Abstract
We designed 0D, 1D, and 2D supramolecular assemblies made of diaryliodonium salts (functioning as double σ-hole donors) and carboxylates (as σ-hole acceptors). The association was based on two charge-supported halogen bonds (XB), which occurred between IIII sites of the iodonium cations and the carboxylate anions. The sequential introduction of the carboxylic groups in the aryl ring of the benzoic acid added a dimension to the 0D supramolecular organization of the benzoate, which furnished 1D-chained and 2D-layered structures when terephthalate and trimesate anions, correspondingly, were applied as XB acceptors. The structure-directing XB were studied using DFT calculations under periodic boundary conditions and were followed by the one-electron-potential analysis and the Bader atoms-in-molecules topological analysis of electron density. These theoretical methods confirmed the existence of the XB and verified the philicities of the interaction partners in the designed solid-state structures.
1. Introduction
Halogen bonding (abbreviated as XB) [1,2], as a part of the spectrum of “unorthodox” [3] noncovalent interactions, is a subject of growing interest in crystal engineering [4,5,6], biomedical science [7,8,9,10], ion and molecular recognition [11,12,13,14], noncovalent catalysis [15,16,17,18], and many other fields [19]. The particular interest in XB lies in the area of crystal engineering and the high directionality of XB is a main factor for the rational design of the targeted supramolecular architectures.
Modern XB-based crystal engineering mainly utilizes monovalent halogen organic compounds, exhibiting one σ-hole per one halogen(I) site. In the vast majority of instances, these atoms form two-center XB. For the crystal design of higher-dimensional arrays, polyhalogenated XB donors—in which every halogen site provides one σ-hole for the appropriate XB—should be applied.
A suitable alternative to the polyhalogenated compounds is a hypervalent halogen species such as a diaryliodonium salt-bearing IIII site as a double σ-hole donor [20,21,22]. Diaryliodonium salts have already been used for the control of solid-state reactions [23], the stabilization of copper(I) complexes [24], the design of extended supramolecular arrays [25,26,27], XB-involving catalysis [17,18,28,29,30], and for the preparation of iodonium-based porous materials [31].
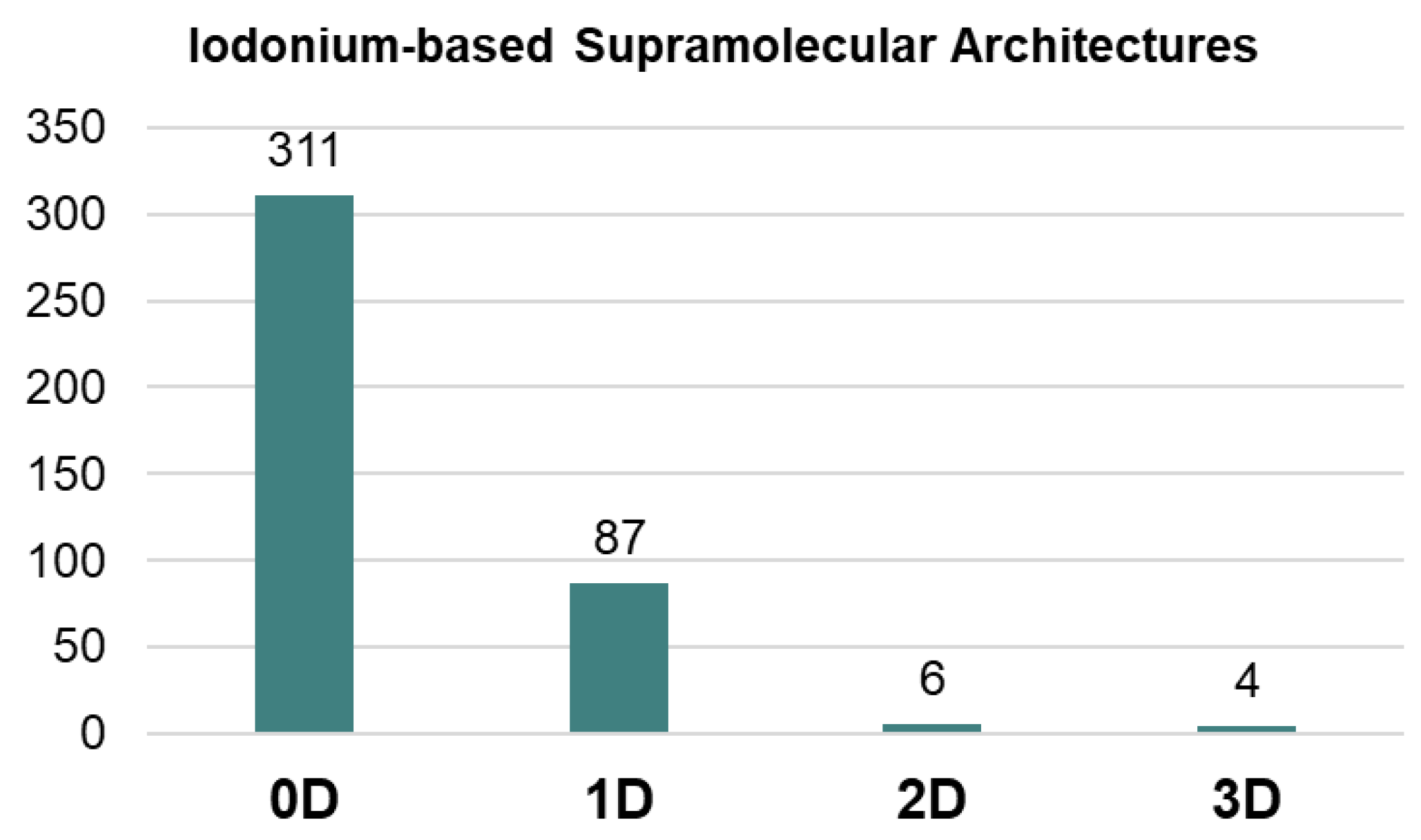
Our processing of the Cambridge Structural Database (CSD) showed that highly dimensional supramolecular architectures, namely 2D layers and 3D frameworks, based on iodonium species are still quite rare (<3%; Figure 1). The most common motifs in the supramolecular organization of iodonium species are 0D clusters or 1D-chained arrays (scattered examples) (Figure 1). The utilization of iodonium cations as tectons for the rational construction of highly dimensional supramolecular architectures is poorly studied and, in fact, it is limited by our findings in the design of halogen-bonded 1D chains of solid iodonium disulfonates [25]. Notably, in the case of iodonium sulfonates, we also obtained a few 2D-layered structures from the uncontrolled crystal growth.
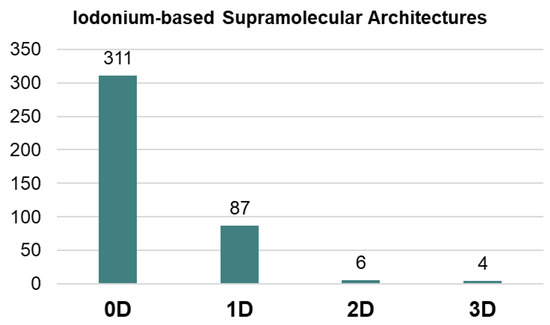
Figure 1.
Supramolecular arrays of different dimension from CSD.
Inspired by our success in the rational design of 1D-chained architectures from iodonium disulfonates, we extended this approach to other salts, namely iodonium carboxylates—benzoates, terephthalates, and trimesates. These derivatives of benzoic acid are commercially available and they have been repeatedly employed in the syntheses and design of metal–organic frameworks [32]. In comparison with iodonium sulfonates, the structures of the corresponding carboxylates are poorly studied and available examples are limited only to iodonium acetates; trifluoroacetates (~20 structures); and to one structure of an iodonium benzoate [33].
