The Influence of the Solid Solution Formation on Purification of L-Menthol from the Enantiomer Mixture by Three-Phase Crystallization
Abstract
:1. Introduction
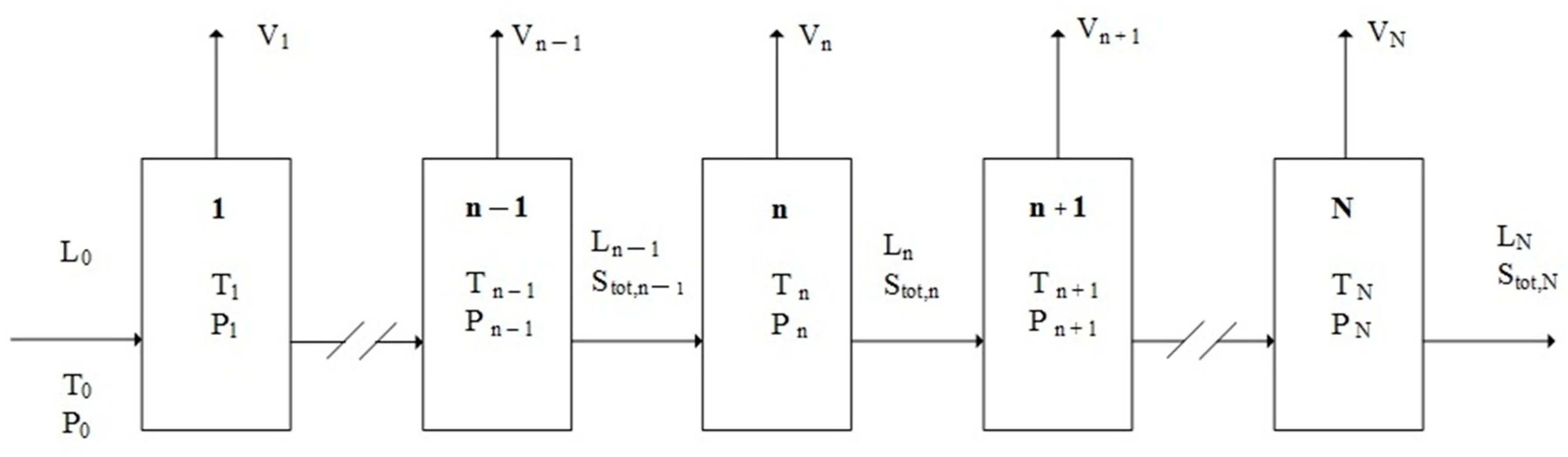
2. Principle of TPC
3. TPC Model
4. Experimental Section
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Notation | |
| Experimental purity of L-menthol in the final product, dimensionless | |
| Calculated purity of L-menthol in the final product, dimensionless | |
| Mass of the initial liquid feed, | |
| Mass of the liquid out of stage , | |
| Pressure in stage , | |
| Saturated vapor pressure of component-j, | |
| Triple-point pressure of component-j, | |
| Ideal gas constant, | |
| Mass of the solid solution enriched with L-menthol formed in stage , | |
| Total amount of the solid solution enriched with L-menthol formed from stage 1 to stage , | |
| Temperature in stage , | |
| Boiling temperature of component-j, | |
| Melting temperature of component-j, | |
| Time, | |
| Mass of the vapor formed in stage , | |
| Total amount of vapor formed and removed from stage 1 to stage , | |
| Measured weight of the final product at the end of TPC, | |
| Calculated weight of the final product at the end of TPC, | |
| Initial mole fraction of component-j in the liquid feed, dimensionless | |
| Mole fraction of component-j in the remaining liquid in stage , dimensionless | |
| Mole fraction of component-j in the vapor phase formed in stage , dimensionless | |
| Mole fraction of component-j in the solid solution formed in stage , dimensionless | |
| Average mole fraction of component-j in the solid solution at the end of T, dimensionless | |
| Experimental purity of L-menthol for the final product at the end of the sweating experiment, dimensionless | |
| Heat of melting for component-j (>0), | |
| Heat of vaporization for component-j (>0), | |
| Amount of liquid removed at the end of the sweating experiment, | |
| Greek Letters | |
| Activity coefficient of component-j in liquid, dimensionless | |
| Subscript | |
| In the initial feed | |
| At the end of TPC | |
| In stage | |
| In the last stage | |
References
- Eccles, R. Menthol and related cooling compounds. J. Pharm. Pharmacol. 1994, 46, 618–630. [Google Scholar] [CrossRef]
- Hamasaki, K.; Kato, K.; Watanabe, T.; Yoshimura, Y.; Nakazawa, H.; Yamamoto, A.; Matsunaga, A. Determination of L-menthol in pharmaceutical products by high performance liquid chromatography with polarized photometric detection. J. Pharm. Biomed. Anal. 1998, 16, 1275–1280. [Google Scholar] [PubMed]
- Galeotti, N.; Mannelli, L.D.C.; Mazzanti, G.; Bartolini, A.; Ghelardini, C. Menthol a natural analgesic compound. Neurosci. Lett. 2002, 322, 145–148. [Google Scholar] [PubMed]
- Leffingwell, J.C.; Shackelford, R.E. Laevo-menthol-syntheses and organoleptic properties. Cosmet. Perfum. 1974, 89, 69–89. [Google Scholar]
- Lokotsch, W.; Fritsche, K.; Syldatk, C. Resolution of D. L-methanol by interesterification with triacetin using the free and immobilized lipase of candida cylindracea. Appl. Microbiol. Biotechnol. 1989, 31, 467–472. [Google Scholar]
- Wang, D.L.; Nag, A.; Lee, G.C.; Shaw, J.F. Factors affecting the resolution of dl-menthol by immobilized lipase-catalyzed esterification in organic solvent. J. Agric. Food. Chem. 2002, 50, 262–265. [Google Scholar] [CrossRef]
