Production of Bacterial Exopolysaccharides: Xanthan and Bacterial Cellulose
Abstract
:1. Introduction
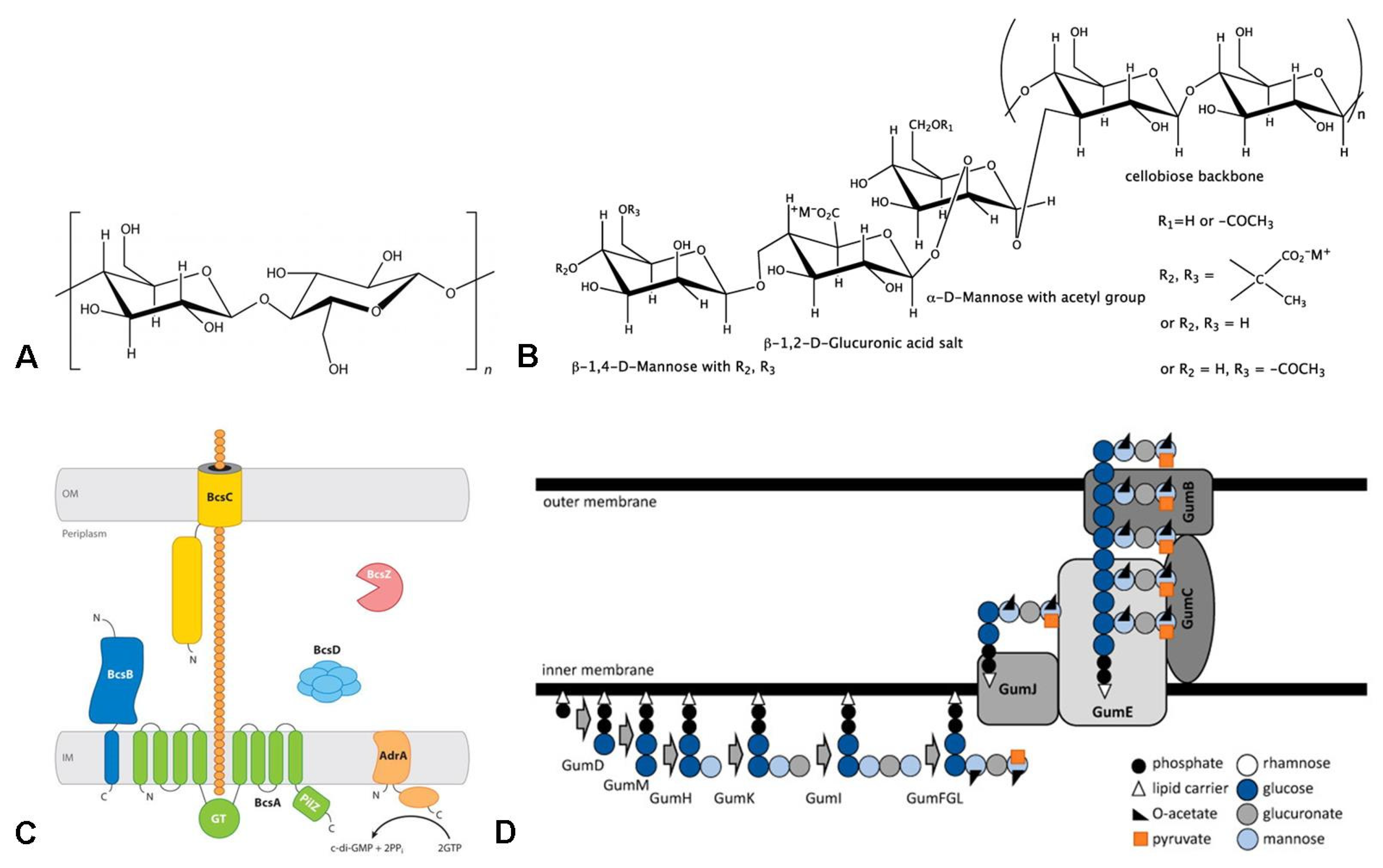
2. Properties and Biosynthesis of BC and Xanthan
3. Strategies for Cost-Effective Production of Xanthan and BC
3.1. BC and Xanthan Production from Wastes
3.2. Technologies for Cost-Effective Production of Xanthan and BC
3.2.1. Co-Cultivation of EPS-producing Bacteria
3.2.2. Biocatalytic Technologies
3.2.3. Genetic and Metabolic Engineering
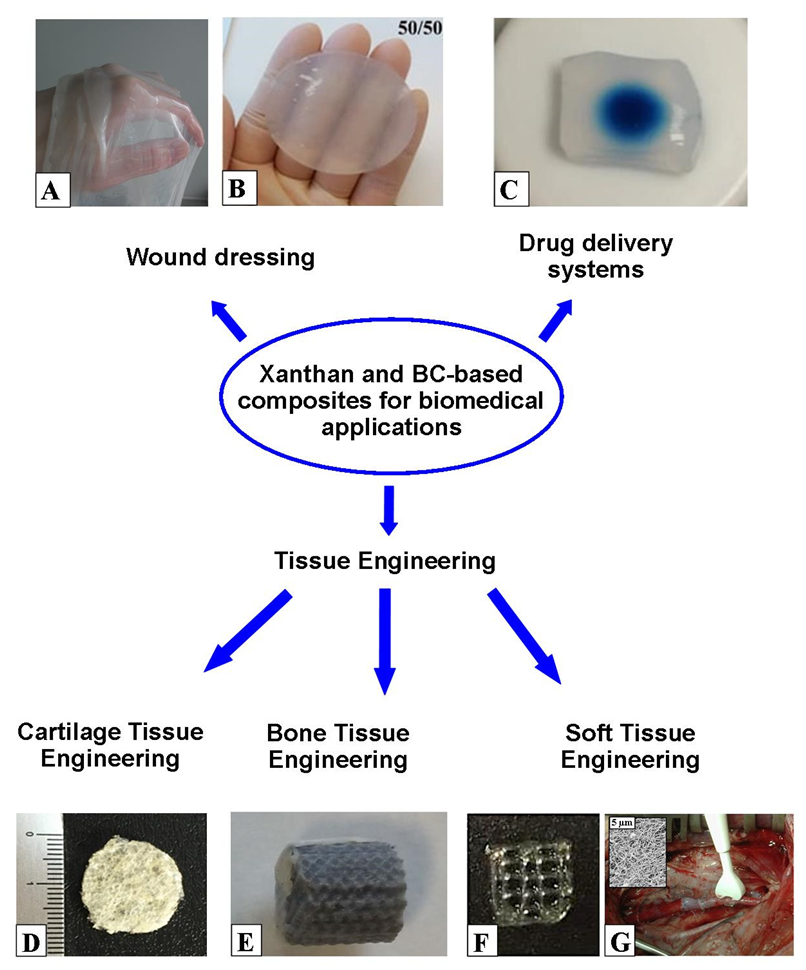
4. Applications of BC and Xanthan
5. Conclusions and Further Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, N.; Dey, P. Bacterial Exopolysaccharides as Emerging Bioactive Macromolecules: From Fundamentals to Applications. Res. Microbiol. 2022, 29, 104024. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Farrokh, P. Recent advances in the biological activities of microbial exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Purohit, S.R. Microbial exopolysaccharide: Sources, stress conditions, properties and application in food and environment: A comprehensive review. Int. J. Biol. Macromol. 2023, 242, 124925. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, V.; Bhatt, A.K.; Banu, J.R.; Kumar, V.; Kumar, G.; Yang, Y.H.; Bhatia, S.K. Microbial Exopolysaccharide Composites in Biomedicine and Healthcare: Trends and Advances. Polymers 2023, 15, 1801. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.A.-G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent Advancements in Microbial Polysaccharides: Synthesis and Applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2019, 60, 1475–1495. [Google Scholar] [CrossRef]
- Hussain, A.; Zia, K.M.; Tabasum, S.; Noreen, A.; Ali, M.; Iqbal, R.; Zuber, M. Blends and composites of exopolysaccharides; properties and applications: A review. Int. J. Biol. Macromol. 2017, 94, 10–27. [Google Scholar] [CrossRef]
- Aditya, T.; Allain, J.P.; Jaramillo, C.; Restrepo, A.M. Surface Modification of Bacterial Cellulose for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 610. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.H.; Abou Elhassayeb, H.E.; El-Sayed, W.M.M. Potential functions and applications of diverse microbial exopolysaccharides in marine environments. J. Genet. Eng. Biotechnol. 2022, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Revin, V.V.; Liyas’kina, E.V.; Sapunova, N.B.; Bogatyreva, A.O. Isolation and characterization of the strains producing bacterial cellulose. Microbiology 2020, 14, 86–95. [Google Scholar] [CrossRef]
- Gromovykh, T.I.; Sadykova, V.S.; Lutcenko, S.V.; Dmitrenok, A.S.; Feldman, N.B.; Danilchuk, T.N.; Kashirin, V.V. Bacterial cellulose synthesized by Gluconacetobacter hansenii for medical applications. Appl. Biochem. Microbiol. 2017, 53, 60–67. [Google Scholar] [CrossRef]
- Volova, T.; Prudnikova, S.V.; Sukovatyi, A.G.; Shishatskaya, E.I. Production and properties of bacterial cellulose by the strain Komagataeibacter xylinus B-12068. Appl. Microbiol. Biotechnol. 2018, 102, 7417–7428. [Google Scholar] [CrossRef]
- Semjonovs, P.; Ruklisha, M.; Paegle, L.; Saka, M.; Treimane, R.; Skute, M.; Rozenberga, L.; Vikele, L.; Sabovics, M.; Cleenwerck, I. Cellulose synthesis by Komagataeibacter rhaeticus strain P 1463 isolated from Kombucha. Appl. Microbiol. Biotechnol. 2017, 101, 1003–1012. [Google Scholar] [CrossRef]
- Jacek, P.; da Silva, F.A.S.; Dourado, F.; Bielecki, S.; Gama, M. Optimization and characterization of bacterial nanocellulose produced by Komagataeibacter rhaeticus K3. Carbohydr. Polym. Technol. Appl. 2021, 2, 100022. [Google Scholar]
- Cepec, E.; Trček, J. Antimicrobial Resistance of Acetobacter and Komagataeibacter Species Originating from Vinegars. Int. J. Environ. Res. Public Health 2022, 19, 463. [Google Scholar] [CrossRef]
- Naloka, K.; Yukphan, P.; Matsutani, M.; Matsushita, K.; Theeragool, G. Komagataeibacter diospyri sp. nov., a novel species of thermotolerant bacterial nanocellulose-producing bacterium. Int. J. Syst. Evol. Microbiol. 2020, 70, 251–258. [Google Scholar] [CrossRef]
- Hollensteiner, J.; Poehlein, A.; Kloskowski, P.; Ali, T.T.; Daniel, R. Genome Sequence of Komagataeibacter saccharivorans Strain JH1, Isolated from Fruit Flies. Microbiol. Resour. Announc. 2020, 9, e00098-20. [Google Scholar] [CrossRef] [PubMed]
- Greser, A.B.; Avcioglu, N.H. Optimization and Physicochemical Characterization of Bacterial Cellulose by Komagataeibacter nataicola and Komagataeibacter maltaceti Strains Isolated from Grape, Thorn Apple and Apple Vinegars. Arch. Microbiol. 2022, 204, 465. [Google Scholar] [CrossRef] [PubMed]
- Liyaskina, E.V.; Rakova, N.A.; Kitykina, A.A.; Rusyaeva, V.V.; Toukach, P.V.; Fomenkov, A.; Vainauskas, S.; Roberts, R.J.; Revin, V.V. Production and сharacterization of the exopolysaccharide from strain Paenibacillus polymyxa 2020. PLoS ONE 2021, 16, e0253482. [Google Scholar] [CrossRef] [PubMed]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Food 2022, 11, 156. [Google Scholar] [CrossRef]
- Khalil, M.A.; Sonbol, F.I.; Al-Madboly, L.A.; Aboshady, T.A.; Alqurashi, A.S.; Ali, S.S. Exploring the Therapeutic Potentials of Exopolysaccharides Derived from Lactic Acid Bacteria and Bifidobacteria: Antioxidant, Antitumor, and Periodontal Regeneration. Front. Microbiol. 2022, 13, 803688. [Google Scholar] [CrossRef]
- Miyamoto, J.; Shimizu, H.; Hisa, K.; Matsuzaki, C.; Inuki, S.; Ando, Y.; Nishida, A.; Izumi, A.; Yamano, M.; Ushiroda, C.; et al. Host metabolic benefits of prebiotic exopolysaccharides produced by Leuconostoc mesenteroides. Gut Microbes 2023, 15, 2161271. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.; Tian, Y. Biological activities and applications of exopolysaccharides produced by lactic acid bacteria: A mini-review. World J. Microbiol. Biotechnol. 2023, 39, 155. [Google Scholar] [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from marine and marine extremophilic bacteria: Structures, properties, ecological roles and applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic exopolysaccharides: A review and new perspectives on engineering strategies and applications. Carbohydr. Polym. 2019, 5, 8–26. [Google Scholar] [CrossRef]
- Isfahani, F.M.; Tahmourespour, A.; Hoodaji, M.; Ataabadi, M.; Mohammadi, A. Characterizing the new bacterial isolates of high yielding exopolysaccharides under hypersaline conditions. J. Clean. Prod. 2018, 185, 922–928. [Google Scholar] [CrossRef]
- Pallach, M.; Marchetti, R.; Di Lorenzo, F.; Fabozzi, A.; Giraud, E.; Gully, D.; Paduano, L.; Molinaro, A.; D’Errico, G.; Silipo, A. Zymomonas mobilis exopolysaccharide structure and role in high ethanol tolerance. Carbohydr. Polym. 2018, 201, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Secor, P.R.; Michaels, L.A.; Bublitz, D.C.; Jennings, L.K.; Singh, P.K. The Depletion Mechanism Actuates Bacterial Aggregation by Exopolysaccharides and Determines Species Distribution & Composition in Bacterial Aggregates. Front. Cell Infect. Microbiol. 2022, 12, 869736. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Balducci, E.; Papi, F.; Capialbi, D.E.; Del Bino, L. Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens. Int. J. Mol. Sci. 2023, 24, 4030. [Google Scholar] [CrossRef]
- Liu, M.; Liu, L.; Jia, S. Complete genome analysis of Gluconacetobacter xylinus CGMCC 2955 for elucidating bacterial cellulose biosynthesis and metabolic regulation. Sci. Rep. 2018, 8, 6266. [Google Scholar] [CrossRef]
- Balíková, K.; Farkas, B.; Matúš, P.; Urík, M. Prospects of Biogenic Xanthan and Gellan in Removal of Heavy Metals from Contaminated Waters. Polymers 2022, 14, 5326. [Google Scholar] [CrossRef]
- Manan, S.; Wajid Ullah, M.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Yu, M.; Shen, M.; Wang, Q.; Yu, Y.; Xie, J. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. Int. J. Biol. Macromol. 2019, 121, 743–751. [Google Scholar] [CrossRef]
- Chakraborty, I.; Sen, I.K.; Mondal, S.; Rout, D.; Bhanja, S.K.; Maity, G.N.; Maity, P. Bioactive polysaccharides from natural sources: A review on the antitumor and immunomodulating activities. Biocatal. Agric. Biotechnol. 2019, 22, 101425. [Google Scholar] [CrossRef]
- Noufal, Z.M.; Sivaperumal, P.; Elumalai, P.J. Extraction, characterization, and anticancer potential of extracellular polymeric substances from marine actinobacteria of Streptomyces species. Adv. Pharm. Technol. Res. 2022, 13 (Suppl. S1), S125–S129. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Shah, M.D.; Shah, L.; Lee, P.C.; Khan, I. Bacterial polysaccharides-A big source for prebiotics and therapeutics. Front. Nutr. 2022, 9, 1031935. [Google Scholar] [CrossRef] [PubMed]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Subhash, M.; Jadhav, A.; Jana, S. Sustainable production of microbial polysaccharide xanthan gum from supplemental subststrate. Int. J. Sci. Res. 2015, 4, 9–11. [Google Scholar]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of bacterial cellulose from alternative cheap and waste resources: A step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Revin, V.V.; Liyaskina, E.V.; Parchaykina, M.V.; Kuzmenko, T.P.; Urgaeva, I.V.; Revin, V.D.; Ullah, M.W. Bacterial Cellulose-Based Polymer Nanocomposites: A Review. Polymers 2022, 14, 4670. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.M.; Im, J.; Cho, G.C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 100385. [Google Scholar] [CrossRef]
- Furtado, I.F.S.P.C.; Sydney, E.B.; Rodrigues, S.A.; Sydney, A.C.N. Xanthan gum: Applications, challenges, and advantages of this asset of biotechnological origin. Biotechnol. Res. Innov. 2022, 6, e202204. [Google Scholar] [CrossRef]
- Berninger, T.; Dietz, N.; González López, Ó. Water-soluble polymers in agriculture: Xanthan gum as eco-friendly alternative to synthetics. Microb. Biotechnol. 2021, 14, 1881–1896. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Yano, H.; Nogi, M.; Eichhorn, S.J. An estimation of the young’s modulus of bacterial cellulose filaments. Cellulose 2008, 15, 507–513. [Google Scholar] [CrossRef]
- Choi, C.N.; Song, H.J.; Kim, M.J.; Chang, M.H.; Kim, S.J. Properties of bacterial cellulose produced in a pilot-scale spherical type bubble column bioreactor. Korean J. Chem. Eng. 2009, 26, 136–140. [Google Scholar] [CrossRef]
