Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients
Abstract
:1. Introduction
2. Results
2.1. Clinical, Inflammatory, and Endothelial Characteristics of Patients
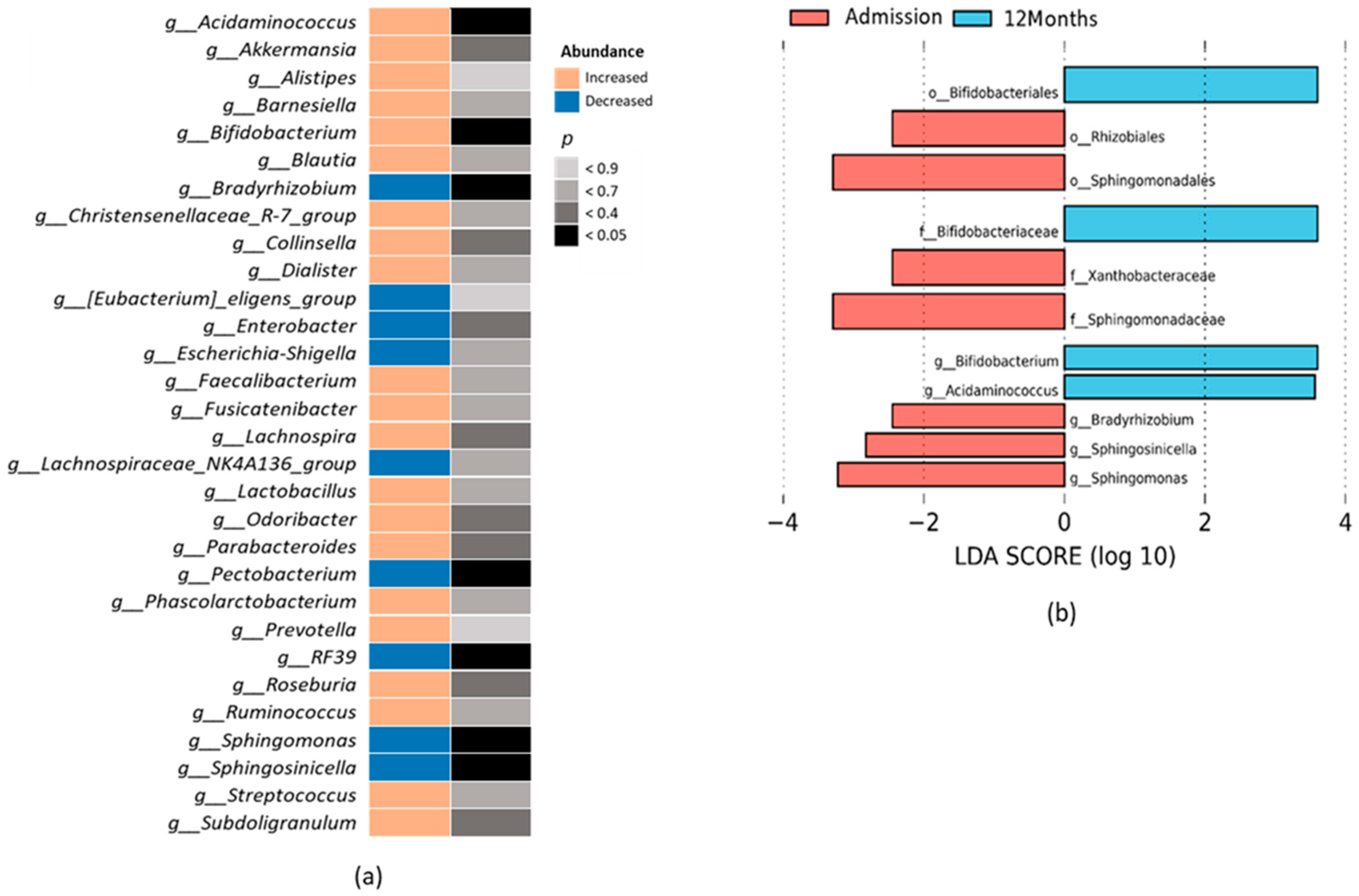
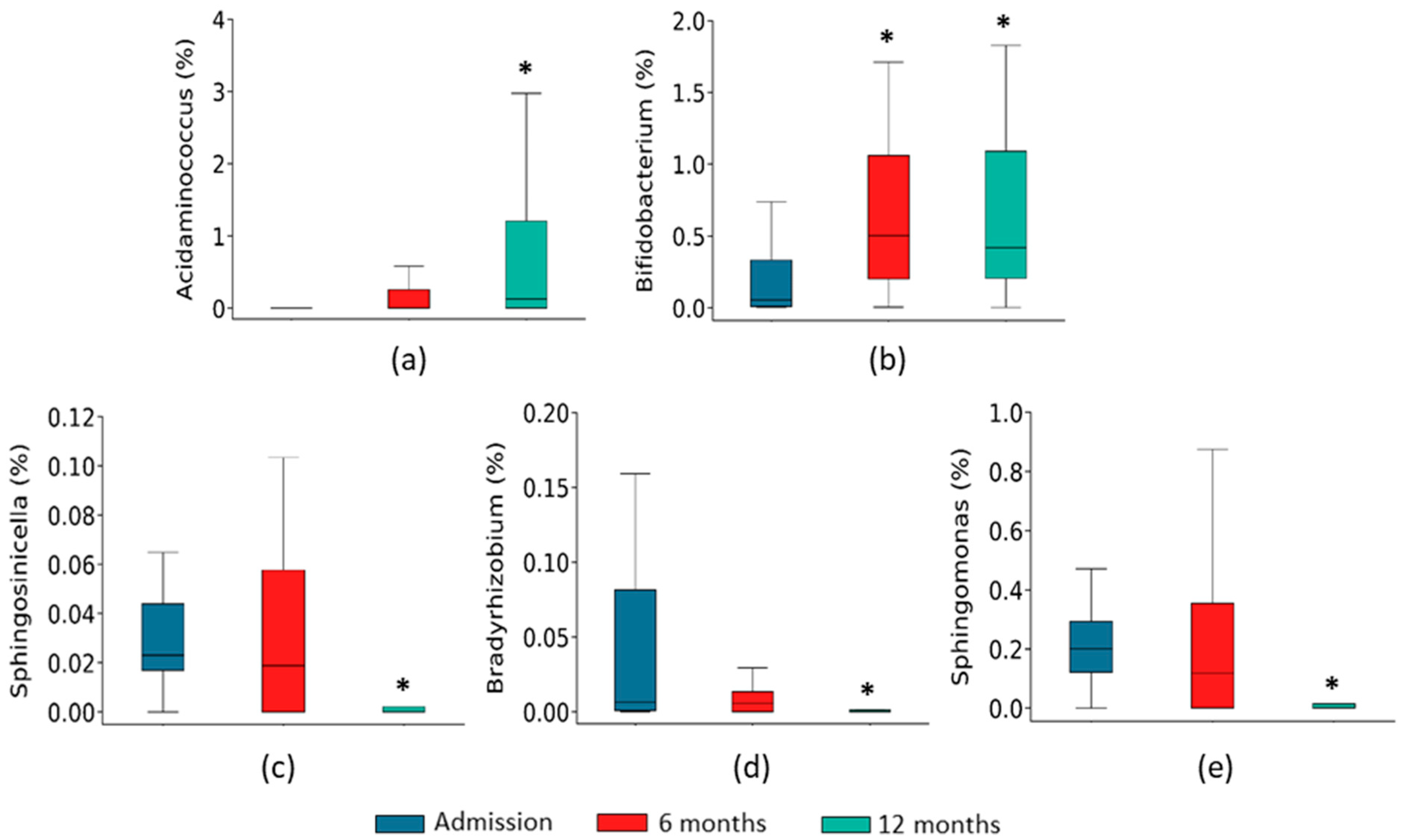
2.2. Gut Microbiota Composition Associated with the Course of HF
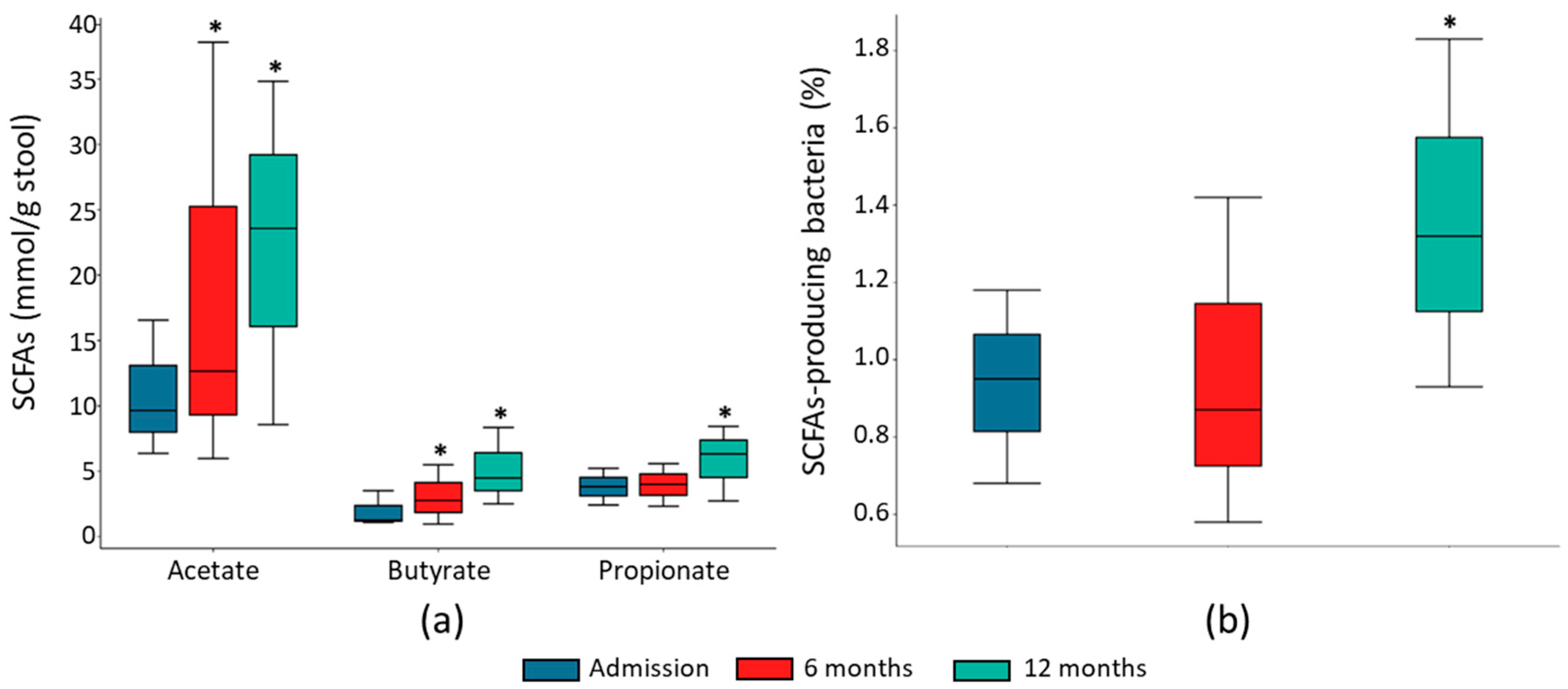
2.3. Fecal SCFA Concentration Associated with HF Course
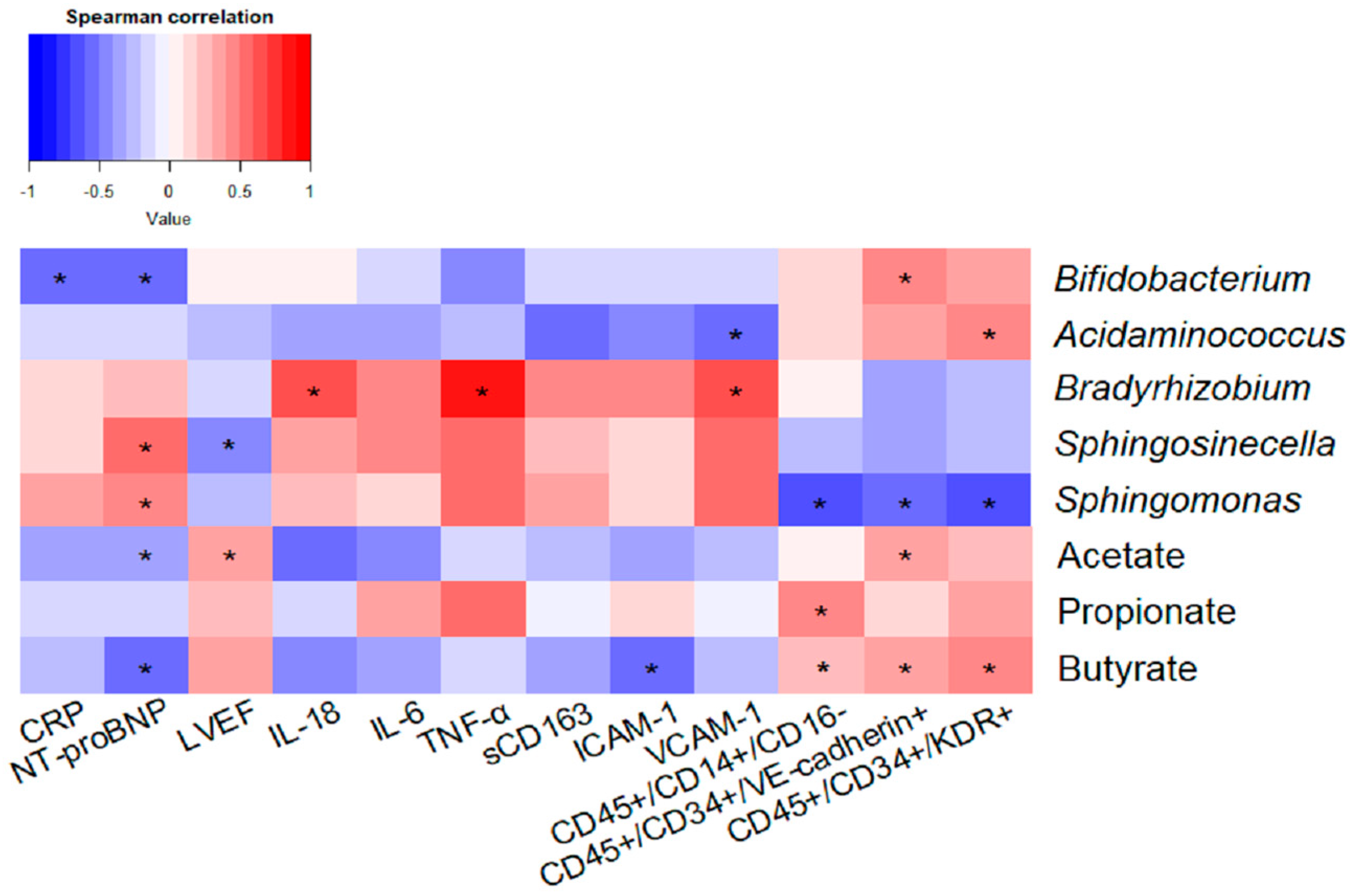
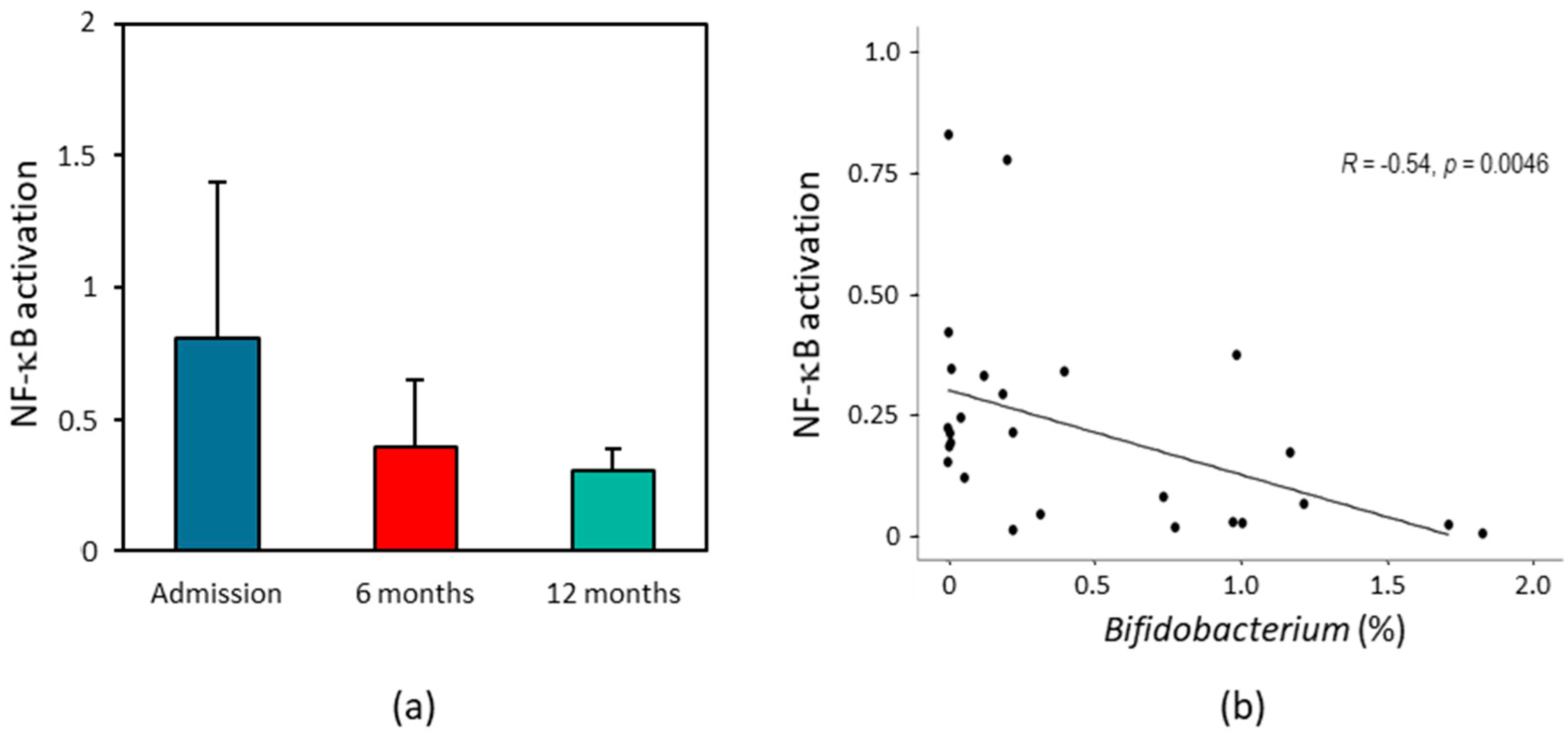
2.4. Impact of Taxonomic Biomarkers and SCFAs on Clinical, Inflammatory and Endothelial Function Markers
2.5. Effect of HF Patients’ Fecal Supernatant on Intestinal Inflammation
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Plasma Collection and Quantification of Proinflammatory Biomarkers
4.3. Flow Cytometry Analysis
4.4. Fecal Sample Collection and DNA Extraction
4.5. 16S rRNA Gene Sequencing and Bioinformatics Analysis
4.6. Measurement of SCFAs
4.7. In Vitro NF-kB Activation Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Seferović, P.M.; Vardas, P.; Jankowska, E.A.; Maggioni, A.P.; Timmis, A.; Milinković, I.; Polovina, M.; Gale, C.P.; Lund, L.H.; Lopatin, Y.; et al. National Heart Failure Societies of the ESC member countries. The heart failure association atlas: Heart failure epidemiology and management statistics 2019. Eur. J. Heart Fail. 2021, 23, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in heart failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Alem, M.M. Endothelial dysfunction in chronic heart failure: Assessment, findings, significance, and potential therapeutic targets. Int. J. Mol. Sci. 2019, 20, 3198. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Madan, S.; Mehra, M.R. Gut dysbiosis and heart failure: Navigating the universe within. Eur. J. Heart Fail. 2020, 22, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; O’Donnell, J.A.; Nakai, M.E.; Nanayakkara, S.; Vizi, D.; Carter, K.; Dean, E.; Ribeiro, R.V.; Yiallourou, S.; Carrington, M.J.; et al. The gut microbiome of heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2021, 10, e020654. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Mayerhofer, C.C.K.; Vestad, B.; Broch, K.; Awoyemi, A.; Storm-Larsen, C.; Ueland, T.; Yndestad, A.; Hov, J.R.; Trøseid, M. Gut microbiota signature in heart failure defined from profiling of 2 independent cohors. J. Am. Coll. Cardiol. 2018, 71, 1184–1186. [Google Scholar] [CrossRef]
- Sun, W.; Du, D.; Fu, T.; Han, Y.; Li, P.; Ju, H. Alterations of the gut microbiota in patients with severe chronic heart failure. Front. Microbiol. 2022, 12, 813289. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef]
- Mamic, P.; Heidenreich, P.A.; Hedlin, H.; Tennakoon, L.; Staudenmayer, K.L. Hospitalized patients with heart failure and common bacterial infections: A Nationwide Analysis of concomitant Clostridium difficile infection rates and in-hospital mortality. J. Card. Fail. 2016, 22, 891–900. [Google Scholar] [CrossRef]
- Yuzefpolskaya, M.; Bohn, B.; Nasiri, M.; Zuver, A.M.; Onat, D.D.; Royzman, E.A.; Nwokocha, J.; Mabasa, M.; Pinsino, A.; Brunjes, D.; et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. J. Heart Lung Transplant. 2020, 39, 880–890. [Google Scholar] [CrossRef]
- Luedde, M.; Winkler, T.; Heinsen, F.A.; Ruhlemann, M.C.; Spehlmann, M.E.; Bajrovic, A.; Lieb, W.; Franke, A.; Ott, S.J.; Frey, N. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017, 4, 282–290. [Google Scholar] [CrossRef]
- Huang, Z.; Mei, X.; Jiang, Y.; Chen, T.; Zhou, Y. Gut microbiota in heart failure patients with preserved ejection fraction (GUMPTION Study). Front. Cardiovasc. Med. 2022, 8, 803744. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Kamo, T.; Akazawa, H.; Suda, W.; Saga-Kamo, A.; Shimizu, Y.; Yagi, H.; Liu, Q.; Nomura, S.; Naito, A.T.; Takeda, N.; et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS ONE 2017, 12, e0174099. [Google Scholar] [CrossRef]