- Brady, D.; Reddy, S.; Mboniswa, B.; Steenkamp, L.H.; Rousseau, A.L.; Parkinson, C.J.; Chaplin, J.; Mitra, R.K.; Moutlana, T.; Marai, S.F.; et al. Biocatalytic enantiomeric resolution of L-menthol from an eight isomeric menthol mixture through transesterification. J. Mol. Catal. B-Enzym. 2012, 75, 1–10. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.R.; Xu, G.; Wu, J.P. Highly diastereoselective acylation of L-menthol by a lipase from stenotrophomonas maltophilia CGMCC 4254. Biochem. Eng. J. 2016, 109, 81–87. [Google Scholar]
- Craveiro, R.; Meneses, L.; Durazzo, L.; Rocha, A.; Silva, J.M.; Reis, R.L.; Barreiros, S.; Duarte, A.R.C.; Paiva, A. Deep eutectic solvents for enzymatic esterification of racemic menthol. ACS. Sustain. Chem. Eng. 2019, 7, 19943–19950. [Google Scholar] [CrossRef]
- Kim, K.J.; Ulrich, J. Impurity distribution in a solid-liquid interface during static layer crystallization. J. Colloid. Interface. Sci. 2002, 252, 161–168. [Google Scholar] [CrossRef]
- Ulrich, J.; Glade, H. Melt Crystallization: Fundamentals, Equipment and Applications; Shaker Verlag: Düren, Germany, 2003. [Google Scholar]
- Jiang, X.; Hou, B.; He, G.; Wang, J. Falling film melt crystallization (I): Model development, experimental validation of crystal layer growth and impurity distribution process. Chem. Eng. Sci. 2012, 84, 120–133. [Google Scholar] [CrossRef]
- Beierling, T.; Osiander, J.; Sadowski, G. Melt crystallization of isomeric long-chain aldehydes from hydroformylation. Sep. Purif. Technol. 2013, 118, 13–24. [Google Scholar]
- Micovic, J.; Beierling, T.; Lutze, P.; Sadowski, G.; Gorak, A. Design of hybrid distillation/melt crystallization processes for separation of close boiling mixtures. Chem. Eng. Process Process Intensif. 2013, 67, 16–24. [Google Scholar]
- Jiang, X.; Li, M.; He, G.; Wang, J. Research progress and model development of crystal layer growth and impurity distribution in layer melt crystallization: A review. Ind. Eng. Chem. Res. 2014, 53, 13211–13227. [Google Scholar]
- Fukui, K.; Fujikawa, T.; Satone, H.; Yamamoto, T.; Maeda, K. Application of solute distribution theory to melt crystallization of fatty acids. Chem. Eng. Sci. 2016, 143, 114–121. [Google Scholar]
- Ahmad, M.; Ulrich, J. Separation of complex feed streams of a product by layer melt crystallization. Chem. Eng. Technol. 2016, 39, 1341–1345. [Google Scholar] [CrossRef]
- Yazdanpanah, N.; Myerson, A.; Trout, B. Mathematical modeling of layer crystallization on a cold column with recirculation. Ind. Eng. Chem. Res. 2016, 55, 5019–5029. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Su, W.; Wang, H. Research progress of hybrid distillation crystallization technology. Chem. Eng. Technol. 2018, 41, 894–1904. [Google Scholar] [CrossRef]
- Ioannou, I.S.; Kontos, S.S.; Koutsoukos, P.G.; Paraskeva, C.A. Mathematical modeling and experimental coupling of solution layer crystallization on a vertically cold surface. Sep. Purif. Technol. 2018, 197, 8–17. [Google Scholar]
- Wang, T.; Li, X.; Dong, J. Ethylene glycol purification by melt crystallization: Removal of short-chain glycol impuritie. Ind. Eng. Chem. Res. 2020, 59, 8805–8812. [Google Scholar] [CrossRef]