- Jadczak, K.; Ochędzan-Siodłak, W. Bacterial cellulose: Biopolymer with novel medical applications. J. Biomater. Appl. 2023, 38, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.D.S.; Castro, G.R. Bacterial Cellulose-Based Materials as Dressings for Wound Healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.; Ghica, M.V.; Tudoroiu, E.-E.; Ionescu, D.G.; Dinu-Pîrvu, C.E. Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials 2022, 15, 1054. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.V.; Gayathri, V.; Samanta, D.; Sampath, S. Macromolecules Bacterial Cellulose: A Promising Biopolymer with Interesting Properties and Applications. Int. J. Biol. Macromol. 2022, 220, 435–461. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J. The Nanofication and Functionalization of Bacterial Cellulose and Its Applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef]
- Ullah, M.W.; Rojas, O.J.; McCarthy, R.R.; Yang, G. Editorial: Nanocellulose: A Multipurpose Advanced Functional Material. Front. Bioeng. Biotechnol. 2021, 9, 738779. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ahmad, F.; Fatima, A.; Shah, N.; Yasir, S.; Ahmad, M.W.; Manan, S.; Ullah, M.W. Ex situ Synthesis and Characterization of High Strength Multipurpose Bacterial Cellulose- Aloe vera Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 601988. [Google Scholar] [CrossRef]
- Wu, M.; Qu, J.; Shen, Y.; Dai, X.; Wei, W.; Shi, Z.; Li, G.; Ma, T. Gel properties of xanthan containing a single repeating unit with saturated pyruvate produced by an engineered Xanthomonas campestris CGMCC 15155. Food Hydrocoll. 2019, 87, 747–757. [Google Scholar] [CrossRef]
- Fitzpatrick, P.; Meadows, J.; Ratcliffe, I.; Williams, P.A. Control of the properties of xanthan/glucomannan mixed gels by varying xanthan fine structure. Carbohydr. Polym. 2013, 92, 1018–1025. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef]
- Moffat, J.; Morris, V.J.; Al-Assaf, S.; Gunning, A.P. Visualisation of xanthan conformation by atomic force microscopy. Carbohydr. Polym. 2016, 148, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F. Advances in xanthan gum production, modifications and its applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Al-Muhanna, M.K.; Anwar, N.; Hasnain, S.; Nayak, A.K. Chapter 1–Synthesis of tailor-made polysaccharides: An overview. In Tailor-Made Polysaccharides in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–27. [Google Scholar]
- Şen, E.; Demirci, A.S.; Palabiyik, I. Xanthan gum characterization and production kinetics from pomace of Vitis vinifera. J. Food Process. Preserv. 2022, 46, e17098. [Google Scholar] [CrossRef]
- Maurya, D.K.; Kumar, A.; Chaurasiya, U.; Hussain, T.; Singh, S.K. Modern era of microbial biotechnology: Opportunities and future prospects. Microbiomes Plant Health Panoply Appl. 2020, 1, 317–343. [Google Scholar]
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem. 2023, 399, 133967. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.L.; Tang, D.J.; Dubrow, Z.E.; Bogdanove, A.; An, S.Q. Xanthomonas campestris Pathovars. Trends Microbiol. 2021, 29, 182–183. [Google Scholar] [CrossRef]
- Gumus, T.; Sukru Demirci, A.; Mirik, M.; Arici, M.; Aysan, Y. Xanthan gum production of Xanthomonas spp. isolated from different plants. Food Sci. Biotechnol. 2010, 19, 201–206. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Asadollahi, M.A.; Zamani, A.; Biria, D. Production of xanthan gum by free and immobilized cells of Xanthomonas campestris and Xanthomonas pelargonii. Int. J. Biol. Macromol. 2016, 82, 751–756. [Google Scholar] [CrossRef]
- Ramezani, A.; Jafari, M.; Goodarzi, T.; Alavi, S.M.; Salmanian, A.H.; Azin, M. Lactose consuming strains of Xanthomonas citri subsp. citri (Xcc) insight into the emergence of natural field resources for xanthan gum production. World J. Microbiol. Biotechnol. 2014, 30, 1511–1517. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Jedrzejczak-Krzepkowska, M.; Ludwicka, K. Comparative Analysis of Bacterial Cellulose Membranes Synthesized by Chosen Komagataeibacter Strains and Their Application Potential. Int. J. Mol. Sci. 2022, 23, 3391. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, J.Y.; Cha, M.Y.; Kang, H.C. Characteristics of biocellulose by Gluconobacter uchimurae GYS15. J. Soc. Cosmet. Sci. Korea 2016, 42, 247–255. [Google Scholar] [CrossRef]
- Nie, W.; Zheng, X.; Feng, W.; Liu, Y.; Li, Y.; Liang, X. Characterization of Bacterial Cellulose Produced by Acetobacter Pasteurianus MGC-N8819 Utilizing Lotus Rhizome. LWT 2022, 165, 113763. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable bacterial cellulose production by Achromobacter using mango peel waste. Microb. Cell Fact. 2023, 22, 24. [Google Scholar] [CrossRef]
- Barnhart, D.M.; Su, S.; Farrand, S.K. A signaling pathway involving the diguanylate cyclase CelR and the response regulator DivK controls cellulose synthesis in Agrobacterium tumefaciens. J. Bacteriol. 2014, 196, 1257–1274. [Google Scholar] [CrossRef]
- Gao, G.; Ji, K.; Zhang, Y.; Liu, X.; Dai, X.; Zhi, B.; Cao, Y.; Liu, D.; Wu, M.; Li, G.; et al. Microbial enhanced oil recovery through deep profile control using a conditional bacterial cellulose-producing strain derived from Enterobacter sp. FY-07. Microb. Cell. Fact. 2020, 19, 59. [Google Scholar] [CrossRef]
- Ardré, M.; Dufour, D.; Rainey, P.B. Causes and Biophysical Consequences of Cellulose Production by Pseudomonas fluorescens SBW25 at the Air-Liquid Interface. J. Bacteriol. 2019, 201, e00110-19. [Google Scholar] [CrossRef]
- Pérez-Mendoza, D.; Romero-Jiménez, L.; Rodríguez-Carvajal, M.Á.; Lorite, M.J.; Muñoz, S.; Olmedilla, A.; Sanjuán, J. The Role of Two Linear β-Glucans Activated by c-di-GMP in Rhizobium etli CFN42. Biology 2022, 11, 1364. [Google Scholar] [CrossRef]
- Fratty, I.S.; Shachar, D.; Katsman, M.; Yaron, S. The activity of BcsZ of Salmonella Typhimurium and its role in Salmonella-plants interactions. Front. Cell. Infect. Microbiol. 2022, 12, 967796. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Bhavikatti, J.S.; Muddapur, U.M.; Yaraguppi, D.A.; Mulla, S.I. Statistical Optimization and Characterization of Bacterial Cellulose Produced by Isolated Thermophilic Bacillus licheniformis Strain ZBT2. Carbohydr. Res. 2020, 491, 107979. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Deng, Y.; Wei, Q. Research progress of the biosynthetic strains and pathways of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab071. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.T.; Morgan, J.L.; Zimmer, J. A molecular description of cellulose biosynthesis. Annu. Rev. Biochem. 2015, 84, 895–921. [Google Scholar] [CrossRef] [PubMed]
- Ryngajłło, M.; Jędrzejczak-Krzepkowska, M.; Kubiak, K.; Ludwicka, K.; Bielecki, S. Towards control of cellulose biosynthesis by Komagataeibacter using systems-level and strain engineering strategies: Current progress and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 6565–6585. [Google Scholar] [CrossRef]
- Ross, P.; Weinhouse, H.; Aloni, Y.; Michaeli, D.; Weinberger-Ohana, P.; Mayer, R.; Braun, S.; de Vroom, E.; van der Marel, G.A.; van Boom, J.H.; et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 1987, 325, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Buldum, G.; Mantalaris, A. Systematic Understanding of Recent Developments in Bacterial Cellulose Biosynthesis at Genetic, Bioprocess and Product Levels. Int. J. Mol. Sci. 2021, 22, 7192. [Google Scholar] [CrossRef]
- Bimmer, M.; Mientus, M.; Klingl, A.; Ehrenreich, A.; Liebl, W. The Roles of the Various Cellulose Biosynthesis Operons in Komagataeibacter hansenii ATCC 23769. Appl. Environ. Microb. 2022, 88, e02460-21. [Google Scholar] [CrossRef]
- Kondo, T.; Nakamura, Y.; Nojima, S.; Yao, M.; Imai, T. The BcsD subunit of type I bacterial cellulose synthase interacts dynamically with the BcsAB catalytic core complex. FEBS Lett. 2022, 596, 3069–3086. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Letisse, F.; Lindley, N.; Roux, G. Development of a Phenomenological Modeling Approach for Prediction of Growth and Xanthan Gum Production Using Xanthomonas campestris. Biotechnol. Prog. 2003, 19, 822–827. [Google Scholar] [CrossRef]
- Soleymanpour, Z.; Nikzad, M.; Talebnia, F.; Niknezhad, V. Xanthan gum production from acid hydrolyzed broomcorn stem as a sole carbon source by Xanthomonas campestris. 3 Biotech 2018, 8, 296. [Google Scholar] [CrossRef]
- Ramos, L.C.; Jesus, M.S.; Pires, P.; Fontes-Junior, A.S.; Nunes, E.S.; Santos, K.S.; Teixeira, J.A.; Padilha, F.F.; Ruzene, D.S.; Silva, D.P. Optimization of Xanthan Gum Production by Demerara Sugar Using Response Surface Methodology. Sustainability 2023, 15, 5080. [Google Scholar] [CrossRef]
- Vaishnav, A.; Upadhyay, K.; Koradiya, M.; Tipre, D.; Dave, S. Valorisation of fruit waste for enhanced exopolysaccharide production by Xanthomonas campestries using statistical optimisation of medium and process. Food Biosci. 2022, 46, 101608. [Google Scholar] [CrossRef]
- Son, J.; Lee, K.H.; Lee, T.; Kim, H.S.; Shin, W.H.; Oh, J.M.; Koo, S.M.; Yu, B.J.; Yoo, H.Y.; Park, C. Enhanced Production of Bacterial Cellulose from Miscanthus as Sustainable Feedstock through Statistical Optimization of Culture Conditions. Int. J. Environ. Res. Public Health 2022, 19, 866. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; Mohammed, A.B.A.; El-Malkey, S.E. Bacterial nanocellulose production using Cantaloupe juice, statistical optimization and characterization. Sci. Rep. 2023, 13, 51. [Google Scholar] [CrossRef]
- Cielecka, I.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Response surface methodology-based improvement of the yield and differentiation of properties of bacterial cellulose by metabolic enhancers. Int. J. Biol. Macromol. 2021, 187, 584–593. [Google Scholar] [CrossRef]
- El-Gendi, H.; Salama, A.; El-Fakharany, E.M.; Saleh, A.K. Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydr. Polym. 2023, 302, 120383. [Google Scholar] [CrossRef]
- Suwanposri, A.; Yukphan, P.; Yamada, Y.; Ochaikul, D. Statistical optimisation of culture conditions for biocellulose production by Komagataeibacter sp. PAP1 using soya bean whey. Maejo Int. J. Sci. Technol. 2014, 8, 1–14. [Google Scholar]
- Rocha, A.R.F.D.S.; Venturim, B.C.; Ellwanger, E.R.A.; Pagnan, C.S.; Silveira, W.B.D.; Martin, J.G.P. Bacterial cellulose: Strategies for its production in the context of bioeconomy. J. Basic. Microbiol. 2023, 63, 257–275. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Kadier, A.; Ilyas, R.A.; Huzaifah, M.R.M.; Harihastuti, N.; Sapuan, S.M.; Harussani, M.M.; Azlin, M.N.M.; Yuliasni, R.; Ibrahim, R.; Atikah, M.S.N.; et al. Use of Industrial Wastes as SustainableNutrient Sources for Bacterial Cellulose (BC) Production:Mechanism, Advances, and Future Perspectives. Polymers 2021, 13, 3365. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Fatima, A.; Manan, S.; Khattak, W.A.; Ullah, M.W.; Guang, Y. Potential of Food and Agro-Industrial Wastes for Cost-Effective Bacterial Cellulose Production: An Updated Review of Literature. ES Food Agrofor. 2023, 13, 905. [Google Scholar] [CrossRef]
