Overview of Tumor Heterogeneity in High-Grade Serous Ovarian Cancers
Abstract
:1. Introduction
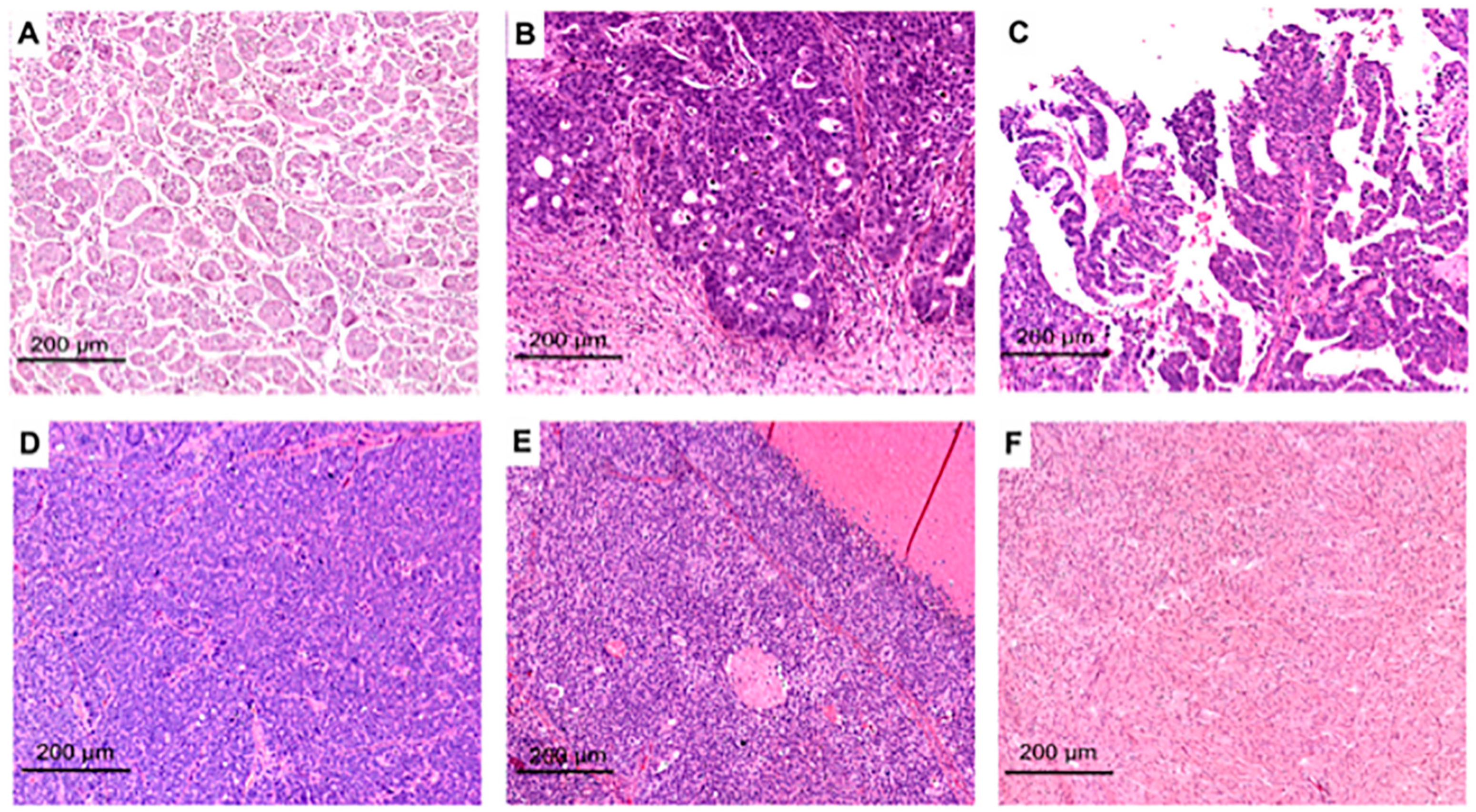
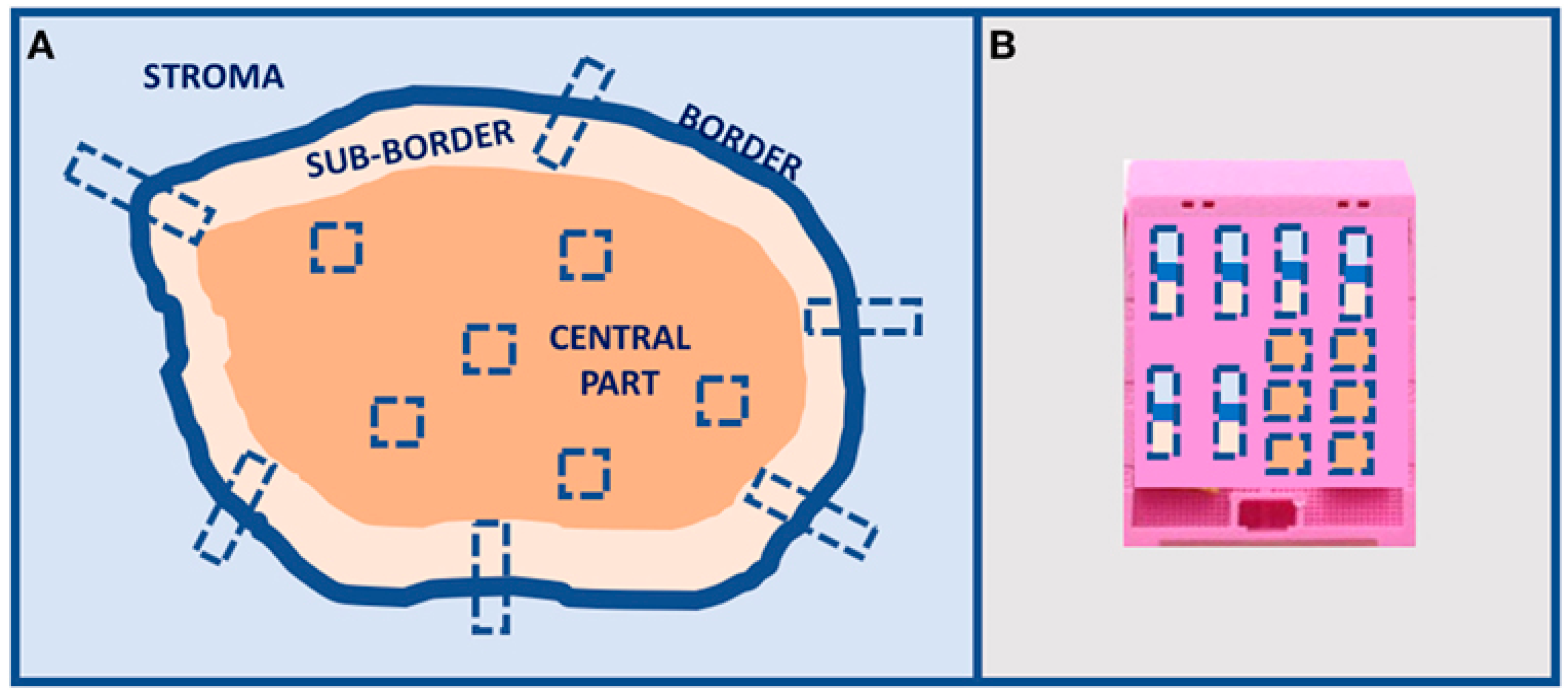
2. Morpho-Histological ITH of HGSOC
3. Clonal ITH of HGSOC
4. Non-Clonal ITH of HGSOC
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization; International Agency for Research on Cancer. Female Genital Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Prat, J.; D’Angelo, E.; Espinosa, I. Ovarian carcinomas: At least five different diseases with distinct histological features and molecular genetics. Hum. Pathol. 2018, 80, 11–27. [Google Scholar] [CrossRef]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Azzalini, E.; Barbazza, R.; Stanta, G.; Giorda, G.; Bortot, L.; Bartoletti, M.; Puglisi, F.; Canzonieri, V.; Bonin, S. Histological patterns and intra-tumor heterogeneity as prognostication tools in high grade serous ovarian cancers. Gynecol. Oncol. 2021, 163, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Morden, C.R.; Farrell, A.C.; Sliwowski, M.; Lichtensztejn, Z.; Altman, A.D.; Nachtigal, M.W.; McManus, K.J. Chromosome instability is prevalent and dynamic in high-grade serous ovarian cancer patient samples. Gynecol. Oncol. 2021, 161, 769–778. [Google Scholar] [CrossRef]
- Takaya, H.; Nakai, H.; Takamatsu, S.; Mandai, M.; Matsumura, N. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci. Rep. 2020, 10, 2757. [Google Scholar] [CrossRef]
- Stewart, M.D.; Merino Vega, D.; Arend, R.C.; Baden, J.F.; Barbash, O.; Beaubier, N.; Collins, G.; French, T.; Ghahramani, N.; Hinson, P.; et al. Homologous Recombination Deficiency: Concepts, Definitions, and Assays. Oncologist 2022, 27, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Stanta, G.; Bonin, S. Overview on clinical relevance of intra-tumor heterogeneity. Front. Med. 2018, 5, 85. [Google Scholar] [CrossRef]
- Stanta, G.; Bonin, S. A Practical Approach to Tumor Heterogeneity in Clinical Research and Diagnostics. Pathobiology 2018, 85, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Stanta, G.; Jahn, S.W.; Bonin, S.; Hoefler, G. Tumour heterogeneity: Principles and practical consequences. Virchows Arch. 2016, 469, 371–384. [Google Scholar] [CrossRef]
- Soslow, R.A.; Han, G.; Park, K.J.; Garg, K.; Olvera, N.; Spriggs, D.R.; Kauff, N.D.; Levine, D.A. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod. Pathol. 2012, 25, 625–636. [Google Scholar] [CrossRef]
- Azzalini, E.; Abdurakhmanova, N.; Parisse, P.; Bartoletti, M.; Canzonieri, V.; Stanta, G.; Casalis, L.; Bonin, S. Cell-stiffness and morphological architectural patterns in clinical samples of high grade serous ovarian cancers. Nanomedicine 2021, 37, 102452. [Google Scholar] [CrossRef]
