Articular Cartilage—From Basic Science Structural Imaging to Non-Invasive Clinical Quantitative Molecular Functional Information for AI Classification and Prediction
Abstract
1. Introduction
2. What Are the Targets for Imaging of Articular Cartilage?
2.1. Current Commonly Used Histological Reference for Articular Cartilage Imaging
2.2. The Importance of the Extracellular Matrix (ECM) in Function and as an Imaging Target
2.3. The Importance of the Zonal Structure in Function and as Imaging Target in Healthy and Injured Articular Cartilage
2.4. The Importance of the Structure and Function as Imaging Targets in OA/Early OA
2.5. Imaging Cartilage Thickness
2.6. Imaging Articular Chondrocyte Volume, Shape, Phenotypes, and Spatial Arrangements
2.7. Imaging Articular Chondrocyte Viability, Hypocellularity, and Cell Death
2.8. Imaging the Pericellular Matrix (PCM)/Chondron
2.9. Calcified Zone, Tidemark, and Imaging Hypertrophy
2.10. Imaging Subchondral Bone
3. Quantitative MRI for Non-Invasive Assessment of Articular Cartilage Composition, Microstructure, and Function
4. Selected Non-Destructive Quantitative Articular Cartilage Live-Imaging Methods
5. The Articular Cartilage Image as Non-Invasive Functional Information for AI Classification of Degeneration and Prediction of OA Progression
6. Introducing a Future-Oriented, AI-Supported, Non-Destructive Quantitative Optical Biopsy for Early Disease Detection
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, N.; Wang, R.; Zhang, X.; Li, X.; Liang, Y.; Ding, Z. Spatially-resolved proteomics and transcriptomics: An emerging digital spatial profiling approach for tumor microenvironment. Vis. Cancer Med. 2021, 2, 1. [Google Scholar] [CrossRef]
- Khella, C.M.; Asgarian, R.; Horvath, J.M.; Rolauffs, B.; Hart, M.L. An Evidence-Based Systematic Review of Human Knee Post-Traumatic Osteoarthritis (PTOA): Timeline of Clinical Presentation and Disease Markers, Comparison of Knee Joint PTOA Models and Early Disease Implications. Int. J. Mol. Sci. 2021, 22, 1996. [Google Scholar] [CrossRef]
- Khella, C.M.; Horvath, J.M.; Asgarian, R.; Rolauffs, B.; Hart, M.L. Anti-Inflammatory Therapeutic Approaches to Prevent or Delay Post-Traumatic Osteoarthritis (PTOA) of the Knee Joint with a Focus on Sustained Delivery Approaches. Int. J. Mol. Sci. 2021, 22, 8005. [Google Scholar] [CrossRef] [PubMed]
- Tschaikowsky, M.; Selig, M.; Brander, S.; Balzer, B.N.; Hugel, T.; Rolauffs, B. Proof-of-concept for the detection of early osteoarthritis pathology by clinically applicable endomicroscopy and quantitative AI-supported optical biopsy. Osteoarthr. Cartil. 2021, 29, 269–279. [Google Scholar] [CrossRef]
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2021, 9, 603408. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Kurz, B.; Hart, M.L.; Rolauffs, B. Mechanical Articular Cartilage Injury Models and Their Relevance in Advancing Therapeutic Strategies. Adv. Exp. Med. Biol. 2023, 1402, 107–124. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Custers, R.J.; Creemers, L.B.; Verbout, A.J.; van Rijen, M.H.; Dhert, W.J.; Saris, D.B. Reliability, reproducibility and variability of the traditional Histologic/Histochemical Grading System vs the new OARSI Osteoarthritis Cartilage Histopathology Assessment System. Osteoarthr. Cartil. 2007, 15, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Nims, R.J.; Dicks, A.; Wu, C.L.; Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018, 71–72, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; de Girolamo, L.; Filardo, G.; Oliveira, J.M.; Orth, P.; Pape, D.; Reboul, P. Basic science of osteoarthritis. J. Exp. Orthop. 2016, 3, 22. [Google Scholar] [CrossRef]