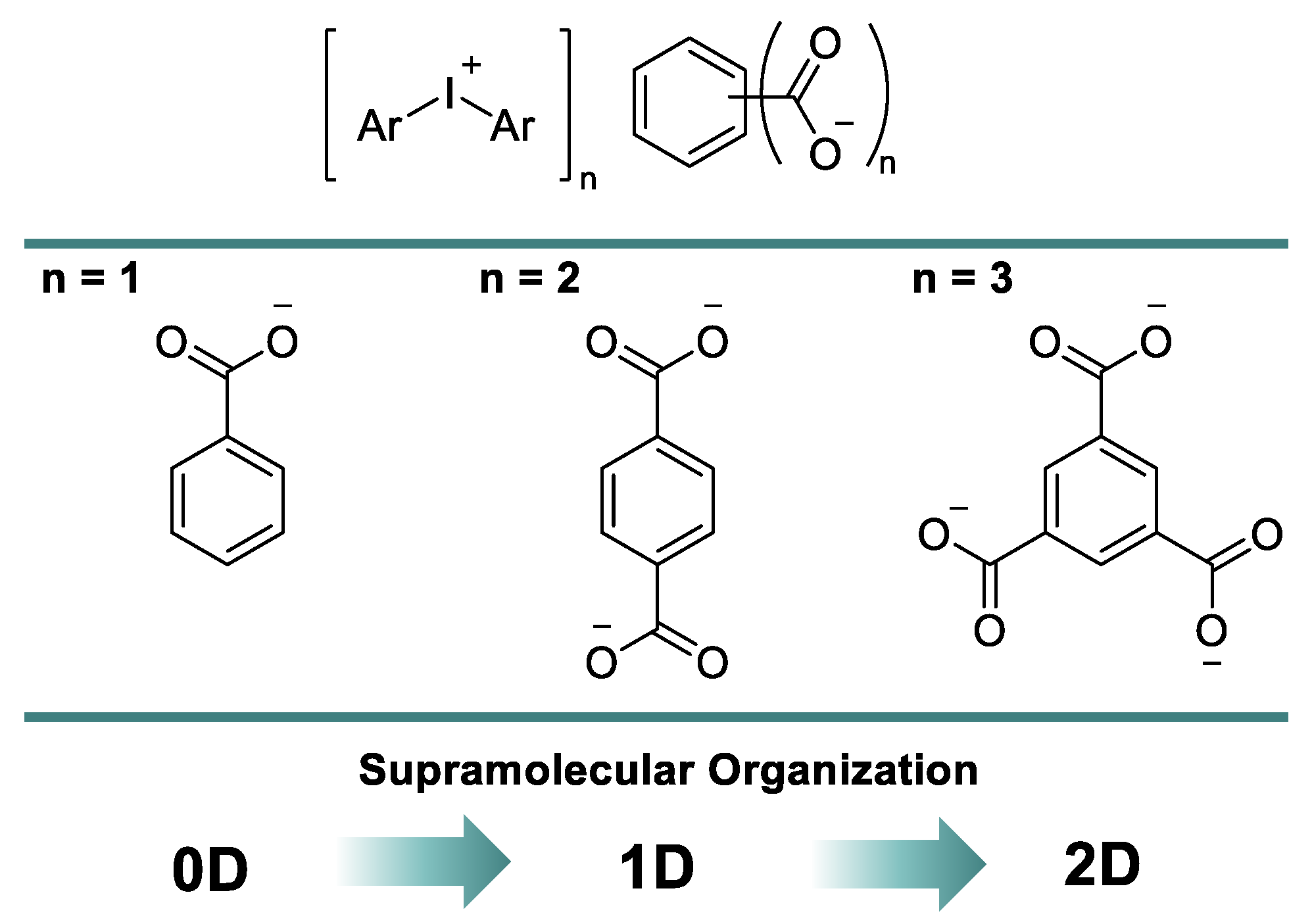
In this report, we assumed that the sequential introduction of carboxylic groups in the aryl ring of benzoic acid could increase the dimension of corresponding XB-based supramolecular assemblies. In this way, one could design different supramolecular architectures using a variation of a carboxylate anion of iodonium salts and obtain 0D clusters for benzoates, 1D chains for terephthalates, and 2D layers for trimesates (Figure 2). All of our findings are detailed in the following sections.
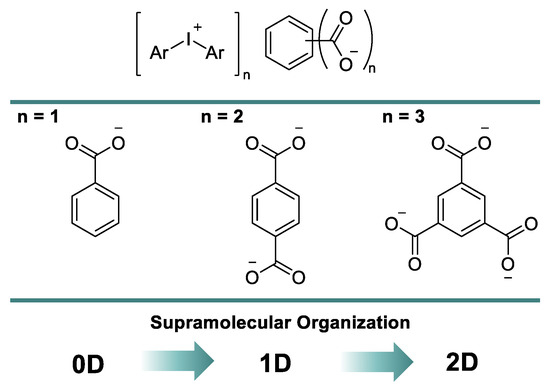
Figure 2.
Adding a dimension to the supramolecular organization of iodonium carboxylates.
2. Results and Discussion
2.1. Synthesis and Crystal Growth
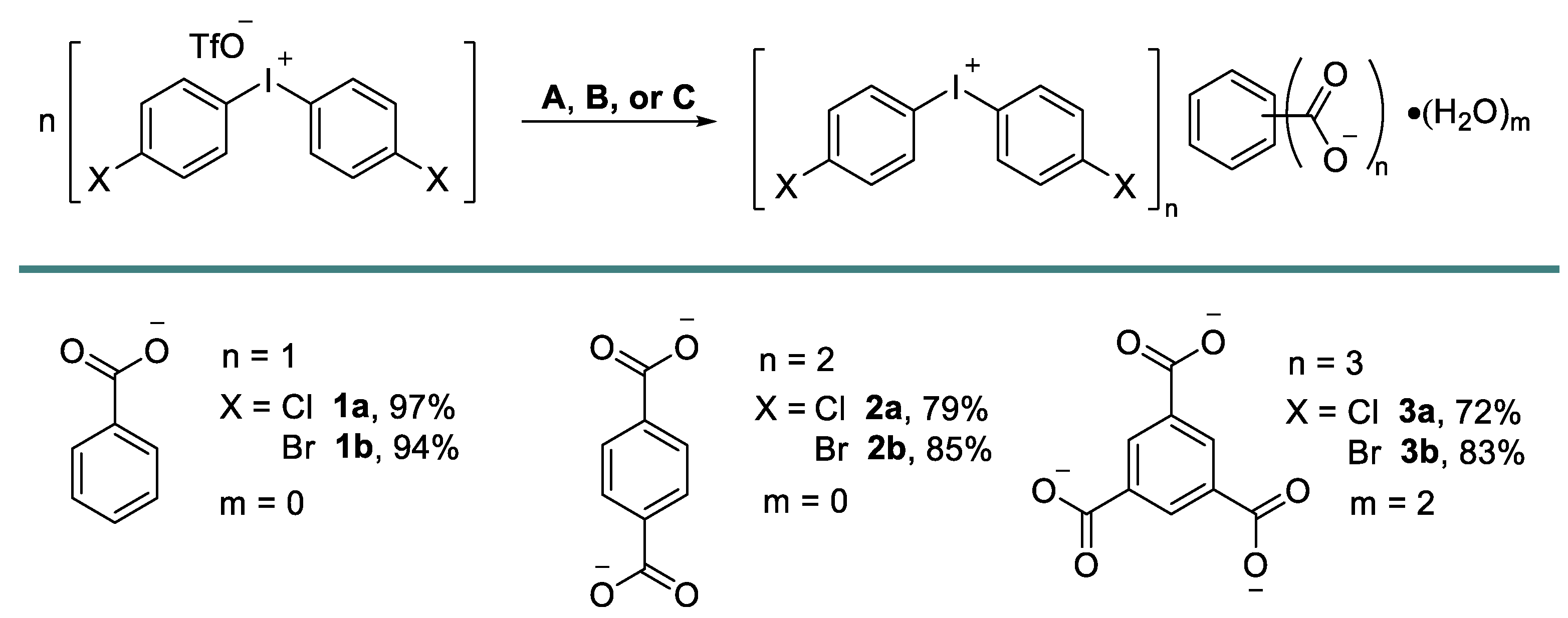
Iodonium carboxylates [33] were prepared in high isolated yields via the anion metathesis of potassium, or the Bu4N salts of corresponding carboxylates (TBA carboxylates), and iodonium triflates (Figure 3). Notably, the deviation from the reported benzoate load or concentration variations led to the contamination of the resulting product by triflates. The role of the solvent was also important as, for example, the change of solvent to neat MeCN or neat MeOH did not lead to the precipitation of the pure products.
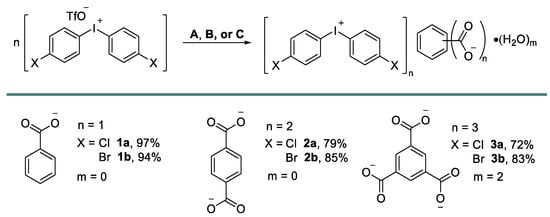
Figure 3.
Preparation of 1–3. Conditions A: iodonium triflate (1 equiv.), potassium benzoate (3 equiv.), MeOH/H2O; Conditions B: iodonium triflate (2.2 equiv.), (Bu4N+)2C6H4(COO−)2 (1 equiv.), MeOH/H2O; Conditions C: iodonium triflate (3.3 equiv.), (Bu4N+)3C6H3(COO−)3 (1 equiv.), MeCN/H2O.
Crystals of 1 were grown on the slow evaporation of its MeOH solution at room temperature in air. Crystals of 2 and 3 were prepared via the co-crystallization of iodonium triflate with TBA carboxylates from aqueous MeCN, also at room temperature in air.