- Murakami, R.; Matsumura, N.; Mandai, M.; Yoshihara, K.; Tanabe, H.; Nakai, H.; Yamanoi, K.; Abiko, K.; Yoshioka, Y.; Hamanishi, J.; et al. Establishment of a Novel Histopathological Classification of High-Grade Serous Ovarian Carcinoma Correlated with Prognostically Distinct Gene Expression Subtypes. Am. J. Pathol. 2016, 186, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; McCluggage, W.G.; Gilks, C.B. High-grade serous carcinoma of tubo-ovarian origin: Recent developments. Histopathology 2017, 71, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; McGuire, V.A.; Felberg, A.; Sieh, W.; Whittemore, A.S.; Longacre, T.A. Prediction of BRCA1 germline mutation status in women with ovarian cancer using morphology-based criteria: Identification of a BRCA1 ovarian cancer phenotype. Am. J. Surg. Pathol. 2012, 36, 1170–1177. [Google Scholar] [CrossRef]
- Reyes, M.C.; Arnold, A.G.; Kauff, N.D.; Levine, D.A.; Soslow, R.A. Invasion patterns of metastatic high-grade serous carcinoma of ovary or fallopian tube associated with BRCA deficiency. Mod. Pathol. 2014, 27, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Y.R.; Ducie, J.A.; Arnold, A.G.; Kauff, N.D.; Vargas-Alvarez, H.A.; Sala, E.; Levine, D.A.; Soslow, R.A. Invasion Patterns of Metastatic Extrauterine High-grade Serous Carcinoma with BRCA Germline Mutation and Correlation With Clinical Outcomes. Am. J. Surg. Pathol. 2016, 40, 404–409. [Google Scholar] [CrossRef]
- Reid, B.M.; Vyas, S.; Chen, Z.; Chen, A.; Kanetsky, P.A.; Permuth, J.B.; Sellers, T.A.; Saglam, O. Morphologic and molecular correlates of EZH2 as a predictor of platinum resistance in high-grade ovarian serous carcinoma. BMC Cancer 2021, 21, 714. [Google Scholar] [CrossRef]
- Lakis, S.; Kotoula, V.; Koliou, G.A.; Efstratiou, I.; Chrisafi, S.; Papanikolaou, A.; Zebekakis, P.; Fountzilas, G. Multisite Tumor Sampling Reveals Extensive Heterogeneity of Tumor and Host Immune Response in Ovarian Cancer. Cancer Genom. Proteom. 2020, 17, 529–541. [Google Scholar] [CrossRef]
- Uner, H.; Demir, M.; Goksuluk, D.; Kars, A.; Uner, M.; Usubutun, A. Evidence for Diverse Prognosis in High-Grade Serous Ovarian Carcinoma: Solid, Pseudoendometrioid, and Transitional-Like; So-Called “SET Morphology” and Progesterone Receptor Status. Turk Patoloji Derg. 2022, 38, 240–250. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, L.; Zhang, M.; Luo, Y.; Ma, X. Integration of histopathological images and multi-dimensional omics analyses predicts molecular features and prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 2021, 163, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Laury, A.R.; Blom, S.; Ropponen, T.; Virtanen, A.; Carpen, O.M. Artificial intelligence-based image analysis can predict outcome in high-grade serous carcinoma via histology alone. Sci. Rep. 2021, 11, 19165. [Google Scholar] [CrossRef]
- Azarianpour, S.; Corredor, G.; Bera, K.; Leo, P.; Fu, P.; Toro, P.; Joehlin-Price, A.; Mokhtari, M.; Mahdi, H.; Madabhushi, A. Computational image features of immune architecture is associated with clinical benefit and survival in gynecological cancers across treatment modalities. J. Immunother. Cancer 2022, 10, e003833. [Google Scholar] [CrossRef]
- Nawaz, S.; Trahearn, N.A.; Heindl, A.; Banerjee, S.; Maley, C.C.; Sottoriva, A.; Yuan, Y. Analysis of tumour ecological balance reveals resource-dependent adaptive strategies of ovarian cancer. EBioMedicine 2019, 48, 224–235. [Google Scholar] [CrossRef]