- Urbina, L.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Retegi, A. A review of bacterial cellulose: Sustainable production from agricultural waste and applications in various fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Shiram, S.; Venugopal, P.; Tungare, A.; Gondekar, N.; Chatterji, B.P. Optimization of Xanthan Gum Fermentation Utilizing Food Waste. GRD J. Eng. 2021, 6, 19–29. [Google Scholar]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa, A., Jr.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Han, Y.H.; Chen, J.L.; Zhang, D.C.; Shi, X.X.; Ye, Y.X.; Chen, D.L.; Li, M. Insights into bacterial cellulose biosynthesis from different carbon sources and the associated biochemical transformation pathways in Komagataeibacter sp. W1. Polymers 2018, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mohite, A.M.; Sharma, N. Influence of particle size on physical, mechanical, thermal, and morphological properties of tamarind- fenugreek mucilage biodegradable films. Polym. Bull. 2022, 80, 3119–3133. [Google Scholar] [CrossRef]
- Rosalam, S.; England, R. Review of xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzym. Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Ngwabebhoh, F.A.; Saha, N.; Zandraa, O.; Saha, T.; Saha, P. Development of novel biocomposites based on the clean production of microbial cellulose from dairy waste (sour whey). J. Appl. Polym. Sci. 2022, 139, 51433. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef]
- Revin, V.V.; Dolganov, A.V.; Liyaskina, E.V.; Nazarova, N.B.; Balandina, A.V.; Devyataeva, A.A.; Revin, V.D. Characterizing Bacterial Cellulose Produced by Komagataeibacter sucrofermentans H-110 on Molasses Medium and Obtaining a Biocomposite Based on It for the Adsorption of Fluoride. Polymers 2021, 13, 1422. [Google Scholar] [CrossRef] [PubMed]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Preparation and characterization of cellulose nanocrystals from bacterial cellulose produced in sugar beet molasses and cheese whey media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Płoska, J.; Garbowska, M.; Klempová, S.; Stasiak-Różańska, L. Obtaining Bacterial Cellulose through Selected Strains of Acetic Acid Bacteria in Classical and Waste Media. Appl. Sci. 2023, 13, 6429. [Google Scholar] [CrossRef]
- Ghozali, M.; Meliana, Y.; Chalid, M. Synthesis and characterization of bacterial cellulose by Acetobacter xylinum using liquid tapioca waste. Mater. Today Proc. 2021, 44, 2131–2134. [Google Scholar] [CrossRef]
- Gorgieva, S.; Jančič, U.; Cepec, E.; Trček, J. Production efficiency and properties of bacterial cellulose membranes in a novel grape pomace hydrolysate by Komagataeibacter melomenusus AV436T and Komagataeibacter xylinus LMG 1518. Int. J. Biol. Macromol. 2023, 244, 125368. [Google Scholar] [CrossRef]
- Senthilnathan, S.; Rahman, S.S.A.; Pasupathi, S.; Venkatachalam, P.; Karuppiah, S. Stoichiometric analysis and production of bacterial cellulose by Gluconacetobacter liquefaciens using borassus flabellifer L. jiggery. Appl. Biochem. Biotechnol. 2022, 194, 3645–3667. [Google Scholar] [CrossRef]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial Cellulose Production from agricultural Residues by two Komagataeibacter sp. Strains. Bioeng. 2022, 13, 10010–10025. [Google Scholar] [CrossRef]
- Efthymiou, M.-N.; Tsouko, E.C.; Pateraki, A.; Papagiannopoulos, P.; Tzamalis, S.; Pispas, K.; Bethanis, I.; Mantala, A.; Koutinas, A. Property evaluation of bacterial cellulose nanostructures produced from confectionery wastes. Biochem. Eng. J. 2022, 186, 108575. [Google Scholar] [CrossRef]
- Filippi, K.; Papapostolou, H.; Alexandri, M.; Vlysidis, A.; Myrtsi, E.D.; Ladakis, D.; Pateraki, C.; Haroutounian, S.A.; Koutinas, A. Integrated biorefinery development using winery waste streams for the production of bacterial cellulose, succinic acid and value- added fractions. Bioresour. Technol. 2022, 343, 125989. [Google Scholar] [CrossRef]
- Sar, T.; Yesilcimen Akbas, M. Potential use of olive oil mill wastewater for bacterial cellulose production. Bioengineered 2022, 13, 7659–7669. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, F.; Yang, X.-X.; Han, S.-F. Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresour. Technol. 2013, 135, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Sugimura, K.; Yoshioka, M.; Yoshinaga, A.; Kamitakahara, H. Synthesis-property-performance relationships of multifunctional bacterial cellulose composites fermented in situ alkali lignin medium. Carbohydr. Polym. 2021, 252, 117114. [Google Scholar] [CrossRef]
- Luo, M.-T.; Zhao, C.; Huang, C.; Chen, X.-F.; Huang, Q.-L.; Qi, G.-X.; Tian, L.-L.; Xiong, L.; Li, H.-L.; Chen, X.-D. Efficient using durian shell hydrolysate as low-cost substrate for bacterial cellulose production by Gluconacetobacter xylinus. Ind. J. Microbiol. 2017, 57, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, G.; Nogueira, C.R.; Meneguin, A.B.; Trovatti, E.; Silva, M.C.C.; Machado, R.T.A.; Ribeiro, S.J.L.; da Silva Filho, E.C.; Barud, H.S. Development and characterization of bacterial cellulose produced by cashew tree residues as alternative carbon source. Ind. Crops Prod. 2017, 107, 13–19. [Google Scholar] [CrossRef]
- Ogrizek, L.; Lamovšek, J.; Čuš, F.; Leskovšek, M.; Gorjanc, M. Properties of bacterial cellulose produced using white and red grape bagasse as a nutrient source. Processes 2021, 9, 1088. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, H.; Wang, H.; Yu, H. Coproduction of bacterial cellulose and pear vinegar by fermentation of pear peel and pomace. Bioprocess Biosyst. Eng. 2021, 44, 2231–2244. [Google Scholar] [CrossRef]
- Santoso, S.P.; Lin, S.-P.; Wang, T.-Y.; Ting, Y.; Hsieh, C.-W.; Yu, R.-C.; Angkawijaya, A.E.; Soetaredjo, F.E.; Hsu, H.-Y.; Cheng, K.-C. Atmospheric cold plasma-assisted pineapple peel waste hydrolysate detoxification for the production of bacterial cellulose. Int. J. Biol. Macromol. 2021, 175, 526–534. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.-L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Álvarez, J.; Zuluaga, R.; Castro, C.; Gañán, P. A novel approach using conventional methodologies to scale up BNC production using Komagataeibacter medellinensis and rotten banana waste as alternative. Processes 2020, 8, 1469. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, J.; Singh, R.P. Biotransformation of sweet lime pulp waste into high-quality nanocellulose with an excellent productivity using Komagataeibacter europaeus SGP37 under static intermittent fed-batch cultivation. Bioresour. Technol. 2018, 247, 73–80. [Google Scholar] [CrossRef]
- Lotfiman, S.; Awang Biak, D.R.; Beng Ti, T.; Kamarudin, S.; Nikbin, S. Influence of date syrup as a carbon source on bacterial cellulose production by Acetobacter xylinum 0416. Adv. Polym. Technol. 2018, 37, 1085–1091. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Production and characterization of bacterial cellulose from citrus peels. Waste Biomass Valorization 2019, 10, 2165–2175. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Preparation and characterization of bacterial cellulose produced from fruit and vegetable peels by Komagataeibacter hansenii GA2016. Int. J. Biol. Macromol. 2020, 162, 1597–1604. [Google Scholar] [CrossRef]
- Bilgi, E.; Bayir, E.; Sendemir-Urkmez, A.; Esin Hames, E. Optimization of bacterial cellulose production by Gluconacetobacter xylinus using carob and haricot bean. Int. J. Biol. Macromol. 2016, 90, 2–10. [Google Scholar] [CrossRef]
- Xu, S.; Xu, S.; Ge, X.; Tan, L.; Liu, T. Low-cost and highly efficient production of bacterial cellulose from sweet potato residues: Optimization, characterization, and application. Int. J. Biol. Macromol. 2022, 196, 172–179. [Google Scholar] [CrossRef]
- Betlej, I.; Rybak, K.; Nowacka, M.; Antczak, A.; Borysiak, S.; Krochmal-Marczak, B.; Lipska, K.; Boruszewski, P. Structural properties of bacterial cellulose film obtained on a substrate containing sweet potato waste. Crystals 2022, 12, 1191. [Google Scholar] [CrossRef]
- Chaiyachet, O.A.; Wongtham, K.; Sangkasame, K. Bacterial cellulose production from Komagataeibacter xylinus TISTR 1011 and Komagataeibacter nataicola TISTR 975 using yam bean juice as a nutrient source. J. Gen. Appl. Microbiol. 2022, 68, 225–231. [Google Scholar] [CrossRef]
- Taokaew, S.; Nakson, N.; Zhang, X.; Kongklieng, P.; Kobayashi, T. Biotransformation of okara extracted protein to nanocellulose and chitin by Gluconacetobacter xylinus and Bacillus pumilus. Bioresour. Technol. Rep. 2022, 17, 100904. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, K. n alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacter aceti subsp. xylinus ATCC 23770. Carbohydr. Polym. 2008, 72, 545–549. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Azi, F.; Ge, Z.-W.; Liu, Y.-F.; Yin, X.-T.; Dong, M.-S. Bio-conversion of kitchen waste into bacterial cellulose using a new multiple carbon utilizing Komagataeibacter rhaeticus: Fermentation profiles and genome-wide analysis. Int. J. Biol. Macromol. 2021, 191, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Sutthiphatkul, T.; Suwanposri, A.; Ochaikul, D. Optimization of bacterial cellulose production from wastewater of noodle processing by Komagataeibacter sp. PAP1 and bio-cellulose paper production. Walailak J. Sci. Technol. 2020, 17, 1241–1251. [Google Scholar] [CrossRef]
- Rani, M.U.; Appaiah, K.A.A. Production of bacterial cellulose by Gluconacetobacter hansenii UAC09 using coffee cherry husk. J. Food Sci. Technol. 2013, 50, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.K.; Kim, B.S. Bacterial cellulose production from biodiesel-derived crude glycerol, magnetic functionalization, and its application as carrier for lipase immobilization. Int. J. Biol. Macromol. 2020, 153, 902–911. [Google Scholar] [CrossRef]
- Mohsin, A.; Zhang, K.; Hu, J.; Salim-ur-Rehman; Tariq, M.; Zaman, W.Q.; Khan, I.M.; Zhuang, Y.; Guo, M. Optimized Biosynthesis of Xanthan via Effective Valorization of Orange Peels Using Response Surface Methodology: A Kinetic Model Approach. Carbohydr. Polym. 2018, 181, 793–800. [Google Scholar] [CrossRef]
- Gunasekar, V.; Reshma, K.R.; Treesa, G.; Gowdhaman, D.; Ponnusami, V. Xanthan from sulphuric acid treated tapioca pulp: Influence of acid concentration on xanthan fermentation. Carbohydr. Polym. 2014, 102, 669–673. [Google Scholar] [CrossRef]
