Can Pyomelanin Produced by Pseudomonas aeruginosa Promote the Regeneration of Gastric Epithelial Cells and Enhance Helicobacter pylori Phagocytosis?
Abstract
:1. Introduction
2. Results
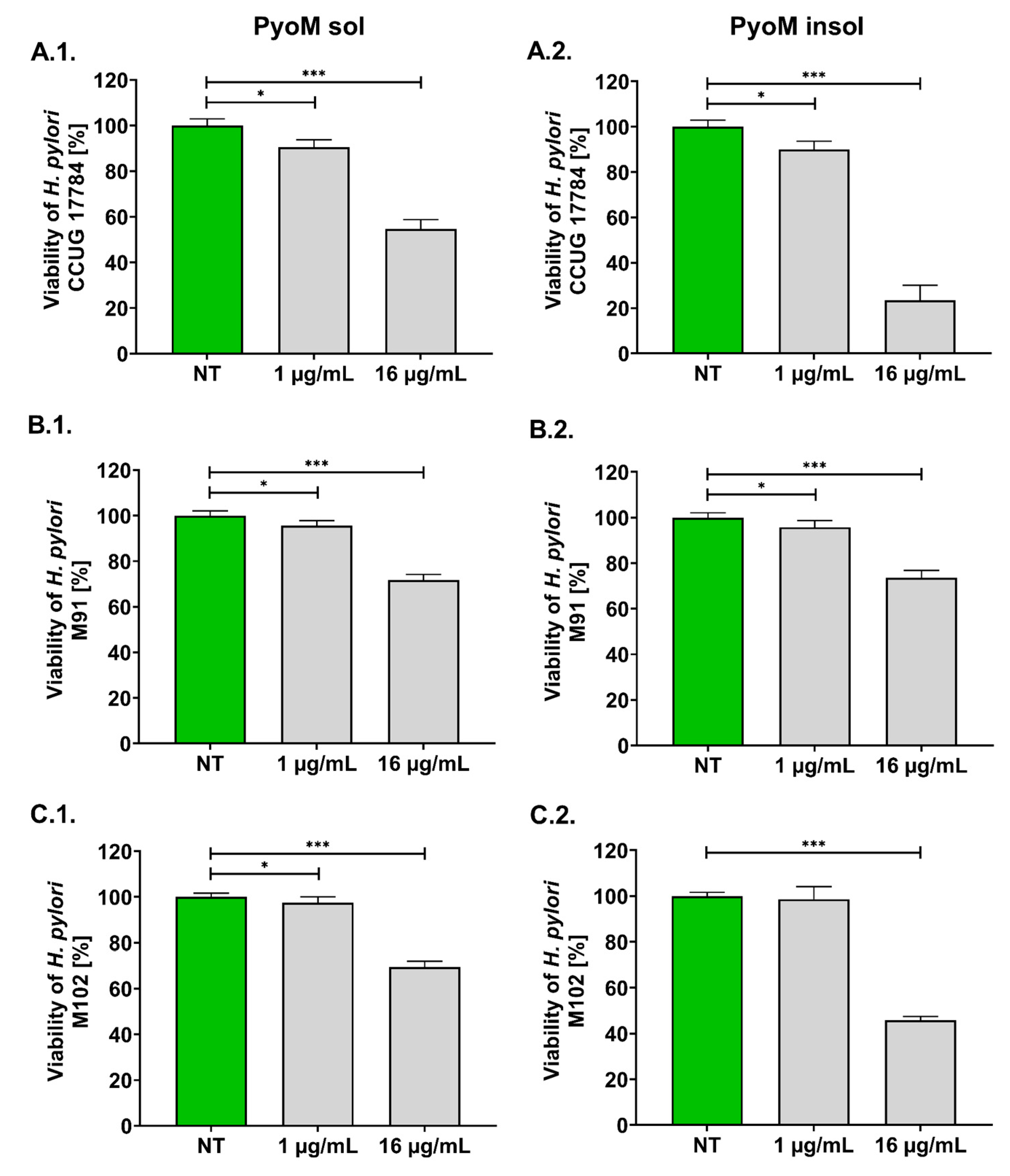
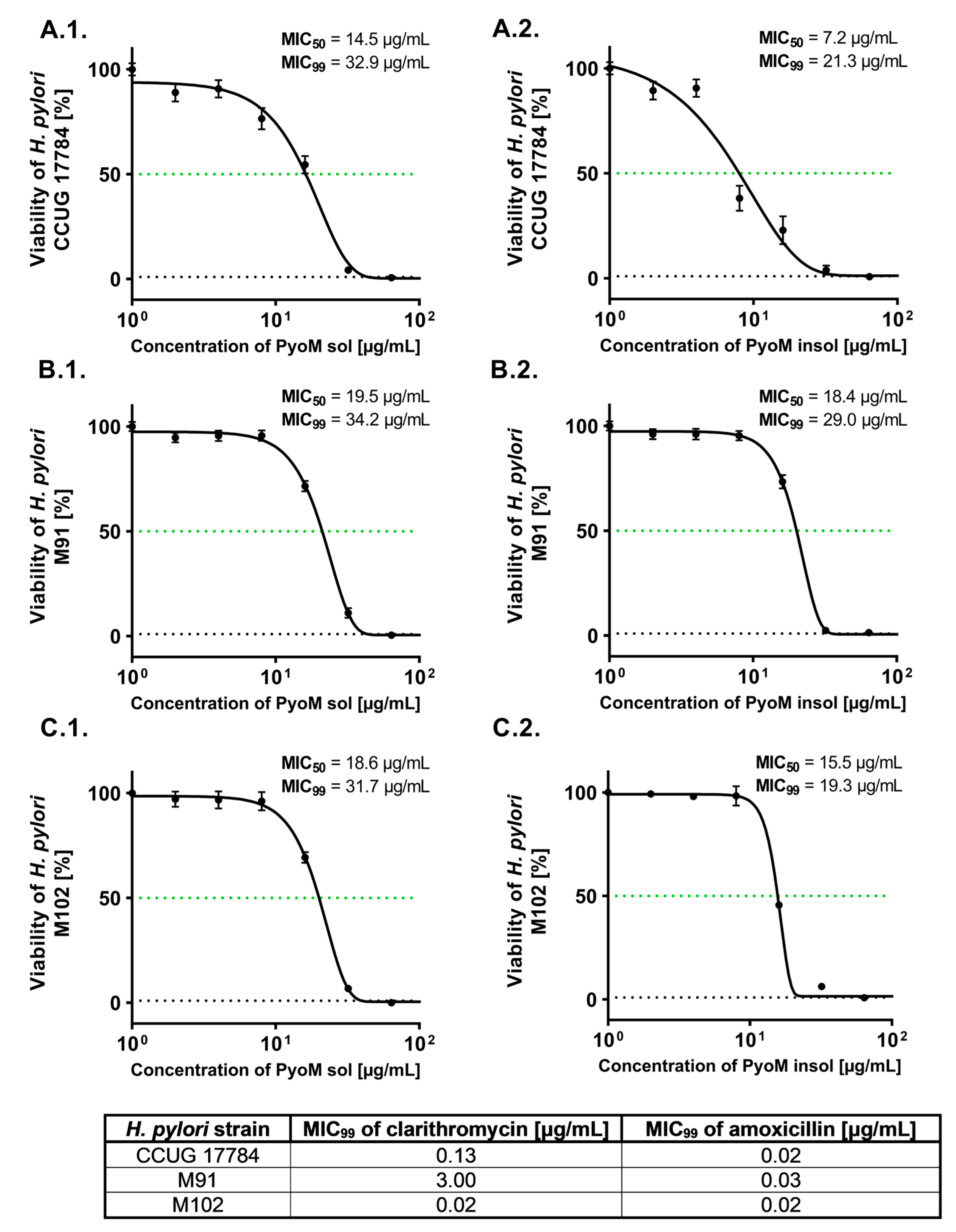
2.1. Antibacterial Activity of PyoM towards H. pylori
2.2. PyoM Neutralizes the Cytotoxic Effect of H. pylori LPS towards Gastric Epithelial Cells and Monocytes
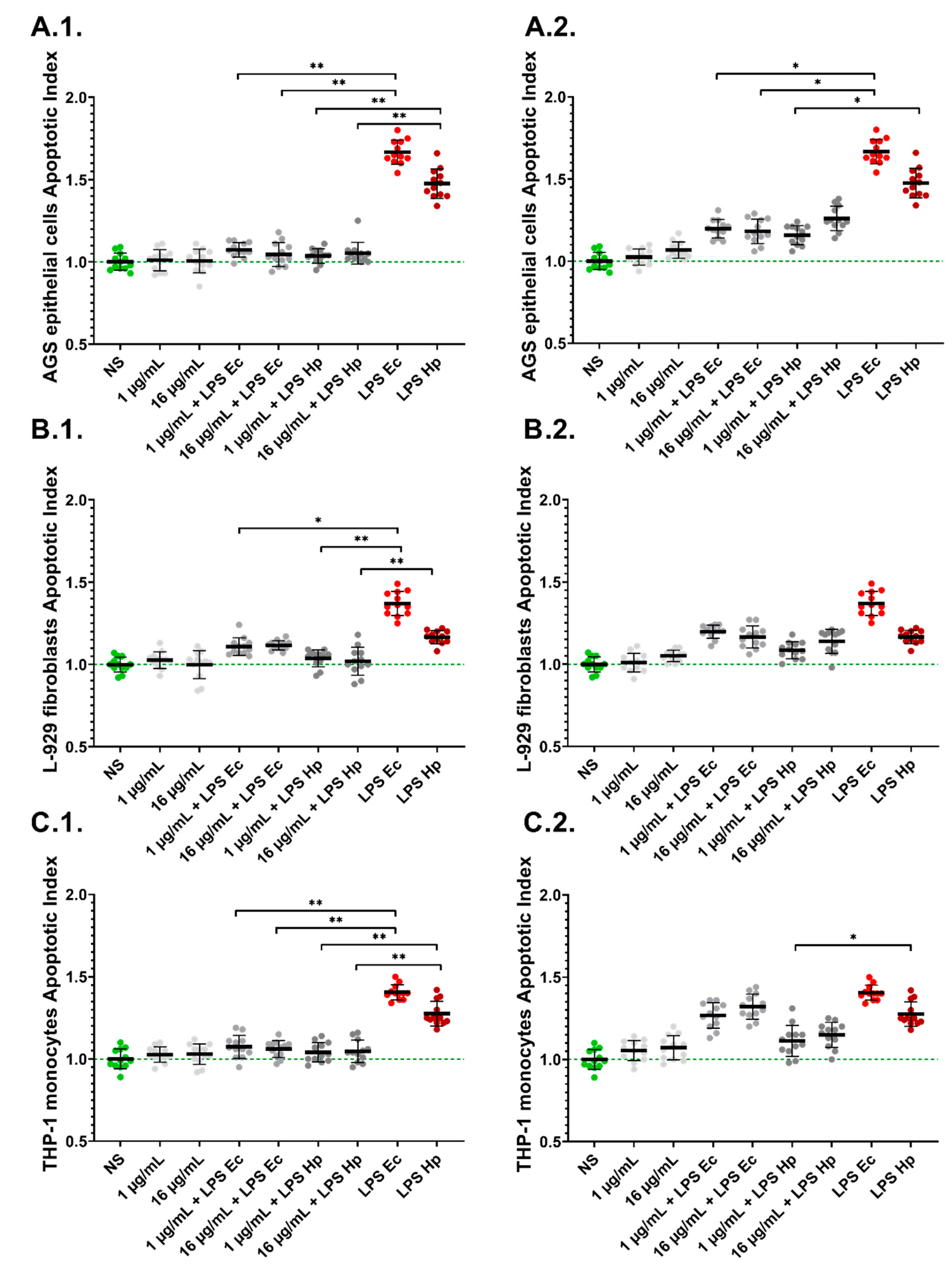
2.3. LPS-Induced Apoptosis Is Diminished in the Presence of PyoMsol
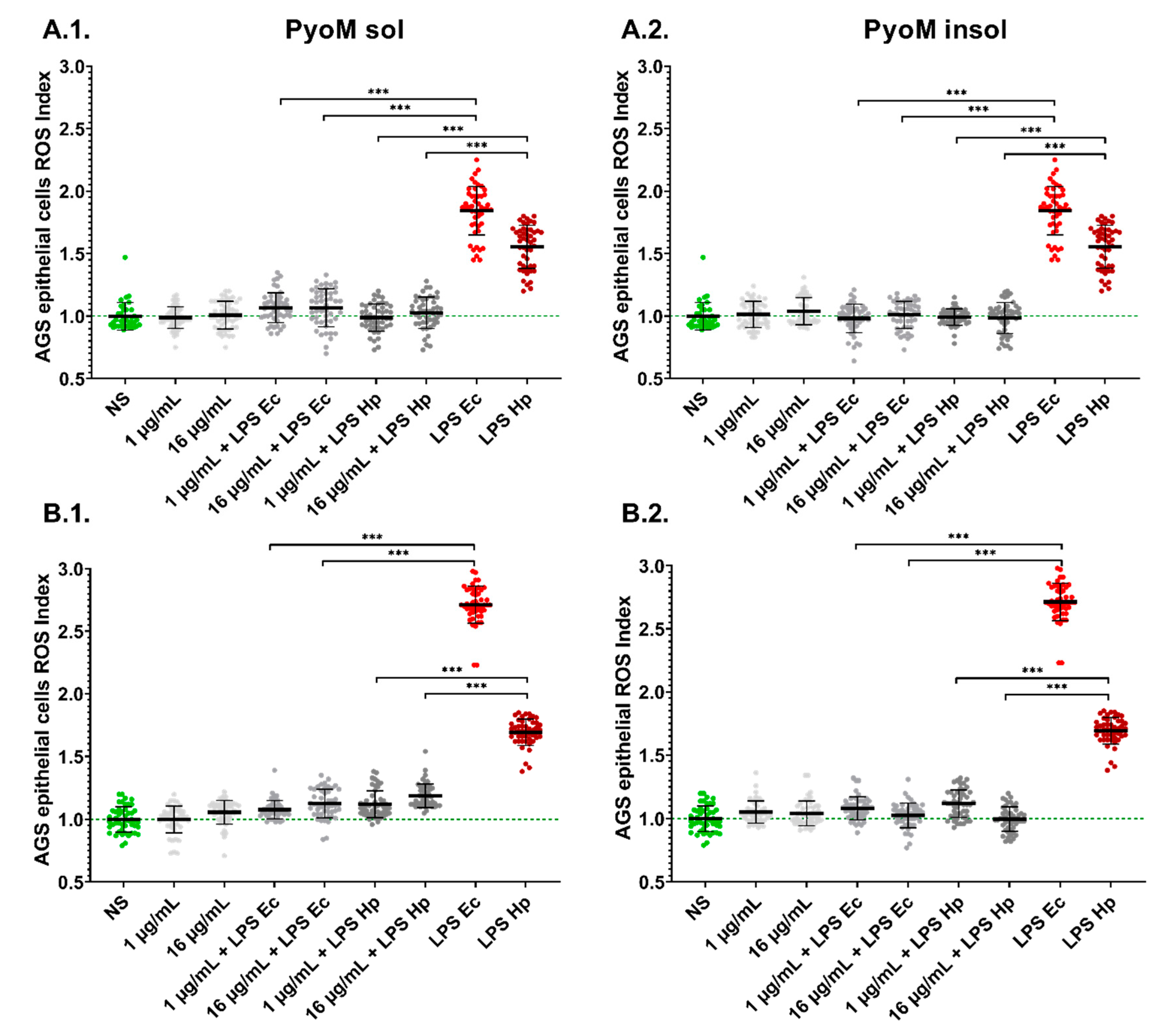
2.4. PyoM Neutralizes Reactive Oxygen Species Produced by Cells Exposed to H. pylori LPS
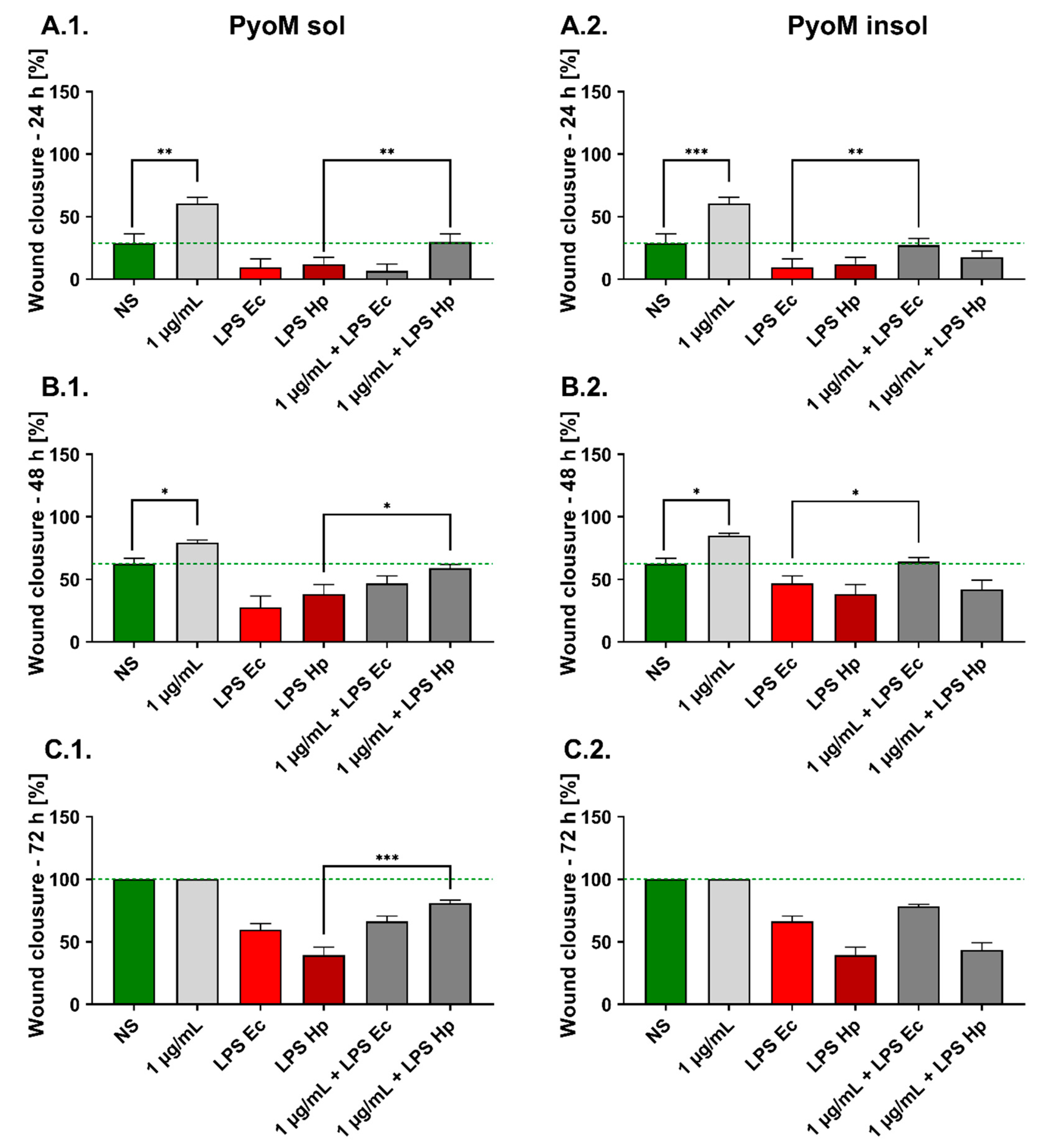
2.5. PyoMsol Promotes the Migration of Gastric Epithelial Cells Affected by H. pylori LPS
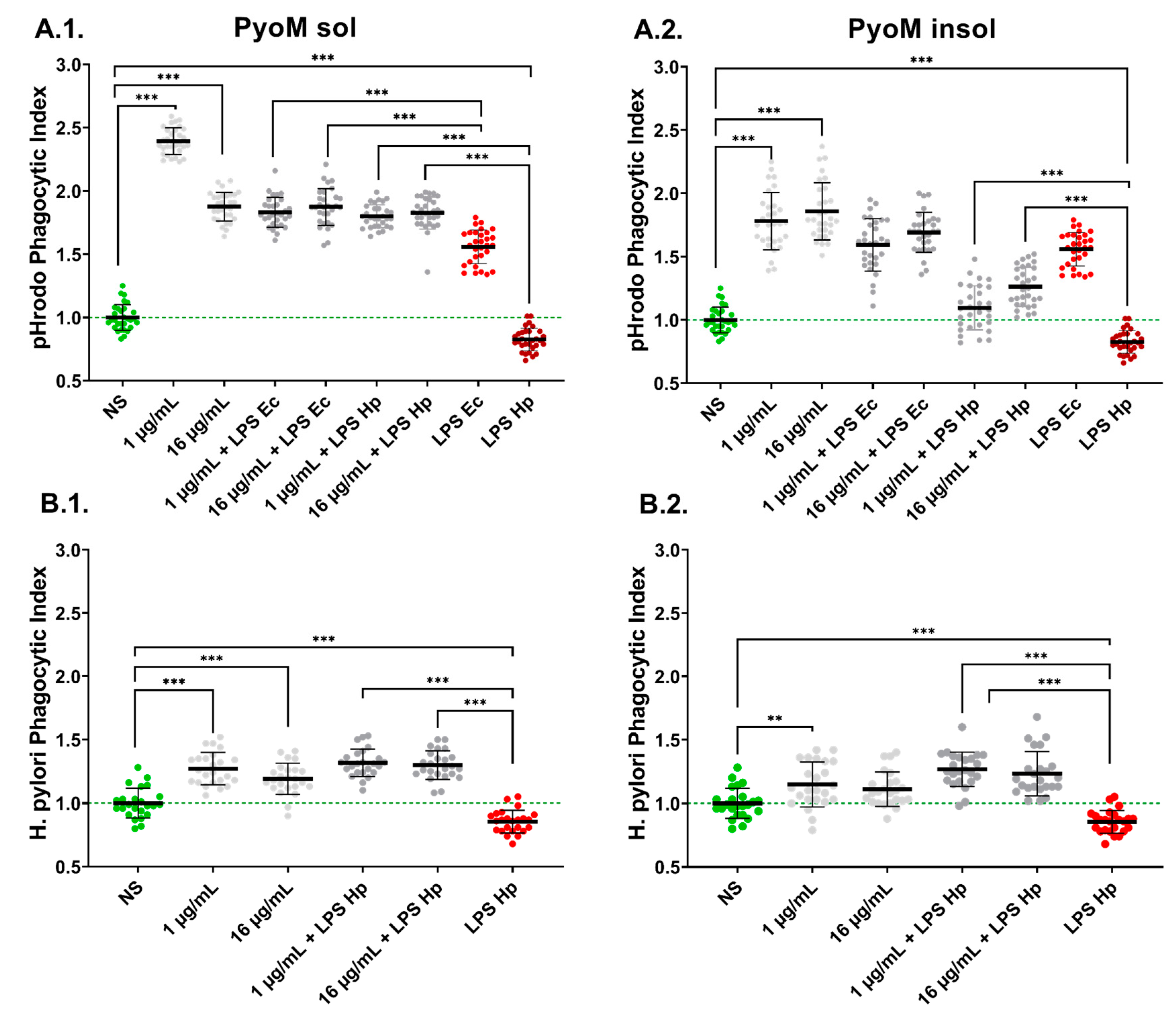
2.6. PyoMsol Enhances the Phagocytic Capacity of Monocytes towards Fluorescently Labelled E. coli or H. pylori
3. Discussion
4. Materials and Methods
4.1. Growth Conditions of Pseudomonas aeruginosa
4.2. Isolation and Purification of PyoMinsol and PyoMsol
4.3. Cell Cultures
4.4. Cell Stimulators
4.5. Antibacterial Activity of PyoMinsol and PyoMsol
4.6. Cell’s Viability Assay
4.7. Reactive Oxygen Species
4.8. Apoptosis
4.9. Wound Healing Assay
4.10. Phagocytosis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baj, J.; Forma, A.; Sitarz, M.; Piero, P.; Garrut, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori virulence factors—Mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 2021, 10, 27. [Google Scholar] [CrossRef]
- Sukri, A.; Hanafiah, A.; Mohamad Zin, N.; Kosai, N.R. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. Apmis 2020, 128, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Tsay, F.W.; Hsu, P.I. H. pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nestegard, O.; Moayeri, B.; Halvorsen, F.A.; Tønnesen, T.; Sørbye, S.W.; Paulssen, E.; Johnsen, K.M.; Goll, R.; Florholmen, J.R.; Melby, K.K. Helicobacter pylori resistance to antibiotics before and after treatment: Incidence of eradication failure. PLoS ONE 2022, 17, e0265322. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Prim. 2023, 9, 19. [Google Scholar] [CrossRef]
- Allen, L.A.H. Phagocytosis and persistence of Helicobacter pylori. Cell Microbiol. 2007, 9, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.T.; Allen, L.A.H. Role of urease in megasome formation and Helicobacter pylori survival in macrophages. J. Leukoc. Biol. 2006, 79, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, H.; Miyamoto, Y.; Akaike, T.; Kubota, T.; Sawa, T.; Okamoto, S.; Maeda, H. Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infect. Immun. 2000, 68, 4378–4383. [Google Scholar] [CrossRef]
- Scott, D.R.; Marcus, E.A.; Wen, Y.; Singh, S.; Feng, J.; Sachs, G. Cytoplasmic histidine kinase (HP0244)-regulated assembly of urease with UreI, a channel for urea and its metabolites, CO2, NH3, and NH4+, is necessary for acid survival of Helicobacter pylori. J. Bacteriol. 2010, 192, 94–103. [Google Scholar] [CrossRef]
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori virulence factors exploiting gastric colonization and its pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori virulence factor cytotoxin-associated gene A (CagA)-mediated gastric pathogenicity. Int. J. Mol. Sci. 2020, 21, 7430. [Google Scholar] [CrossRef]
- Sundqvist, M.O.; Wärme, J.; Hofmann, R.; Pawelzik, S.C.; Bäck, M. Helicobacter pylori virulence factor cytotoxin-associated gene A (CagA) induces vascular calcification in coronary artery smooth muscle cells. Int. J. Mol. Sci. 2023, 24, 5392. [Google Scholar] [CrossRef]
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative stress resulting from Helicobacter pylori infection contributes to gastric carcinogenesis. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 316–322. [Google Scholar] [CrossRef]