- Abel, J.; Jain, S.; Rajan, D.; Padigela, H.; Leidal, K.; Prakash, A.; Conway, J.; Nercessian, M.; Kirkup, C.; Javed, S.A.; et al. Cell-type-specific nuclear morphology predicts genomic instability and prognosis in multiple cancer types. bioRxiv 2023. [Google Scholar] [CrossRef]
- Heindl, A.; Khan, A.M.; Rodrigues, D.N.; Eason, K.; Sadanandam, A.; Orbegoso, C.; Punta, M.; Sottoriva, A.; Lise, S.; Banerjee, S.; et al. Microenvironmental niche divergence shapes BRCA1-dysregulated ovarian cancer morphological plasticity. Nat. Commun. 2018, 9, 3917. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Tiwari, A.; Singh, C.; Walsh, C.; Chuang, F.; Kim, E.; Singh, K.; Dadmanesh, F. High-grade ovarian serous carcinomas: Significant correlation of histologic patterns with IMP3 and E-Cadherin predicting disease recurrence and survival. Ann. Diagn. Pathol. 2019, 40, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Khashaba, M.; Fawzy, M.; Abdel-Aziz, A.; Eladawei, G.; Nagib, R. Subtyping of high grade serous ovarian carcinoma: Histopathological and immunohistochemical approach. J. Egypt Natl. Canc. Inst. 2022, 34, 6. [Google Scholar] [CrossRef] [PubMed]
- Khalique, L.; Ayhan, A.; Weale, M.E.; Jacobs, I.J.; Ramus, S.J.; Gayther, S.A. Genetic intra-tumour heterogeneity in epithelial ovarian cancer and its implications for molecular diagnosis of tumours. J. Pathol. 2007, 211, 286–295. [Google Scholar] [CrossRef]
- Ohsuga, T.; Yamaguchi, K.; Kido, A.; Murakami, R.; Abiko, K.; Hamanishi, J.; Kondoh, E.; Baba, T.; Konishi, I.; Matsumura, N. Distinct preoperative clinical features predict four histopathological subtypes of high-grade serous carcinoma of the ovary, fallopian tube, and peritoneum. BMC Cancer 2017, 17, 580. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, C.; Nakai, H.; Otani, T.; Murakami, R.; Takamura, S.; Takaya, H.; Murakami, K.; Mandai, M.; Matsumura, N. Histopathological subtyping of high-grade serous ovarian cancer using whole slide imaging. J. Gynecol. Oncol. 2023, 34, e47. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, E.; Tierno, D.; Bartoletti, M.; Barbazza, R.; Giorda, G.; Puglisi, F.; Cecere, S.C.; Losito, N.S.; Russo, D.; Stanta, G.; et al. AKT Isoforms Interplay in High-Grade Serous Ovarian Cancer Prognosis and Characterization. Cancers 2022, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.L.; Bateman, N.W.; Barakat, W.; Makohon-Moore, S.; Hood, B.L.; Conrads, K.A.; Zhou, M.; Calvert, V.; Pierobon, M.; Loffredo, J.; et al. Extensive three-dimensional intratumor proteomic heterogeneity revealed by multiregion sampling in high-grade serous ovarian tumor specimens. iScience 2021, 24, 102757. [Google Scholar] [CrossRef]
- Lahtinen, A.; Lavikka, K.; Virtanen, A.; Li, Y.; Jamalzadeh, S.; Skorda, A.; Lauridsen, A.R.; Zhang, K.; Marchi, G.; Isoviita, V.M.; et al. Evolutionary states and trajectories characterized by distinct pathways stratify patients with ovarian high grade serous carcinoma. Cancer Cell 2023, 41, 1103–1117.e1112. [Google Scholar] [CrossRef]