- Ben Salah, R.; Chaari, K.; Besbes, S.; Ktari, N.; Blecker, C.; Deroanne, C.; Attia, H. Optimisation of xanthan gum production by palm date (Phoenix dactylifera L.) juice by-products using response surface methodology. Food Chem. 2010, 121, 627–633. [Google Scholar] [CrossRef]
- Demirci, A.S.; Palabiyik, I.; Apaydın, D.; Mirik, M.; Gumus, T. Xanthan gum biosynthesis using Xanthomonas isolates from waste bread: Process optimization and fermentation kinetics. LWT 2019, 101, 40–47. [Google Scholar] [CrossRef]
- Li, P.; Zeng, Y.; Xie, Y.; Li, X.; Kang, Y.; Wang, Y.; Xie, T.; Zhang, Y. Effect of pretreatment on the enzymatic hydrolysis of kitchen waste for xanthan production. Bioresour. Technol. 2017, 223, 84–90. [Google Scholar] [CrossRef]
- Felicia Katherine, R.; Muthukumaran, C.; Sharmila, G.; Manoj Kumar, N.; Tamilarasan, K.; Jaiganesh, R. Xanthan gum production using jackfruit-seed-powder-based medium: Optimization and characterization. 3 Biotech 2017, 7, 248. [Google Scholar] [CrossRef]
- Jesus, M.; Mata, F.; Batista, R.A.; Ruzene, D.S.; Albuquerque-Júnior, R.; Cardoso, J.C.; Vaz-Velho, M.; Pires, P.; Padilha, F.F.; Silva, D.P. Corncob as Carbon Source in the Production of Xanthan Gum in Different Strains Xanthomonas sp. Sustainability 2023, 15, 2287. [Google Scholar] [CrossRef]
- da Silva, J.A.; Cardoso, L.G.; de Jesus Assis, D.; Gomes, G.V.P.; Oliveira, M.B.P.P.; de Souza, C.O.; Druzian, J.I. Xanthan Gum Production by Xanthomonas campestris pv. campestris IBSBF 1866 and 1867 from Lignocellulosic Agroindustrial Wastes. Appl. Biochem. Biotechnol. 2018, 186, 750–763. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Zhu, L.; Zhan, X. Activation of glycerol metabolism in Xanthomonas campestris by adaptive evolution to produce a high-transparency and low-viscosity xanthan gum from glycerol. Bioresour. Technol. 2016, 211, 390–397. [Google Scholar] [CrossRef]
- Rončević, Z.; Zahović, I.; Danilović, N. Potential of different Xanthomonas campestris strains for xanthan biosynthesis on waste glycerol from biodiesel production. J. Process. Energy Agric. 2020, 24, 62–66. [Google Scholar] [CrossRef]
- de Jesus Assis, D.; Brandão, L.V.; de Sousa Costa, L.; Figueiredo, T.V.B.; Sousa, L.S.; Padilha, F.F.; Druzian, J.I. A Study of the Effects of Aeration and Agitation on the Properties and Production of Xanthan Gum from Crude Glycerin Derived from Biodiesel Using the Response Surface Methodology. Appl. Biochem. Biotechnol. 2014, 172, 2769–2785. [Google Scholar] [CrossRef] [PubMed]
- Moravej, R.; Alavi, S.M.; Azin, M.; Salmanian, A.H. Production and physicochemical characterization of xanthan gum by native lactose consuming isolates of Xanthomonas citri subsp. citri. Ukr. Biochem. J. 2020, 92, 92–102. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Asadollahi, M.A.; Zamani, A.; Biria, D.; Doostmohammadi, M. Optimization of xanthan gum production using cheese whey and response surface methodology. Food Sci. Biotechnol. 2015, 24, 453–460. [Google Scholar] [CrossRef]
- Revin, V.V.; Liyas’kina, E.V.; Pokidko, B.V.; Pimenov, N.V.; Mardanov, A.V.; Ravin, N.V. Characteristics of the New Xanthan-Producing Strain Xanthomonas campestris М 28: Study of the Genome, Cultivation Conditions, and Physicochemical and Rheological Properties of the Polysaccharide. Appl. Biochem. Microbiol. 2021, 57, 356–365. [Google Scholar] [CrossRef]
- Bajić, B.; Rončević, Z.; Puškaš, V.; Miljić, U.; Dodić, S.; Grahovac, J.; Dodić, J. White wine production effluents used for biotechnological production of xanthan. J. Process. Energy Agric. 2015, 19, 52–55. [Google Scholar]
- Costa, L.A.; Campos, M.I.; Druzian, J.I.; de Oliveira, A.M.; de Oliveira Junior, E.N. Biosynthesis of Xanthan Gum from Fermenting Shrimp Shell: Yield and Apparent Viscosity. Int. J. Polym. Sci. 2014, 2014, 273650. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef]
- Kumbhar, J.V.; Rajwade, J.M.; Paknikar, K.M. Fruit peels support higher yield and superior quality bacterial cellulose production. Appl. Microbiol. Biotechnol. 2015, 99, 6677–6691. [Google Scholar] [CrossRef]
- Kuo, C.-H.H.; Huang, C.-Y.Y.; Shieh, C.-J.J.; Wang, H.-M.M.D.; Tseng, C.-Y.Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Khami, S.; Khamwichit, W.; Suwannahong, K.; Sanongraj, W. Characteristics of Bacterial Cellulose Production from Agricultural Wastes. Adv. Mater. Res. 2014, 931–932, 693–697. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Zhu, L.; Zhan, X. Characterization of xanthan gum produced from glycerol by a mutant strain Xanthomonas campestris CCTCC M2015714. Carbohydr. Polym. 2017, 157, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Zikmanis, P.; Kolesovs, S.; Ruklisha, M.; Semjonovs, P. Production of bacterial cellulose from glycerol: The current state and perspectives. Bioresour. Bioprocess. 2021, 8, 116. [Google Scholar] [CrossRef]
- Lu, T.; Gao, H.; Liao, B.; Wu, J.; Zhang, W.; Huang, J.; Liu, M.; Huang, J.; Chang, Z.; Jin, M.; et al. Characterization and optimization of production of bacterial cellulose from strain CGMCC 17276 based on whole genome analysis. Carbohydr. Polym. 2020, 232, 115788. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, G.C.; Liu, M.; Zheng, X.T.; Han, P.P.; Jia, S.R. Metabolic flux analysis of Gluconacetobacter xylinus for bacterial cellulose production. Appl. Microbiol. Biotechnol. 2013, 97, 6189–6199. [Google Scholar] [CrossRef]
- Gayathri, G.; Srinikethan, G. Crude glycerol as a cost effective carbon source for the production of cellulose by K. saccharivorans. Biocatal. Agric. Biotechnol. 2018, 16, 326–330. [Google Scholar] [CrossRef]
- Yang, H.J.; Lee, T.; Kim, J.R.; Choi, Y.-E.; Park, C. Improved production of bacterial cellulose from waste glycerol through investigation of inhibitory effects of crude glycerol-derived compounds by Gluconacetobacter xylinus. J. Ind. Eng. Chem. 2019, 75, 158–163. [Google Scholar] [CrossRef]
- Zikmanis, P.; Kolesovs, S.; Semjonovs, P. Production of biodegradable microbial polymers from whey. Bioresour. Bioprocess. 2020, 7, 36. [Google Scholar] [CrossRef]
- Kolesovs, S.; Semjonovs, P. Production of bacterial cellulose from whey—Current state and prospects. Appl. Microbiol. Biotechnol. 2020, 104, 7723–7730. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, M.; La China, S.; Lasagni, F.; Romeo, F.V.; Pulvirenti, A.; Gullo, M. Acetic acid bacteria in agro-wastes: From cheese whey and olive mill wastewater to cellulose. Appl. Microbiol. Biotechnol. 2023, 107, 3729–3744. [Google Scholar] [CrossRef]
- Jain, S.; Gupta, R.; Jain, S. Development of low cost nutritional beverage from whey. J. Environ. Sci. Toxicol. Food Technol. 2013, 5, 73–88. [Google Scholar] [CrossRef]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef]
- Menchik, P.; Zuber, T.; Zuber, A.; Moraru, C.I. Short communication: Composition of coproduct streams from dairy processing: Acid whey and milk permeate. J. Dairy Sci. 2019, 102, 3978–3984. [Google Scholar] [CrossRef]
- Carreira, P.; Mendes, J.A.; Trovatti, E.; Serafim, L.S.; Freire, C.S.; Silvestre, A.J.; Neto, C.P. Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresour. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial Cellulose Production from Industrial Waste and by-Product Streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef]
- Yang, T.C.; Hu, R.M.; Hsiao, Y.M.; Weng, S.F.; Tseng, Y.H. Molecular genetic analyses of potential β-galactosidase genes in Xanthomonas campestris. J. Mol. Microbiol. Biotechnol. 2003, 6, 145–154. [Google Scholar] [CrossRef]
- Ashraf, S.; Soudi, M.R.; Sadeghizadeh, M. Isolation of a novel mutated strain of Xanthomonas campestris for xanthan production using whey as the sole substrate. Pak. J. Biol. Sci. 2008, 11, 438–442. [Google Scholar] [CrossRef]
- Kamal, F.; Mehrgan, H.; Assadi, M.M.; Mortazavi, S.A. Mutagenesis of Xanthomonas campestris and selection of strains with enhanced xanthan production. Iran. Biomed. J. 2003, 7, 91–98. [Google Scholar]
- Bae, S.; Shoda, M. Bacterial Cellulose Production by Fed-Batch Fermentation in Molasses Medium. Biotechnol. Prog. 2004, 20, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Cakar, F.; Özer, I.; Aytekin, A.Ö.; Sahin, F. Improvement production of bacterial cellulose by semi-continuous process in molasses medium. Carbohydr. Polym. 2014, 106, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.-H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 3491. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.T.A.; Meneguin, A.B.; Sábio, R.M.; Franco, D.F.; Antonio, S.G.; Gutierrez, J.; Tercjak, A.; Berretta, A.A.; Ribeiro, S.J.L.; Lazarini, S.C.; et al. Komagataeibacter rhaeticus grown in sugarcane molasses-supplemented culture medium as a strategy for enhancing bacterial cellulose production. Ind. Crops Prod. 2018, 122, 637–646. [Google Scholar] [CrossRef]
- Tyagi, N.; Suresh, S. Production of cellulose from sugarcane molasses using Gluconacetobacter intermedius SNT-1: Optimization &characterization. J. Clean. Prod. 2016, 112, 71–80. [Google Scholar]
- Jung, H.I.; Lee, O.M.; Jeong, J.H.; Jeon, Y.D.; Park, K.H.; Kim, H.S.; An, W.G.; Son, H.J. Production and characterization of cellulose by Acetobacter sp. V6 using a cost-effective molasses-corn steep liquor medium. Appl. Biochem. Biotechnol. 2010, 162, 486–497. [Google Scholar] [CrossRef]
- Öz, Y.E.; Kalender, M. A novel static cultivation of bacterial cellulose production from sugar beet molasses: Series static culture (SSC) system. Int. J. Biol. Macromol. 2023, 225, 1306–1314. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielińska, M. Distillery Stillage: Characteristics, Treatment, and Valorization. Appl. Biochem. Biotechnol. 2020, 192, 770–793. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J.; Hao, X. Fertilizer potential of thin stillage from wheat-based ethanol production. Bioenerg. Res. 2014, 7, 1421–1429. [Google Scholar] [CrossRef]
- Ratanapariyanuch, K.; Shen, J.; Jia, Y.; Tyler, R.T.; Shim, Y.Y.; Reaney, M.J. Rapid NMR method for the quantification of organic compounds in thin stillage. J. Agric. Food Chem. 2011, 59, 10454–10460. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Liu, R.H. Thin stillage supplementation greatly enhances bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2012, 90, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Liu, R.H. Cost-effective production of bacterial cellulose in static cultures using distillery wastewater. J. Biosci. Bioeng. 2013, 115, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Huang, C.; Li, X.M.; Chen, X.F.; Wang, B.; Wang, C.; Zeng, X.A.; Chen, X.D. Acetone-Butanol-Ethanol (ABE) Fermentation Wastewater Treatment by Oleaginous Yeast Trichosporon cutaneum. Appl. Biochem. Biotechnol. 2015, 176, 563–571. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.-Y.; Xiong, L.; Guo, H.-J.; Luo, J.; Wang, B.; Zhang, H.-R.; Lin, X.; Chen, X.-D. Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett. Appl. Microbiol. 2015, 60, 491–496. [Google Scholar] [CrossRef]