- Cheok, Y.Y.; Tan, G.M.Y.; Lee, C.Y.Q.; Abdullah, S.; Looi, C.Y.; Wong, W.F. Innate immunity crosstalk with Helicobacter pylori: Pattern recognition receptors and cellular responses. Int. J. Mol. Sci. 2022, 23, 7561. [Google Scholar] [CrossRef]
- Mnich, E.; Kowalewicz-Kulbat, M.; Sicińska, P.; Hinc, K.; Obuchowski, M.; Gajewski, A.; Moran, A.P.; Chmiela, M. Impact of Helicobacter pylori on the healing process of the gastric barrier. World J. Gastroenterol. 2016, 22, 7536–7558. [Google Scholar] [CrossRef]
- Hug, I.; Couturier, M.R.; Rooker, M.M.; Taylor, D.E.; Stein, M.; Feldman, M.F. Helicobacter pylori lipopolysaccharide is synthesized via a novel pathway with an evolutionary connection to protein N-glycosylation. PLoS Pathog. 2010, 6, e1000819. [Google Scholar] [CrossRef] [PubMed]
- Sijmons, D.; Guy, A.J.; Walduck, A.K.; Ramsland, P.A. Helicobacter pylori and the role of lipopolysaccharide variation in innate immune evasion. Front. Immunol. 2022, 13, 868225. [Google Scholar] [CrossRef]
- Rudnicka, W.; Czkwianianc, E.; Płaneta-Małecka, I.; Jurkiewicz, M.; Wiśniewska, M.; Cieślikowski, T.; Rózalska, B.; Wadström, T.; Chmiela, M. A potential double role of anti-Lewis X antibodies in Helicobacter pylori-associated gastroduodenal diseases. FEMS Microbiol. Immunol. 2001, 30, 121–125. [Google Scholar] [CrossRef]
- Jain, U.; Saxena, K.; Chauhan, N. Helicobacter pylori induced reactive oxygen species: A new and developing platform for detection. Helicobacter 2021, 26, e12796. [Google Scholar] [CrossRef]
- Godavarthy, P.K.; Puli, C. From antibiotic resistance to antibiotic renaissance: A new era in Helicobacter pylori treatment. Cureus 2023, 15, e36041. [Google Scholar] [CrossRef] [PubMed]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance—From biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [PubMed]