- Handley, K.F.; Sims, T.T.; Bateman, N.W.; Glassman, D.; Foster, K.I.; Lee, S.; Yao, J.; Yao, H.; Fellman, B.M.; Liu, J.; et al. Classification of High-Grade Serous Ovarian Cancer Using Tumor Morphologic Characteristics. JAMA Netw. Open 2022, 5, e2236626. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.I.; Handley, K.F.; Glassman, D.; Sims, T.T.; Javadi, S.; Palmquist, S.M.; Saleh, M.M.; Fellman, B.M.; Fleming, N.D.; Bhosale, P.R.; et al. Characterizing morphologic subtypes of high-grade serous ovarian cancer by CT: A retrospective cohort study. Int. J. Gynecol. Cancer 2023, 33, 937–943. [Google Scholar] [CrossRef]
- Eckert, M.A.; Pan, S.; Hernandez, K.M.; Loth, R.M.; Andrade, J.; Volchenboum, S.L.; Faber, P.; Montag, A.; Lastra, R.; Peter, M.E.; et al. Genomics of Ovarian Cancer Progression Reveals Diverse Metastatic Trajectories Including Intraepithelial Metastasis to the Fallopian Tube. Cancer Discov. 2016, 6, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.L.; Brenton, J.D. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol. 2011, 12, 1169–1174. [Google Scholar] [CrossRef]
- Donehower, L.A.; Soussi, T.; Korkut, A.; Liu, Y.; Schultz, A.; Cardenas, M.; Li, X.; Babur, O.; Hsu, T.-K.; Lichtarge, O.; et al. Integrated Analysis of TP53 Gene and Pathway Alterations in the Cancer Genome Atlas. Cell Rep. 2019, 28, 1370–1384.e1375. [Google Scholar] [CrossRef]
- Cunnea, P.; Curry, E.W.; Christie, E.L.; Nixon, K.; Kwok, C.H.; Pandey, A.; Wulandari, R.; Thol, K.; Ploski, J.; Morera-Albert, C.; et al. Spatial and temporal intra-tumoral heterogeneity in advanced HGSOC: Implications for surgical and clinical outcomes. Cell Rep. Med. 2023, 4, 101055. [Google Scholar] [CrossRef]
- Masoodi, T.; Siraj, S.; Siraj, A.K.; Azam, S.; Qadri, Z.; Parvathareddy, S.K.; Tulbah, A.; Al-Dayel, F.; AlHusaini, H.; AlOmar, O.; et al. Genetic heterogeneity and evolutionary history of high-grade ovarian carcinoma and matched distant metastases. Br. J. Cancer 2020, 122, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.C.; Lheureux, S.; Karakasis, K.; Burnier, J.V.; Bruce, J.P.; Clouthier, D.L.; Danesh, A.; Quevedo, R.; Dowar, M.; Hanna, Y.; et al. Landscape of genomic alterations in high-grade serous ovarian cancer from exceptional long- and short-term survivors. Genome Med. 2018, 10, 81. [Google Scholar] [CrossRef]
- Mittempergher, L. Genomic Characterization of High-Grade Serous Ovarian Cancer: Dissecting Its Molecular Heterogeneity as a Road Towards Effective Therapeutic Strategies. Curr. Oncol. Rep. 2016, 18, 44. [Google Scholar] [CrossRef]
- Filippova, O.T.; Selenica, P.; Pareja, F.; Vahdatinia, M.; Zhu, Y.; Pei, X.; Riaz, N.; Long Roche, K.; Chi, D.S.; Abu-Rustum, N.R.; et al. Molecular characterization of high-grade serous ovarian cancers occurring in younger and older women. Gynecol. Oncol. 2021, 161, 545–552. [Google Scholar] [CrossRef]