- Jazini, M.; Fereydouni, E.; Karimi, K. Microbial xanthan gum production from alkali-pretreated rice straw. RSC Adv. 2017, 7, 3507–3514. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 1–32. [Google Scholar] [CrossRef]
- Baptista, S.L.; Cunha, J.T.; Romaní, A.; Domingues, L. Xylitol Production from Lignocellulosic Whole Slurry Corn Cob by Engineered Industrial Saccharomyces Cerevisiae PE-2. Bioresour. Technol. 2018, 267, 481–491. [Google Scholar] [CrossRef]
- Aleshina, L.A.; Gladysheva, E.K.; Budaeva, V.V.; Mironova, G.F.; Skiba, E.A.; Sakovich, G.V. X-ray Diffraction Data on the Bacterial Nanocellulose Synthesized by Komagataeibacter xylinus B-12429 and B-12431 Microbial Producers in Miscanthus- and Oat Hull-Derived Enzymatic Hydrolyzates. Crystallogr. Rep. 2022, 67, 391–397. [Google Scholar] [CrossRef]
- Skiba, E.A.; Gladysheva, E.K.; Budaeva, V.V.; Aleshina, L.A.; Sakovich, G.V. Yield and quality of bacterial cellulose from agricultural waste. Cellulose 2022, 29, 1543–1555. [Google Scholar] [CrossRef]
- Cavka, A.; Guo, X.; Tang, S.-J.J.; Winestrand, S.; Jönsson, L.J.; Hong, F. Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol. Biofuels 2013, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Kiziltas, E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Harini, K.; Chandra Mohan, C. Isolation and characterization of micro and nanocrystalline cellulose fibers from the walnut shell, corncob and sugarcane bagasse. Int. J. Biol. Macromol. 2020, 163, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, J.; Panchal, R.; Patel, D.; Goswami, D. Production and Characterization of Xanthan Gum by Xanthomonas Campestris Using Sugarcane Bagasse as Sole Carbon Source. In Biotechnology and Biological Sciences; CRC Press: Boca Raton, FL, USA, 2019; pp. 363–367. [Google Scholar]
- Demirci, A.S.; Arici, M.; Gumus, T. Xanthan Gum Production from Hydrolyzed Rice Bran as a Carbon Source by Xanthomonas spp. Korean J. Microbiol. Biotechnol. 2012, 40, 356–363. [Google Scholar] [CrossRef]
- Ozdal, M.; Kurbanoglu, E.B. Valorisation of Chicken Feathers for Xanthan Gum Production Using Xanthomonas Campestris MO-03. J. Genet. Eng. Biotechnol. 2018, 16, 259–263. [Google Scholar] [CrossRef]
- Soltaninejad, A.; Jazini, M.; Karimi, K. Biorefinery for Efficient Xanthan Gum, Ethanol, and Biogas Production from Potato Crop 17. Residues. Biomass Bioenergy 2022, 158, 106354. [Google Scholar] [CrossRef]
- Rončević, Z.; Grahovac, J.; Dodić, S.; Vučurović, D.; Dodić, J. Utilisation of Winery Wastewater for Xanthan Production in Stirred Tank Bioreactor: Bioprocess Modelling and Optimisation. Food Bioprod. Process. 2019, 117, 113–125. [Google Scholar] [CrossRef]
- Seto, A.; Saito, Y.; Matsushige, M.; Kobayashi, H.; Sasaki, Y.; Tonouchi, N.; Tsuchida, T.; Yoshinaga, F.; Ueda, K.; Beppu, T. Effective cellulose production by a coculture of Gluconacetobacter xylinus and Lactobacillus mali. Appl Microbiol Biotechnol. 2006, 73, 915–921. [Google Scholar] [CrossRef]
- Czaja, W.; Romanovicz, D.; Brown, R. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Liu, K.; Catchmark, J.M. Enhanced mechanical properties of bacterial cellulose nanocomposites produced by co-culturing Gluconacetobacter hansenii and Escherichia coli under static conditions. Carbohydr. Polym. 2019, 219, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, N.B.; Liyaskina, E.V.; Revin, V.V. The Production of Bacterial Cellulose by Co-Cultivation of Komagataeibacter sucrofermentans with Dextran Producers Leuconostoc mesenteroides and Xanthan Xanthomonas campestris. Tomsk State Univ. J. Biol. 2022, 60, 23–42. [Google Scholar] [CrossRef]
- Brugnoli, M.; Mazzini, I.; La China, S.; De Vero, L.; Gullo, M. A Microbial Co-Culturing System for Producing Cellulose-Hyaluronic Acid Composites. Microorganisms 2023, 11, 1504. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I. Biocatalysts in Synthesis of Microbial Polysaccharides: Properties and Development Trends. Catalysts 2022, 12, 1377. [Google Scholar] [CrossRef]
- Xie, Z.; Meng, K.; Yang, X.; Liu, J.; Yu, J.; Zheng, C.; Cao, W.; Liu, H. Identification of a quorum sensing system regulating capsule polysaccharide production and biofilm formation in Streptococcus zooepidemicus. Front. Cell. Infect. Microbiol. 2019, 9, 121. [Google Scholar] [CrossRef]
- Wang, X.L.; Chen, N.; Li, J.; Han, C.F.; Wang, S.; Hao, L.M.; Jia, S.R.; Han, P.P. The effects of quorum sensing molecule farnesol on the yield and activity of extracellular polysaccharide from Grifola frondosa in liquid fermentation. Int. J. Biol. Macromol. 2021, 191, 377–384. [Google Scholar] [CrossRef]
- Zheng, T.; Jing, M.; Gong, T.; Yan, J.; Wang, X.; Xu, M.; Zhou, X.; Zeng, J.; Li, Y. Regulatory mechanisms of exopolysaccharide synthesis and biofilm formation in Streptococcus mutans. J. Oral Microbiol. 2023, 15, 2225257. [Google Scholar] [CrossRef]
- Steiner, E.; Shilling, R.E.; Richter, A.M.; Schmid, N.; Fazli, M.; Kaever, V.; Jenal, U.; Tolker-Nielsen, T.; Eberl, L. The BDSF quorum sensing receptor RpfR regulates Bep exopolysaccharide synthesis in Burkholderia cenocepacia via interaction with the transcriptional regulator BerB. NPJ Biofilms Microbiomes 2022, 8, 93. [Google Scholar] [CrossRef]
- Bergmaier, D.; Champagne, C.P.; Lacroix, C. Exopolysaccharide production during batch cultures with free and immobilized Lactobacillus rhamnosus RW-9595M. J. Appl. Microbiol. 2003, 95, 1049–1057. [Google Scholar] [CrossRef]
- EL-Gizawy, S.A.; Barakat, O.S.; Sharaf, O.M.; EL-Shafei, K.; Fathy, F.A.; EL-Sayed, H.S. Effect of growth conditions on the production of exopolysaccharides by microencapsulated Lactobacillus bulgaricus and use it to improve quality of Kareish cheese. J. Appl. Sci. Res. 2013, 9, 1097–1109. [Google Scholar]
- Nugroho, D.A.; Aji, P. Characterization of nata de coco produced by fermentation of immobilized Acetobacter xylinum. Agric. Agric. Sci. Procedia 2015, 3, 278–282. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. “Deceived” concentrated immobilized cells as biocatalyst for intensive bacterial cellulose production from various sources. Catalysts 2018, 8, 33. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Vaishnavi, T.; Vidyasri, G.S.; Sathya, K.; Priyanka, P.; Venkatachalam, P.; Karuppiah, S. Production of bacterial cellulose using Gluconacetobacter kombuchae immobilized on Luffa aegyptiaca support. Sci. Rep. 2021, 11, 2912. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ullah, M.W.; Ul-Islam, M.; Khan, S.; Jang, J.H.; Park, J.K. Self-assembly of bio-cellulose nanofibrils through intermediate phase in a cell-free enzyme system. Biochem. Eng. J. 2019, 142, 135–144. [Google Scholar] [CrossRef]
- Ullah, M.W.; Manan, S.; Ul-Islam, M.; Khattak, W.A.; Khan, K.A.; Liu, J.; Yang, G.; Sun, J. Cell-free systems for biosynthesis: Towards a sustainable and economical approach. Green Chem. 2023, 25, 4912–4940. [Google Scholar] [CrossRef]
- Hur, D.H.; Choi, W.S.; Kim, T.Y.; Lee, S.Y.; Park, J.H.; Jeong, K.J. Enhanced production of bacterial cellulose in Komagataeibacter xylinus via tuning of biosynthesis genes with synthetic RBS. J. Microbiol. Biotechnol. 2020, 30, 1430–1435. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Tsai, M.L.; Chen, C.W.; Di Dong, C. Genetic modification for enhancing bacterial cellulose production and its applications. Bioengineered 2021, 12, 6793–6807. [Google Scholar] [CrossRef]
- Moradi, M.; Jacek, P.; Farhangfar, A.; Guimarães, J.T.; Forough, M. The role of genetic manipulation and in situ modifications on production of bacterial nanocellulose: A review. Int. J. Biol. Macromol. 2021, 183, 635–650. [Google Scholar] [CrossRef]
- Kuo, C.H.; Teng, H.Y.; Lee, C.K. Knockout of glucose dehydrogenase gene in Gluconacetobacter xylinus for bacterial cellulose production enhancement. Biotechnol. Bioprocess Eng. 2015, 20, 18–25. [Google Scholar] [CrossRef]
- Al-Janabi, S.S.; Shawky, H.; El-Waseif, A.A.; Farrag, A.A.; Abdelghany, T.M.; El-Ghwas, D.E. Stable, efficient, and cost-effective system for the biosynthesis of recombinant bacterial cellulose in Escherichia coli DH5_ platform. J. Gen. Eng. Biotechnol. 2022, 20, 90. [Google Scholar]
- Yang, F.; Cao, Z.; Li, C.; Chen, L.; Wu, G.; Zhou, X.; Hong, F.F. A recombinant strain of Komagataeibacter xylinus ATCC 23770 for production of bacterial cellulose from mannose-rich resources. New Biotechnol. 2023, 76, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.D.; Kim, T.Y.; Kim, H.U.; Shim, W.Y.; Ryu, J.Y.; Park, J.H.; Lee, S.Y. Genomic and metabolic analysis of Komagataeibacter xylinus DSM 2325 producing bacterial cellulose nanofiber. Biotechnol Bioeng. 2019, 116, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Rao, K.; Zo, S.; Shin, E.; Han, S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Huang, L.-H.; Zhao, X.-Q.; Li, W.-C.; Wang, Y.-Y.; Jia, S.-R.; Zhong, C. Bacterial cellulose and its potential for biomedical applications. Biotechnol. Adv. 2021, 53, 107856. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent advances and applications of bacterial cellulose in biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced Functional Materials Based on Nanocellulose for Pharmaceutical/Medical Applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Pandit, A.; Kumar, R. A Review on Production, Characterization and Application of Bacterial Cellulose and Its Biocomposites. J. Polym. Environ. 2021, 29, 2738–2755. [Google Scholar] [CrossRef]
- Qian, H.; Liu, J.; Wang, X.; Pei, W.; Fu, C.; Ma, M.; Huang, C. The state-of-the-art application of functional bacterial cellulose-based materials in biomedical fields. Carbohydr. Polym. 2023, 300, 120252. [Google Scholar] [CrossRef]
- Tang, K.Y.; Heng, J.Z.X.; Chai, C.H.T.; Chan, C.Y.; Low, B.Q.L.; Chong, S.M.E.; Loh, H.Y.; Li, Z.; Ye, E.; Loh, X.J. Modified Bacterial Cellulose for Biomedical Applications. Chem. Asian J. 2022, 17, 202200598. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.U.; Yoon, K.R. Xanthan gum-derived materials for applications in environment and eco-friendly materials: A review. J. Environ. Chem. Eng. 2020, 9, 104702. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Use of xanthan gum for whole cell immobilization and its impact in bioremediation—A review. Bioresour. Technol. 2022, 351, 126918. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Cubas, A.L.V.; Provin, A.P.; Dutra, A.R.A.; Mouro, C.; Gouveia, I.C. Advances in the Production of Biomaterials through Kombucha Using Food Waste: Concepts, Challenges, and Potential. Polymers 2023, 15, 1701. [Google Scholar] [CrossRef] [PubMed]
