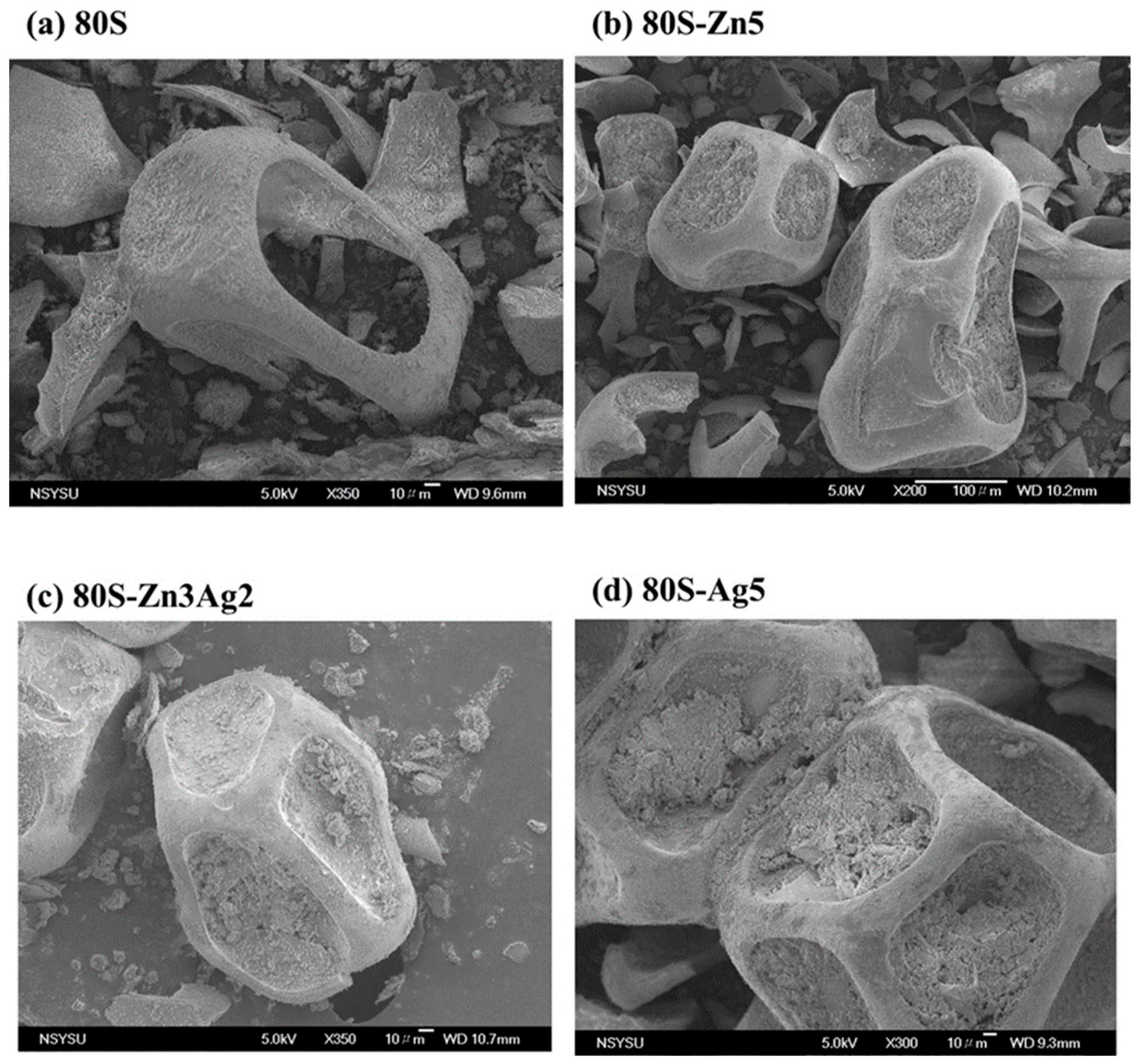
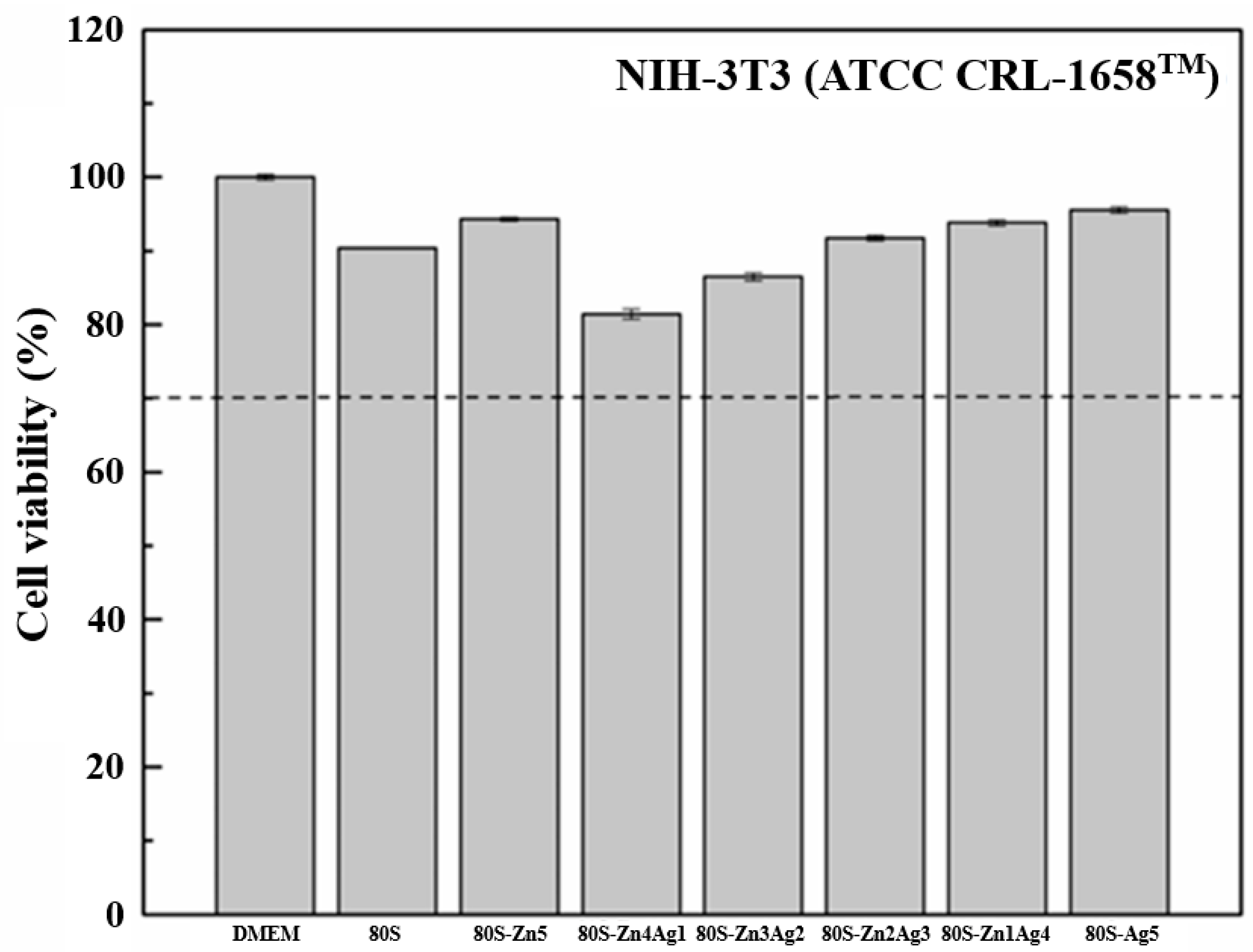
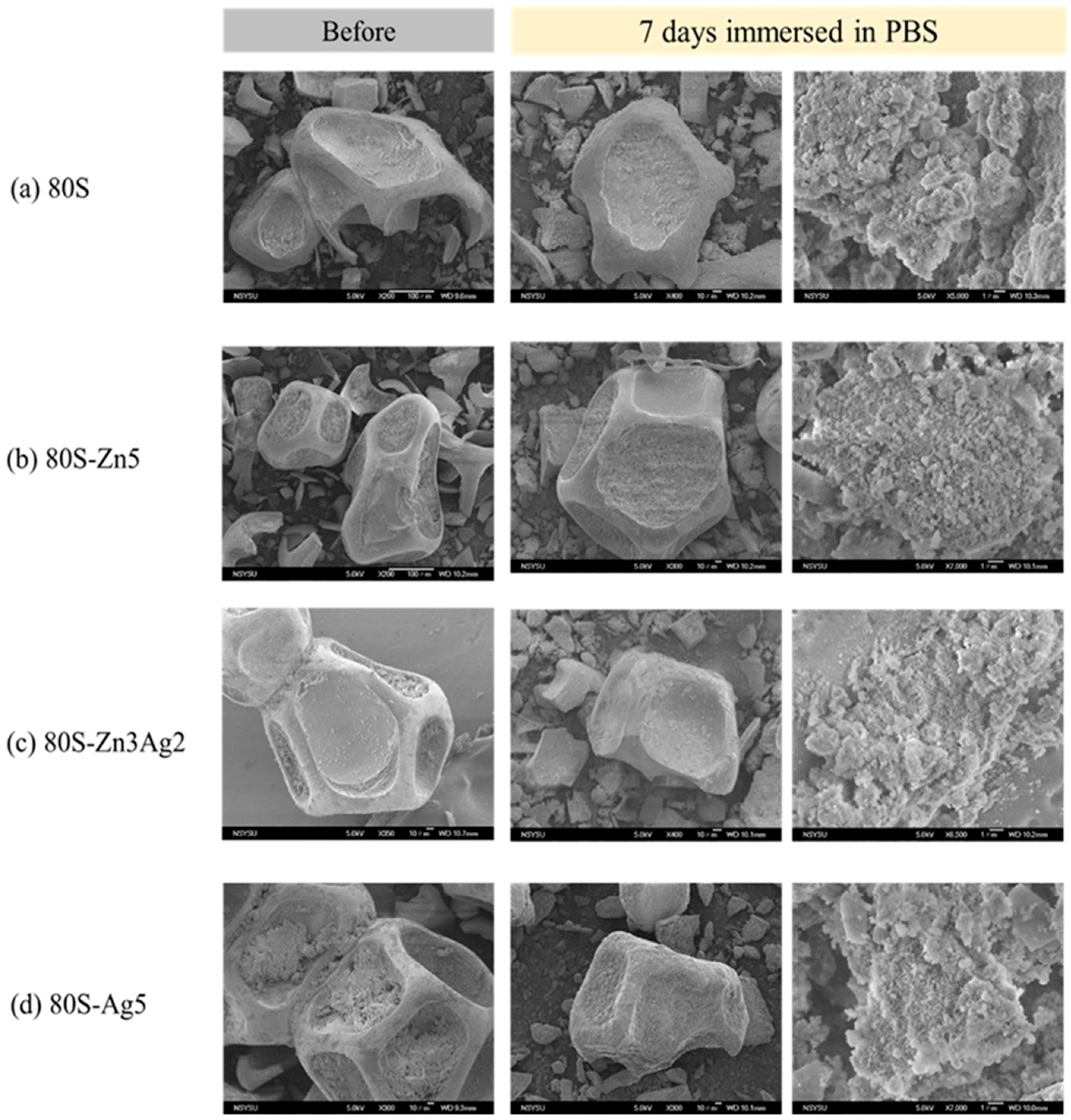
2.4. Bioactivity of 80S-ZnAg
3. Discussion
4. Materials and Methods
4.1. Preparation of 80S-ZnAg
4.2. Characterization of 80S-ZnAg
4.3. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
4.4. Solid-State Magic Angle Spinning Nuclear Magnetic Resonance (29Si MAS NMR)
4.5. Morphology of 80S-ZnAg
4.6. Bacterial Strains
4.7. Disk Diffusion
4.8. In Vitro Kinetic and Colony-Forming Assays
4.9. Bioactivity of 80S-ZnAg
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michno, A.; Nowak, A.; Królicki, L. Review of contemporary knowledge of osteomyelitis diagnosis. World Sci. News 2018, 92, 272–282. [Google Scholar]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Pajares-Chamorro, N.; Shook, J.; Hammer, N.D.; Chatzistavrou, X. Resurrection of antibiotics that methicillin-resistant Staphylococcus aureus resists by silver-doped bioactive glass-ceramic microparticles. Acta Biomater. 2019, 96, 537–546. [Google Scholar] [CrossRef]
- Lowy, F. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Boucher, H.W.; Corey, G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46 (Suppl. 5), S344–S349. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Medicine, Focus: Infectious diseases: Vancomycin resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar] [PubMed]
- Rôças, I.N.; Siqueira, J.F., Jr.; Santos, K.R. Association of Enterococcus faecalis with different forms of periradicular diseases. J. Endod. 2004, 30, 315–320. [Google Scholar] [CrossRef]