- Martins, F.C.; Santiago, I.; Trinh, A.; Xian, J.; Guo, A.; Sayal, K.; Jimenez-Linan, M.; Deen, S.; Driver, K.; Mack, M.; et al. Combined image and genomic analysis of high-grade serous ovarian cancer reveals PTEN loss as a common driver event and prognostic classifier. Genome Biol. 2014, 15, 526. [Google Scholar] [CrossRef] [PubMed]
- Stronach, E.A.; Paul, J.; Timms, K.M.; Hughes, E.; Brown, K.; Neff, C.; Perry, M.; Gutin, A.; El-Bahrawy, M.; Steel, J.H.; et al. Biomarker Assessment of HR Deficiency, Tumor BRCA1/2 Mutations, and CCNE1 Copy Number in Ovarian Cancer: Associations with Clinical Outcome Following Platinum Monotherapy. Mol. Cancer Res. 2018, 16, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Patch, A.M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef]
- Martins, F.C.; Couturier, D.-L.; Paterson, A.; Karnezis, A.N.; Chow, C.; Nazeran, T.M.; Odunsi, A.; Gentry-Maharaj, A.; Vrvilo, A.; Hein, A.; et al. Clinical and pathological associations of PTEN expression in ovarian cancer: A multicentre study from the Ovarian Tumour Tissue Analysis Consortium. Br. J. Cancer 2020, 123, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, E.N.; Mullen, M.M.; Spies, N.C.; Li, T.; Inkman, M.; Zhang, J.; Martins-Rodrigues, F.; Hagemann, I.S.; McCourt, C.K.; Thaker, P.H.; et al. Genetic characterization of primary and metastatic high-grade serous ovarian cancer tumors reveals distinct features associated with survival. Commun. Biol. 2023, 6, 688. [Google Scholar] [CrossRef]
- van Wagensveld, L.; van Baal, J.O.A.M.; Timmermans, M.; Gaillard, D.; Borghuis, L.; Coffelt, S.B.; Rosenberg, E.H.; Lok, C.A.R.; Nijman, H.W.; Kooreman, L.F.S.; et al. Homologous Recombination Deficiency and Cyclin E1 Amplification Are Correlated with Immune Cell Infiltration and Survival in High-Grade Serous Ovarian Cancer. Cancers 2022, 14, 5965. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.C.; Couturier, D.L.; de Santiago, I.; Sauer, C.M.; Vias, M.; Angelova, M.; Sanders, D.; Piskorz, A.; Hall, J.; Hosking, K.; et al. Clonal somatic copy number altered driver events inform drug sensitivity in high-grade serous ovarian cancer. Nat. Commun. 2022, 13, 6360. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.F.; Ng, C.K.; Cooke, S.L.; Newman, S.; Temple, J.; Piskorz, A.M.; Gale, D.; Sayal, K.; Murtaza, M.; Baldwin, P.J.; et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: A phylogenetic analysis. PLoS Med. 2015, 12, e1001789. [Google Scholar] [CrossRef]
- Vishwakarma, R.; McManus, K.J. Chromosome Instability; Implications in Cancer Development, Progression, and Clinical Outcomes. Cancers 2020, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Blagden, S.P. Harnessing Pandemonium: The Clinical Implications of Tumor Heterogeneity in Ovarian Cancer. Front. Oncol. 2015, 5, 149. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Z.; Tian, L.; Zheng, Y.; Wu, L.; Guo, Y.; Li, X.; Li, Y.; Shen, H.; Lai, Y.; et al. Dualistic classification of high grade serous ovarian carcinoma has its root in spatial heterogeneity. J. Adv. Res. 2023, 48, 213–225. [Google Scholar] [CrossRef]