- Kamal, T.; Ul-Islam, M.; Fatima, A.; Ullah, M.W.; Manan, S. Cost-Effective Synthesis of Bacterial Cellulose and Its Applications in the Food and Environmental Sectors. Gels 2022, 8, 552. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Jiang, Z.; Ngai, T. Recent Advances in Chemically Modified Cellulose and Its Derivatives for Food Packaging Applications: A Review. Polymers 2022, 14, 1533. [Google Scholar] [CrossRef]
- Singh, A.K.; Itkor, P.; Lee, Y.S. State-of-the-Art Insights and Potential Applications of Cellulose-Based Hydrogels in Food Packaging: Advances towards Sustainable Trends. Gels 2023, 9, 433. [Google Scholar] [CrossRef]
- Atta, O.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, W.M.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Cielecka, I.; Szustak, M.; Kalinowska, H.; Gendaszewska-Darmach, E.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Glycerolplasticized bacterial nanocellulose-based composites with enhanced flexibility and liquid sorption capacity. Cellulose 2019, 26, 5409–5426. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef] [PubMed]
- de Amorim, J.D.P.; da Silva Junior, C.J.G.; de Medeiros, A.D.M.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.M.; Costa, A.F.S.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef]
- Meng, S.; Wu, H.; Xiao, D.; Lan, S.; Dong, A. Recent advances in bacterial cellulose-based antibacterial composites for infected wound therapy. Carbohydr. Polym. 2023, 316, 121082. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Ilyas, S.; Tamrin, T.; Radecka, I.; Swingler, S.; Gupta, A.; Stamboulis, A.G.; Gea, S. Bioactive bacterial cellulose wound dressings for burns with collagen in-situ and chitosan ex-situ impregnation. Int. J. Biol. Macromol. 2023, 230, 123118. [Google Scholar] [CrossRef]
- Tang, S.; Gong, Z.; Wang, Z.; Gao, X.; Zhang, X. Multifunctional hydrogels for wound dressings using xanthan gum and polyacrylamide. Int. J. Biol. Macromol. 2022, 217, 944–955. [Google Scholar] [CrossRef]
- Singh, S.; Nwabor, O.F.; Sukri, D.M.; Wunnoo, S.; Dumjun, K.; Lethongkam, S.; Kusolphat, P.; Hemtanon, N.; Klinprathum, K.; Sunghan, J.; et al. Poly (vinyl alcohol) copolymerized with xanthan gum/hypromellose/sodium carboxymethyl cellulose dermal dressings functionalized with biogenic nanostructured materials for antibacterial and wound healing application. Int. J. Biol. Macromol. 2022, 216, 235–250. [Google Scholar] [CrossRef]
- Gutierrez-Reyes, J.E.; Caldera-Villalobos, M.; Claudio-Rizo, J.A.; Cabrera-Munguía, D.A.; Becerra-Rodriguez, J.J.; Soriano-Corral, F.; Herrera-Guerrero, A. Smart collagen/xanthan gum-based hydrogels with antibacterial effect, drug release capacity and excellent performancein vitrobioactivity for wound healing application. Biomed. Mater. 2023, 18, 035011. [Google Scholar] [CrossRef] [PubMed]
- Unalan, I.; Schruefer, S.; Schubert, D.W.; Boccaccini, A.R. 3D-Printed Multifunctional Hydrogels with Phytotherapeutic Properties: Development of Essential Oil-Incorporated ALG-XAN Hydrogels for Wound Healing Applications. ACS Biomater. Sci. Eng. 2023, 9, 4149–4167. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chitrakar, B.; Liu, Z.; Ming, X.; Xu, D.; Mo, H.; Shi, C.; Zhu, X.; Hu, L.; Li, H. Preparation and characterization of 3D-printed antibacterial hydrogel with benzyl isothiocyanate. Int. J. Bioprint. 2023, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Miguel, S.P.; Araujo, A.R.T.S.; de Jesús Valle, M.J.; Sánchez Navarro, A.; Correia, I.J.; Ribeiro, M.P.; Coutinho, P. Xanthan Gum–Konjac Glucomannan Blend Hydrogel for Wound Healing. Polymers 2020, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ghosh, B.; Sarkar, K. Nanocellulose as sustainable biomaterials for drug delivery. Sens. Int. 2022, 3, 100135. [Google Scholar] [CrossRef]
- Mensah, A.; Chen, Y.; Christopher, N.; Wei, Q. Membrane Technological Pathways and Inherent Structure of Bacterial Cellulose Composites for Drug Delivery Bioengineering. Bioengineering 2022, 9, 3. [Google Scholar]
- Hasan, N.; Rahman, L.; Kim, S.-H.; Cao, J.; Arjuna, A.; Lallo, S.; Jhun, B.H.; Yoo, J.-W. Recent advances of nanocellulose in drug delivery systems. J. Pharm. Investig. 2020, 50, 553–572. [Google Scholar] [CrossRef]
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural Polysaccharide-Based Nanodrug Delivery Systems for Treatment of Diabetes. Polymers 2022, 14, 3217. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, Y.; Xia, M.; Du, H.; Lin, Z.; Li, B.; Liu, H. Nanocellulose-Based Composite Materials Used in Drug Delivery Systems. Polymers 2022, 14, 2648. [Google Scholar] [CrossRef]
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.-H.; Ismadji, S. Nanocelluloses: Sources, Pretreatment, Isolations, Modification, and Its Application as the Drug Carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef]
- Chung, C.K.; Beekmann, U.; Kralisch, D.; Bierau, K.; Chan, A.; Ossendorp, F.; Cruz, L.J. Bacterial Cellulose as Drug Delivery System for Optimizing Release of Immune Checkpoint Blocking Antibodies. Pharmaceutics 2022, 14, 1351. [Google Scholar] [CrossRef] [PubMed]
- Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents. Pharmaceutics 2023, 15, 402. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.; Gurudiwan, P.; Juyal, D. Formulation and Evaluation of Besifloxacin Loaded In Situ Gel For Ophthalmic Delivery. Pharm. Biosci. J. 2018, 6, 36–40. [Google Scholar] [CrossRef]
- Garg, R.; Kumar, V.; Sharma, V. Design and Characterization of Flucytosine Loaded Bioadhesive In Situ Ophthalmic Gel for Improved Bioavailability. Pharm. Biosci. J. 2019, 7, 17–20. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/Xanthan Gum Based Hydrogels as Potential Carrier for an Antiviral Drug: Fabrication, Characterization, and Safety Evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef]
- Ribeiro, M.; Boudoukhani, M.; Belmonte-Reche, E.; Genicio, N.; Sillankorva, S.; Gallo, J.; Rodríguez-Abreu, C.; Moulai-Mostefa, N.; Bañobre-López, M. Xanthan-Fe3O4 Nanoparticle Composite Hydrogels for Non-Invasive Magnetic Resonance Imaging and Magnetically Assisted Drug Delivery. ACS Appl. Nano Mater. 2021, 4, 7712–7729. [Google Scholar] [CrossRef]
- Inoue, B.S.; Streit, S.; Schneider, A.L.D.S.; Meier, M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020, 148, 1098–1108. [Google Scholar] [CrossRef]
- Ul, S.; Ul-islam, M.; Ahsan, H.; Bilal, M.; Shehzad, A.; Fatima, A.; Kyung, J.; Sup, Y. Potential applications of bacterial cellulose and its composites for cancer treatment. Int. J. Biol. Macromol. 2020, 168, 301–309. [Google Scholar]
- Cacicedo, M.L.; Islan, G.A.; León, I.E.; Álvarez, V.A.; Chourpa, I.; Allard-Vannier, E.; Castro, G.R. Bacterial cellulose hydrogel loaded with lipid nanoparticles for localized cancer treatment. Colloids Surf. B Biointerfaces 2018, 170, 596–608. [Google Scholar] [CrossRef]
- Zhang, L.K.; Du, S.; Wang, X.; Jiao, Y.; Yin, L.; Zhang, Y.; Guan, Y.Q. Bacterial cellulose based composites enhanced transdermal drug targeting for breast cancer treatment. Chem. Eng. J. 2019, 370, 749–759. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Li, X.-H.; He, P.-Y.; Qi, F.-Y.; Ullah, M.W.; Li, S.-J.; Liu, Y.-T.; Bu, L.-L.; Yang, G.; Sun, Z.-J. Implantable versatile oxidized bacterial cellulose membrane for postoperative HNSCC treatment via photothermal-boosted immunotherapy. Nano Res. 2023, 16, 951–963. [Google Scholar] [CrossRef]
- Trombino, S.; Serini, S.; Cassano, R.; Calviello, G. Xanthan gum-based materials for omega-3 PUFA delivery: Preparation, characterization and antineoplastic activity evaluation. Carbohydr. Polym. 2019, 208, 431–440. [Google Scholar] [CrossRef]
- Webster, T.J.; Singh, S.; Kotla, N.G.; Tomar, S.; Maddiboyina, B.; Sharma, D.; Sunnapu, O. A nanomedicine-promising approach to provide an appropriate colon-targeted drug delivery system for 5-fluorouracil. Int. J. Nanomed. 2015, 10, 7175–7182. [Google Scholar] [CrossRef]
- Anwar, M.; Pervaiz, F.; Shoukat, H.; Noreen, S.; Shabbir, K.; Majeed, A.; Ijaz, S. Formulation and evaluation of interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym. Bull. 2021, 78, 59–80. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, J.; Ni, C. Preparation of redox responsive modified xanthan gum nanoparticles and the drug controlled release. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 994–1001. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Wen, Q.; Ni, C. Preparation of xanthan gum nanogels and their pH/redox responsiveness in controlled release. J. Appl. Polym. Sci. 2019, 136, 6–11. [Google Scholar] [CrossRef]
- Anghel, N.; Apostol, I.; Dinu, M.V.; Dimitriu, C.D.; Spiridon, I.; Verestiuc, L. Xanthan-Based Materials as a Platform for Heparin Delivery. Molecules 2023, 28, 2757. [Google Scholar] [CrossRef]
- Gorgieva, S. Bacterial Cellulose as a Versatile Platform for Research and Development of Biomedical Materials. Processes 2020, 8, 624. [Google Scholar] [CrossRef]
- da Silva, I.G.R.; dos Santos Pantoja, B.T.; Almeida, G.H.D.R.; Carreira, A.C.O.; Miglino, M.A. Bacterial Cellulose and ECM Hydrogels: An Innovative Approach for Cardiovascular Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 3955. [Google Scholar] [CrossRef]
- Raut, M.P.; Asare, E.; Syed Mohamed, S.M.D.; Amadi, E.N.; Roy, I. Bacterial Cellulose-Based Blends and Composites: Versatile Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2023, 24, 986. [Google Scholar] [CrossRef]
- Andersson, J.; Stenhamre, H.; Bäckdahl, H.; Gatenholm, P. Behavior of human chondrocytes in engineered porous bacterial cellulose scaffolds. J. Biomed. Mater. Res. A 2010, 94, 1124–1132. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Yin, N.; Stilwell, M.D.; Santos, T.M.; Wang, H.; Weibel, D.B. Agarose particle-templated porous bacterial cellulose and its application in cartilage growth in vitro. Acta Biomater. 2015, 12, 129–138. [Google Scholar] [CrossRef]
- Wu, J.; Yin, N.; Chen, S.; Weibel, D.B.; Wang, H. Simultaneous 3D cell distribution and bioactivity enhancement of bacterial cellulose (BC) scaffold for articular cartilage tissue engineering. Cellulose 2019, 26, 2513–2528. [Google Scholar] [CrossRef]
- Xun, X.; Li, Y.; Zhu, X.; Zhang, Q.; Lu, Y.; Yang, Z.; Wan, Y.; Yao, F.; Deng, X.; Luo, H. Fabrication of Robust, Shape Recoverable, Macroporous Bacterial Cellulose Scaffolds for Cartilage Tissue Engineering. Macromol. Biosci. 2021, 2021, 2100167. [Google Scholar] [CrossRef]