2.2. General Consideration of the XRD Structures
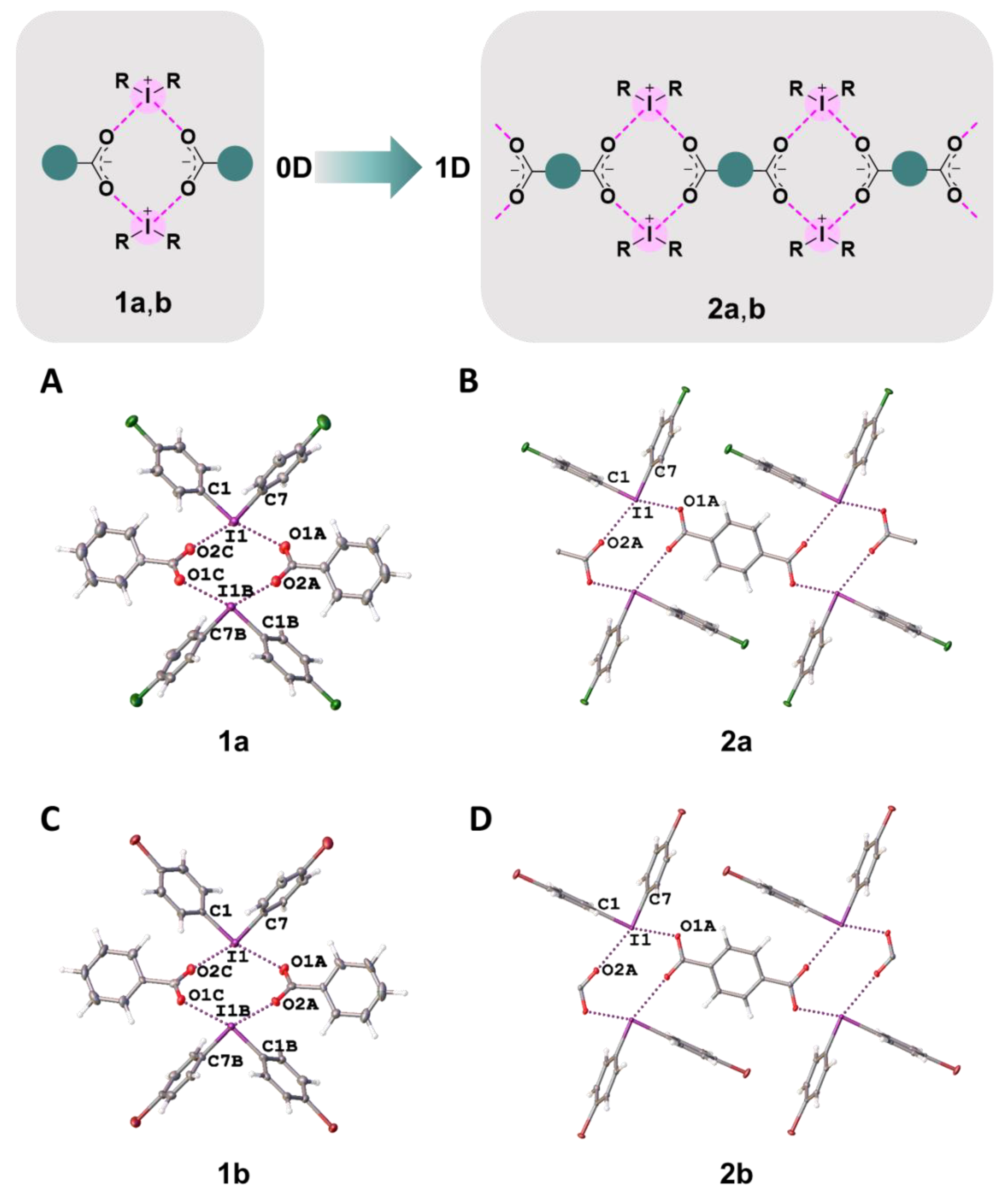
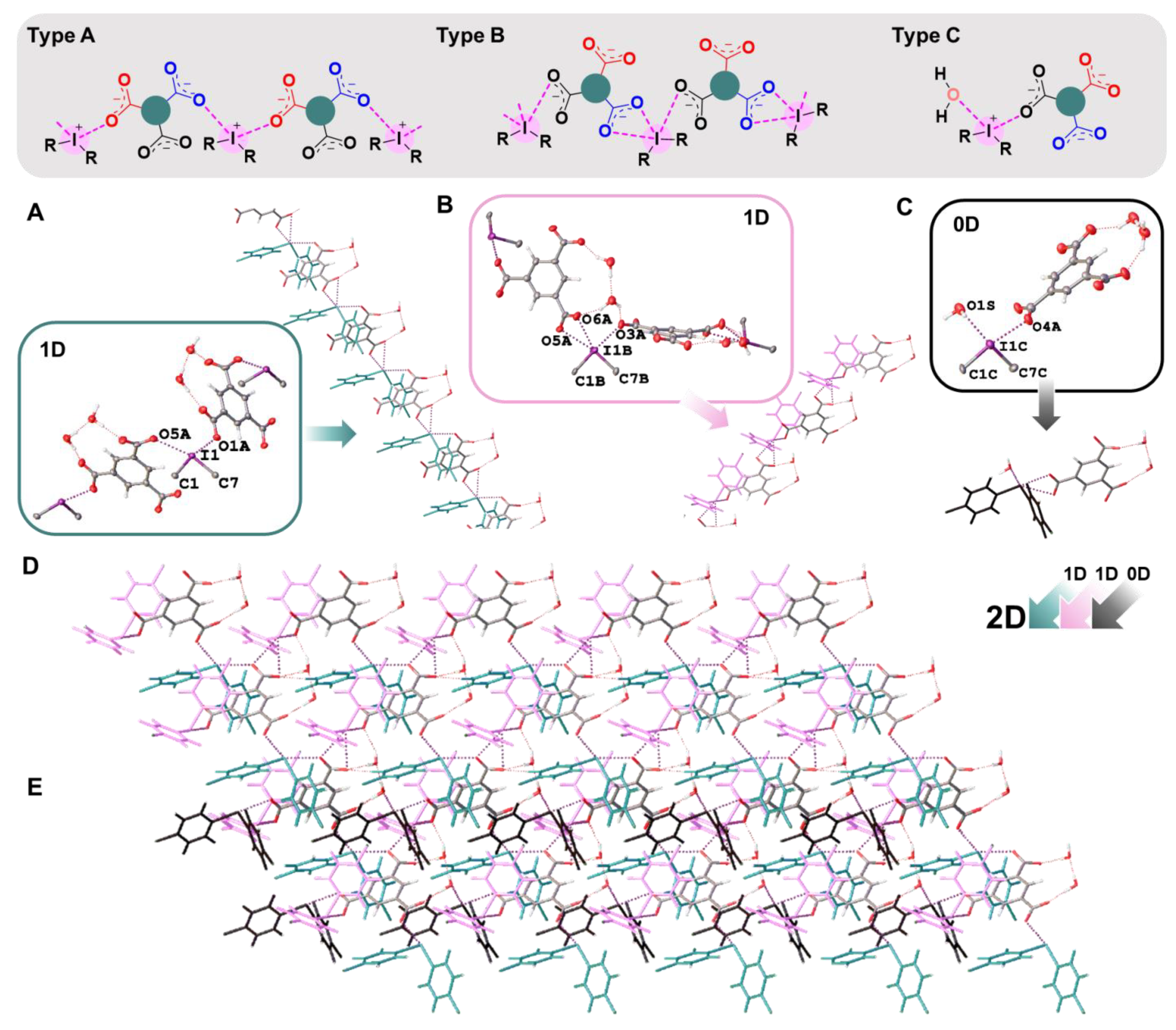
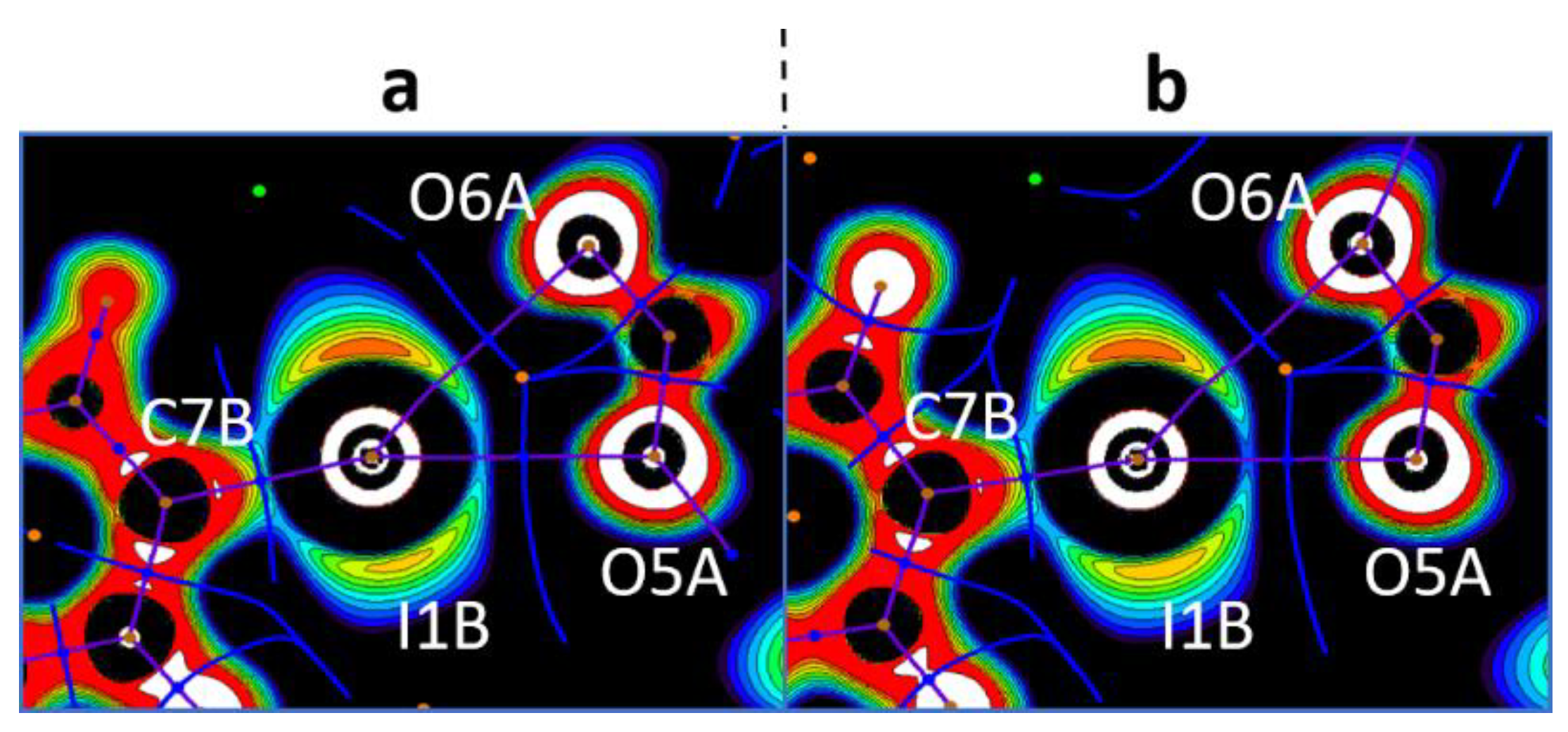
In the crystal structures of 1a,b–3a,b, the hypervalent I-atom forms two I∙∙∙O XBs with either the O-atoms of two carboxylic groups (Figure 4 and Figure 5A,B), or with the O-atom of a carboxylic group and the O-atom of a water molecule (Figure 5C). All these I∙∙∙O interactions fulfill the IUPAC geometrical criteria [1] for the identification of XB (d(I∙∙∙O) = 2.5–3.0 Å vs. ∑vdW O + I = 3.5 Å [34]; ∠C–I∙∙∙X = 163–175°; Table 1). The only deviation from the general trend is the structures of 3a,b (Figure 5B), in which the IIII site is involved in the bifurcated C7B–I1B∙∙∙O5A(O6A) XB of the type μ-I∙∙∙(O,O) (for more information on bifurcated XBs, see refs. [35,36,37,38]). The bifurcation is realized for iodonium cations of Type B (Figure 5B; hereinafter crystallographically independent iodonium cations in the same structure are defined as Type A, B, or C; Table 1) and the occurrence of the bifurcated XB was confirmed using appropriate DFT calculations (Section 2.4).