- Geistlinger, L.; Oh, S.; Ramos, M.; Schiffer, L.; LaRue, R.S.; Henzler, C.M.; Munro, S.A.; Daughters, C.; Nelson, A.C.; Winterhoff, B.J.; et al. Multiomic Analysis of Subtype Evolution and Heterogeneity in High-Grade Serous Ovarian Carcinoma. Cancer Res. 2020, 80, 4335–4345. [Google Scholar] [CrossRef]
- Cardenas, H.; Fang, F.; Jiang, G.; Perkins, S.M.; Zhang, C.; Emerson, R.E.; Hutchins, G.; Keer, H.N.; Liu, Y.; Matei, D.; et al. Methylomic Signatures of High Grade Serous Ovarian Cancer. Epigenetics 2021, 16, 1201–1216. [Google Scholar] [CrossRef]
- Reyes, H.D.; Devor, E.J.; Warrier, A.; Newtson, A.M.; Mattson, J.; Wagner, V.; Duncan, G.N.; Leslie, K.K.; Gonzalez-Bosquet, J. Differential DNA methylation in high-grade serous ovarian cancer (HGSOC) is associated with tumor behavior. Sci. Rep. 2019, 9, 17996. [Google Scholar] [CrossRef]
- Chan, D.W.; Lam, W.Y.; Chen, F.; Yung, M.M.H.; Chan, Y.S.; Chan, W.S.; He, F.; Liu, S.S.; Chan, K.K.L.; Li, B.; et al. Genome-wide DNA methylome analysis identifies methylation signatures associated with survival and drug resistance of ovarian cancers. Clin. Epigenetics 2021, 13, 142. [Google Scholar] [CrossRef]
- Silva, R.; Glennon, K.; Metoudi, M.; Moran, B.; Salta, S.; Slattery, K.; Treacy, A.; Martin, T.; Shaw, J.; Doran, P.; et al. Unveiling the epigenomic mechanisms of acquired platinum-resistance in high-grade serous ovarian cancer. Int. J. Cancer 2023, 153, 120–132. [Google Scholar] [CrossRef]
- Nishiyama, A.; Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021, 37, 1012–1027. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Chen, R.; Yue, H.; Li, W.; Wu, B.; Bai, Y.; Zhu, G.; Lu, X. DNA methylation-based profiling reveals distinct clusters with survival heterogeneity in high-grade serous ovarian cancer. Clin. Epigenetics 2021, 13, 190. [Google Scholar] [CrossRef]
- Bitler, B.G.; Bailey, C.A.; Yamamoto, T.M.; McMellen, A.; Kim, H.; Watson, Z.L. Targeting BRPF3 moderately reverses olaparib resistance in high grade serous ovarian carcinoma. Mol. Carcinog. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Watson, Z.L.; Yamamoto, T.M.; McMellen, A.; Kim, H.; Hughes, C.J.; Wheeler, L.J.; Post, M.D.; Behbakht, K.; Bitler, B.G. Histone methyltransferases EHMT1 and EHMT2 (GLP/G9A) maintain PARP inhibitor resistance in high-grade serous ovarian carcinoma. Clin. Epigenetics 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Kukita, A.; Sone, K.; Oda, K.; Hamamoto, R.; Kaneko, S.; Komatsu, M.; Wada, M.; Honjoh, H.; Kawata, Y.; Kojima, M.; et al. Histone methyltransferase SMYD2 selective inhibitor LLY-507 in combination with poly ADP ribose polymerase inhibitor has therapeutic potential against high-grade serous ovarian carcinomas. Biochem. Biophys. Res. Commun. 2019, 513, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; George, J.; Wang, C.; Budden, T.; Tan, T.Z.; Chiu, D.S.; Kommoss, S.; Leong, H.S.; Chen, S.; Intermaggio, M.P.; et al. Development and Validation of the Gene Expression Predictor of High-grade Serous Ovarian Carcinoma Molecular SubTYPE (PrOTYPE). Clin. Cancer Res. 2020, 26, 5411–5423. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.H.; Kim, S.I.; Shin, J.Y.; Kim, H.S.; Chung, H.H.; Kim, J.W.; Lee, M.; Seo, J.S. Classification of High-Grade Serous Ovarian Carcinoma by Epithelial-to-Mesenchymal Transition Signature and Homologous Recombination Repair Genes. Genes 2021, 12, 1103. [Google Scholar] [CrossRef]