- Kanumuru, N.R.; Subbaiah, R. Bacterial efficacy of Ca(OH)2 against E. faecalis compared with three dental lasers on root canal dentin- an in vitro study. J. Clin. Diagn. Res. 2014, 8, ZC135–ZC137. [Google Scholar] [PubMed]
- Mohammadi, Z.; Dummer, P.M.H. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, H.; Dhillon, J.S.; Batra, M.; Saini, M. MTA versus Biodentine: Review of literature with a comparative analysis. J. Clin. Diagn. Res. 2017, 11, ZG01–ZG05. [Google Scholar] [CrossRef]
- Dalmia, S.; Gaikwad, A.; Samuel, R.; Aher, G.; Gulve, M.; Kolhe, S. Antimicrobial efficacy of different endodontic sealers against Enterococcus faecalis: An in vitro study. J. Int. Soc. Prev. Community Dent. 2018, 8, 104–109. [Google Scholar] [CrossRef]
- Zhang, D.; Hupa, M.; Hupa, L. In situ pH within particle beds of bioactive glasses. Acta Biomater. 2008, 4, 149820081505. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chen, H.J.; Liu, H.C.; Kang, S.H.; Lee, B.S.; Lin, F.H.; Lin, H.P.; Lin, C.P. A novel mesoporous biomaterial for treating dentin hypersensitivity. J. Dent. Res. 2010, 89, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Combes, C.; Drouet, C.; Cazalbou, S.; Grossin, D.; Brouillet, F.; Sarda, S. Surface properties of biomimetic nanocrystalline apatites: Applications in biomaterials. Prog. Cryst. Growth Charact. Mater. 2014, 60, 63–73. [Google Scholar] [CrossRef]
- Chen, X.; Meng, Y.; Li, Y.; Zhao, N. Investigation on bio-mineralization of melt and sol–gel derived bioactive glasses. Appl. Surf. Sci. 2008, 255, 562–564. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef]
