Catecholamines Promote Ovarian Cancer Progression through Secretion of CXC-Chemokines
Abstract
:1. Introduction
2. Results
2.1. Catecholamines Induce Invasion of Ovarian Cancer Cells
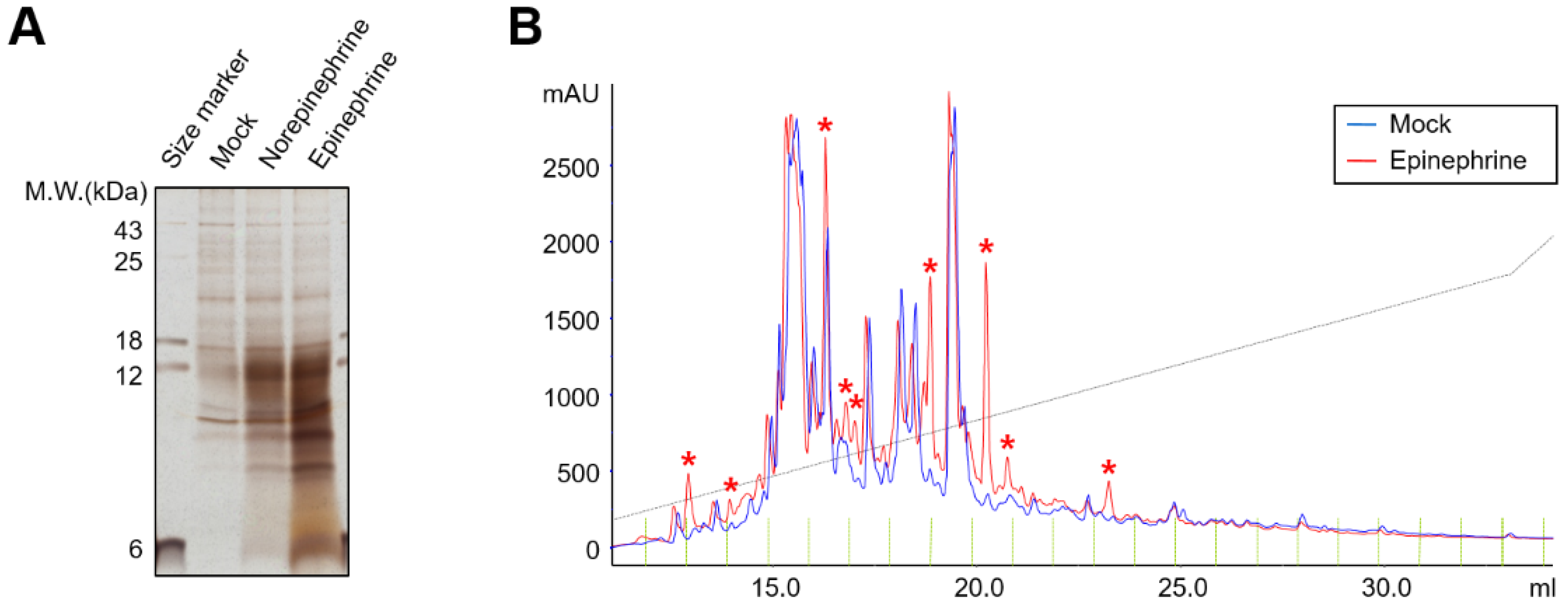
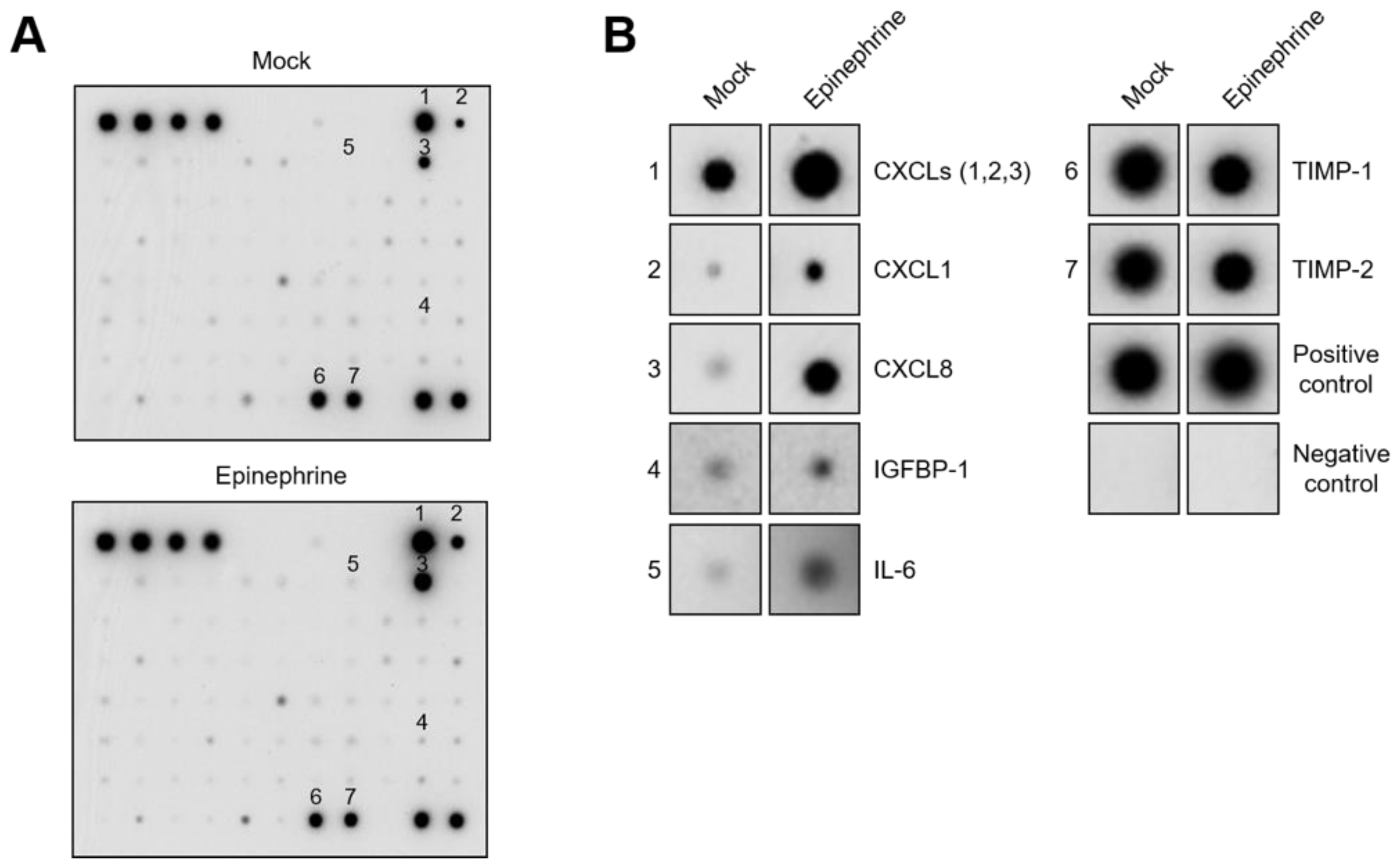
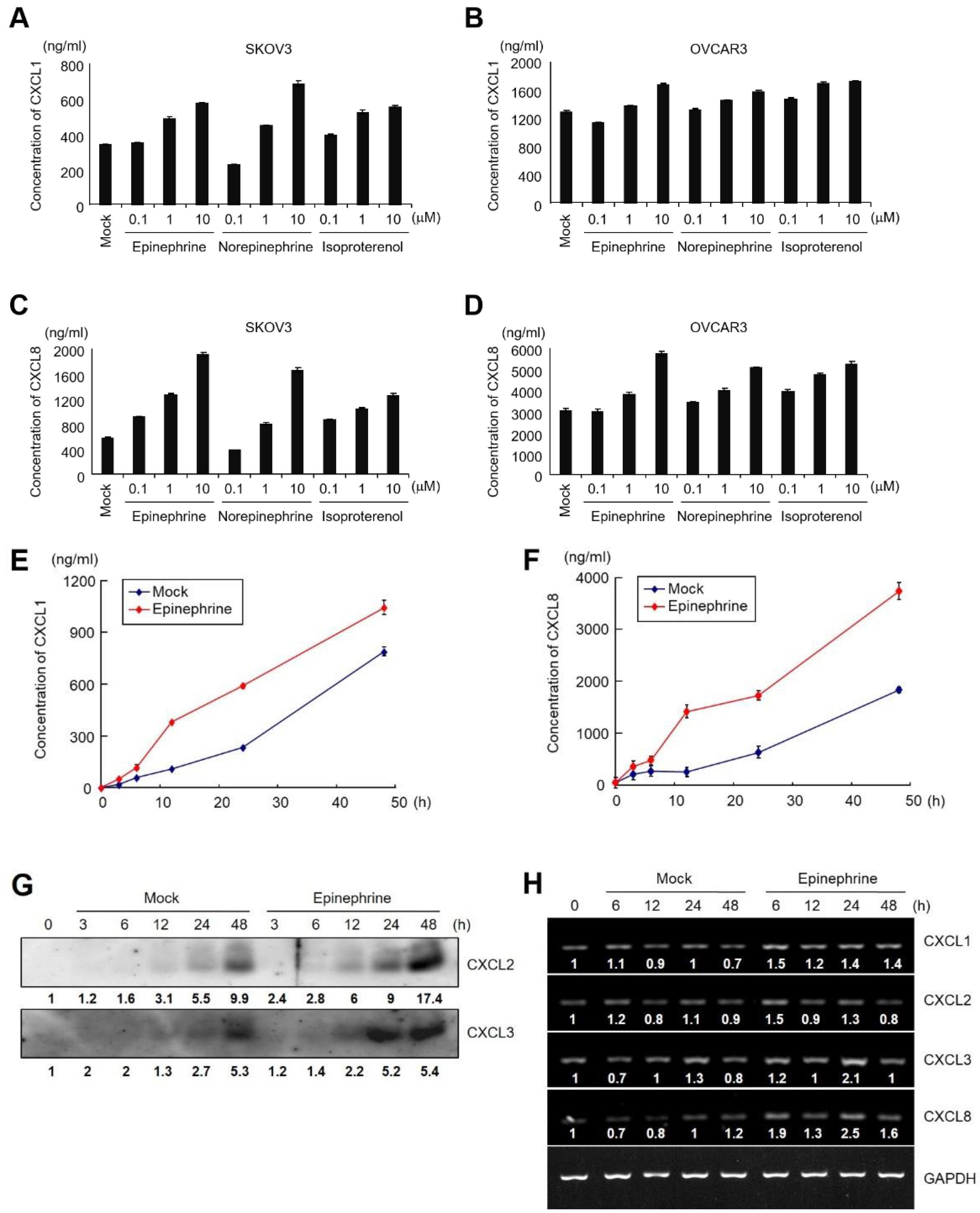
2.2. Catecholamines Induce the Secretion of CXC-Chemokines in SKOV3 Cells
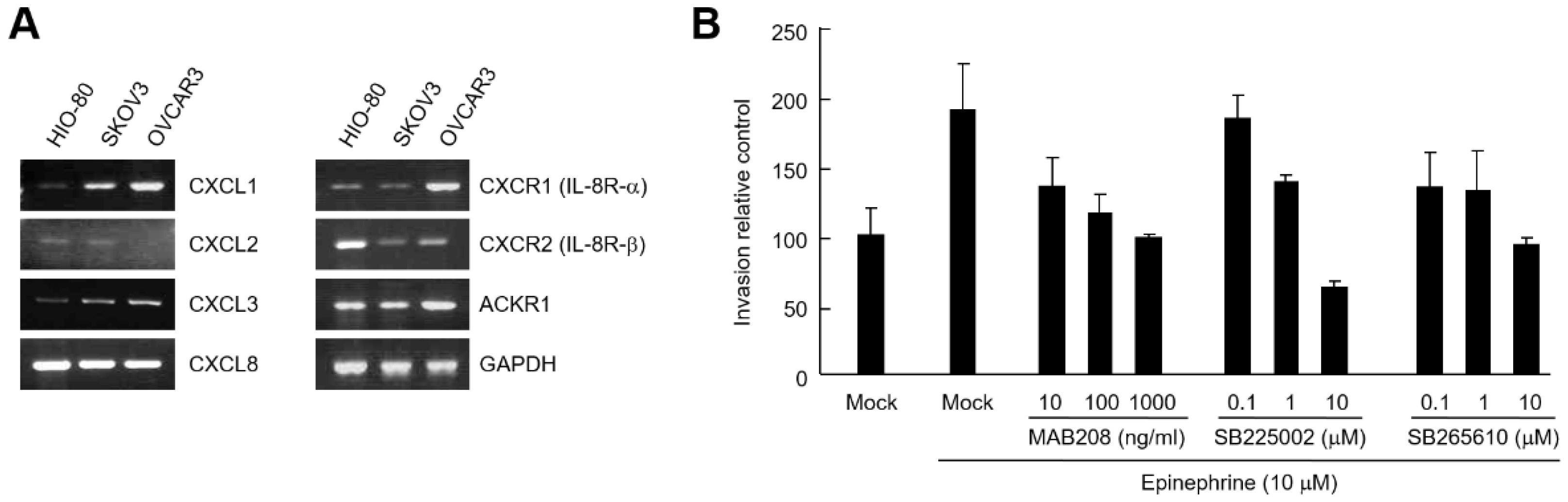
2.3. CXCL8 Secreted by Ovarian Cancer Cells Induces Cell Invasion
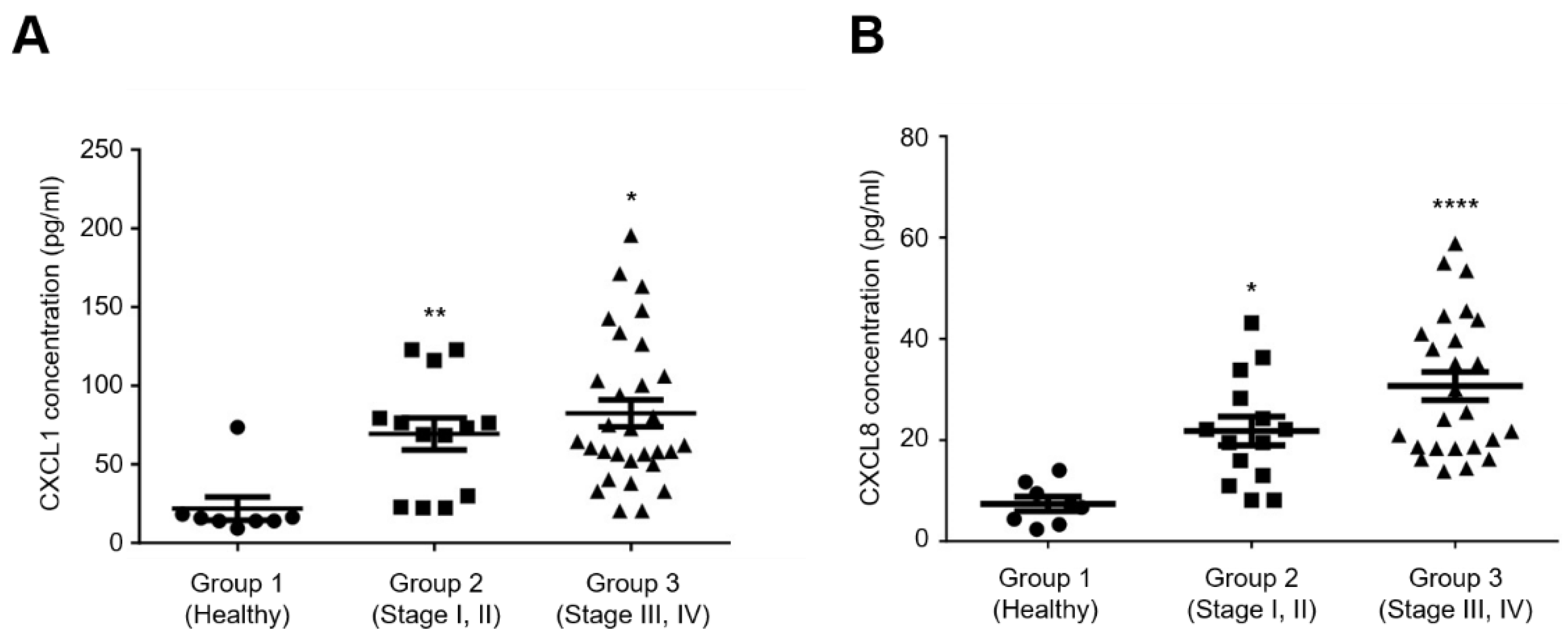
2.4. Elevated Serum Levels of CXCL1 and CXCL8 Are Associated with Advanced Clinical Stages of Ovarian Cancer
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Antibody, and Chemicals
4.2. RT-PCR
4.3. Cell Invasion Assay
4.4. High-Performance Liquid Chromatography (HPLC)
4.5. Cytokine Antibody Array
4.6. Silver Staining and Western Blotting
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Clinical Samples
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Segerstrom, S.C.; Miller, G.E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef] [PubMed]
- Degroote, C.; Schwaninger, A.; Heimgartner, N.; Hedinger, P.; Ehlert, U.; Wirtz, P.H. Acute Stress Improves Concentration Performance. Exp. Psychol. 2020, 67, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, S.; Ning, B.; Huang, T.; Li, Y.; Wei, Y. Stress and cancer: The mechanisms of immune dysregulation and management. Front. Immunol. 2022, 13, 1032294. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A. The effects of chronic stress on health: New insights into the molecular mechanisms of brain-body communication. Future Sci. OA 2015, 1, FSO23. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, Y.; Luo, M.; Hu, X.; Li, H.; Liu, Q.; Zou, Z. Chronic stress in solid tumor development: From mechanisms to interventions. J. Biomed. Sci. 2023, 30, 8. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Park, P.S.; Lodowski, D.T.; Palczewski, K. Activation of G protein-coupled receptors: Beyond two-state models and tertiary conformational changes. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 107–141. [Google Scholar] [CrossRef]
- Ma, B.; Shatsky, M.; Wolfson, H.J.; Nussinov, R. Multiple diverse ligands binding at a single protein site: A matter of pre-existing populations. Protein Sci. 2002, 11, 184–197. [Google Scholar] [CrossRef]
- Serebryany, E.; Zhu, G.A.; Yan, E.C. Artificial membrane-like environments for in vitro studies of purified G-protein coupled receptors. Biochim. Biophys. Acta 2012, 1818, 225–233. [Google Scholar] [CrossRef]
- Ma, A.W.; Dong, J.Y.; Ma, D.; Wells, J.W. Cleavage-resistant fusion proteins of the M(2) muscarinic receptor and Galpha(i1). Homotropic and heterotropic effects in the binding of ligands. Biochim. Biophys. Acta 2011, 1810, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.C.; Li, J.M.; Lee, K.F.; Huang, Y.C.; Wang, K.C.; Lai, H.C.; Cheng, C.C.; Kuo, Y.H.; Shi, C.S. Selective beta2-AR Blockage Suppresses Colorectal Cancer Growth Through Regulation of EGFR-Akt/ERK1/2 Signaling, G1-Phase Arrest, and Apoptosis. J. Cell Physiol. 2016, 231, 459–472. [Google Scholar] [CrossRef]
