Thermodynamic Origin of Negative Thermal Expansion Based on a Phase Transition-Type Mechanism in the GdF3-TbF3 System
Abstract
:1. Introduction
2. Results and Discussion
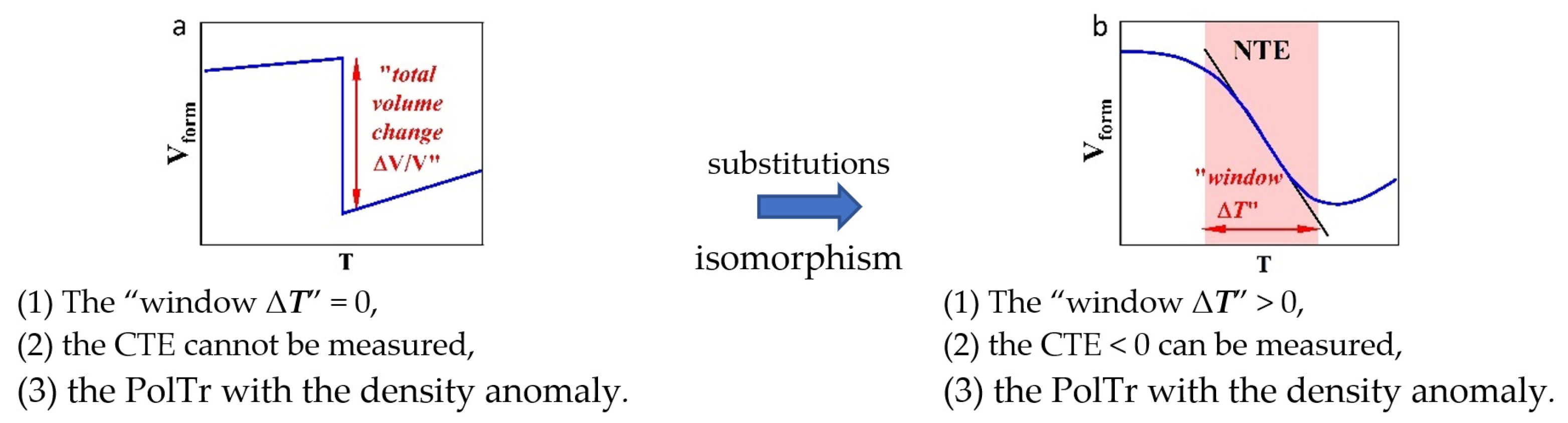
2.1. Signs of NTE-II Materials
2.1.1. The Qualitative Description of the NTE-II Materials Signs
2.1.2. Signs of NTE-II in Ionic REE Fluorides
2.2. Two-Component NTE-II Materials with Adjustable Parameters in the GdF3-TbF3 System
2.2.1. Choosing a Model System
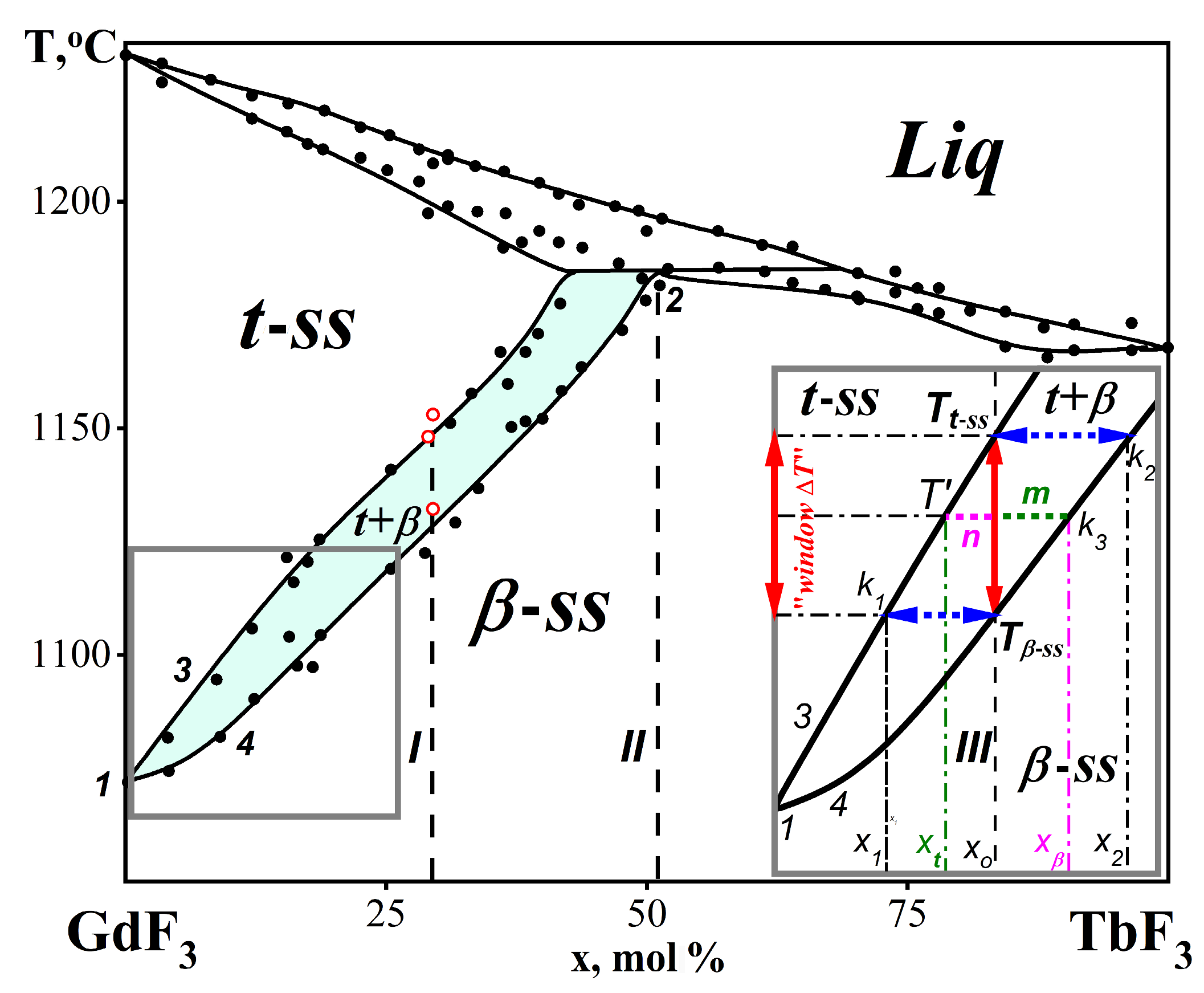
2.2.2. The Method of Calculating the Phase Composition of an NTE-II Material on the Example of the GdF3-TbF3 Phase Diagram
The Single-Phase β-Gd1−xTbxF3-ss Area without NTE
The Two-Phase (β + t)-Gd1−xTbxF3 Area with NTE-II
The Single-Phase t-Gd1−xTbxF3-ss Area without NTE
2.3. NTE-II in the GdF3-TbF3 System
2.3.1. The ΔV/V = f(T) Dependencies at the PolTr in Gd1−xTbxF3
The PolTr in GdF3 and Gd0.49Tb0.51F3 and NTE-II
The PolTr in Two-Phase (β + t)-Gd1−xTbxF3 Composites and NTE-II Materials
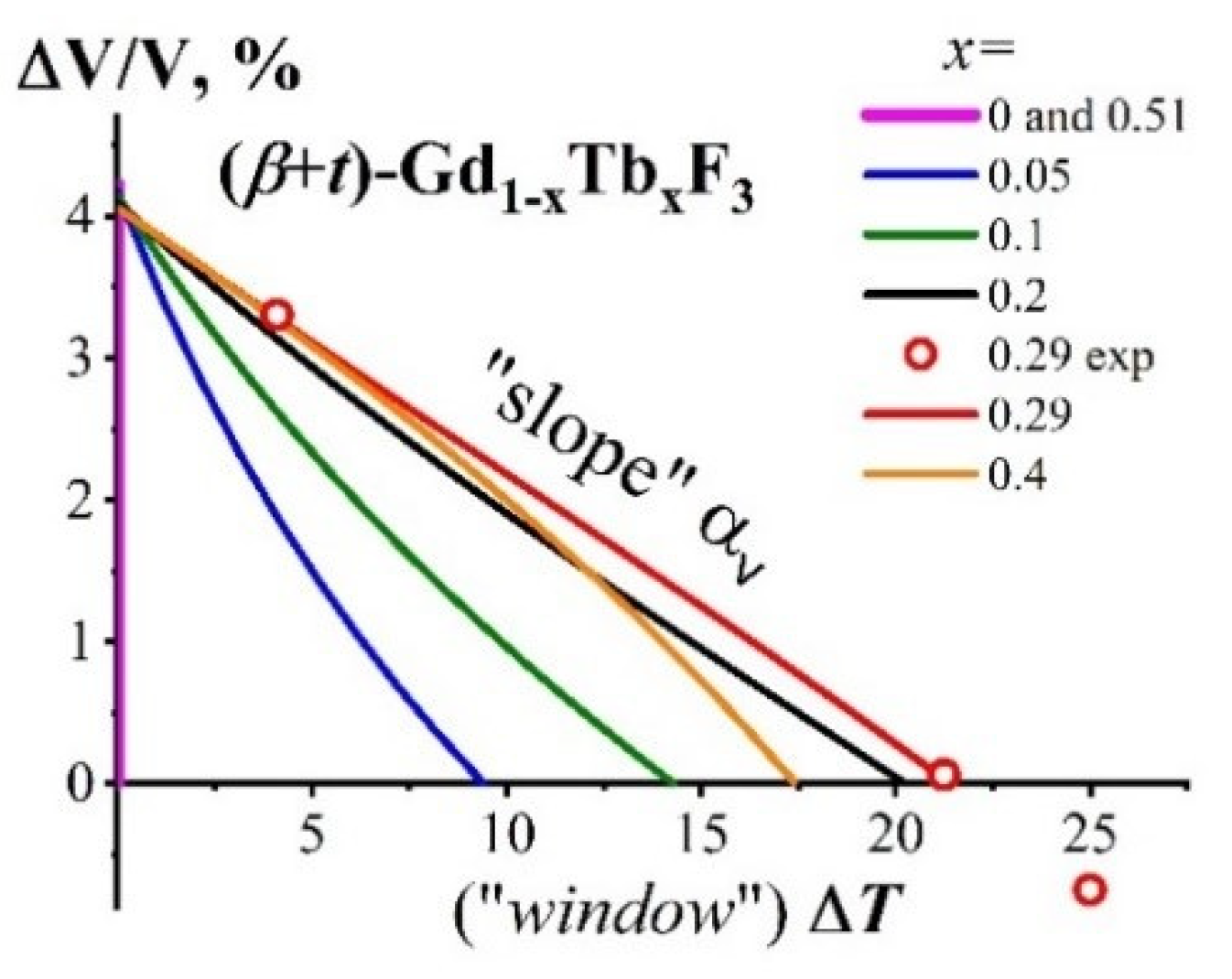
The PolTr in the Two-Phase Composite Gd0.71Tb0.29F3 (Gross Composition) According to the Phase Diagram and the NTE-II Parameters
2.3.2. The ΔV/V = f(ΔT) Dependence for the (β + t)-Gd1−xTbxF3 Composite
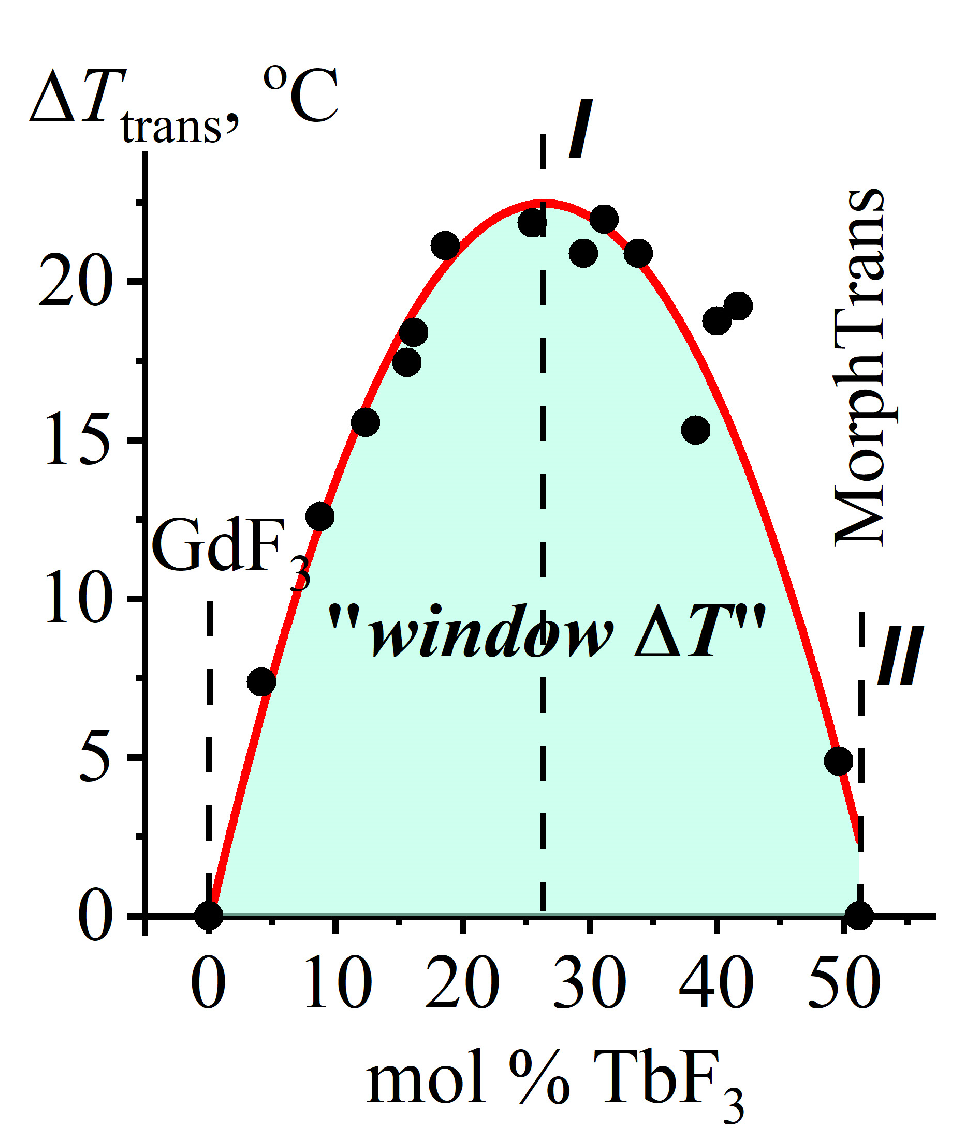
2.3.3. The Dependence of ΔTtrans (the “Window ΔT”) on a Chemical Composition for (β + t)-Gd1−xTbxF3 Composites
3. Materials and Methods
Two-Component NTE-II Materials in RF3-R′F3 and MF2-RF3 Systems (M = Ca, Sr, Ba; R = La − Lu)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takenaka, K. Progress of Research in Negative Thermal Expansion Materials: Paradigm Shift in the Control of Thermal Expansion. Front. Chem. 2018, 6, 267. [Google Scholar] [CrossRef]