- Kitai, T.; Kirsop, J.; Tang, W.H. Exploring the microbiome in heart failure. Curr. Heart Fail. Rep. 2016, 13, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Fromentin, S.; Forslund, S.K.; Chechi, K.; Aron-Wisnewsky, J.; Chakaroun, R.; Nielsen, T.; Tremaroli, V.; Ji, B.; Prifti, E.; Myridakis, A.; et al. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 2022, 28, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Calabrés, E.; Ortega-Hernández, A.; Modrego, J.; Gómez-Gordo, R.; Caro-Vadillo, A.; Rodríguez-Bobada, C.; González, P.; Gómez-Garre, D. Gut microbiota profile identifies transition from compensated cardiac hypertrophy to heart failure in hypertensive rats. Hypertension 2020, 76, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.; Finkel, T. Circulating endothelial progenitor cells, vascular function and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Geerlings, S.Y.; Kostopoulos, W.M.; Belzer, C. Akkermansia muciniphila in the human gastrointestinal tract: When, where, and how? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef]
- Robert, C.; Chassard, C.; Lawson, P.A.; Bernalier-Donadille, A. Bacteroides cellulosilyticus sp. Nov., a cellulolytic bacterium from the human gut microbial community. Int. J. Syst. Evol. Microbiol. 2007, 57 Pt 7, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Taylor, T.D.; Ohno, H.; Hattori, M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 2012, 3, 449–454. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, L.; Xin, Y.; Xu, Z.; He, H.; Kong, J. Oxygen-inducible conversion of lactate to acetate in heterofermentative Lactobacillus brevis ATCC 367. Appl. Environ. Microbiol. 