- Hamadouche, M.; Meunier, A.; Greenspan, D.C.; Blanchat, C.; Zhong, J.P.; La Torre, G.P.; Sedel, L. Long-term in vivo bioactivity and degradability of bulk sol-gel bioactive glasses. J. Biomed. Mater. Res. 2001, 54, 560–566. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, W.; Xu, L.; Ye, Q.; Zhou, H.; Berg, C.; Yuan, H.; Li, J.; Xia, W. Sequential macrophage transition facilitates endogenous bone regeneration induced by Zn-doped porous microcrystalline bioactive glass. J. Mater. Chem. B 2021, 9, 2885–2898. [Google Scholar] [CrossRef]
- Perez, R.; Sanchez-Salcedo, S.; Lozano, D.; Heras, C.; Esbrit, P.; Vallet-Regi, M.; Salinas, A.J. Osteogenic effect of ZnO-mesoporous glasses loaded with osteostatin. Nanomaterials 2018, 8, 592. [Google Scholar] [CrossRef]
- Lalzawmliana, V.; Anand, A.; Roy, M.; Kundu, B.; Nandi, S.K. Mesoporous bioactive glasses for bone healing and biomolecules delivery. Mater. Sci. Eng. 2020, 106, 110180. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous bioactive glasses: Relevance of their porous structure compared to that of classical bioglasses. Biomed. Glas. 2015, 1, 140–150. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Zhang, Y.; Ji, L.; Huang, H.; Zhu, Y. Synthesis of monodispersed mesoporous bioactive glass nanospheres for bone repair. Mater. Lett. 2016, 171, 259–262. [Google Scholar]
- Wu, X.; Meng, G.; Wang, S.; Wu, F.; Huang, W.; Gu, Z. Zn and Sr incorporated 64S bioglasses: Material characterization, in-vitro bioactivity and mesenchymal stem cell responses. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 242–250. [Google Scholar] [CrossRef]
- Atkinson, I.; Anghel, E.; Predoana, L.; Mocioiu, O.; Jecu, L.; Raut, I.; Munteanu, C.; Culita, D.; Zaharescu, M. Influence of ZnO addition on the structural, in vitro behavior and antimicrobial activity of sol–gel derived CaO–P2O5–SiO2 bioactive glasses. Ceram. Int. 2016, 42, 3033–3045. [Google Scholar] [CrossRef]