- Sobolev, B.P. The Rare Earth Trifluorides, Part 1: The High Temperature Chemistry of the Rare Earth Trifluorides; Institute of Crystallography: Moscow, Russia; Institut d’Estudis Catalans: Barcelona, Spain, 2000. [Google Scholar]
- Greis, O.; Haschke, J.M. Rare Earth Fluorides. In Handbook on the Physics and Chemistry of Rare Earths; K.A. Gscheidner, K.A., Eyring, L.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1982; Volume 5, pp. 387–460. [Google Scholar]
- Spedding, F.H.; Daane, A.H. The Rare Earths; John Willey and Sons, Inc.: New York, NY, USA, 1961; 394p. [Google Scholar]
- Greve, B.K.; Martin, K.L.; Lee, P.; Chupas, P.J.; Chapman, K.W.; Wilkinson, A.P. Pronounced Negative Thermal Expansion from a Simple Structure: Cubic ScF3. J. Am. Chem. Soc. 2010, 132, 15496–15498. [Google Scholar] [CrossRef]
- Kasatkin, L.; Gulina, N.; Platonova, V.; Tolstoy, P.; Murin, I. Strong negative thermal expansion in the hexagonal polymorph of ScF3. CrystEngComm 2018, 20, 2768. [Google Scholar] [CrossRef]
- Lazar, P.; Bučko, T.; Hafner, J. Negative thermal expansion of ScF3: Insights from density-functional molecular dynamics in the isothermal-isobaric ensemble. Phys. Rev. 2015, B92, 224302. [Google Scholar] [CrossRef]
- Yang, C.; Tong, P.; Lin, J.C.; Guo, X.G.; Zhang, K.; Wang, M.; Wu, Y.; Lin, S.; Huang, P.C.; Xu, W.; et al. Size effects on negative thermal expansion in cubic ScF3. Appl. Phys. Lett. 2016, 109, 023110. [Google Scholar] [CrossRef]
- Greis, O.; Cader, M.S.R. Polymorphism of high purity rare earth trifluorides. Thermochim. Acta 1985, 87, 145–150. [Google Scholar] [CrossRef]