- Choi, M.J.; Cho, K.H.; Lee, S.; Bae, Y.J.; Jeong, K.J.; Rha, S.Y.; Choi, E.J.; Park, J.H.; Kim, J.M.; Lee, J.S.; et al. hTERT mediates norepinephrine-induced Slug expression and ovarian cancer aggressiveness. Oncogene 2015, 34, 3402–3412. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, A.S.; Dorniak, P.L.; Sadaoui, N.C.; Kang, Y.; Lin, T.; Armaiz-Pena, G.; Wu, S.Y.; Rupaimoole, R.; Allen, J.K.; Gharpure, K.M.; et al. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene 2016, 35, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.K.; Armaiz-Pena, G.N.; Nagaraja, A.S.; Sadaoui, N.C.; Ortiz, T.; Dood, R.; Ozcan, M.; Herder, D.M.; Haemmerle, M.; Gharpure, K.M.; et al. Sustained Adrenergic Signaling Promotes Intratumoral Innervation through BDNF Induction. Cancer Res. 2018, 78, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J. Beta blockers, norepinephrine, and cancer: An epidemiological viewpoint. Clin. Epidemiol. 2012, 4, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.V. Role for catecholamines in tumor progression: Possible use for beta-blockers in the treatment of cancer. Cancer Biol. Ther. 2010, 10, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.J.; Hedrick, J.; Zlotnik, A.; Siani, M.A.; Thompson, D.A.; Butcher, E.C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 1998, 279, 381–384. [Google Scholar] [CrossRef]
- Huber, A.R.; Kunkel, S.L.; Todd, R.F., 3rd; Weiss, S.J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 1991, 254, 99–102. [Google Scholar] [CrossRef]
- Butcher, E.C. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell 1991, 67, 1033–1036. [Google Scholar] [CrossRef]
- Pulai, J.I.; Chen, H.; Im, H.J.; Kumar, S.; Hanning, C.; Hegde, P.S.; Loeser, R.F. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J. Immunol. 2005, 174, 5781–5788. [Google Scholar] [CrossRef]