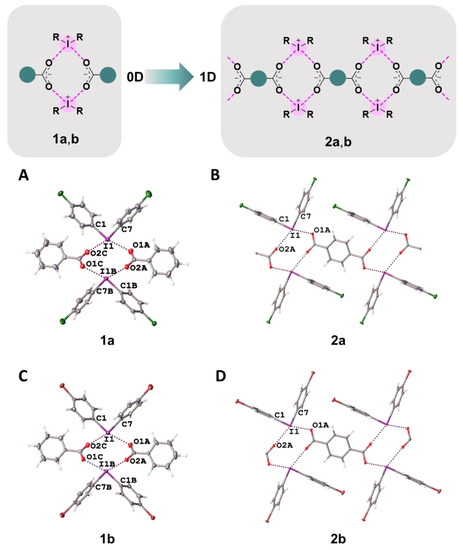
Figure 4.
Graphical representation of 1a,b and 2a,b (top). Fragments of the crystal structures of 1a (A), 2a (B), 1b (C), and 2b (D) (middle and bottom).
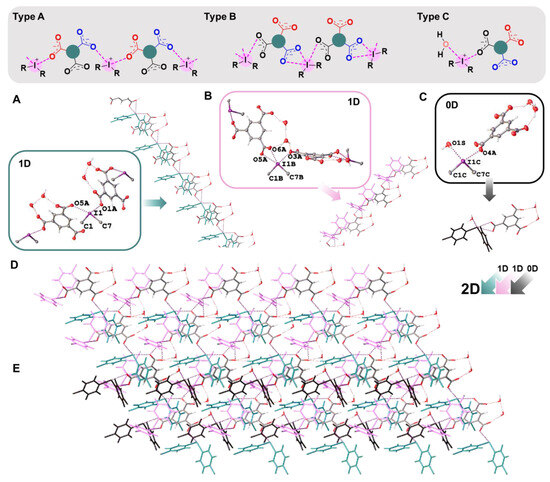
Figure 5.
Graphical presentation of major motifs in 3a,b (top). Fragments of the crystal structures 3a and 3b. (A,B): 1D chain from the assembly of the iodonium cations (A—Type A; B—Type B) with trimesate anion; (C): 0D structure from the assembly of iodonium cations (Type C) with trimesate anion; (D): 2D layer from the assembly of iodonium cations (Types A and B) with trimesate anion; (E): 2D layer from the assembly of iodonium cations (Types A, B, and C) with trimesate anion (middle and bottom).

Table 1.
Geometrical parameters of XBs in the structures of 1a,b–3a,b.
In the structures of 1a,b–3a,b, the mean value of normalized contacts (Nc 0.78) for the I∙∙∙O XB, involving the carboxylic group which acted as an XB acceptor, agreed well with the Nc mean value (Nc 0.79) for other iodonium carboxylates from CSD. Further inspection of CSD and the comparison of I∙∙∙O XBs, including the carboxylic (this work) or a sulfonate group (accessed in CSD), revealed that Nc values for iodonium carboxylates (our data 0.78; CSD data: mean value 0.79) are lower than those for iodonium sulfonates (mean value 0.82). This comparison indirectly indicates that the carboxylate systems provide stronger XBs, probably due to a more localized negative charge on the carboxylate function (bearing two electronegative O-atoms), rather than that on the sulfonate group (featuring three O-atoms).
All pairs of structures (namely, 1a and 1b, 2a and 2b, and 3a and 3b) of the salts bearing p-Cl (for 1a–3a) and p-Br substituents (1b–3b) in the arene rings provided examples of the isostructural exchange [39,40,41,42] (Figure S1). The counterions did not affect this exchange and, furthermore, crystal packings were the same for the p-Cl and p-Br substituents. Previously, we reported a relevant isostructural exchange in symmetrical [43] and unsymmetrical [23] iodonium salts bearing p-Cl and p-Br substituents in arenes of iodonium cations.
Notably, in the structures of 1a,b and 3a,b, the halogens of the arene rings formed additional X∙∙∙X (X = Cl or Br). These XBs occurred between a σ-hole of one halogen and an electron belt of another halogen atom. However, these interactions were characterized by rather large Nc values (~1.00), indicating that they were very weak (Table S1). The angles ∠C–X∙∙∙X (163–177°) were close to 180°, and this, in combination with the results of the DFT calculations (Section 2.4), allowed the attribution of these interactions to XBs, according to the IUPAC classification [44]. In comparison with the C–X∙∙∙X XBs, stronger C–X∙∙∙O XBs were observed in the structures of 3a,b, in which the Nc was noticeably lower than 1.00 (X = Cl, Nc = 0.94; X = Br, Nc = 0.91), although the angles ∠C–X∙∙∙O (~155°; Table S1) deviated from linearity.
2.3. XRD Structures: Supramolecular Assembly
In 1a,b and 2a,b, two cationic and two anionic species assembled into heterotetrameric motifs via four I∙∙∙O XBs (Figure 4). Similar heterotetrameric motifs were found in a large number of iodonium salts, in particular, in the structure of iodonium benzoate (CSD refcode: TUDWEX) [33]. In the cases of benzoates 1a,b, the crystal structures exhibited 0D organization, while the addition of one carboxylic group in terephthalates 2a,b increased the dimensionality providing assembly into 1D chains by linking the heterotetrameric motifs to the phenylene bridges, –C6H4– (Figure 4).
We earlier reported a relevant self-assembly of iodonium disulfonates, where heterotetrameric motifs were linked by naphthalene bridges [25]. Apart from 1D chains, the studied iodonium disulfonates formed 2D-layered structures [25]. However, occurrence of the 2D systems happened occasionally, depending on cation, anion, and crystallization conditions.
We assumed that for the triple-charged anion (namely, trimesate anion), the occurrence of 2D layers was more favorable due to the branching of the supramolecular assembly by a larger number of XB-accepting sites in the same functionality. According to our expectations, the replacement of doubly-charged terephthalates in 2a,b, to triply-charged trimesates in 3a,b, led to the addition of a dimension and accomplished the 2D-layered architecture. The structures of 3a,b included one trimesate anion, three crystallographically independent iodonium cations (Types A–C), and two water molecules; the latter were linked to a trimesate anion by a hydrogen bond (namely, O2A···H–O1S–H···O2S and O6A···H–O2S–H···O3A, Figure S2).
In general, the analysis of the crystal structures of 3a,b revealed five different XBs with trimesate anions, namely four two-center and one three-center bifurcated XBs. Thus, 2D layers in 3a,b—depending on the identity of the iodonium cation (Figure 5A–C)—exhibited three basic motifs. Type A and B cations formed 1D chains with trimesate anions (Figure 5A,B), whilst Type C iodonium cation formed a 0D cluster, including one trimesate anion and one H2O (Figure 5C). Both 1D-chained motifs displayed a similar architecture, where one trimesate anion interacted with two anions of one type (Type A: I1···O1A and I1···O5A XBs; Type B: I1B···O3A and I1B···O5A(O6A) XBs, Figure 5A,B). A combination of 1D chains (Types A and B) led to an XB net-like organization (Figure 5D), in which trimesate anions functioned as nodes. Each trimesate anion additionally interacted with Type C cations, so the 0D clusters motif was woven into XB net-like 2D layers (Figure 5E).
2.4. Theoretical Calculations
To closely interrogate the observed XB contacts, we performed appropriate DFT calculations, which were based on the experimentally determined XRD coordinates and performed under the periodic boundary conditions (crystal models, PBE [45]-D3 [46,47] level of theory, and the DZVP-MOLOPT-SR-GTH/SZV-MOLOPT-SR-GTH [48] bases within the Gaussian/plane wave (GPW) [49] methodology in CP2K). The DZVP-MOLOPT-SR-GTH basis set was used for all atoms in the structures of 2a and 2b. In view of software limitations for the structures exhibiting large unit cell volumes (>2000 Å3), the same approach for 1a, 1b, 3a, and 3b was not able to be performed and, hence, the calculations were conducted using the DZVP-MOLOPT-SR-GTH basis set for halogen atoms; O-atoms; and for the C-atom which is covalently bound to halogen or O-atoms; and also for H-atoms covalently bound to oxygen. The SZV-MOLOPT-SR-GTH basis set was used for the remaining H- and C-atoms.