- Mansmann, M. Die Kristallstruktur von Lanthantrifluorid. Zeitschrift fur Krist. 1965, 122, 375–398. [Google Scholar] [CrossRef]
- Schlyter, K. On the crystal of fluorides of the tysonite (LaF3) type. Arkiv Kemi. 1953, 5, 73–82. [Google Scholar]
- Zalkin, A.; Templeton, D.H.; Hopkins, T.E. The Atomic Parameters in the Lanthanum Trifluoride Structure. Inorgan. Chem. 1966, 5, 1466–1468. [Google Scholar] [CrossRef]
- Greaves, C.; Fender, B.E.F. The structure of α-UO3 by neutron and electron diffraction. Acta Cryst. 1972, B28, 3609–3614. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Garashina, L.S.; Fedorov, P.P.; Tkachenko, N.L.; Seiranian, K.B. Polymorphism and crystallographic properties of yttrium and rare earth trifluorides. Sov. Phys. Crystallogr. 1974, 18, 473. [Google Scholar]
- Sobolev, B.P.; Sulyanova, E.A. Promethium trifluoride: Polymorphism and the structure of modifications on the model crystal 61(Ce0.5Gd0.5)F3. Part I. Structural and chemical modeling of “pseudo 61PmF3” in a short quasi-system “from CeF3 to GdF3”. CrystEngComm 2022, 24, 7602–7610. [Google Scholar] [CrossRef]
- Sulyanova, E.A.; Sobolev, B.P. Two-component rare earth fluoride materials with negative thermal expansion based on phase transition-type mechanism in 50 RF3-R’F3 (R = La-Lu) systems. Int. J. Mol. Sci. 2023, 24, 14000. [Google Scholar]
- Kurnakov, N.S. The Introduction to Physicochemical Analysis, 4th ed.; Akad. Nauk SSSR: Moscow, Russia, 1940; 564p. (In Russian) [Google Scholar]
- Weigel, F.; Scherer, V. Die Chemie des Prometiums III. Zur Kenntnis einiger Prometium (III) -halogenide und der Oxidhaloganide. Radiochim. Acta 1967, 7, 40–46. [Google Scholar]