- Mohammadi, H.; Mequanint, K.; Herzog, W. Computational aspects in mechanical modeling of the articular cartilage tissue. Proc. Inst. Mech. Eng. H 2013, 227, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Soltz, M.A.; Ateshian, G.A. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann. Biomed. Eng. 2000, 28, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, J.M.P.; Wilson, W.; Ito, K.; van Donkelaar, C.C. Relative contribution of articular cartilage’s constitutive components to load support depending on strain rate. Biomech. Model. Mechanobiol. 2017, 16, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Turunen, M.J.; Finnila, M.A.; Joukainen, A.; Kroger, H.; Saarakkala, S.; Korhonen, R.K.; Tanska, P. Structure-Function Relationships of Healthy and Osteoarthritic Human Tibial Cartilage: Experimental and Numerical Investigation. Ann. Biomed. Eng. 2020, 48, 2887–2900. [Google Scholar] [CrossRef]
- Li, L.P.; Buschmann, M.D.; Shirazi-Adl, A. Strain-rate dependent stiffness of articular cartilage in unconfined compression. J. Biomech. Eng. 2003, 125, 161–168. [Google Scholar] [CrossRef]
- Bartell, L.R.; Fortier, L.A.; Bonassar, L.J.; Cohen, I. Measuring microscale strain fields in articular cartilage during rapid impact reveals thresholds for chondrocyte death and a protective role for the superficial layer. J. Biomech. 2015, 48, 3440–3446. [Google Scholar] [CrossRef]
- Wu, J.P.; Kirk, T.B.; Zheng, M.H. Study of the collagen structure in the superficial zone and physiological state of articular cartilage using a 3D confocal imaging technique. J. Orthop. Surg. Res. 2008, 3, 29. [Google Scholar] [CrossRef]
- McLeod, M.A.; Wilusz, R.E.; Guilak, F. Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy. J. Biomech. 2013, 46, 586–592. [Google Scholar] [CrossRef]
- Rolauffs, B.; Kurz, B.; Felka, T.; Rothdiener, M.; Uynuk-Ool, T.; Aurich, M.; Frank, E.; Bahrs, C.; Badke, A.; Stockle, U.; et al. Stress-vs-time signals allow the prediction of structurally catastrophic events during fracturing of immature cartilage and predetermine the biomechanical, biochemical, and structural impairment. J. Struct. Biol. 2013, 183, 501–511. [Google Scholar] [CrossRef]
- Rolauffs, B.; Muehleman, C.; Li, J.; Kurz, B.; Kuettner, K.E.; Frank, E.; Grodzinsky, A.J. Vulnerability of the superficial zone of immature articular cartilage to compressive injury. Arthritis Rheum. 2010, 62, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Xia, Y. Quantitative zonal differentiation of articular cartilage by microscopic magnetic resonance imaging, polarized light microscopy, and Fourier-transform infrared imaging. Microsc. Res. Tech. 2013, 76, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Haudenschild, A.K.; Sherlock, B.E.; Lagarto, J.; Hu, J.C.; Leach, J.K.; Athanasiou, K.A.; Marcu, L. Zonal characterization of bovine articular cartilage using fluorescence lifetime imaging. In Proceedings of the Optics in the Life Sciences Congress, San Diego, CA, USA, 2 April 2017; p. OmM3D.4. [Google Scholar]
- Chaudhary, R.; Campbell, K.R.; Tilbury, K.B.; Vanderby, R.; Block, W.F.; Kijowski, R.; Campagnola, P.J. Articular cartilage zonal differentiation via 3D Second-Harmonic Generation imaging microscopy. Connect. Tissue Res. 2015, 56, 76–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rolauffs, B.; Williams, J.M.; Grodzinsky, A.J.; Kuettner, K.E.; Cole, A.A. Distinct horizontal patterns in the spatial organization of superficial zone chondrocytes of human joints. J. Struct. Biol. 2008, 162, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Morini, S.; Pannarale, L.; Braidotti, P.; Marinozzi, A.; Gaudio, E. A morphological study on femoral heads in human hip joint osteoarthrosis. Ital. J. Anat. Embryol. 1996, 101, 29–43. [Google Scholar] [PubMed]
- von der Mark, K.; Kirsch, T.; Nerlich, A.; Kuss, A.; Weseloh, G.; Gluckert, K.; Stoss, H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992, 35, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Huch, K.; Kuettner, K.E.; Dieppe, P. Osteoarthritis in ankle and knee joints. Semin. Arthritis Rheum. 1997, 26, 667–674. [Google Scholar] [CrossRef]