- Capela, M.; Tobaldi, D.; Oliveira, C.; Pereira, A.; Duarte, A.; Seabra, M.; Fernandes, M. Bioactivity and antibacterial activity against E. coli of calcium-phosphate-based glasses: Effect of silver content and crystallinity. Ceram. Int. 2017, 43, 13800–13809. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Salvatori, R.; Maisetta, G.; Batoni, G.; Cannillo, V. Zinc containing bioactive glasses with ultra-high crystallization temperature, good biological performance and antibacterial effects. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109910. [Google Scholar] [CrossRef]
- Akhtach, S.; Tabia, Z.; El Mabrouk, K.; Bricha, M.; Belkhou, R. A comprehensive study on copper incorporated bio-glass matrix for its potential antimicrobial applications. Ceram. Int. 2021, 47, 424–433. [Google Scholar] [CrossRef]
- Cho, Y.-E.; Lomeda, R.-A.R.; Ryu, S.-H.; Sohn, H.-Y.; Shin, H.-I.; Beattie, J.H.; Kwun, I.-S. Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats. Nutr. Res. Pract. 2007, 1, 113–119. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Kanchanadumkerng, P.; Ruenraroengsak, P. Multifunctional Zn and Ag co-doped bioactive glass nanoparticles for bone therapeutic and regeneration. Sci. Rep. 2023, 13, 6775. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Kucuk, O.; Sarkar, F.H. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004, 37, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, N.Z.; Rink, L. Zinc in infection and inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Li, Y.-J.; Wong, K.W.; Huang, Y.-C.; Chien, C.-S.; Shih, C.