- Greis, O.; Petzel, T. Ein Beitrag zur Strukturchemie der Seltenerd-Trifluoride. Z. Anorgan. Allgem. Chem. 1974, 403, 1–22. [Google Scholar] [CrossRef]
- Chareev, D.A. Phase Diagrams, Part 1; International University of Nature, Society and Human “Dubna”: Dubna, Russia, 2013; 60p. (In Russian) [Google Scholar]
- Nagel, L.E.; O’Keeffe, M. Highly-conducting fluorides related to fluorite and tysonite. In Fast Ion Transport in Solids; Van Gool, W., Ed.; North-Holland Publishing: Amsterdam, The Netherlands, 1973; pp. 165–172. [Google Scholar]
- Roos, A.; van de Pol, F.C.M.; Keim, R.; Schoonman, J. Ionic conductivity in tysonite-type solid solutions La1−xBaxF3-x. Solid State Ion. 1984, 13, 191–203. [Google Scholar] [CrossRef]
- Potanin, A.A. Solid-state chemical current source based on fluorine-ionic conductor with structure-type of lanthanum fluoride. J. Russ. Chem. Soc. 2001, 45, 58–63. (In Russian) [Google Scholar]
- Sinitsyna, V.V.; Lipsa, O.; Privalova, A.F.; Fujaraa, F.; Murin, I.V. Transport properties of LaF3 fast ionic conductor studied by field gradient NMR and impedance spectroscopy. J. Phys. Chem. Solid. 2003, 64, 1201–1205. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Sorokin, N.I.; Bolotina, N.B. Nonstoichiometric Single Crystals M1−xRxF2+x and R1−yMyF3−y (M = Ca, Sr, Ba: R = Rare Earth Elements) as Fluorine-Ionic Conductive Solid Electrolytes. In Photonic & Electronic Properties of Fluoride Materials. Vol. 1 in Serie: Progress in Fluorine Science; Tressaud, A., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 21, pp. 465–491. [Google Scholar]