2017, 83, e01659-17. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A.; Schleicher, L.; Deusch, S.; Gätgens, J.; Steuber, J.; Seifer, J. Short-chain fatty acids modulate metabolic pathways and membrane lipids in Prevotella bryantii B14. Proteomes 2020, 8, 28. [Google Scholar] [CrossRef]
- Rey, F.E.; Faith, J.J.; Bain, J.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; Gordon, J.I. Dissecting the in vivo metabolic potential of two human gut acetogens. J. Biol. Chem. 2010, 285, 22082–22090. [Google Scholar] [CrossRef]
- Chang, S.C.; Shen, M.H.; Liu, C.Y.; Pu, C.M.; Hu, J.M.; Huang, C.J. A gut butyrate-producing bacterium Butyricicoccus pullicaecorum regulates short-chain fatty acid transporter and receptor to reduce the progression of 1,2-dimethylhydrazine-associated colorectal cancer. Oncol. Lett. 2020, 20, 327. [Google Scholar] [CrossRef]
- Mallott, E.K.; Amato, K.R. Butyrate production pathway abundances are similar in human and nonhuman primate gut microbiomes. Mol. Biol. Evol. 2022, 39, msab279. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, S.; Furuya, H.; Nagashima, K.; Asanuma, N.; Hino, T. Oral administration of butyrivibrio fibrisolvens, a butyrate-producing bacterium, decreases the formation of aberrant crypt foci in the colon and rectum of mice. J. Nutr. 2005, 135, 2878–2883. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic butyrate-producing communities in humans: An overview using omics data. mSystems 2017, 2, e00130-17. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, I.S.; Pereira, J.A.; Barbosa, S.C.; Gonsalez-Machado, M.S.; Carlos, D. Akkermansia muciniphila and gut immune system: A good friendship that attenuates inflammatory bowel disease, obesity, and diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef]