- Jia, S.; Jing, B.; Gao, Z.; Gong, J.; Wang, J.; Rohani, S. Melt crystallization of 2,4-dinitrochlorobenzene: Purification and process parameters evaluation. Sep. Purif. Technol. 2021, 259, 118140. [Google Scholar] [CrossRef]
- Ding, S.; Huang, X.; Yin, Q.; Wang, N.; Wang, T.; Dong, Y.; Chen, Y.; Hao, H. Static layer crystallization: Effects of impurities on the growth behaviors of crystal layers. Sep. Purif. Technol. 2021, 279, 119764. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Li, S. Purification of 2-pyrrolidone by falling film melt crystallization. Ind. Eng. Chem. Res. 2021, 60, 13286–13292. [Google Scholar] [CrossRef]
- Jia, S.; Gao, Z.; Tian, N.; Li, Z.; Gong, J.; Wang, J.; Rohani, S. Review of melt crystallization in the pharmaceutical field, towards crystal engineering and continuous process development. Chem. Eng. Res. Des. 2021, 166, 268–280. [Google Scholar]
- Zhang, B.; Yang, L.; Wang, H.; Shen, C.; Li, Y.; Cheng, J.; Yang, C. Experiment and modeling of static layer melt crystallization in a crystallizer with an inner cooling tube. J. Cryst. Growth 2022, 93, 126739. [Google Scholar] [CrossRef]
- Feng, H.; Wang, N.; Huang, X.; Wang, T.; Zhou, L.; Hao, H. Recent progress in melt crystallization. Chem. Eng. Res. Des. 2023, 190, 268–281. [Google Scholar] [CrossRef]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolutions; John Wiley & Sons Inc.: New York, NY, USA, 1981. [Google Scholar]
- Palovics, E.; Madarasz, J.; Pokol, G.; Fogassy, E.; Banhegyi, D.F. Economic separations of organic acidic or basic enantiomeric mixtures—A protocol suggestion. Int. J. Mol. Sci. 2023, 24, 846. [Google Scholar] [CrossRef]
- Shiau, L.D.; Wen, C.C.; Lin, B.S. Separation and purification of p-xylene from the mixture of m-xylene and p-xylene by distillative freezing. Ind. Eng. Chem. Res. 2005, 44, 2258–2265. [Google Scholar] [CrossRef]
- Shiau, L.D.; Wen, C.C.; Lin, B.S. Application of distillative freezing in the separation of o-xylene and p-xylene. AIChE J. 2006, 52, 1962–1967. [Google Scholar] [CrossRef]
- Shiau, L.D.; Wen, C.C.; Lin, B.S. Separation of p-xylene from the multicomponent xylene system by stripping crystallization. AIChE J. 2008, 54, 337–342. [Google Scholar] [CrossRef]
- Shiau, L.D.; Yu, C.C. Separation of the benzene/cyclohexane mixture by stripping crystallization. Sep. Purif. Technol. 2009, 66, 422–464. [Google Scholar] [CrossRef]
- Shiau, L.D.; Liu, K.F.; Hsu, Y.C. Chiral purification of S-ibuprofen from ibuprofen enantiomers by stripping crystallization. Chem. Eng. Res. Des. 2017, 117, 301–308. [Google Scholar] [CrossRef]
- Shiau, L.D. Product yield, purity, and effective distribution coefficient in stripping crystallization of R-2-amino-1-phenylethanol from the enantiomer mixture. Cryst. Growth. Des. 2020, 20, 1328–1336. [Google Scholar] [CrossRef]
- Shiau, L.D. Purification of m-xylene from the mixed xylenes by stripping crystallization. Sep. Purif. Technol. 2021, 255, 117688. [Google Scholar] [CrossRef]
- Shiau, L.D.; Wang, P.C. Chiral purification of S-2-phenylpropionic acid from an enantiomer mixture by stripping crystallization. Ind. Eng. Chem. Res. 2022, 61, 10224–10232. [Google Scholar] [CrossRef]