- Chirshev, E.; Hojo, N.; Bertucci, A.; Sanderman, L.; Nguyen, A.; Wang, H.; Suzuki, T.; Brito, E.; Martinez, S.R.; Castanon, C.; et al. Epithelial/mesenchymal heterogeneity of high-grade serous ovarian carcinoma samples correlates with miRNA let-7 levels and predicts tumor growth and metastasis. Mol. Oncol. 2020, 14, 2796–2813. [Google Scholar] [CrossRef]
- Ferri-Borgogno, S.; Zhu, Y.; Sheng, J.; Burks, J.K.; Gomez, J.A.; Wong, K.K.; Wong, S.T.C.; Mok, S.C. Spatial Transcriptomics Depict Ligand–Receptor Cross-talk Heterogeneity at the Tumor-Stroma Interface in Long-Term Ovarian Cancer Survivors. Cancer Res. 2023, 83, 1503–1516. [Google Scholar] [CrossRef]
- Nath, A.; Cosgrove, P.A.; Mirsafian, H.; Christie, E.L.; Pflieger, L.; Copeland, B.; Majumdar, S.; Cristea, M.C.; Han, E.S.; Lee, S.J.; et al. Evolution of core archetypal phenotypes in progressive high grade serous ovarian cancer. Nat. Commun. 2021, 12, 3039. [Google Scholar] [CrossRef] [PubMed]
- Izar, B.; Tirosh, I.; Stover, E.H.; Wakiro, I.; Cuoco, M.S.; Alter, I.; Rodman, C.; Leeson, R.; Su, M.J.; Shah, P.; et al. A single-cell landscape of high-grade serous ovarian cancer. Nat. Med. 2020, 26, 1271–1279. [Google Scholar] [CrossRef]
- Stur, E.; Corvigno, S.; Xu, M.; Chen, K.; Tan, Y.; Lee, S.; Liu, J.; Ricco, E.; Kraushaar, D.; Castro, P.; et al. Spatially resolved transcriptomics of high-grade serous ovarian carcinoma. iScience 2022, 25, 103923. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yan, C.; Xu, D.; Zhang, Z.; Li, K.; Li, X.; Zhou, M.; Hao, D. Immuno-genomic characterisation of high-grade serous ovarian cancer reveals immune evasion mechanisms and identifies an immunological subtype with a favourable prognosis and improved therapeutic efficacy. Br. J. Cancer 2022, 126, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fang, Y.; Chen, K.; Li, S.; Tang, S.; Ren, Y.; Cen, Y.; Fei, W.; Zhang, B.; Shen, Y.; et al. Single-Cell RNA Sequencing Reveals the Tissue Architecture in Human High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2022, 28, 3590–3602. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Zhang, W.; Fan, J.; Zhou, Y.; Li, W.; Yin, J.; Yang, X.; Guo, E.; Li, X.; et al. Spatial heterogeneity of infiltrating T cells in high-grade serous ovarian cancer revealed by multi-omics analysis. Cell Rep. Med. 2022, 3, 100856. [Google Scholar] [CrossRef]