- Dinakaran, V.; Rathinavel, A.; Pushpanathan, M.; Sivakumar, R.; Gunasekaran, P.; Rajendhran, J. Elevated levels of circulating DNA in cardiovascular disease patients: Metagenomic profiling of microbiome in the circulation. PLoS ONE 2014, 9, e105221. [Google Scholar] [CrossRef]
- Marques da Silva, R.; Caugant, D.A.; Eribe, E.R.; Aas, J.A.; Lingaas, P.S.; Geiran, O.; Tronstad, L.; Olsen, I. Bacterial diversity in aortic aneurysms determined by 16S ribosomal RNA gene analysis. J. Vasc. Surg. 2006, 44, 1055–1060. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, H.; Lu, H.; Chen, X.; Zhou, L.; Zhang, J.; Wu, J.; Dong, C. Associations between peripheral blood microbiome and the risk of hypertension. Am. J. Hypertens. 2021, 34, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Shumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef]
- Sandek, A.; Swidsinski, A.; Schroedl, W.; Watson, A.; Valentova, M.; Herrmann, R.; Scherbakov, N.; Cramer, L.; Rauchhaus, M.; Grosse-Herrenthey, A.; et al. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J. Am. Coll. Cardiol. 2014, 64, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.P.; Grisanti, L.A. The dynamic interplay between cardiac inflammation and fibrosis. Front. Physiol. 2020, 11, 529075. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Zuo, L.; Zhu, W.; Wang, B.; Li, Q.; Li, J. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br. J. Nutr. 2013, 109, 1990–1998. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Dwivedi, G.; O’Gara, F.; Caparros-Martin, J.; Ward, N.C. The gut microbiome and cardiovascular disease: Current knowledge and clinical potential. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H923–H938. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple facets of NF-kappaB in the heart: To be or not to NF-kappaB. Circ. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, H.M.; Yang, K.M.; Kim, S.A.; Kim, S.K.; An, M.J.; Park, J.J.; Lee, S.K.; Kim, T.I.; Kim, W.H.; et al. Bifidobacterium lactis inhibits NF-kappaB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice. Inflamm. Bowel Dis. 2010, 16, 1514–1525. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Cheng, Y.; Liu, X.; Huang, Y. Secreted factors from Bifidobacterium animalis subsp. lactis inhibit NF-κB-mediated interleukin-8 gene expression in Caco-2 cells. Appl. Environ. Microbiol. 2011, 77, 8171–8174. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Barba-Ostria, C.; Simancas-Racines, D.; Guamán, L.P. Protective role of butyrate in obesity and diabetes: New insights. Front. Nutr. 2022, 9, 1067647. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, K.; Wei, J.; Ding, Y.; Wang, X.; Hou, H.; Wu, J.; Liu, T.; Wang, B.; Cao, H. Gut microbiota-derived short-chain fatty acids regulate gastrointestinal tumor immunity: A novel therapeutic strategy? Front. Immunol. 2023, 14, 1158200. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Ortega-Madueño, I.; Modrego, J.; Gómez-Gordo, R.; Ortega-Hernández, A.; Pérez de Isla, L.; Muñoz, J.C.; Nieto, M.L.; Gómez-Garre, D. Relationship between the coronary artery calcium quantification and gut microbiota composition in subjects without previous cardiovascular disease: A pilot study. Clin. Investig. Arterioscler. 2022, 34, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, B.L.; Wilson, A.S.; O’Keefe, S.J.D.; Ramaboli, M.C.; Nesengani, L.T. A simplified method for the quantitation of short-chain fatty acids in human stool. Anal. Biochem. 2021, 612, 114016. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Gonçalves, T.; Martins, Z.E.; Bäuerl, C.; Cortés-Macías, E.; Collado, M.C.; Ferreira, I. Mycotoxin interactions along the gastrointestinal tract: In vitro semi-dynamic digestion and static colonic fermentation of a contaminated meal. Toxins 2022, 14, 28. [Google Scholar] [CrossRef] [PubMed]