- Behzadi, P.; Baráth, Z.; Gajdács, M. It’s not easy being green: A narrative review on the microbiology, virulence and therapeutic prospects of multidrug-resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 42. [Google Scholar] [CrossRef]
- Urbaniak, M.M.; Gazińska, M.; Rudnicka, K.; Płociński, P.; Nowak, M.; Chmiela, M. In vitro and in vivo biocompatibility of natural and synthetic Pseudomonas aeruginosa pyomelanin for potential biomedical applications. Int. J. Mol. Sci. 2023, 24, 7846. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. New insight into melanin for food packaging and biotechnology applications. Crit. Rev. Food Sci. Nutr. 2021, 62, 4629–4655. [Google Scholar] [CrossRef] [PubMed]
- Lorquin, F.; Ziarelli, F.; Amouric, A.; Di Giorgio, C.; Robin, M.; Piccerelle, P.; Lorquin, J. Production and properties of non-cytotoxic pyomelanin by laccase and comparison to bacterial and synthetic pigments. Sci. Rep. 2021, 11, 8538. [Google Scholar] [CrossRef]
- Fonseca, É.; Freitas, F.; Caldart, R.; Morgado, S.; Vicente, A.C. Pyomelanin biosynthetic pathway in pigment-producer strains from the pandemic Acinetobacter baumannii IC-5. Mem. Inst. Oswaldo Cruz. 2020, 115, 1–6. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive oxygen species in pathogen clearance: The killing mechanisms, the adaption response, and the side effects. Front. Microbiol. 2021, 11, 622534. [Google Scholar] [CrossRef] [PubMed]
- Tapia, C.V.; Falconer, M.; Tempio, F.; Falcón, F.; López, M.; Fuentes, M.; Alburquenque, C.; Amaro, J.; Bucarey, S.A.; Di Nardo, A. Melanocytes and melanin represent a first line of innate immunity against Candida albicans. Med. Mycol. 2014, 52, 445–452. [Google Scholar] [CrossRef]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic. Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar]