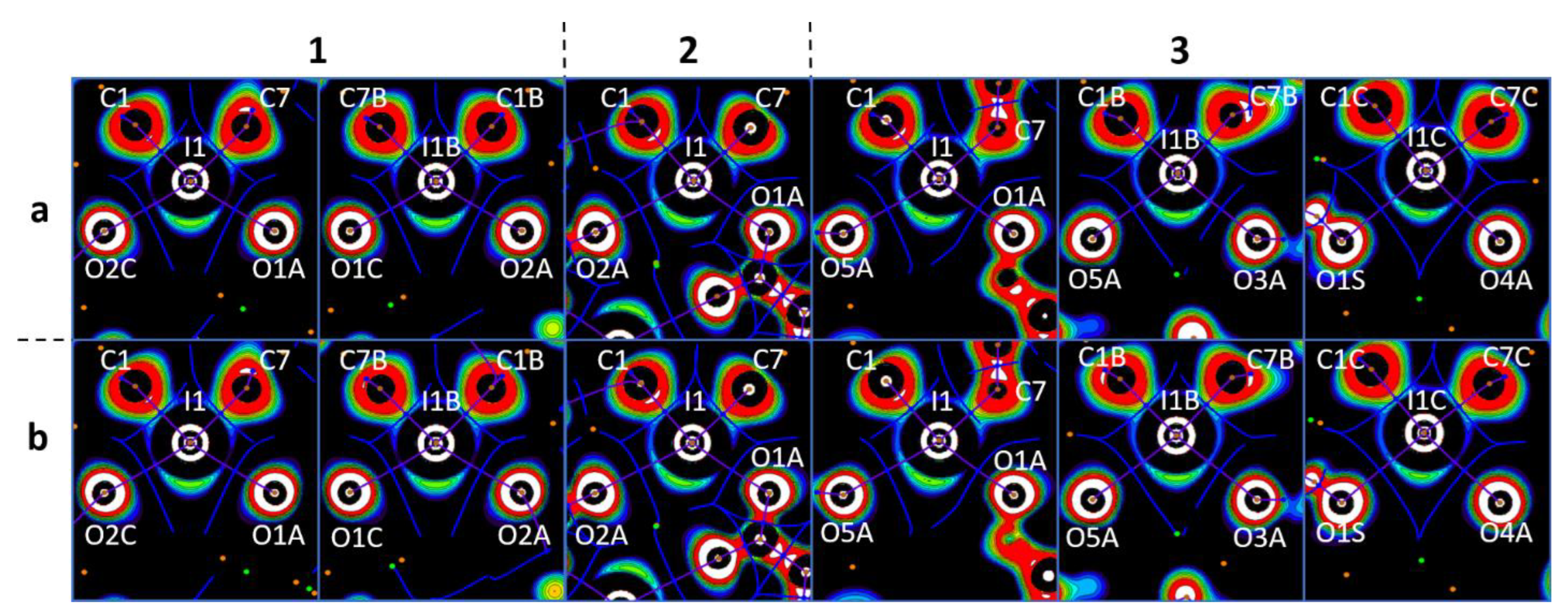
The existence and noncovalent nature of the studied interactions was confirmed by the topological analysis of electron density within the Bader quantum theory of atoms in molecules (QTAIM analysis) [50,51,52,53]. Bond critical points (3, −1) (BCPs) between the iodonium I-atoms and the carboxylate O-atoms (including the bifurcate I···OCO interactions in 3a and 3b) were found, and they are gathered in Table 2. In addition, BCPs were detected between the Cl- (or Br) atoms in the structures of 1a, 1b, 3a, and 3b; between the Cl- (Br) atoms and the π-systems of the aromatic rings in 2a and 2b; and between the Cl- (Br) atoms and the carboxylate O-atoms in 3a and 3b (Table S2). Finally, BCPs between the H-atoms of H2O molecules and the O atoms of carboxylate were also identified.

Table 2.
Parameters in (3, −1) bond critical points (the electron density with sign of λ2 sign(λ2)ρ(r) in e/bohr3, Laplacian of electron density ∇2ρ(r) in e/bohr5, the local electronic energy density Hb, local electronic potential energy density V(r), local electronic kinetic energy density G(r) in Hartree/bohr3) corresponding to the I∙∙∙O XBs in crystal models of all structures.
The obtained BCP values of sign(λ2) ρ(r) were negative and small, and their considerations point to the attractive and noncovalent nature of the interactions under study [54]. Furthermore, these interactions can also be classified as noncovalent because of their close to zero positive energy density values (0.0002−0.0023 Hartree/bohr3); the balance of the Lagrangian kinetic energy G(r); and the potential energy density V(r) (−G(r)/V(r) > 1) at the corresponding BCPs [53]. In some cases, when d(I···O) < 3 Å or d(H···O) < 1.85 Å, the energy density values were negative, and this indicated a certain degree of covalency in the occurrence of these contacts.
To confirm the philicities [55,56,57] (the property of atom(s) to function as electron donor(s) (nucleophile(s)) or electron acceptor(s) (electrophile(s)) of the coformers, we computed one-electron-potential (OEP) [58,59] projections with assigned critical points and bond paths from ρ(r) QTAIM analysis (Figure 6). The OEP-based approach has previously been used [60,61,62] for the visualization of shared and lone electron pairs. In particular, this method has been applied to various diaryliodonium systems and many other relevant systems [25]. The OEP approach is more useful than the electron localization function (ELF) [63,64,65] method considering that the former does not directly depend on the wave function. Consequently, one can calculate OEP in any area using the electron density function (EDF) for core electrons [66].
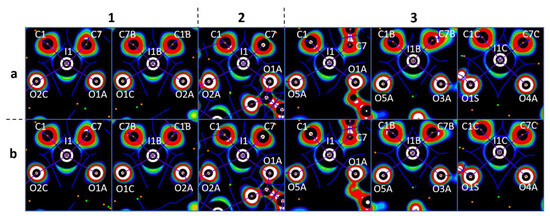
Figure 6.
Visualization of the OEP projections through the O···I···O planes for the crystal models (numbers above the figure represent the anion structures and letters means the cation structures). Contour lines are drawn from −0.25 to 0.25 OEP value with 0.05 step and with additional −0.60 contour line; the color range is white (<−0.60), from red (−0.25) to purple (0.25), and black (>0.25). QTAIM ρ(r) topological pale brown nuclear (3, −3), blue bond (3, −1), orange ring (3, +1), and green cell (3, +3) critical points are drawn with purple bond paths and blue interbasin paths.
In all cases, the I···O bond paths passed between the I−C shared and iodine lone pair areas with positive OEP, namely through iodine σ-holes, and through the lone pair areas of the carboxylate O-atoms. This observation allowed the accurate determination of the philicities of the I- and O-atoms in the studied XBs, particularly the electrophilicity of the iodonium centers and the nucleophilicity of the carboxylate O-sites. The same pattern detected in the monofurcate was also detected for the bifurcate I···OCO interactions. In the latter case, bond paths were located between the lone pair areas around the iodonium I-atoms. This observation confirmed their electrophilicity toward the carboxylate O-atoms (Figure 7). Likewise, the analysis of the OEP projections verified the electrophilicity of the Cl- (Br) atoms; the nucleophilicity of the Cl- (Br) atoms; the C-atoms of the aromatic rings; and the O-atoms of carboxylate in X···X, X···C, and X···O (X = Cl, Br) interactions.
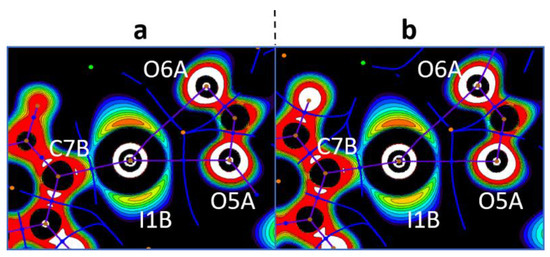
Figure 7.
Visualization of the OEP projections through the O···I···O planes for the bifurcate I···OCO interactions (the crystal models 3a (a) and 3b (b)). Contour lines are drawn from −0.25 to 0.25 OEP value with 0.05 step and with additional −0.60 contour line; the color range is white (<−0.60), from red (−0.25) to purple (0.25), and black (>0.25). QTAIM ρ(r) topological pale brown nuclear (3, −3), blue bond (3, −1), orange ring (3, +1), and green cell (3, +3) critical points are drawn with purple bond paths and blue interbasin paths.
To summarize the computational results, we confirmed the occurrence of the I···O XBs and the Cl···Cl (Br···Br), Cl···C (Br···C) and Cl···O (Br···O) XBs, proved their noncovalent nature (albeit with a small covalent contribution), and determined the philicities of the coformers in the solid supramolecular assemblies.
3. Materials and Methods
3.1. General Information
All reagents and solvents were obtained from commercial sources and used without further purification. Iodonium salts were obtained using the previously reported procedure [1]. Melting points were measured on a BUCHI M-560 apparatus (BUCHI Labortechnik AG, Flawil, St. Gallen, Switzerland) in capillaries and were not corrected. The NMR spectra were recorded on Bruker Avance III HD (400 MHz) (Bruker Corp., Billerica, MA, USA). The 1H NMR spectra were recorded at 400 MHz and the 13C NMR spectra were recorded at 100 MHz. Chemical shifts were reported in parts per million (ppm). The 1H and 13C chemical shifts were referenced relative to the residual solvent signal. High-resolution mass spectra (HRMS) were recorded using electrospray ionization (ESI) methods on a Bruker micrOTOF spectrometer (Bruker Corp., Billerica, MA, USA) equipped with an ESI source. Elemental CHNS analysis was obtained on an elemental analyzer Thermo Flash EA 2000 (Thermo Fisher Scientific, Rockford, IL, USA), and sulfanilamide was used as a standard. Drying of the samples for elemental analysis was carried out at 80 °C to constant weight in a dry argon atmosphere using combined TG-DSC analysis on an SDT Q600 thermal analyzer (TA Instruments New Castle, DE, USA).