- Vazquez-Garcia, I.; Uhlitz, F.; Ceglia, N.; Lim, J.L.P.; Wu, M.; Mohibullah, N.; Niyazov, J.; Ruiz, A.E.B.; Boehm, K.M.; Bojilova, V.; et al. Ovarian cancer mutational processes drive site-specific immune evasion. Nature 2022, 612, 778–786. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, A.; Cybulska, P.; Mager, K.L.; Koplev, S.; Cast, O.; Couturier, D.L.; Memon, D.; Selenica, P.; Nikolovski, I.; Mazaheri, Y.; et al. Unraveling tumor-immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat. Genet. 2020, 52, 582–593. [Google Scholar] [CrossRef]
- Kelliher, L.; Lengyel, E. Understanding Long-Term Survival of Patients with Ovarian Cancer-The Tumor Microenvironment Comes to the Forefront. Cancer Res. 2023, 83, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Lecker, L.S.M.; Berlato, C.; Maniati, E.; Delaine-Smith, R.; Pearce, O.M.T.; Heath, O.; Nichols, S.J.; Trevisan, C.; Novak, M.; McDermott, J.; et al. TGFBI Production by Macrophages Contributes to an Immunosuppressive Microenvironment in Ovarian Cancer. Cancer Res. 2021, 81, 5706–5719. [Google Scholar] [CrossRef]
- El-Arabey, A.A.; Denizli, M.; Kanlikilicer, P.; Bayraktar, R.; Ivan, C.; Rashed, M.; Kabil, N.; Ozpolat, B.; Calin, G.A.; Salama, S.A.; et al. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell. Signal. 2020, 68, 109539. [Google Scholar] [CrossRef] [PubMed]
- Pejovic, T.; Fitch, K.; Mills, G. Ovarian cancer recurrence: “Is the definition of platinum resistance modified by PARP inhibitors and other intervening treatments?”. Cancer Drug Resist. 2022, 5, 451–458. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients with Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2023, 41, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiao, X.; Feng, J.; Yan, R.; Xi, J. Machine learning-assisted analysis of epithelial mesenchymal transition pathway for prognostic stratification and immune infiltration assessment in ovarian cancer. Front. Endocrinol. 2023, 14, 1196094. [Google Scholar] [CrossRef]
- Montfort, A.; Barker-Clarke, R.J.; Piskorz, A.M.; Supernat, A.; Moore, L.; Al-Khalidi, S.; Böhm, S.; Pharoah, P.; McDermott, J.; Balkwill, F.R.; et al. Combining measures of immune infiltration shows additive effect on survival prediction in high-grade serous ovarian carcinoma. Br. J. Cancer 2020, 122, 1803–1810. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzalini, E.; Stanta, G.; Canzonieri, V.; Bonin, S. Overview of Tumor Heterogeneity in High-Grade Serous Ovarian Cancers. Int. J. Mol. Sci. 2023, 24, 15077. https://doi.org/10.3390/ijms242015077
Azzalini E, Stanta G, Canzonieri V, Bonin S. Overview of Tumor Heterogeneity in High-Grade Serous Ovarian Cancers. International Journal of Molecular Sciences. 2023; 24(20):15077. https://doi.org/10.3390/ijms242015077
Chicago/Turabian StyleAzzalini, Eros, Giorgio Stanta, Vincenzo Canzonieri, and Serena Bonin. 2023. "Overview of Tumor Heterogeneity in High-Grade Serous Ovarian Cancers" International Journal of Molecular Sciences 24, no. 20: 15077. https://doi.org/10.3390/ijms242015077
APA StyleAzzalini, E., Stanta, G., Canzonieri, V., & Bonin, S. (2023). Overview of Tumor Heterogeneity in High-Grade Serous Ovarian Cancers. International Journal of Molecular Sciences, 24(20), 15077. https://doi.org/10.3390/ijms242015077