- Dieudonné, B.; Chable, J.; Mauvy, F.; Fourcade, S.; Durand, E.; Lebraud, E.; Leblanc, M.; Legein, C.; Body, M.; Maisonneuve, V.; et al. Exploring the Sm1–xCaxF3–x Tysonite Solid Solution as a Solid-State Electrolyte: Relationships between Structural Features and F- Ionic Conductivity. J. Phys. Chem. 2015, 119, 25170–25179. [Google Scholar] [CrossRef]
- Mori, K.; Morita, Y.; Saito, T.; Kamiyama, T.; Otomo, T.; Abe, T.; Fukunaga, T. Structural and Electrochemical Properties of Tysonite Ce0.95A0.05F2.95 (A = Mg, Ca, Sr, and Ba): Fast-Fluoride-Ion-Conducting Solid Electrolytes. J. Phys. Chem. C 2020, 124, 18452–18461. [Google Scholar] [CrossRef]
- Toma, O.; Rotella, H.; Dahab, H.; Maisonneuve, V.; Boulard, B. Tysonite-type solid state electrolyte for fluoride ion batteries: Highly dense thin film by PVD processing. J. Alloys Compd. 2021, 862, 158683. [Google Scholar] [CrossRef]
- Belli, P.; Bernabei, R.; Cerulli, R.; Dai, C.J.; Danevich, F.A.; Incicchittid, A.; Kobychev, V.V.; Ponkratenko, O.A.; Prosperi, D.; Tretyak, V.I.; et al. Performances of a CeF3 crystal scintillator and its application to the search for rare processes. Nucl. Instr. Meth. Phys. Res. 2003, 498, 352–361. [Google Scholar] [CrossRef]
- Nikl, M.; Yoshikawa, A.; Satonaga, T.; Kamada, K.; Sato, H.; Solovieva, N.; Beitlerova, A.; Fukuda, T. Scintillation characteristics of PrF3:Ce single crystal. Phys. Stat. Sol. 2004, 201, R108–R110. [Google Scholar] [CrossRef]
- Feller, R.K.; Purdy, G.M.; Ortiz-Acosta, D.; Stange, S.; Li, A.; McKigney, E.A.; Esch, E.I.; Muenchausen, R.E.; Gilbertson, R.; Bacrania, M.; et al. Large-scale synthesis of CexLa1−xF3 nanocomposite scintillator materials. J. Mater. Chem. 2011, 21, 5716–5722. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; Agamaljan, N.R.; Denisenko, G.A.; Sarkisov, S.E.; Fedorov, P.P. Spectroscopy and laser emission of disordered GdF3-CaF2:Nd3+ trigonal crystals. Phys. Stat. Sol. 1982, 70A, 397–406. [Google Scholar] [CrossRef]