- Amieva, M.R.; El-Omar, E.M. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 2008, 134, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Link, A.; Selgrad, M. Helicobacter pylori: Perspectives and time trends. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 628–638. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Vasanthabharathi, V.; Lakshminarayanan, R.; Jayalakshmi, S. Melanin production from marine Streptomyces Afr. J. Biotechnol. 2011, 10, 11224–11234. [Google Scholar]
- Zerrad, A.; Anissi, J.; Ghanam, J.; Sendide, K. Antioxidant and antimicrobial activities of melanin produced by a Pseudomonas balearica strain. J. Biotech. Let. 2014, 5, 87–94. [Google Scholar]
- Xu, C.; Li, J.; Yang, L.; Shi, F.; Yang, L.; Ye, M. Antibacterial activity and a membrane damage mechanism of Lachnum YM30 melanin against Vibrio parahaemolyticus and Staphylococcus aureus. Food Control 2017, 73, 1445–1451. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Saber, W.I.A. Natural melanin: Current trends, and future approaches, with especial reference to microbial source. Polymers 2022, 14, 1339. [Google Scholar] [CrossRef]
- Żądło, A.; Sarna, T. Interaction of iron ions with melanin. Acta Biochim. Pol. 2019, 66, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Waidner, B.; Greiner, S.; Odenbreit, S.; Kavermann, H.; Velayudhan, J.; Stähler, F.; Guhl, J.; Bissé, E.; van Vliet, A.H.; Andrews, S.C.; et al. Essential role of ferritin Pfr in Helicobacter pylori iron metabolism and gastric colonization. Infect. Immun. 2002, 70, 3923–3929. [Google Scholar] [CrossRef]
- Lorquin, F.; Piccerelle, P.; Orneto, C.; Robin, M.; Lorquin, J. New insights and advances on pyomelanin production: From microbial synthesis to applications. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac013. [Google Scholar] [CrossRef]