- Wolf, M.J.; Hoos, A.; Bauer, J.; Boettcher, S.; Knust, M.; Weber, A.; Simonavicius, N.; Schneider, C.; Lang, M.; Sturzl, M.; et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell 2012, 22, 91–105. [Google Scholar] [CrossRef]
- Yang, G.; Rosen, D.G.; Liu, G.; Yang, F.; Guo, X.; Xiao, X.; Xue, F.; Mercado-Uribe, I.; Huang, J.; Lin, S.H.; et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin. Cancer Res. 2010, 16, 3875–3886. [Google Scholar] [CrossRef] [PubMed]
- Merritt, W.M.; Lin, Y.G.; Spannuth, W.A.; Fletcher, M.S.; Kamat, A.A.; Han, L.Y.; Landen, C.N.; Jennings, N.; De Geest, K.; Langley, R.R.; et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J. Natl. Cancer Inst. 2008, 100, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Colon-Echevarria, C.B.; Ortiz, T.; Maldonado, L.; Hidalgo-Vargas, M.J.; Perez-Morales, J.; Aquino-Acevedo, A.N.; Herrera-Noriega, R.; Bonilla-Claudio, M.; Castro, E.M.; Armaiz-Pena, G.N. Zoledronic Acid Abrogates Restraint Stress-Induced Macrophage Infiltration, PDGF-AA Expression, and Ovarian Cancer Growth. Cancers 2020, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Saha, P.; Chatterjee, B.; Srivastava, A.K. Chemokines driven ovarian cancer progression, metastasis and chemoresistance: Potential pharmacological targets for cancer therapy. Semin. Cancer Biol. 2022, 86, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Strange, K.S.; Kerr, L.R.; Andrews, H.N.; Emerman, J.T.; Weinberg, J. Psychosocial stressors and mammary tumor growth: An animal model. Neurotoxicol Teratol. 