- Krych, A.J.; Saris, D.B.F.; Stuart, M.J.; Hacken, B. Cartilage Injury in the Knee: Assessment and Treatment Options. J. Am. Acad. Orthop. Surg. 2020, 28, 914–922. [Google Scholar] [CrossRef]
- Oakley, S.P.; Portek, I.; Szomor, Z.; Turnbull, A.; Murrell, G.A.; Kirkham, B.W.; Lassere, M.N. Accuracy and reliability of arthroscopic estimates of cartilage lesion size in a plastic knee simulation model. Arthroscopy 2003, 19, 282–289. [Google Scholar] [CrossRef]
- Kurz, B.; Lemke, A.K.; Fay, J.; Pufe, T.; Grodzinsky, A.J.; Schunke, M. Pathomechanisms of cartilage destruction by mechanical injury. Ann. Anat. 2005, 187, 473–485. [Google Scholar] [CrossRef]
- Tschaikowsky, M.; Brander, S.; Barth, V.; Thomann, R.; Rolauffs, B.; Balzer, B.N.; Hugel, T. The articular cartilage surface is impaired by a loss of thick collagen fibers and formation of type I collagen in early osteoarthritis. Acta Biomater. 2022, 146, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, F.U.; Karydis, A.; Lee, B.S.; Deguchi, T.; Kim, D.G.; Cho, H. Understanding Early-Stage Posttraumatic Osteoarthritis for Future Prospects of Diagnosis: From Knee to Temporomandibular Joint. Curr. Osteoporos. Rep. 2021, 19, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Pastoureau, P.C.; Hunziker, E.B.; Pelletier, J.P. Cartilage, bone and synovial histomorphometry in animal models of osteoarthritis. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S106–S112. [Google Scholar] [CrossRef]
- Lee, L.S.; Chan, P.K.; Fung, W.C.; Chan, V.W.K.; Yan, C.H.; Chiu, K.Y. Imaging of knee osteoarthritis: A review of current evidence and clinical guidelines. Musculoskelet. Care 2021, 19, 363–374. [Google Scholar] [CrossRef]
- Eckstein, F.; Reiser, M.; Englmeier, K.H.; Putz, R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging—From image to data, from data to theory. Anat. Embryol. 2001, 203, 147–173. [Google Scholar] [CrossRef]
- Eckstein, F.; Siedek, V.; Glaser, C.; Al-Ali, D.; Englmeier, K.H.; Reiser, M.; Graichen, H. Correlation and sex differences between ankle and knee cartilage morphology determined by quantitative magnetic resonance imaging. Ann. Rheum. Dis. 2004, 63, 1490–1495. [Google Scholar] [CrossRef]
- Hall, A.C. The Role of Chondrocyte Morphology and Volume in Controlling Phenotype-Implications for Osteoarthritis, Cartilage Repair, and Cartilage Engineering. Curr. Rheumatol. Rep. 2019, 21, 38. [Google Scholar] [CrossRef]
- Karim, A.; Amin, A.K.; Hall, A.C. The clustering and morphology of chondrocytes in normal and mildly degenerate human femoral head cartilage studied by confocal laser scanning microscopy. J. Anat. 2018, 232, 686–698. [Google Scholar] [CrossRef]
- von der Mark, K.; Gauss, V.; von der Mark, H.; Müller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]
- Dessau, W.; Vertel, B.M.; von der Mark, H.; von der Mark, K. Extracellular matrix formation by chondrocytes in monolayer culture. J. Cell Biol. 1981, 90, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Platt, D.; Wells, T.; Bayliss, M.T. Proteoglycan metabolism of equine articular chondrocytes cultured in alginate beads. Res. Vet. Sci. 1997, 62, 39–47. [Google Scholar] [CrossRef]
- Hart, M.L.; Lauer, J.C.; Selig, M.; Hanak, M.; Walters, B.; Rolauffs, B. Shaping the Cell and the Future: Recent Advancements in Biophysical Aspects Relevant to Regenerative Medicine. J. Funct. Morphol. Kinesiol. 2018, 3, 2. [Google Scholar] [CrossRef]
- Che, H.; Selig, M.; Rolauffs, B. Micro-patterned cell populations as advanced pharmaceutical drugs with precise functional control. Adv. Drug Deliv. Rev. 2022, 184, 114169. [Google Scholar] [CrossRef]