- Kaminskii, A.A. Laser Crystals, Their Physics and Properties, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1991; 457p. [Google Scholar]
- Glushkova, T.M.; Kiselev, D.F.; Firsova, M.M.; Shtirkova, A.P.; Krivandina, E.A.; Zhmurova, Z.I. Refractometry of single crystals of solid solutions La1−xSrxF3−x (0 < x < 0.15) with tysonite structure. Optichesk. Zhurn. 1997, 64, 31–33. (In Russian) [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulyanova, E.A.; Sobolev, B.P. Thermodynamic Origin of Negative Thermal Expansion Based on a Phase Transition-Type Mechanism in the GdF3-TbF3 System. Int. J. Mol. Sci. 2023, 24, 14944. https://doi.org/10.3390/ijms241914944
Sulyanova EA, Sobolev BP. Thermodynamic Origin of Negative Thermal Expansion Based on a Phase Transition-Type Mechanism in the GdF3-TbF3 System. International Journal of Molecular Sciences. 2023; 24(19):14944. https://doi.org/10.3390/ijms241914944
Chicago/Turabian StyleSulyanova, Elena A., and Boris P. Sobolev. 2023. "Thermodynamic Origin of Negative Thermal Expansion Based on a Phase Transition-Type Mechanism in the GdF3-TbF3 System" International Journal of Molecular Sciences 24, no. 19: 14944. https://doi.org/10.3390/ijms241914944
APA StyleSulyanova, E. A., & Sobolev, B. P. (2023). Thermodynamic Origin of Negative Thermal Expansion Based on a Phase Transition-Type Mechanism in the GdF3-TbF3 System. International Journal of Molecular Sciences, 24(19), 14944. https://doi.org/10.3390/ijms241914944