2000, 22, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Ragan, A.R.; Lesniak, A.; Bochynska-Czyz, M.; Kosson, A.; Szymanska, H.; Pysniak, K.; Gajewska, M.; Lipkowski, A.W.; Sacharczuk, M. Chronic mild stress facilitates melanoma tumor growth in mouse lines selected for high and low stress-induced analgesia. Stress 2013, 16, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Smith, M.; Lutgendorf, S.K.; Sood, A.K. Impact of stress on cancer metastasis. Future Oncol. 2010, 6, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, D.; Sarkar, C.; Basu, B.; Dasgupta, P.S.; Basu, S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009, 69, 3727–3730. [Google Scholar] [CrossRef]
- Melik-Parsadaniantz, S.; Rostene, W. Chemokines and neuromodulation. J. Neuroimmunol. 2008, 198, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Raman, D.; Sobolik-Delmaire, T.; Richmond, A. Chemokines in health and disease. Exp. Cell Res. 2011, 317, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Entschladen, F.; Drell, T.L.t.; Lang, K.; Joseph, J.; Zaenker, K.S. Neurotransmitters and chemokines regulate tumor cell migration: Potential for a new pharmacological approach to inhibit invasion and metastasis development. Curr. Pharm. Des. 2005, 11, 403–411. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Liu, Z.Q.; Wei, J.; Liu, Y.; Hu, L.S. Contribution of endothelial progenitor cells to neovascularization (Review). Int. J. Mol. Med. 2012, 30, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Petit, I.; Jin, D.; Rafii, S. The SDF-1-CXCR4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends Immunol. 2007, 28, 299–307. [Google Scholar] [CrossRef]