| Admission | 12 Months | |
|---|---|---|
| Age, years | 67.6 ± 4.1 | 68.3 ± 4.3 |
| Male/female, n | 7/11 | - |
| Smokers, n (%) | 8 (44.4) | - |
| T2DM, n (%) | 7 (38.9) | - |
| Dyslipidemia, n (%) | 10 (55.6) | - |
| CHD, n (%) | 2 (11.1) | - |
| CVD, n (%) | 1 (5.6) | - |
| PAD, n (%) | 1 (5.6) | - |
| SBP, mmHg | 136 ± 4 | 129 ± 9 |
| DBP, mmHg | 83 ± 4 | 77 ± 4 |
| HR, bpm | 91.3 ± 5.3 | 61.4 ± 2.7 |
| NT-proBNP, pg/mL | 7,081 ± 3544 | 358 ± 69 |
| LVEF, % | 36.2 ± 4.3 | 56.7 ± 3.5 |
| Patients with LVEF > 40%, n (%) | 7 (38.9) | 13 (81.2) |
| LVEF in patients with pLVEF *, % | 56.4 ± 4.3 | 62.7 ± 5.6 |
| LVEF in patients with rLVEF *, % | 26.5 ± 2.8 | 52.1 ± 4.4 |
| NYHA class, n (%) | ||
| I/II | 0 (0) | 15 (93.8) |
| III | 12 (66.7) | 1 (6.2) |
| IV | 6 (33.3) | 0 (0) |
| Medications, n (%) | ||
| ACEI/ARB | 7 (38.9) | 13 (81.3) |
| Statins | 7 (38.9) | 8 (50.0) |
| Diuretics | 2 (11.1) | 10 (62.5) |
| β-blockers | 4 (22.2) | 14 (87.5) |
| MRAs | 0 (0) | 8 (50.0) |
| OADs | 6 (33.3) | 9 (56.3) |
| Admission | After 12 Months | |
|---|---|---|
| Circulating proinflammatory biomarkers | ||
| D-dimer, µg/mL | 795.7 (493.2–1217.7) | 563.2 (362.6–703.8) |
| ICAM-1, µg/mL | 512.3 (480.6–596.8) | 427.5 (374.9–480.2) * |
| IL-1β, ng/mL | 0.3 (0.2–0.5) | 0.3 (0.2–0.4) |
| IL-6, ng/mL | 2.9 (2.4–4.5) | 2.3 (1.6–3.4) * |
| IL-18, ng/mL | 186 (132.3–228.3) | 158 (109.9–193.5) * |
| CRP, mg/L | 7.5 (3.7–22.2) | 2.9 (2.7–5.3) * |
| sCD14, mg/mL | 1.4 (1.3–1.6) | 1.4 (1.2–1.6) |
| sCD163, mg/mL | 0.8 (0.7–1.1) | 0.6 (0.5–0.8) * |
| TNF-α, ng/mL | 8.9 (6.4–9.6) | 7.3 (5.4–8.3) * |
| VCAM-1, mg/mL | 1.1 (0.8–1.3) | 0.8 (0.7–1.0) * |
| Monocyte subset populations | ||
| Classical CD45+/CD14+/CD16−, % | 65.3 (43.7–68.0) | 76.5 (66.9–87.0) * |
| Intermediate CD45+/CD14+/CD16+, % | 18.7 (16.1–30.4) | 16.1 (7.9–23.5) |