- Gajewski, A.Ł.; Gawrysiak, M.; Krupa, A.; Rechciński, T.; Chałubiński, M.; Gonciarz, W.; Chmiela, M. Accumulation of deleterious effects in gastric epithelial cells and vascular endothelial cells in vitro in the milieu of Helicobacter pylori components, 7-ketocholesterol and acetylsalicylic acid. Int. J. Mol. Sci. 2022, 23, 6355. [Google Scholar] [CrossRef]
- Hill, B. Gastric mucosal barrier: Evidence for Helicobacter pylori ingesting gastric surfactant and deriving protection from it. Gut 1993, 34, 588–593. [Google Scholar] [CrossRef]
- Von Herbay, A.; Rudi, J. Role of apoptosis in gastric epithelial turnover. Microsc. Res. Tech. 2000, 48, 303–311. [Google Scholar] [CrossRef]
- Cover, T.L.; Krishna, U.S.; Israel, D.A.; Peek, R.M. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 1996, 38, 498–501. [Google Scholar]
- Gonciarz, W.; Krupa, A.; Hinc, K.; Obuchowski, M.; Moran, A.P.; Gajewski, A.; Chmiela, M. The effect of Helicobacter pylori infection and different H. pylori components on the proliferation and apoptosis of gastric epithelial cells and fibroblasts. PLoS ONE 2019, 14, e0220636. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Z.; Minohara, Y.; Fan, X.J.; Wang, J.; Reyes, V.E.; Patel, J.; Dirden-Kramer, B.; Boldogh, I.; Ernst, P.B.; Crowe, S.E. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect. Immun. 2007, 75, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ramanujam, K.S.; Wong, A.; Fantry, G.T.; Drachenberg, C.B.; James, S.P.; Meltzer, S.J.; Wilson, K.T. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology 1999, 116, 1319–1329. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, L.; Rong, S.; Qu, H.; Zhang, Y.; Chang, D.; Pan, H.; Wang, W. Relation between gastric cancer and protein oxidation, DNA damage, and lipid peroxidation. Oxid. Med. Cell. Longev. 2013, 2013, 543760. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our current understanding of a universal biological process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Grębowska, A.; Moran, A.P.; Matusiak, A.; Bak-Romaniszyn, L.; Czkwianianc, E.; Rechciński, T.; Walencka, M.; Płaneta-Małecka, I.; Rudnicka, W.; Chmiela, M. Anti-phagocytic activity of Helicobacter pylori lipopolysaccharide (LPS)-possible modulation of the innate immune response to these bacteria. Pol. J. Microbiol. 2008, 3, 185–192. [Google Scholar]
- Fox, S.; Ryan, K.A.; Berger, A.H.; Petro, K.; Das, S.; Crowe, S.E.; Ernst, P.B. The role of C1q in recognition of apoptotic epithelial cells and inflammatory cytokine production by phagocytes during Helicobacter pylori infection. J. Inflamm. 2015, 12, 1–13. [Google Scholar] [CrossRef]
- Gonciarz, W.; Chmiela, M.; Kost, B.; Piątczak, E.; Brzeziński, M. Stereocomplexed microparticles loaded with Salvia cadmica Boiss. extracts for enhancement of immune response towards Helicobacter pylori. Sci. Rep. 2023, 13, 7039. [Google Scholar] [CrossRef]
- Chmiela, M.; Czkwianianc, E.; Wadstrom, T.; Rudnicka, W. Role of Helicobacter pylori surface structures in bacterial interaction with macrophages. Gut 1997, 40, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Alviano, D.S.; Franzen, A.J.; Travassos, L.R.; Holandino, C.; Rozental, S.; Ejzemberg, R.; Alviano, C.S.; Rodrigues, M.L. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 2004, 72, 229–237. [Google Scholar] [CrossRef]
- Borlace, G.N.; Keep, S.J.; Prodoehl, M.J.; Jones, H.F.; Butler, R.N.; Brooks, D.A. A role for altered phagosome maturation in the long-term persistence of Helicobacter pylori infection. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, F.; Wu, Y.; He, Y.; Kong, Q.; Sang, H. Fungal melanin-induced metabolic reprogramming in macrophages is crucial for inflammation. J. Med. Mycol. 2023, 33, 101359. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Duarte-Oliveira, C.; Campos, C.F.; Aimanianda, V.; Ter Horst, R.; Leite, L.; Mercier, T.; Pereira, P.; Fernández-García, M.; Antunes, D.; et al. Phagosomal removal of fungal melanin reprograms macrophage metabolism to promote antifungal immunity. Nat. Commun. 2020, 11, 2282. [Google Scholar] [CrossRef]