- Gu, L.; Li, T.; Song, X.; Yang, X.; Li, S.; Chen, L.; Liu, P.; Gong, X.; Chen, C.; Sun, L. Preparation and characterization of methacrylated gelatin/bacterial cellulose composite hydrogels for cartilage tissue engineering. Regen. Biomater. 2020, 7, 195–202. [Google Scholar] [CrossRef]
- Horbert, V.; Boettcher, J.; Foehr, P.; Kramer, F.; Udhardt, U.; Bungartz, M.; Brinkmann, O.; Burgkart, R.H.; Klemm, D.O.; Kinne, R.W. Laser perforation and cell seeding improve bacterial nanocellulose as a potential cartilage implant in the in vitro cartilage punch model. Cellulose 2019, 26, 647–664. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Zhang, W.; Sun, Z.; Li, Y.; Yang, M.; Zeng, D.; Peng, B.; Zheng, W.; Jiang, X.; et al. Reverse reconstruction and bioprinting of bacterial cellulose-based functional total intervertebral disc for therapeutic implantation. Small 2018, 14, 1702582. [Google Scholar] [CrossRef]
- Tazi, N.; Zhang, Z.; Messaddeq, Y.; Almeida-Lopes, L.; Zanardi, L.M.; Levinson, D.; Rouabhia, M. Hydroxyapatite bioactivated bacterial cellulose promotes osteoblast growth and the formation of bone nodules. AMB Express 2012, 2, 61. [Google Scholar] [CrossRef]
- Dubey, S.; Mishra, R.; Roy, P.; Singh, R.P. 3-D macro/microporous-nanofibrous bacterial cellulose scaffolds seeded with BMP-2 preconditioned mesenchymal stem cells exhibit remarkable potential for bone tissue engineering. Int. J. Biol. Macromol. 2021, 167, 934–946. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef]
- Klinthoopthamrong, N.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 2020, 149, 51–59. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, J.; Zhao, S.; Luo, H.; Yang, Z.; Gama, M.; Zhang, Q.; Su, D.; Wan, Y. Biocompatibility evaluation of bacterial cellulose as a scaffold material for tissue-engineered corneal stroma. Cellulose 2020, 27, 2775–2784. [Google Scholar] [CrossRef]
- Wippermann, J.; Schumann, D.; Klemm, D.; Kosmehl, H.; Salehi-Gelani, S.; Wahlers, T. Preliminary Results of Small Arterial Substitute Performed with a New Cylindrical Biomaterial Composed of Bacterial Cellulose. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 592–596. [Google Scholar] [CrossRef]
- Pértile, R.; Moreira, S.; Andrade, F.; Domingues, L.; Gama, M. Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol. Prog. 2012, 28, 526–532. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, S.E.; Jo, I.S.; Kim, S.M.; Kang, D.H.; Cho, S.P.; Park, J.B.; Hong, B.H.; Yoon, M.H. Multiscale modulation of nanocrystalline cellulose hydrogel via nanocarbon hybridization for 3D neuronal bilayer formation. Small 2017, 13, 1700331. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; Goodarzi, V.; Mousavi, S.M.; Khonakdar, H.A.; Zamanlui, S. Lightweight aerogels based on bacterial cellulose/silver nanoparticles/ polyaniline with tuning morphology of polyaniline and application in soft tissue engineering. Int. J. Biol. Macromol. 2020, 152, 57–67. [Google Scholar] [CrossRef]
- Sämfors, S.; Karlsson, K.; Sundberg, J.; Markstedt, K.; Gatenholm, P. Biofabrication of bacterial nanocellulose scaffolds with complex vascular structure. Biofabrication 2019, 11, 045010. [Google Scholar] [CrossRef]
- Jabbari, F.; Babaeipour, V.; Bakhtiari, S. Bacterial cellulose-based composites for nerve tissue engineering. Int. J. Biol. Macromol. 2022, 217, 120–130. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Fooladi, S.; Nematollahi, M.H.; Rabiee, N.; Iravani, S. Bacterial Cellulose-Based Materials: A Perspective on Cardiovascular Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2023, 9, 2949–2969. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Bao, L.; Li, G.; Chen, L.; Hong, F.F. Vascular cells responses to controlled surface structure and properties of bacterial nanocellulose artificial blood vessel after mercerization. Carbohydr. Polym. 2023, 306, 120572. [Google Scholar] [CrossRef] [PubMed]
- Anton-Sales, I.; D’Antin, J.C.; Fernández-Engroba, J.; Charoenrook, V.; Laromaine, A.; Roig, A.; Michael, R. Bacterial nanocellulose as a corneal bandage material: A comparison with amniotic membrane. Biomater. Sci. 2020, 8, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, C.; Cai, Q.; Bao, X.; Tang, L.; Ao, H.; Liu, J.; Jin, M.; Zhou, Y.; Wan, Y.; et al. Studies on Bacterial Cellulose/Poly (Vinyl Alcohol) Hydrogel Composites as Tissue-Engineered Corneal Stroma. Biomed. Mater. 2020, 15, 035022. [Google Scholar] [CrossRef]
- Yang, J.; Du, M.; Wang, L.; Li, S.; Wang, G.; Yang, X.; Zhang, L.; Fang, Y.; Zheng, W.; Yang, G.; et al. Bacterial cellulose as a supersoft neural interfacing substrate. ACS Appl. Mater. Interfaces 2018, 10, 33049–33059. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Yang, J.; Zhu, R.; Zhang, Z.; Li, Y. Development and biocompatibility evaluation of biodegradable bacterial cellulose as a novel peripheral nerve scaffold. J. Biomed. Mater. Res. Part A 2018, 106, 1288–1298. [Google Scholar] [CrossRef]
- Dydak, K.; Junka, A.; Szymczyk, P.; Chodaczek, G.; Toporkiewicz, M.; Fijałkowski, K.; Dudek, B.; Bartoszewicz, M. Development and biological evaluation of Ti6Al7Nb scaffold implants coated with gentamycin-saturated bacterial cellulose biomaterial. PLoS ONE 2018, 13, e0205205. [Google Scholar] [CrossRef]
- Zuliani, C.C.; Damas, I.I.; Andrade, K.C.; Westin, C.B.; Moraes, A.M.; Coimbra, I.B. Chondrogenesis of human amniotic fluid stem cells in Chitosan-Xanthan scaffold for cartilage tissue engineering. Sci. Rep. 2021, 11, 3063. [Google Scholar] [CrossRef]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Barbosa, L.R.S.; Petri, D.F.S. Synthesis and characterization of xanthan–hydroxyapatite nanocomposites for cellular uptake. Mater. Sci. Eng. C 2014, 37, 195–203. [Google Scholar] [CrossRef]
- Barbosa, R.M.; da Rocha, D.N.; Bombaldi de Souza, R.F.; Santos, J.L.; Ferreira, J.R.M.; Moraes, Â.M. Cell-Friendly Chitosan-Xanthan Gum Membranes Incorporating Hydroxyapatite Designed for Periodontal Tissue Regeneration. Pharmaceutics 2023, 15, 705. [Google Scholar] [CrossRef]
- Souza, A.P.C.; Neves, J.G.; Navarro da Rocha, D.; Lopes, C.C.; Moraes, Â.M.; Correr-Sobrinho, L.; Correr, A.B. Chitosan/Xanthan membrane containing hydroxyapatite/Graphene oxide nanocomposite for guided bone regeneration. J. Mech. Behav. Biomed. Mater. 2022, 136, 105464. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.P.; Neves, J.G.; Navarro da Rocha, D.; Lopes, C.C.; Moraes, Â.M.; Correr-Sobrinho, L.; Correr, A.B. Chitosan/Xanthan/Hydroxyapatite-graphene oxide porous scaffold associated with mesenchymal stem cells for dentin-pulp complex regeneration. J. Biomater. Appl. 2023, 37, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Muduli, C.; Senanayak, S.P.; Goswami, L. Graphite nanopowder incorporated xanthan gum scaffold for effective bone tissue regeneration purposes with improved biomineralization. Int. J. Biol. Macromol. 2023, 234, 123724. [Google Scholar] [CrossRef] [PubMed]
- Piola, B.; Sabbatini, M.; Gino, S.; Invernizzi, M.; Renò, F. 3D Bioprinting of Gelatin–Xanthan Gum Composite Hydrogels for Growth of Human Skin Cells. Int. J. Mol. Sci. 2022, 23, 539. [Google Scholar] [CrossRef] [PubMed]
- Decarli, M.C.; Seijas-Gamardo, A.; Morgan, F.L.C.; Wieringa, P.; Baker, M.B.; Silva, J.V.L.; Moraes, Â.M.; Moroni, L.; Mota, C. Bioprinting of Stem Cell Spheroids Followed by Post-Printing Chondrogenic Differentiation for Cartilage Tissue Engineering. Adv. Healthcare Mater. 2023, 12, 2203021. [Google Scholar] [CrossRef]
- Salama, A.; Abouzeid, R.; Leong, W.S.; Jeevanandam, J.; Samyn, P.; Dufresne, A.; Bechelany, M.; Barhoum, A. Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration. Nanomaterials 2021, 11, 3008. [Google Scholar] [CrossRef]
- Shi, R.-J.; Wang, T.; Lang, J.-Q.; Zhou, N.; Ma, M.-G. Multifunctional Cellulose and Cellulose-Based (Nano) Composite Adsorbents. Front. Bioeng. Biotechnol. 2022, 10, 891034. [Google Scholar] [CrossRef]
- Shoukat, A.; Wahid, F.; Khan, T.; Siddique, M.; Nasreen, S.; Yang, G.; Ullah, M.W.; Khan, R. Titanium oxide-bacterial cellulose bioadsorbent for the removal of lead ions from aqueous solution. Int. J. Biol. Macromol. 2019, 129, 965–971. [Google Scholar] [CrossRef]
- Hosseini, H.; Mousavi, S.M. Bacterial cellulose/polyaniline nanocomposite aerogels as novel bioadsorbents for removal of hexavalent chromium: Experimental and simulation study. J. Clean. Prod. 2021, 278, 123817. [Google Scholar] [CrossRef]
- Mensah, A.; Lv, P.; Narh, C.; Huang, J.; Wang, D.; Wei, Q. Sequestration of Pb (II) Ions from Aqueous Systems with Novel Green Bacterial Cellulose Graphene Oxide Composite. Materials 2019, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Varsha, M.; Senthil Kumar, P.; Senthil Rathi, B. A Review on Recent Trends in the Removal of Emerging Contaminants from Aquatic Environment Using Low-Cost Adsorbents. Chemosphere 2022, 287, 132270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yadav, S.; Heinlein, T.; Konjik, V.; Breitzke, H.; Buntkowsky, G.; Schneider, J.J.; Zhang, K. Ultra-light nanocomposite aerogels of bacterial cellulose and reduced graphene oxide for specific absorption and separation of organic liquids. RSC Adv. 2014, 4, 21553–21558. [Google Scholar] [CrossRef]
- Zhu, H.-Y.; Fu, Y.-Q.; Jiang, R.; Jiang, J.-H.; Xiao, L.; Zeng, G.-M.; Zhao, G.-M.; Wang, Y. Adsorption Removal of congo Red onto Magnetic cellulose/Fe3O4/activated Carbon Composite: Equilibrium, Kinetic and Thermodynamic Studies. Chem. Eng. J. 2011, 173, 494–502. [Google Scholar] [CrossRef]
- Galdino, C.J.S.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.; Almeida, F.C.; Costa, A.F.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process. Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Paulauskiene, T.; Uebe, J.; Ziogas, M. Cellulose aerogel composites as oil sorbents and their regeneration. PeerJ 2021, 9, e11795. [Google Scholar] [CrossRef]
- Ieamviteevanich, P.; Daneshvar, E.; Eshaq, G.; Puro, L.; Mongkolthanaruk, W.; Pinitsoontorn, S.; Bhatnagar, A. Synthesis and Characterization of a Magnetic Carbon Nanofiber Derived from Bacterial Cellulose for the Removal of Diclofenac fromWater. ACS Omega 2022, 7, 7572–7584. [Google Scholar] [CrossRef]
- Parizadeh, P.; Moeinpour, F.; Mohseni-Shahri, F.S. Anthocyanin-induced color changes in bacterial cellulose nanofibers for the accurate and selective detection of Cu(II) in water samples. Chemosphere 2023, 326, 138459. [Google Scholar] [CrossRef]
- Sorze, A.; Valentini, F.; Dorigato, A.; Pegoretti, A. Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications. Molecules 2023, 28, 1952. [Google Scholar] [CrossRef]