-J. Evaluation of in vitro bioactivity and angiogenesis-promoting effect for mesoporous bioactive glass codoped with copper and silver. J. Non-Cryst. Solids 2023, 613, 122371. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Chávez-Reyes, A.; Castillo, E.C.; García-Rivas, G.; Ortega-Rivera, O.A.; Salinas, E.; Ortiz-Martínez, M.; Gómez-Flores, S.L.; Peña-Martínez, J.A.; Pepi-Molina, A. Synergistic antimicrobial effects of silver/transition-metal combinatorial treatments. Sci. Rep. 2017, 7, 903. [Google Scholar] [CrossRef] [PubMed]
- Bednar, J.; Svoboda, L.; Rybkova, Z.; Dvorsky, R.; Malachova, K.; Stachurova, T.; Matysek, D.; Foldyna, V. Antimicrobial synergistic effect between Ag and Zn in Ag-ZnO·mSiO2 silicate composite with high specific surface area. Nanomaterials 2019, 9, 1265. [Google Scholar] [CrossRef]
- Azizabadi, N.; Azar, P.A.; Tehrani, M.S.; Derakhshi, P. Synthesis and characteristics of gel-derived SiO2-CaO-P2O5-SrO-Ag2O-ZnO bioactive glass: Bioactivity, biocompatibility, and antibacterial properties. J. Non-Cryst. Solids 2021, 556, 120568. [Google Scholar] [CrossRef]
- Shahrabi, S.; Hesaraki, S.; Moemeni, S.; Khorami, M. Structural discrepancies and in vitro nanoapatite formation ability of sol–gel derived glasses doped with different bone stimulator ions. Ceram. Int. 2011, 37, 2737–2746. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, F.; Li, J. Effect of B2O3 on structure of glassy F-free CaO-SiO2-B2O3 systems by 29Si MAS NMR and Raman spectroscopy. JOM 2020, 72, 1414–1421. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Shruti, S.; Salinas, A.J.; Malavasi, G.; Menabue, L.; Vallet-Regi, M. In vitro antibacterial capacity and cytocompatibility of SiO2–CaO–P2O5 meso-macroporous glass scaffolds enriched with ZnO. J. Mater. Chem. 2014, 2, 4836–4847. [Google Scholar] [CrossRef]