- Allinen, M.; Beroukhim, R.; Cai, L.; Brennan, C.; Lahti-Domenici, J.; Huang, H.; Porter, D.; Hu, M.; Chin, L.; Richardson, A.; et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004, 6, 17–32. [Google Scholar] [CrossRef]
- Vaday, G.G.; Peehl, D.M.; Kadam, P.A.; Lawrence, D.M. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate 2006, 66, 124–134. [Google Scholar] [CrossRef]
- Loberg, R.D.; Day, L.L.; Harwood, J.; Ying, C.; St John, L.N.; Giles, R.; Neeley, C.K.; Pienta, K.J. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia 2006, 8, 578–586. [Google Scholar] [CrossRef]
- Darash-Yahana, M.; Pikarsky, E.; Abramovitch, R.; Zeira, E.; Pal, B.; Karplus, R.; Beider, K.; Avniel, S.; Kasem, S.; Galun, E.; et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004, 18, 1240–1242. [Google Scholar] [CrossRef]
- Payne, A.S.; Cornelius, L.A. The role of chemokines in melanoma tumor growth and metastasis. J. Investig. Dermatol. 2002, 118, 915–922. [Google Scholar] [CrossRef]

| Primer Name (or Gene Name) | Primer Sequences 5′-3′ | |
|---|---|---|
| Sense | Antisense | |
| Human α1A adrenergic receptor | GAAGGGCAACACAAGGACAT | CAGGAGGATTGGTCTTTGGA |
| Human α1B adrenergic receptor | CCTGAGGATCCATTCCAAGA | CGGTAGAGCGATGAAGAAGG |
| Human α1D adrenergic receptor | AGAAGAAAGCGGCCAAGACT | GCTGGAACAGGGGTAGATGA |
| Human α2A adrenergic receptor | CTACGTGCGCATCTACCAGA | AGACGAGCTCTCCTCCAGGT |
| Human α2B adrenergic receptor | TTCTTTGCTCCTTGCCTCAT | ACTTCGAGTGTCCGTTGACC |
| Human α2C adrenergic receptor | TCCGTCGAGTTCTTCCTGTC | GCTGAAGAAGAAGGGGAACC |
| Human β1 adrenergic receptor | CTGCTACAACGACCCCAAGT | GCAGCTGTCGATCTTCTTCA |
| Human β2 adrenergic receptor | CTGCGCAGGTCTTCTTTGA | CTTGATGGCCCACAAAGTCT |