- Guimarães, D.T.; de Oliveira Barros, M.; de Araújo E Silva, R.; Silva, S.M.F.; de Almeida, J.S.; de Freitas Rosa, M.; Gonçalves, L.R.B.; Brígida, A.I.S. Superabsorbent bacterial cellulose film produced from industrial residue of cashew apple juice processing. Int. J. Biol. Macromol. 2023, 242, 124405. [Google Scholar] [CrossRef]
- Sudirgo, M.M.; Surya, R.A.; Kristianto, H.; Prasetyo, S.; Sugih, A.K. Application of xanthan gum as coagulant-aid for decolorization of synthetic Congo red wastewater. Heliyon 2023, 9, e15011. [Google Scholar] [CrossRef] [PubMed]
- Gbadamosi, A.; Patil, S.; Kamal, M.S.; Adewunmi, A.A.; Yusuff, A.S.; Agi, A.; Oseh, J. Application of Polymers for Chemical Enhanced Oil Recovery: A Review. Polymers 2022, 14, 1433. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.O.; Carrilho, E.; Machado, S.A.S.; Sgobbi, L.F. Bacterial cellulose-based electrochemical sensing platform: A smart material for miniaturized biosensors. Electrochim. Acta 2020, 349, 136341. [Google Scholar] [CrossRef]
- Yu, H.; Tian, Y.; Dirican, M.; Fang, D.; Yan, C.; Xie, J.; Jia, D.; Liu, Y.; Li, C.; Cui, M.; et al. Flexible, transparent and tough silver nanowire/nanocellulose electrodes for flexible touch screen panels. Carbohydr. Polym. 2021, 273, 118539. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67. [Google Scholar] [CrossRef]
- Kotsiri, Z.; Vidic, J.; Vantarakis, A. Applications of Biosensors for Bacteria and Virus Detection in Food and Water–A Systematic Review. J. Environ. Sci. 2022, 111, 367–379. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Gonzales, K.N.; Sari, R.M.; Gea, S. Bacterial cellulose based biosensors. Med. Devices Sens. 2020, 3, e10102. [Google Scholar] [CrossRef]
- Faraco, T.A.; Fontes, M.L.; Paschoalin, R.T.; Claro, A.M.; Gonçalves, I.S.; Cavicchioli, M.; Farias, R.L.; Cremona, M.; Ribeiro, S.J.L.; Barud, H.D.S.; et al. Review of Bacterial Nanocellulose as Suitable Substrate for Conformable and Flexible Organic Light-Emitting Diodes. Polymers 2023, 15, 479. [Google Scholar] [CrossRef]
- de Assis, S.C.; Morgado, D.L.; Scheidt, D.T.; de Souza, S.S.; Cavallari, M.R.; Ando Junior, O.H.; Carrilho, E. Review of Bacterial Nanocellulose-Based Electrochemical Biosensors: Functionalization, Challenges, and Future Perspectives. Biosensors 2023, 13, 142. [Google Scholar] [CrossRef]
- Prilepskii, A.; Nikolaev, V.; Klaving, A. Conductive bacterial cellulose: From drug delivery to flexible electronics. Carbohydr. Polym. 2023, 313, 120850. [Google Scholar] [CrossRef]
- Pan, X.; Li, J.; Ma, N.; Ma, X.; Gao, M. Bacterial cellulose hydrogel for sensors. Chem. Eng. J. 2023, 461, 142062. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Lin, X.; Lu, J.; Huang, Z.; Sun, P.; Zhang, Y.; Xu, X.; Li, Q.; Liu, H. Dual-network polyvinyl alcohol/polyacrylamide/xanthan gum ionic conductive hydrogels for flexible electronic devices. Int. J. Biol. Macromol. 2023, 233, 123573. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ren, Y.; Liang, Y.; Li, Y. Semi-IPN ionogel based on poly (ionic liquids)/xanthan gum for highly sensitive pressure sensor. Int. J. Biol. Macromol. 2022, 223, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Tomić, N.Z.; Ghodhbane, M.; Matouk, Z.; AlShehhi, N.; Busà, C. Enhancement of Self-Healing Efficacy of Conductive Nanocomposite Hydrogels by Polysaccharide Modifiers. Polymers 2023, 15, 516. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B. Effect of gums on the rheological, microstructural and extrusion printing characteristics of mashed potatoes. Int. J. Biol. Macromol. 2018, 117, 1179–1187. [Google Scholar] [CrossRef]
- Azam, R.S.M.; Zhang, M.; Bhandari, B.; Yang, C. Effect of different gums on features of 3D Printed Object Based on Vitamin-D enriched orange concentrate. Food Biophys. 2018, 13, 250–262. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Ullah, M.W.; Booth, P.; Pei, E.; Yang, G. The use of bacterial polysaccharides in bioprinting. Biotechnol. Adv. 2019, 37, 107448. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Li, J.; Moeinzadeh, S.; Kim, C.; Pan, C.C.; Weale, G.; Kim, S.; Abrams, G.; James, A.W.; Choo, H.; Chan, C.; et al. Development and systematic characterization of GelMA/alginate/PEGDMA/xanthan gum hydrogel bioink system for extrusion bioprinting. Biomaterials 2023, 293, 121969. [Google Scholar] [CrossRef]
- Li, J.; Shelby, T.; Alizadeh, H.V.; Shelby, H.; Yang, Y.P. Development and characterization of an automated active mixing platform for hydrogel bioink preparation. Int. J. Bioprint. 2023, 9, 705. [Google Scholar] [CrossRef]
- Hassan, M.; Dave, K.; Chandrawati, R.; Dehghani, F.; Gomes, V.G. 3D printing of biopolymer nanocomposites for tissue engineering: Nanomaterials, processing and structure-function relation. Eur. Polym. J. 2019, 121, 109340. [Google Scholar] [CrossRef]
- Cakmak, A.M.; Unal, S.; Sahin, A.; Oktar, F.N.; Sengor, M.; Ekren, N.; Gunduz, O.; Kalaskar, D.M. 3D printed polycaprolactone/gelatin/bacterial cellulose/hydroxyapatite composite scaffold for bone tissue engineering. Polymers 2020, 12, 1962. [Google Scholar] [CrossRef] [PubMed]
- Aki, D.; Ulag, S.; Unal, S.; Sengor, M.; Ekren, N.; Lin, C.-C.; Yilmazer, H.; Ustundag, C.B.; Kalaskar, D.M.; Gunduz, O. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater. Des. 2020, 196, 109094. [Google Scholar] [CrossRef]
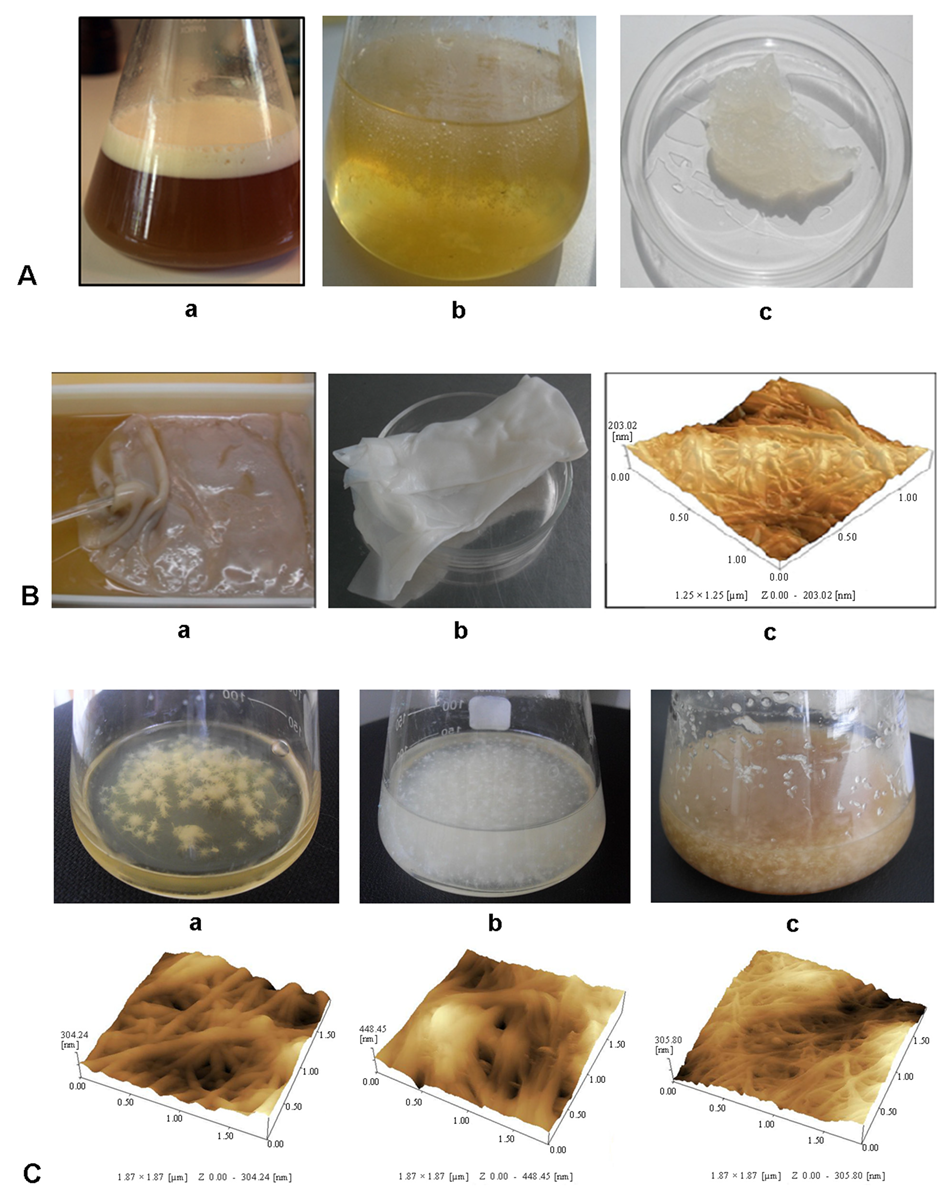

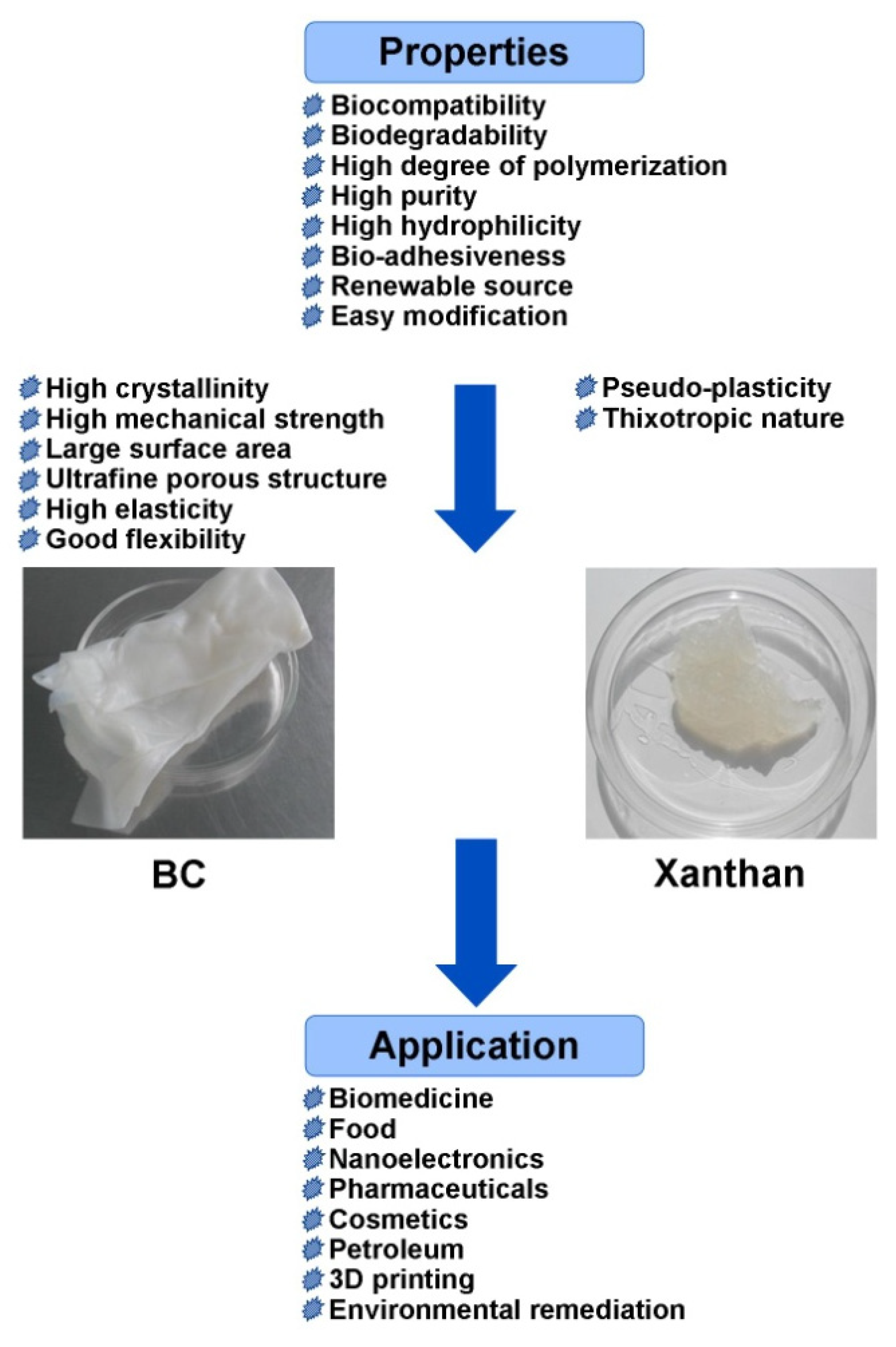
| Bacterial Strains | Nutrient Source | Production Titer (g/L) | Reference |
|---|---|---|---|
| Komagataeibacter sp. PAP1 | soya bean whey | 4.10 | [98] |
| K. xylinus CCM 3611 | sour whey + cane sugar + black tea | 18.5–23 | [111] |
| K. sucrofermentans B-11267 | cheese whey | 5.45 | [112] |
| thin stillage | 6.19 | ||
| sugar beet molasses | 2.9 | [113] | |
| G. xylinus PTCC 1734 | cheese whey treated with β-galactosidase | 3.55 | [114] |
| K. xylinus K2G30 | acid whey | 2.99 | [115] |
| G. xylinus FC01 | molasses | 0.572 | [116] |
| K. melomenusus AV436T | grape pomace hydrolysate | 1.24 | [117] |
| G. liquefaciens | jaggery | 17.79 | [118] |
| Komagataeibacter sp. CCUG73629 and Komagataeibacter sp. CCUG73630 | corncob and sugarcane bagasse | 1.2–1.6 | [119] |
| K. sucrofermentans DSM 15973 | confectionery wastes | 5.7 | [120] |
| K. sucrofermentans DSM 15973 | winery waste streams (grape pomace, stalks, wine lees) | 8–11.6 | [121] |
| K. xylinus BCRC12334 | olive oil mill wastewater | 0.65–5.33 | [122] |
| G. xylinus ATCC 23770 | wheat straw | 8.3 | [123] |
| K. sucrofermentas ATCC 700178 | alkali lignin | 3.2 | [124] |
| G. xylinus CH001 | durian shell | 2.67 | [125] |
| K. rhaeticus | cashew crop residues | 2.3–6 | [126] |
| K. xylinus DSM 6513 | red grapes bagasse | 0.548 | [127] |
| K. rhaeticus M12 | peer peal and pomace | 10.94 | [128] |
| K. xylinus | pineapple waste | 3.82 | [129] |
| G. swingsii | pineapple peel | 2.8 | [130] |
| K. medellinensis NBRC 3288 | rotten banana waste | 3.23 | [131] |
| K. europaeus SGP37 | sweet lime pulp | 26.2 | [132] |
| G. xylinus 0416 MARDI | date | 5.8 | [133] |
| K. hansenii GA2016 | citrus peels | 0.392 | [134,135] |
| G. xylinus ATCC 700178 | carob and haricot bean | 1.8–3.2 | [136] |
| G. xylinus 1.1812 | sweet potato | 11.35 | [137] |
| G. xylinus BPR2001 | sweet potato peel | 2.3–4.2 | [138] |
| K. xylinus TISTR 1011 | yam bean | 0.47 | [139] |
| G. xylinus BPR2001 | okara | 2.3 | [140] |
| G. aceti ATCC 23770 | konjac powder | 2.12 | [141] |
| K. rhaeticus K15 | kitchen waste | 4.76 | [142] |
| Komagataeibacter sp. PAP1 | noodle wastewater | 11.76 | [143] |
| G. hansenii UAC09 | coffee cherry husk | 8.2 | [144] |
| G. xylinus KCCM 41431 (ATCC 11142) | pure glycerol crude glycerol | 3.4 2.93 | [145] |
| Bacterial Strains | Nutrient Source | Production Titer (g/L) | Reference |
|---|---|---|---|
| X. campestris | orange peels hydrolysate | 30.19 | [146] |
| X. campestris | carrot peels extract | 40.88 | [104] |
| pumpkin peels extract | 31.4 | ||
| X. campestris NCIM 2954 | tapioca pulp hydrolysate | 7.1 | [147] |
| X. campestris NRRL B-1459 | date juice | 43.35 | [148] |
| X. campestris pv. vesicatoria | waste bread hydrolysate | 14.3 | [149] |
| X. campestris LRELP-1 | kitchen waste hydrolysate | 4.09–6.46 | [150] |
| X. campestris NCIM 2961 | jackfruit seed powder | 51.62 | [151] |
| Xanthomonas sp. 629 | hemicellulose fractions from the alkaline extraction of corncob | 8.37 | [152] |
| X. campestris pv. campestris 1866 | cocoa husks | 4.48 | [153] |
| X. campestris pv. campestris 1867 | acid hydrolyzed broomcorn stem | 3.89 | [91] |
| X. campestris | 8.9 | ||
| X. campestris CCTCC M2015714 | glycerol | 11.0 | [154] |
| X. campestris Xp 3–1 | crude glycerol | 7.67 | [155] |
| X. campestris pv. mangiferaeindicae 2103 | crude glycerol | 5.59 | [156] |
| X. citri/NIGEB-386 | cheese whey | 22.7 | [157] |
| X. campestris | cheese whey | 16.4 | [158] |
| X. pelargonii | 12.8 | ||
| X. campestris M 28 | molasses | 28 | [159] |
| X. campestris ATCC 13951 | wastewaters during the washing operations of the crusher | 4.004 | [160] |
| wastewaters during the washing operations of the press | 7.596 | ||
| wastewaters during the washing operations of the tanks after clarification of must | 10.67 | ||
| wastewaters during the washing operations of the fermentation | 7.488 | ||
| X. campestris pv. manihotis 1182 | 2% concentration of the shrimp shell used in the aqueous extract | 2.64 | [161] |
| X. campestris pv. campestris 254 | 2.60 | ||
| X. campestris pv. campestris 629 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revin, V.V.; Liyaskina, E.V.; Parchaykina, M.V.; Kurgaeva, I.V.; Efremova, K.V.; Novokuptsev, N.V. Production of Bacterial Exopolysaccharides: Xanthan and Bacterial Cellulose. Int. J. Mol. Sci. 2023, 24, 14608. https://doi.org/10.3390/ijms241914608
Revin VV, Liyaskina EV, Parchaykina MV, Kurgaeva IV, Efremova KV, Novokuptsev NV. Production of Bacterial Exopolysaccharides: Xanthan and Bacterial Cellulose. International Journal of Molecular Sciences. 2023; 24(19):14608. https://doi.org/10.3390/ijms241914608
Chicago/Turabian StyleRevin, Viktor V., Elena V. Liyaskina, Marina V. Parchaykina, Irina V. Kurgaeva, Kristina V. Efremova, and Nikolai V. Novokuptsev. 2023. "Production of Bacterial Exopolysaccharides: Xanthan and Bacterial Cellulose" International Journal of Molecular Sciences 24, no. 19: 14608. https://doi.org/10.3390/ijms241914608
APA StyleRevin, V. V., Liyaskina, E. V., Parchaykina, M. V., Kurgaeva, I. V., Efremova, K. V., & Novokuptsev, N. V. (2023). Production of Bacterial Exopolysaccharides: Xanthan and Bacterial Cellulose. International Journal of Molecular Sciences, 24(19), 14608. https://doi.org/10.3390/ijms241914608