- Shih, C.-J.; Lu, P.-S.; Chen, W.-C.; Chiang, Y.-W.; Chien, C.-S. Evaluation of gentamicin encapsulated hierarchically meso-macroporous silica-based calcium phosphates glass powders. Ceram. Int. 2014, 40, 15019–15025. [Google Scholar] [CrossRef]
- Lázaro, G.S.; Santos, S.C.; Resende, C.X.; Santos, E.A.D. Individual and combined effects of the elements Zn, Mg and Sr on the surface reactivity of a SiO2·CaO·Na2O·P2O5. Bioglass Syst. 2014, 386, 19–28. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tseng, S.-P.; Wu, S.-M.; Shih, C.-J. Compounds, Structure-dependence of anti-methicillin-resistant Staphylococcus aureus (MRSA) activity on ZnO-containing bioglass. J. Alloys Compd. 2020, 848, 156487. [Google Scholar] [CrossRef]
- Goh, E.G.; Xu, X.; McCormick, P.G. Effect of particle size on the UV absorbance of zinc oxide nanoparticles. Scr. Mater. 2014, 78, 49–52. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, L.; Kim, E.J.; Hahn, S.H. Electronic structure and optical properties of Zn(OH)2: LDA+ U calculations and intense yellow luminescence. RSC Adv. 2015, 5, 87496–87503. [Google Scholar] [CrossRef]
- Baino, F.; Yamaguchi, S. The use of simulated body fluid (SBF) for assessing materials bioactivity in the context of tissue engineering: Review and challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef]
- Calahoo, C.; Wondraczek, L. Ionic glasses: Structure, properties and classification. J. Non-Cryst. Solids X 2020, 8, 100054. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Kung, J.-C.; Tseng, S.-P.; Chen, W.-C.; Wu, S.-M.; Shih, C.-J. Effects of AgNPs on the structure and anti-methicillin resistant Staphylococcus aureus (MRSA) properties of SiO2-CaO-P2O5 bioactive glass. J. Non-Cryst. Solids 2021, 553, 120492. [Google Scholar] [CrossRef]