3.2. X-ray Structure Determinations
X-ray diffraction data were collected at 100 K on a XtaLAB Synergy (Rigaku Oxford Diffraction, Oxford, UK), single-source at home/near, HyPix diffractometer using Cu Kα (λ = 1.54184 Å; 3a,b) and a Tongda TD-5000 diffractometer (Dandong Tongda Science and Technology, Dangdong, China) using Mo Kα (λ = 0.71073; 1a,b; 2a,b). The structures were solved with the ShelXT (Shelx, Göttingen, Germany) [67] structure solution program using Intrinsic Phasing and refined with the ShelXL (Shelx, Göttingen, Germany) [68] refinement package incorporated in the OLEX2 program package (OlexSys Ltd., Durham, UK) [69] using Least Squares minimization. The XRD data and structural refinement parameters are summarized in Table S3. The hydrogen atoms in all structures were placed in ideally calculated positions according to neutron diffraction statistical data [70] and were refined as colliding atoms with parameters of relative isotropic displacement. Supplementary crystallographic data have been deposited at Cambridge Crystallographic Data Centre (CCDC 2291471–2291473, 2291475–2291477) and can be obtained free of charge via www.ccdc.cam.ac.uk/data request/cif (accessed on 30 August 2023).
3.3. Computational Details
Single-point DFT calculations under periodic boundary conditions were conducted using the mixed Gaussian/plane-wave (GPW) [49] basis set with 350 plane-wave; 50 Ry relative plane-wave cutoffs for the auxiliary grid; and the PBE [45]-D3 [46,47] level of theory for all studied crystals (1 × 1 × 1 cells) using the CP2K-8.1 program [71,72,73,74,75,76,77]. The PBE-D3 level of theory was previously applied for most of the CP2K calculations performed under 3D periodic boundary conditions [78,79,80,81,82,83,84,85,86,87]. In the structures of 2a and 2b, the DZVP-MOLOPT-SR-GTH basis set was applied for all atoms. However, to achieve 1.0 × 10−6 Hartree convergence for the self-consistent-field cycle in the Γ-point approximation, for the structures of 1a, 1b, 3a, and 3b, a combination of the DZVP-MOLOPT-SR-GTH and the SZV-MOLOPT-SR-GTH basis sets was applied. A similar methodology has previously been used for the studies of various halogen-bonded systems [88,89,90]. In some cases, the starting fractional coordinates were shifted along one (for 2b) or two (for 2a) translation vectors by 0.5 to move the heterotetrameric fragments (consisting of two anions and two cations) to the center of the cell. One-electron-potential (OEP) [58,59] analysis and Bader atoms in molecules topological analysis of electron density (QTAIM) [50,51,52,53] were performed and visualized in Multiwfn 3.8 [91]. The pseudopotential core areas were modeled by the inner code of Multiwfn 3.8 [66] for the OEP and QTAIM analyses.
3.4. Synthetic Procedures
3.4.1. Preparation of Diaryliodonium Benzoates 1
A solution of a diaryliodonium trifluoromethanesulfonate [92] (1 mmol) in methanol/water mixture (1 mL) was added dropwise to a solution of potassium benzoate (3 mmol, 481 mg) in water (5 mL) at RT. The reaction mixture was stirred for 30 min and the precipitate formed was filtered off and washed with water (3 × 5 mL). The prepared diaryliodonium benzoates 1 were dried under reduced pressure.
3.4.2. Preparation of Diaryliodonium Terephtalates 2
To a solution of a diaryliodonium trifluoromethanesulfonate [92] (2.2 mmol) in methanol/water mixture (5 mL, 1:1), the solution of tetrabutylammonium terephthalate (1 mmol, 649 mg) in methanol (1 mL) was added dropwise at RT. The reaction mixture was stirred for 30 min and the precipitate formed was filtered off and washed with water (3 × 5 mL). The prepared diaryliodonium terephthalates 2 were dried under reduced pressure.
3.4.3. Preparation of Diaryliodonium Trimesates 3
To a solution of tetrabutylammonium trimesate (1 mmol, 934 mg) in water/acetonitrile mixture (5 mL, 1:1), a solution of diaryliodonium trifluoromethanesulfonate [92] (3.3 mmol) in acetonitrile (5 mL) was added dropwise at RT. The reaction mixture was stirred for 30 min and the precipitate formed was filtered off and washed with water (3 × 5 mL). The prepared diaryliodonium trimesates 3 were dried under reduced pressure.
4. Conclusions
We utilized the iodonium carboxylates for the design of halogen-bonded supramolecular assemblies (0D, 1D, and 2D). Iodonium cations acted as double σ-hole XB donors, while the carboxylate anions functioned as efficient XB acceptors. The increase in the number of carboxylic groups led to the addition of a dimension to the supramolecular assemblies. Thus, the association of iodonium benzoates furnished a 0D cluster, whilst the use of the terephthalate species and the trimesate species furnished 1D-chained or 2D-layered structures, correspondingly. To model the solid-state electron wave function, DFT calculations under periodic boundary conditions were performed. A topological analysis of the electron density revealed the bond critical points for interionic XBs and, in the cases of 3a and 3b, for bifurcated I···(OCO) XBs. The projections of one-electron potential, which verified the electron pair positions, confirmed the electrophilicity of the XB donors.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241914642/s1.
Author Contributions
Conceptualization, N.S.S., P.S.P. and V.Y.K.; methodology, N.S.S.; software, D.M.I., A.A.I. and I.I.F.; validation, N.S.S., P.S.P. and V.Y.K.; formal analysis, N.S.S.; investigation, A.D.R., I.I.F., A.A.I. and A.I.L.; resources, P.S.P. and M.S.Y.; data curation, N.S.S. and D.M.I.; writing—original draft preparation, N.S.S., D.M.I. and I.I.F.; writing—review and editing, P.S.P. and V.Y.K.; visualization, I.I.F., A.D.R. and N.S.S.; supervision, P.S.P. and V.Y.K.; project administration, P.S.P.; funding acquisition, P.S.P. and N.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work represents an integration of two diverse projects: the crystal engineering parts were conducted under the Russian Science Foundation project No. 23-73-10091, while the synthetic and theoretical parts were performed under the “Mega-grant” project (No. 075-15-2021-585) of the Ministry of Science and Higher Education of the Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request from the authors.