| Non-classical CD45+/CD14low/CD16+, % | 9.5 (6.8–10.6) | 4.5 (3.1–12.5) |
| Circulating EPCs | ||
| CD45+/CD34+/VE-cadherin+, % | 0.05 (0.02–0.06) | 0.25 (0.10–0.76) * |
| CD45+/CD34+/KDR+, % | 0.02 (0.01–0.04) | 0.10 (0.06–0.7) * |
| SCFA | SCFA-Producing Bacteria | References |
|---|---|---|
| Acetate | Akkermansia, Bacteroides, Bifidobacterium, Lactobacillus, Prevotella, Ruminococcus | [26,27,28,29,30,31] |
| Butyrate | Anaerostipes, Butyricicoccus, Butyricimonas, Butyrivibrio, Coprococcus, Eubacterium, Faecalibacterium, Flavonifractor, Odoribacter, Roseburia | [32,33,34,35,36] |
| Propionate | Akkermansia, Roseburia, Veillonella, Phascolarctobacterium, Bacteroides, Coprococcus, Dialister, Prevotella | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrego, J.; Ortega-Hernández, A.; Goirigolzarri, J.; Restrepo-Córdoba, M.A.; Bäuerl, C.; Cortés-Macías, E.; Sánchez-González, S.; Esteban-Fernández, A.; Pérez-Villacastín, J.; Collado, M.C.; et al. Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients. Int. J. Mol. Sci. 2023, 24, 13892. https://doi.org/10.3390/ijms241813892
Modrego J, Ortega-Hernández A, Goirigolzarri J, Restrepo-Córdoba MA, Bäuerl C, Cortés-Macías E, Sánchez-González S, Esteban-Fernández A, Pérez-Villacastín J, Collado MC, et al. Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients. International Journal of Molecular Sciences. 2023; 24(18):13892. https://doi.org/10.3390/ijms241813892
Chicago/Turabian StyleModrego, Javier, Adriana Ortega-Hernández, Josebe Goirigolzarri, María Alejandra Restrepo-Córdoba, Christine Bäuerl, Erika Cortés-Macías, Silvia Sánchez-González, Alberto Esteban-Fernández, Julián Pérez-Villacastín, María Carmen Collado, and et al. 2023. "Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients" International Journal of Molecular Sciences 24, no. 18: 13892. https://doi.org/10.3390/ijms241813892