- Tam, K.; Djurišić, A.; Chan, C.; Xi, Y.; Tse, C.; Leung, Y.; Chan, W.; Leung, F.; Au, D. Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Film. 2008, 516, 6167–6174. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Yuan, Q.; Xu, A.; Zhang, Z.; Chen, Z.; Wan, L.; Shi, X.; Lin, S.; Yuan, Z.; Deng, L. Bioactive silver doped hydroxyapatite composite coatings on metal substrates: Synthesis and characterization. Mater. Chem. Phys. 2018, 218, 130–139. [Google Scholar] [CrossRef]
- Fujino, S.; Tokunaga, H.; Saiz, E.; Tomsia, A.P. Fabrication and characterization of bioactive glass coatings on Co-Cr implant alloys. Mater. Trans. 2004, 45, 1147–1151. [Google Scholar] [CrossRef]
- Koohkan, R.; Hooshmand, T.; Tahriri, M.; Mohebbi-Kalhori, D. Synthesis, characterization and in vitro bioactivity of mesoporous copper silicate bioactive glasses. Ceram. Int. 2018, 44, 2390–2399. [Google Scholar] [CrossRef]
- Yang, T.Y.; Hsieh, Y.J.; Lu, P.L.; Lin, L.; Wang, L.C.; Wang, H.Y.; Tsai, T.H.; Shih, C.J.; Tseng, S.P. In vitro and in vivo assessments of inspired Ag/80S bioactive nanocomposites against carbapenem-resistant Klebsiella pneumoniae. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 125, 112093. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yetter, A.B. Correlation of visual in vitro cytotoxicity ratings of biomaterials with quantitative in vitro cell viability measurements. Cell Biol. Toxicol. 2008, 24, 315–319. [Google Scholar] [CrossRef]