Acknowledgments
The authors are grateful to the Center for X-ray Diffraction Studies, the Magnetic Resonance Research Center, and the Center for Chemical Analysis and Materials Research (all belonging to Saint Petersburg State University) for the physicochemical studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the Halogen Bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cotelle, Y.; Sakai, N.; Matile, S. Unorthodox Interactions at Work. J. Am. Chem. Soc. 2016, 138, 4270–4277. [Google Scholar] [CrossRef] [PubMed]
- Teyssandier, J.; Mali, K.S.; De Feyter, S. Halogen Bonding in Two-Dimensional Crystal Engineering. ChemistryOpen 2020, 9, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef]
- Li, B.; Zang, S.Q.; Wang, L.Y.; Mak, T.C.W. Halogen Bonding: A Powerful, Emerging Tool for Constructing High-Dimensional Metal-Containing Supramolecular Networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Scholfield, M.R.; Vander Zanden, C.M.; Carter, M.; Ho, P.S. Halogen Bonding (X-Bonding): A Biological Perspective. Protein Sci. 2013, 22, 139–152. [Google Scholar] [CrossRef]
- Riel, A.M.S.; Rowe, R.K.; Ho, E.N.; Carlsson, A.-C.C.; Rappé, A.K.; Berryman, O.B.; Ho, P.S. Hydrogen Bond Enhanced Halogen Bonds: A Synergistic Interaction in Chemistry and Biochemistry. Acc. Chem. Res. 2019, 52, 2870–2880. [Google Scholar] [CrossRef]
- Ho, P.S. Biomolecular Halogen Bonds. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2014; pp. 241–276. [Google Scholar]
- Mendez, L.; Henriquez, G.; Sirimulla, S.; Narayan, M. Looking Back, Looking Forward at Halogen Bonding in Drug Discovery. Molecules 2017, 22, 1397. [Google Scholar] [CrossRef]
- Pancholi, J.; Beer, P.D. Halogen Bonding Motifs for Anion Recognition. Coord. Chem. Rev. 2020, 416, 213281. [Google Scholar] [CrossRef]
- Hein, R.; Beer, P.D. Halogen Bonding and Chalcogen Bonding Mediated Sensing. Chem. Sci. 2022, 13, 7098–7125. [Google Scholar] [CrossRef] [PubMed]
- Tay, H.M.; Tse, Y.C.; Docker, A.; Gateley, C.; Thompson, A.L.; Kuhn, H.; Zhang, Z.; Beer, P.D. Halogen-Bonding Heteroditopic [2]Catenanes for Recognition of Alkali Metal/Halide Ion Pairs. Angew. Chem. Int. Ed. 2023, 62, e202214785. [Google Scholar] [CrossRef] [PubMed]
- Docker, A.; Guthrie, C.H.; Kuhn, H.; Beer, P.D. Modulating Chalcogen Bonding and Halogen Bonding Sigma-Hole Donor Atom Potency and Selectivity for Halide Anion Recognition. Angew. Chem. Int. Ed. 2021, 60, 21973–21978. [Google Scholar] [CrossRef] [PubMed]
- Sutar, R.L.; Huber, S.M. Catalysis of Organic Reactions through Halogen Bonding. ACS Catal. 2019, 9, 9622–9639. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Reinhard, D.L.; Engelage, E.; Huber, S.M. A Bidentate Iodine(III)-Based Halogen-Bond Donor as a Powerful Organocatalyst**. Angew. Chem. Int. Ed. 2021, 60, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Engelage, E.; Dreger, A.; Weiss, R.; Huber, S.M. Iodine(III) Derivatives as Halogen Bonding Organocatalysts. Angew. Chem. Int. Ed. 2018, 57, 3830–3833. [Google Scholar] [CrossRef]
- Brammer, L.; Peuronen, A.; Roseveare, T.M. Halogen Bonds, Chalcogen Bonds, Pnictogen Bonds, Tetrel Bonds and Other σ-Hole Interactions: A Snapshot of Current Progress. Acta Crystallogr. Sect. C Struct. Chem. 2023, 79, 204–216. [Google Scholar] [CrossRef]
- Robidas, R.; Reinhard, D.L.; Legault, C.Y.; Huber, S.M. Iodine(III)-Based Halogen Bond Donors: Properties and Applications. Chem. Rec. 2021, 21, 1912–1927. [Google Scholar] [CrossRef]
- Catalano, L.; Cavallo, G.; Metrangolo, P.; Resnati, G.; Terraneo, G. Halogen Bonding in Hypervalent Iodine Compounds. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; Volume 373, pp. 289–309. [Google Scholar]
- Cavallo, G.; Murray, J.S.; Politzer, P.; Pilati, T.; Ursini, M.; Resnati, G. Halogen Bonding in Hypervalent Iodine and Bromine Derivatives: Halonium Salts. IUCrJ 2017, 4, 411–419. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Postnikov, P.S.; Suslonov, V.V.; Kissler, T.Y.; Ivanov, D.M.; Yusubov, M.S.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. Diaryliodonium as a Double σ-Hole Donor: The Dichotomy of Thiocyanate Halogen Bonding Provides Divergent Solid State Arylation by Diaryliodonium Cations. Org. Chem. Front. 2020, 7, 2230–2242. [Google Scholar] [CrossRef]
- Semenov, A.V.; Baykov, S.V.; Soldatova, N.S.; Geyl, K.K.; Ivanov, D.M.; Frontera, A.; Boyarskiy, V.P.; Postnikov, P.S.; Kukushkin, V.Y. Noncovalent Chelation by Halogen Bonding in the Design of Metal-Containing Arrays: Assembly of Double σ-Hole Donating Halolium with Cu I-Containing O,O-Donors. Inorg. Chem. 2023, 62, 6128–6137. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Suslonov, V.V.; Ivanov, D.M.; Yusubov, M.S.; Resnati, G.; Postnikov, P.S.; Kukushkin, V.Y. Controlled Halogen-Bond-Involving Assembly of Double-σ-Hole-Donating Diaryliodonium Cations and Ditopic Arene Sulfonates. Cryst. Growth Des. 2023, 23, 413–423. [Google Scholar] [CrossRef]
- Fedorova, I.I.; Soldatova, N.S.; Ivanov, D.M.; Nikiforova, K.; Aliyarova, I.S.; Yusubov, M.S.; Tolstoy, P.M.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y.; et al. Benzothienoiodolium Cations Doubly Bonded to Anions via Halogen–Chalcogen and Halogen–Hydrogen Supramolecular Synthons. Cryst. Growth Des. 2023, 23, 2661–2674. [Google Scholar] [CrossRef]
- Suslonov, V.V.; Soldatova, N.S.; Postnikov, P.S.; Resnati, G.; Kukushkin, V.Y.; Ivanov, D.M.; Bokach, N.A. Diaryliodonium Tetracyanidometallates Self-Assemble into Halogen-Bonded Square-Like Arrays. Cryst. Growth Des. 2022, 22, 2749–2758. [Google Scholar] [CrossRef]
- Wolf, J.; Huber, F.; Erochok, N.; Heinen, F.; Guérin, V.; Legault, C.Y.; Kirsch, S.F.; Huber, S.M. Activation of a Metal-Halogen Bond by Halogen Bonding. Angew. Chem. Int. Ed. 2020, 59, 16496–16500. [Google Scholar] [CrossRef]
- Heinen, F.; Engelage, E.; Cramer, C.J.; Huber, S.M. Hypervalent Iodine(III) Compounds as Biaxial Halogen Bond Donors. J. Am. Chem. Soc. 2020, 142, 8633–8640. [Google Scholar] [CrossRef]
- Boelke, A.; Kuczmera, T.J.; Lork, E.; Nachtsheim, B.J. N-Heterocyclic Iod(Az)Olium Salts–Potent Halogen-Bond Donors in Organocatalysis. Chem. Eur. J. 2021, 27, 13128–13134. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Postnikov, P.S.; Ivanov, D.M.; Semyonov, O.V.; Kukurina, O.S.; Guselnikova, O.; Yamauchi, Y.; Wirth, T.; Zhdankin, V.V.; Yusubov, M.S.; et al. Zwitterionic Iodonium Species Afford Halogen Bond-Based Porous Organic Frameworks. Chem. Sci. 2022, 13, 5650–5658. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Mayer, R.J.; Ofial, A.R.; Mayr, H.; Legault, C.Y. Lewis Acidity Scale of Diaryliodonium Ions toward Oxygen, Nitrogen, and Halogen Lewis Bases. J. Am. Chem. Soc. 2020, 142, 5221–5233. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Matveychuk, Y.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-Involving Bifurcated Halogen Bonding C–Br···η 2 (Cl–Pt). Cryst. Growth Des. 2019, 19, 1364–1376. [Google Scholar] [CrossRef]
- Efimenko, Z.M.; Eliseeva, A.A.; Ivanov, D.M.; Galmés, B.; Frontera, A.; Bokach, N.A.; Kukushkin, V.Y. Bifurcated μ 2-I···(N,O) Halogen Bonding: The Case of (Nitrosoguanidinate)Ni II Cocrystals with Iodine(I)-Based σ-Hole Donors. Cryst. Growth Des. 2021, 21, 588–596. [Google Scholar] [CrossRef]
- Aliyarova, I.S.; Ivanov, D.M.; Soldatova, N.S.; Novikov, A.S.; Postnikov, P.S.; Yusubov, M.S.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Diaryliodonium Cations as Iodine(III)-Based Double-σ-Hole Donors. Cryst. Growth Des. 2021, 21, 1136–1147. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Rozhkov, A.V.; Ananyev, I.V.; Frontera, A.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Two Rhodium(I) Centers as an Integrated σ-Hole Acceptor. JACS Au 2021, 1, 354–361. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Kinzhalov, M.A.; Novikov, A.S.; Ananyev, I.V.; Romanova, A.A.; Boyarskiy, V.P.; Haukka, M.; Kukushkin, V.Y. H 2 C(X)–X···X–(X = Cl, Br) Halogen Bonding of Dihalomethanes. Cryst. Growth Des. 2017, 17, 1353–1362. [Google Scholar] [CrossRef]
- Fotović, L.; Bedeković, N.; Stilinović, V. Isostructural Halogen Exchange and Halogen Bonds: The Case of N-(4-Halogenobenzyl)-3-Halogenopyridinium Halogenides. Cryst. Growth Des. 2022, 22, 1333–1344. [Google Scholar] [CrossRef]
- Buldakov, A.V.; Kinzhalov, M.A.; Kryukova, M.A.; Ivanov, D.M.; Novikov, A.S.; Smirnov, A.S.; Starova, G.L.; Bokach, N.A.; Kukushkin, V.Y. Isomorphous Series of Pd II-Containing Halogen-Bond Donors Exhibiting Cl/Br/I Triple Halogen Isostructural Exchange. Cryst. Growth Des. 2020, 20, 1975–1984. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Sokolov, M.N. Halogen Bonding in Isostructural Co(II) Complexes with 2-Halopyridines. Crystals 2020, 10, 289. [Google Scholar] [CrossRef]
- Aliyarova, I.S.; Tupikina, E.Y.; Soldatova, N.S.; Ivanov, D.M.; Postnikov, P.S.; Yusubov, M.; Kukushkin, V.Y. Halogen Bonding Involving Gold Nucleophiles in Different Oxidation States. Inorg. Chem. 2022, 61, 15398–15407. [Google Scholar] [CrossRef] [PubMed]