- Shiau, L.D. Purification of p-cresol, o-cresol, m-cresol and 2,6-xylenol from the quaternary mixture by three-phase crystallization. Ind. Eng. Chem. Res. 2023, 62, 8010–8020. [Google Scholar] [CrossRef]
- Corvis, Y.; Negrier, P.; Massip, S.; Leger, J.M.; Espeau, P. Insights into the crystal structure, polymorphism and thermal behavior of menthol optical isomers and racemates. CrystEngComm 2012, 14, 7055–7064. [Google Scholar] [CrossRef]
- Stejfa, V.; Bazyleva, A.; Fulem, M.; Rohlicek, J.; Skorepova, E.; Ruzicka, K.; Blokhin, A.V. Polymorphism and thermophysical properties of L- and DL-menthol. J. Chem. Thermodyn. 2019, 131, 524–543. [Google Scholar] [CrossRef]
- Smith, J.M.; Van, N.H.C.; Abbott, M.M. Introduction to Chemical Engineering Thermodynamics, 6th ed.; McGraw-Hill: Singapore, 2001. [Google Scholar]
- Sandler, S.I. Chemical, Biochemical, and Engineering Thermodynamics, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]









| Property | L-Menthol (D-Menthol) |
|---|---|
| Molecular structure |  |
| Molecular weight | 156.27 |
| Tb (°C) | 215.4 |
| Tm (°C) | 42.9 |
| Ptri (Pa) | 33.9 |
| ∆Hm (J/mol) | 1.347 × 104 |
| ∆Hv (J/mol) | 7.28 × 104 |
| n | T (°C) | P (Pa) | (XB)n | (ZB)n | Ln (g) | Sn (g) | Stot,n (g) | Vn (g) | Vtot,n (g) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 38.4 | 21.4 | 0.9 | 0.967 | 5 | 0 | 0 | 0 | 0 |
| 1 | 37.4 | 19.3 | 0.878 | 0.956 | 3.326 | 1.412 | 1.412 | 0.261 | 0.261 |
| 2 | 36.4 | 17.4 | 0.856 | 0.946 | 2.355 | 0.819 | 2.232 | 0.152 | 0.413 |
| 3 | 35.4 | 15.7 | 0.834 | 0.935 | 1.746 | 0.514 | 2.746 | 0.095 | 0.508 |
| 4 | 34.4 | 14.1 | 0.812 | 0.924 | 1.34 | 0.342 | 3.089 | 0.063 | 0.571 |
| 5 | 33.4 | 12.7 | 0.789 | 0.914 | 1.057 | 0.239 | 3.327 | 0.044 | 0.615 |
| 6 | 32.4 | 11.4 | 0.767 | 0.903 | 0.853 | 0.172 | 3.499 | 0.032 | 0.647 |
| 7 | 31.4 | 10.2 | 0.745 | 0.892 | 0.701 | 0.128 | 3.628 | 0.024 | 0.671 |
| 8 | 30.4 | 9.2 | 0.723 | 0.882 | 0.585 | 0.098 | 3.725 | 0.018 | 0.689 |
| 9 | 29.4 | 8.2 | 0.701 | 0.871 | 0.495 | 0.076 | 3.801 | 0.014 | 0.703 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-C.; Yang, S.-C.; Ku, K.-F.; Shiau, L.-D. The Influence of the Solid Solution Formation on Purification of L-Menthol from the Enantiomer Mixture by Three-Phase Crystallization. Int. J. Mol. Sci. 2023, 24, 14933. https://doi.org/10.3390/ijms241914933
Hsu Y-C, Yang S-C, Ku K-F, Shiau L-D. The Influence of the Solid Solution Formation on Purification of L-Menthol from the Enantiomer Mixture by Three-Phase Crystallization. International Journal of Molecular Sciences. 2023; 24(19):14933. https://doi.org/10.3390/ijms241914933
Chicago/Turabian StyleHsu, Yu-Chao, Sheng-Chin Yang, Kai-Fang Ku, and Lie-Ding Shiau. 2023. "The Influence of the Solid Solution Formation on Purification of L-Menthol from the Enantiomer Mixture by Three-Phase Crystallization" International Journal of Molecular Sciences 24, no. 19: 14933. https://doi.org/10.3390/ijms241914933
APA StyleHsu, Y.-C., Yang, S.-C., Ku, K.-F., & Shiau, L.-D. (2023). The Influence of the Solid Solution Formation on Purification of L-Menthol from the Enantiomer Mixture by Three-Phase Crystallization. International Journal of Molecular Sciences, 24(19), 14933. https://doi.org/10.3390/ijms241914933