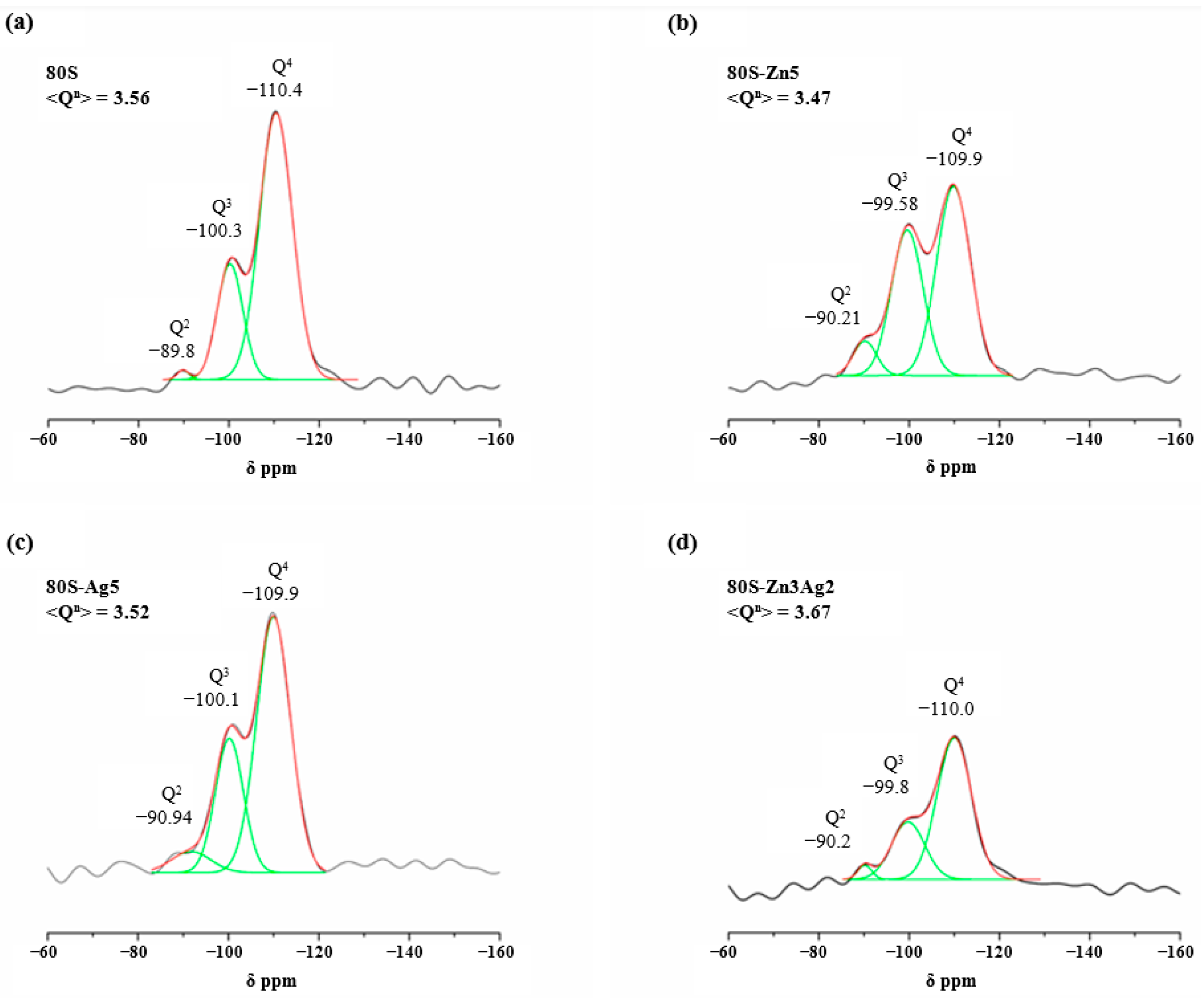


| Q4 | Q3 | Q2 | <Qn> | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CS, ppm | Area (%) | FWHM, ppm | CS, ppm | Area (%) | FWHM, ppm | CS, ppm | Area (%) | FWHM, ppm | ||
| 80S | −110.40 | 57.28 | 8.64 | −100.30 | 41.18 | 6.68 | −89.84 | 1.54 | 3.05 | 3.56 |
| 80S-Zn5 | −109.90 | 54.19 | 8.97 | −99.58 | 39.05 | 8.65 | −90.21 | 6.76 | 3.61 | 3.47 |
| 80S-Ag5 | −109.90 | 59.21 | 8.49 | −100.05 | 34.18 | 7.26 | −90.94 | 6.61 | 6.30 | 3.52 |
| 80S-Zn3Ag2 | −110.00 | 69.88 | 8.97 | −99.76 | 27.32 | 8.65 | −90.19 | 2.80 | 3.61 | 3.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-Y.; Chern, G.-I.; Wang, W.-H.; Shih, C.-J. Synthesis, Characterization, and Bioactivity of Mesoporous Bioactive Glass Codoped with Zinc and Silver. Int. J. Mol. Sci. 2023, 24, 13679. https://doi.org/10.3390/ijms241813679
Yang T-Y, Chern G-I, Wang W-H, Shih C-J. Synthesis, Characterization, and Bioactivity of Mesoporous Bioactive Glass Codoped with Zinc and Silver. International Journal of Molecular Sciences. 2023; 24(18):13679. https://doi.org/10.3390/ijms241813679
Chicago/Turabian StyleYang, Tsung-Ying, Guann-In Chern, Wei-Hsun Wang, and Chi-Jen Shih. 2023. "Synthesis, Characterization, and Bioactivity of Mesoporous Bioactive Glass Codoped with Zinc and Silver" International Journal of Molecular Sciences 24, no. 18: 13679. https://doi.org/10.3390/ijms241813679
APA StyleYang, T.-Y., Chern, G.-I., Wang, W.-H., & Shih, C.-J. (2023). Synthesis, Characterization, and Bioactivity of Mesoporous Bioactive Glass Codoped with Zinc and Silver. International Journal of Molecular Sciences, 24(18), 13679. https://doi.org/10.3390/ijms241813679