| Human β3 adrenergic receptor | TCTTCTCGTGATGCTCTTCG | ATGAGACCCAAGGTGCACAG |
| Human CXCL1 (GRO-α) | AGTCATAGCCACACTCAAGAATG | TGGCCATTTGCTTGGATCCGC |
| Human CXCL2 (GRO-β) | CGCCCAAACCGAAGTCATAGC | GATGCTCAAACACATTAGGCGC |
| Human CXCL3 (GRO-γ) | CGCCCAAACCGAAGTCATAGC | ATCAAGGTGGCTGACACATTATGG |
| Human CXCL8 (IL-8) | GTGCAGTTTTGCCAAGGAGT | CTCTGCACCCAGTTTTCCTT |
| Human CXCR1 | CCACCTGCAGATGAAGATTACA | AGGGACAGATTCATAGACAGT |
| Human CXCR2 | CCAGAATCCCTGGAAATCAA | ACAGGCAGGGCCAGGAGCAA |
| Human ACKR1 | GTCTTGTTGCCATTGGGTTT | GACAACAGCAACAGCTTGGA |
| Human GAPDH | CGGGAAGCTTGTGATCAATGG | GGCAGTGATGGCATGGACTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Chang, H.K.; Lee, Y.M.; Heo, K. Catecholamines Promote Ovarian Cancer Progression through Secretion of CXC-Chemokines. Int. J. Mol. Sci. 2023, 24, 14104. https://doi.org/10.3390/ijms241814104
Kim HJ, Chang HK, Lee YM, Heo K. Catecholamines Promote Ovarian Cancer Progression through Secretion of CXC-Chemokines. International Journal of Molecular Sciences. 2023; 24(18):14104. https://doi.org/10.3390/ijms241814104
Chicago/Turabian StyleKim, Hyun Jung, Ha Kyun Chang, Yul Min Lee, and Kyun Heo. 2023. "Catecholamines Promote Ovarian Cancer Progression through Secretion of CXC-Chemokines" International Journal of Molecular Sciences 24, no. 18: 14104. https://doi.org/10.3390/ijms241814104
APA StyleKim, H. J., Chang, H. K., Lee, Y. M., & Heo, K. (2023). Catecholamines Promote Ovarian Cancer Progression through Secretion of CXC-Chemokines. International Journal of Molecular Sciences, 24(18), 14104. https://doi.org/10.3390/ijms241814104