- United States Patent Application: 0050054626. Available online: https://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220050054626%22.PGNR.&OS=DN/20050054626&RS=DN/20050054626 (accessed on 26 September 2022).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- VandeVondele, J.; Hutter, J. Gaussian Basis Sets for Accurate Calculations on Molecular Systems in Gas and Condensed Phases. J. Chem. Phys. 2007, 127, 114105. [Google Scholar] [CrossRef] [PubMed]
- LIPPERT, B.G.; PARRINELLO, J.H. and M. A Hybrid Gaussian and Plane Wave Density Functional Scheme. Mol. Phys. 1997, 92, 477–488. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: London, UK, 1990; ISBN 9780198558651. [Google Scholar]
- Bader, R.F.W.; Nguyen-Dang, T.T. Quantum Theory of Atoms in Molecules–Dalton Revisited. Adv. Quantum Chem. 1981, 14, 63–124. [Google Scholar]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From Weak to Strong Interactions: A Comprehensive Analysis of the Topological and Energetic Properties of the Electron Density Distribution Involving X-H⋯F-Y Systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Maiti, B.; Sarkar, U. Philicity: A Unified Treatment of Chemical Reactivity and Selectivity. J. Phys. Chem. A 2003, 107, 4973–4975. [Google Scholar] [CrossRef]
- Parsaee, F.; Senarathna, M.C.; Kannangara, P.B.; Alexander, S.N.; Arche, P.D.E.; Welin, E.R. Radical Philicity and Its Role in Selective Organic Transformations. Nat. Rev. Chem. 2021, 5, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, R.H.; Schmauck, J.; Perryman, M.S.; Yue, H.; Riegger, J.; Schweitzer-Chaput, B.; Breugst, M.; Klussmann, M. Philicity of Acetonyl and Benzoyl Radicals: A Comparative Experimental and Computational Study. Chem. Eur. J. 2019, 25, 9088–9097. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G. The Exact One-Electron Model of Molecular Structure. Int. J. Quantum Chem. 1986, 29, 197–204. [Google Scholar] [CrossRef]
- Chan, W.-T.; Hamilton, I.P. Valence Shell Structures in the Distributions of the Laplacian of the Electron Density and the One-Electron Potential for Diatomic Molecules. J. Chem. Phys. 1998, 108, 2473–2485. [Google Scholar] [CrossRef]
- Tsirelson, V.; Stash, A. On Functions and Quantities Derived from the Experimental Electron Density. Acta Crystallogr. Sect. A Found. Crystallogr. 2004, 60, 418–426. [Google Scholar] [CrossRef]
- Bertolotti, F.; Shishkina, A.V.; Forni, A.; Gervasio, G.; Stash, A.I.; Tsirelson, V.G. Intermolecular Bonding Features in Solid Iodine. Cryst. Growth Des. 2014, 14, 3587–3595. [Google Scholar] [CrossRef]
- Bartashevich, E.; Yushina, I.; Kropotina, K.; Muhitdinova, S.; Tsirelson, V. Testing the Tools for Revealing and Characterizing the Iodine–Iodine Halogen Bond in Crystals. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 217–226. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A Simple Measure of Electron Localization in Atomic and Molecular Systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of Chemical Bonds Based on Topological Analysis of Electron Localization Functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. Engl. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Zou, W.; Cai, Z.; Wang, J.; Xin, K. An Open Library of Relativistic Core Electron Density Function for the QTAIM Analysis with Pseudopotentials. J. Comput. Chem. 2018, 39, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Allen, F.H.; Bruno, I.J. Bond Lengths in Organic and Metal-Organic Compounds Revisited: X —H Bond Lengths from Neutron Diffraction Data. Acta Crystallogr. Sect. B Struct. Sci. 2010, 66, 380–386. [Google Scholar] [CrossRef]
- Frigo, M.; Johnson, S.G. The Design and Implementation of FFTW3. Proc. IEEE 2005, 93, 216–231. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and Accurate Density Functional Calculations Using a Mixed Gaussian and Plane Waves Approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
- Hutter, J.; Iannuzzi, M.; Schiffmann, F.; VandeVondele, J. <scp>cp2k:</Scp> Atomistic Simulations of Condensed Matter Systems. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 15–25. [Google Scholar] [CrossRef]
- Borštnik, U.; VandeVondele, J.; Weber, V.; Hutter, J. Sparse Matrix Multiplication: The Distributed Block-Compressed Sparse Row Library. Parallel Comput. 2014, 40, 47–58. [Google Scholar] [CrossRef]
- Schütt, O.; Messmer, P.; Hutter, J.; VandeVondele, J. GPU-Accelerated Sparse Matrix-Matrix Multiplication for Linear Scaling Density Functional Theory. In Electronic Structure Calculations on Graphics Processing Units; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 173–190. [Google Scholar]
- Goerigk, L.; Hansen, A.; Bauer, C.; Ehrlich, S.; Najibi, A.; Grimme, S. A Look at the Density Functional Theory Zoo with the Advanced GMTKN55 Database for General Main Group Thermochemistry, Kinetics and Noncovalent Interactions. Phys. Chem. Chem. Phys. 2017, 19, 32184–32215. [Google Scholar] [CrossRef] [PubMed]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An Electronic Structure and Molecular Dynamics Software Package-Quickstep: Efficient and Accurate Electronic Structure Calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef] [PubMed]
- Golze, D.; Iannuzzi, M.; Hutter, J. Local Fitting of the Kohn–Sham Density in a Gaussian and Plane Waves Scheme for Large-Scale Density Functional Theory Simulations. J. Chem. Theory Comput. 2017, 13, 2202–2214. [Google Scholar] [CrossRef]
- Wang, S.; Lee, J.S.; Wahiduzzaman, M.; Park, J.; Muschi, M.; Martineau-Corcos, C.; Tissot, A.; Cho, K.H.; Marrot, J.; Shepard, W.; et al. A Robust Large-Pore Zirconium Carboxylate Metal–Organic Framework for Energy-Efficient Water-Sorption-Driven Refrigeration. Nat. Energy 2018, 3, 985–993. [Google Scholar] [CrossRef]
- Wang, X.-D.; Huang, Y.-H.; Liao, J.-F.; Jiang, Y.; Zhou, L.; Zhang, X.-Y.; Chen, H.-Y.; Kuang, D.-B. In Situ Construction of a Cs 2 SnI 6 Perovskite Nanocrystal/SnS 2 Nanosheet Heterojunction with Boosted Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 13434–13441. [Google Scholar] [CrossRef]
- Chung, Y.G.; Haldoupis, E.; Bucior, B.J.; Haranczyk, M.; Lee, S.; Zhang, H.; Vogiatzis, K.D.; Milisavljevic, M.; Ling, S.; Camp, J.S.; et al. Advances, Updates, and Analytics for the Computation-Ready, Experimental Metal–Organic Framework Database: CoRE MOF 2019. J. Chem. Eng. Data 2019, 64, 5985–5998. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, W.; Mei, D. Theoretical Characterization of Zeolite Encapsulated Platinum Clusters in the Presence of Water Molecules. Phys. Chem. Chem. Phys. 2021, 23, 23360–23371. [Google Scholar] [CrossRef]
- Bennion, J.C.; Vogt, L.; Tuckerman, M.E.; Matzger, A.J. Isostructural Cocrystals of 1,3,5-Trinitrobenzene Assembled by Halogen Bonding. Cryst. Growth Des. 2016, 16, 4688–4693. [Google Scholar] [CrossRef]
- Oropeza, F.E.; Barawi, M.; Alfonso-González, E.; de la Peña O’Shea, V.A.; Trigo, J.F.; Guillén, C.; Saiz, F.; Villar-Garcia, I.J. Understanding Ultrafast Charge Transfer Processes in SnS and SnS 2: Using the Core Hole Clock Method to Measure Attosecond Orbital-Dependent Electron Delocalisation in Semiconducting Layered Materials. J. Mater. Chem. C 2021, 9, 11859–11872. [Google Scholar] [CrossRef]
- Chadwick, F.M.; Rees, N.H.; Weller, A.S.; Krämer, T.; Iannuzzi, M.; Macgregor, S.A. A Rhodium-Pentane Sigma-Alkane Complex: Characterization in the Solid State by Experimental and Computational Techniques. Angew. Chem. Int. Ed. 2016, 55, 3677–3681. [Google Scholar] [CrossRef]
- Pambudi, F.I.; Prasetyo, N. Insight into the Structure of the Heulandite-Type Zeolite Containing Aromatic Compounds Using Periodic Density Functional Theory. Mater. Today Commun. 2021, 26, 102028. [Google Scholar] [CrossRef]
- Hazra, A.; Bonakala, S.; Adalikwu, S.A.; Balasubramanian, S.; Maji, T.K. Fluorocarbon-Functionalized Superhydrophobic Metal–Organic Framework: Enhanced CO 2 Uptake via Photoinduced Postsynthetic Modification. Inorg. Chem. 2021, 60, 3823–3833. [Google Scholar] [CrossRef] [PubMed]
- Kinzhalov, M.A.; Ivanov, D.M.; Melekhova, A.A.; Bokach, N.A.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y. Chameleonic Metal-Bound Isocyanides: A π-Donating CuI-Center Imparts Nucleophilicity to the Isocyanide Carbon toward Halogen Bonding. Inorg. Chem. Front. 2022, 9, 1655–1665. [Google Scholar] [CrossRef]
- Nieland, E.; Komisarek, D.; Hohloch, S.; Wurst, K.; Vasylyeva, V.; Weingart, O.; Schmidt, B.M. Supramolecular Networks by Imine Halogen Bonding. Chem. Commun. 2022, 58, 5233–5236. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.V.; Kinzhalov, M.A.; Smirnov, A.S.; Cheranyova, A.M.; Ivanov, D.M.; Kukushkin, V.Y.; Bokach, N.A. Polymorph-Dependent Phosphorescence of Cyclometalated Platinum(II) Complexes and Its Relation to Non-Covalent Interactions. ACS Omega 2022, 7, 34454–34462. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Bielawski, M.; Zhu, M.; Olofsson, B. Efficient and General One-Pot Synthesis of Diaryliodonium Triflates: Optimization, Scope and Limitations. Adv. Synth. Catal. 2007, 349, 2610–2618. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).