- Walencka, M.; Gonciarz, W.; Mnich, E.; Gajewski, A.; Stawerski, P.; Knapik-Dabrowicz, A.; Chmiela, M. The microbiological, histological, immunological and molecular determinants of Helicobacter pylori infection in guinea pigs as a convenient animal model to study pathogenicity of these bacteria and the infection dependent immune response of the host. Acta Biochim. Pol. 2015, 62, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.P.; Helander, I.M.; Kosunen, T.U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J. Bacteriol. 1992, 174, 1370–1377. [Google Scholar] [CrossRef]
- Skindersoe, M.E.; Rasmussen, L.; Andersen, L.P.; Krogfelt, K.A. A novel assay for easy and rapid quantification of Helicobacter pylori adhesion. Helicobacter 2015, 20, 199–205. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbaniak, M.M.; Rudnicka, K.; Gościniak, G.; Chmiela, M. Can Pyomelanin Produced by Pseudomonas aeruginosa Promote the Regeneration of Gastric Epithelial Cells and Enhance Helicobacter pylori Phagocytosis? Int. J. Mol. Sci. 2023, 24, 13911. https://doi.org/10.3390/ijms241813911
Urbaniak MM, Rudnicka K, Gościniak G, Chmiela M. Can Pyomelanin Produced by Pseudomonas aeruginosa Promote the Regeneration of Gastric Epithelial Cells and Enhance Helicobacter pylori Phagocytosis? International Journal of Molecular Sciences. 2023; 24(18):13911. https://doi.org/10.3390/ijms241813911
Chicago/Turabian StyleUrbaniak, Mateusz M., Karolina Rudnicka, Grażyna Gościniak, and Magdalena Chmiela. 2023. "Can Pyomelanin Produced by Pseudomonas aeruginosa Promote the Regeneration of Gastric Epithelial Cells and Enhance Helicobacter pylori Phagocytosis?" International Journal of Molecular Sciences 24, no. 18: 13911. https://doi.org/10.3390/ijms241813911