- Selig, M.; Azizi, S.; Walz, K.; Lauer, J.C.; Rolauffs, B.; Hart, M.L. Cell morphology as a biological fingerprint of chondrocyte phenotype in control and inflammatory conditions. Front. Immunol. 2023, 14, 1102912. [Google Scholar] [CrossRef]
- Murray, D.H.; Bush, P.G.; Brenkel, I.J.; Hall, A.C. Abnormal human chondrocyte morphology is related to increased levels of cell-associated IL-1β and disruption to pericellular collagen type VI. J. Orthop. Res. 2010, 28, 1507–1514. [Google Scholar] [CrossRef]
- Lauer, J.C.; Selig, M.; Hart, M.L.; Kurz, B.; Rolauffs, B. Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function. Int. J. Mol. Sci. 2021, 22, 3279. [Google Scholar] [CrossRef]
- Williams, R.; Khan, I.M.; Richardson, K.; Nelson, L.; McCarthy, H.E.; Analbelsi, T.; Singhrao, S.K.; Dowthwaite, G.P.; Jones, R.E.; Baird, D.M.; et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE 2010, 5, e13246. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Brenner, R.E. Pathomechanisms of Posttraumatic Osteoarthritis: Chondrocyte Behavior and Fate in a Precarious Environment. Int. J. Mol. Sci. 2020, 21, 1560. [Google Scholar] [CrossRef]
- Riegger, J.; Palm, H.G.; Brenner, R.E. The functional role of chondrogenic stem/progenitor cells: Novel evidence for immunomodulatory properties and regenerative potential after cartilage injury. Eur. Cells Mater. 2018, 36, 110–127. [Google Scholar] [CrossRef]
- Ji, Q.; Zheng, Y.; Zhang, G.; Hu, Y.; Fan, X.; Hou, Y.; Wen, L.; Li, L.; Xu, Y.; Wang, Y.; et al. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Ann. Rheum. Dis. 2019, 78, 100–110. [Google Scholar] [CrossRef]
- Rolauffs, B.; Williams, J.M.; Aurich, M.; Grodzinsky, A.J.; Kuettner, K.E.; Cole, A.A. Proliferative remodeling of the spatial organization of human superficial chondrocytes distant from focal early osteoarthritis. Arthritis Rheum. 2010, 62, 489–498. [Google Scholar] [CrossRef]
- Aicher, W.K.; Rolauffs, B. The spatial organisation of joint surface chondrocytes: Review of its potential roles in tissue functioning, disease and early, preclinical diagnosis of osteoarthritis. Ann. Rheum. Dis. 2014, 73, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, X.; Zhou, J.; Wei, L. The age-related changes in cartilage and osteoarthritis. BioMed Res. Int. 2013, 2013, 916530. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.; Lauer, J.C.; Hart, M.L.; Rolauffs, B. Mechanotransduction and Stiffness-Sensing: Mechanisms and Opportunities to Control Multiple Molecular Aspects of Cell Phenotype as a Design Cornerstone of Cell-Instructive Biomaterials for Articular Cartilage Repair. Int. J. Mol. Sci. 2020, 21, 5399. [Google Scholar] [CrossRef] [PubMed]
- Tschaikowsky, M.; Neumann, T.; Brander, S.; Haschke, H.; Rolauffs, B.; Balzer, B.N.; Hugel, T. Hybrid fluorescence-AFM explores articular surface degeneration in early osteoarthritis across length scales. Acta Biomater. 2021, 126, 315–325. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.M.; Hauselmann, H.J.; Shintani, N.; Hunziker, E.B. Cell and matrix morphology in articular cartilage from adult human knee and ankle joints suggests depth-associated adaptations to biomechanical and anatomical roles. Osteoarthr. Cartil. 2013, 21, 1904–1912. [Google Scholar] [CrossRef]
- Boehme, K.A.; Rolauffs, B. Onset and progression of human osteoarthritis—Can growth factors, inflammatory cytokines, or differential miRNA expression concomitantly induce proliferation, ECM degradation, and inflammation in articular cartilage? Int. J. Mol. Sci. 2018, 19, 2282. [Google Scholar] [CrossRef]
- David, M.A.; Smith, M.K.; Pilachowski, R.N.; White, A.T.; Locke, R.C.; Price, C. Early, focal changes in cartilage cellularity and structure following surgically induced meniscal destabilization in the mouse. J. Orthop. Res. 2017, 35, 537–547. [Google Scholar] [CrossRef]
- Rolauffs, B.; Rothdiener, M.; Bahrs, C.; Badke, A.; Weise, K.; Kuettner, K.E.; Kurz, B.; Aurich, M.; Grodzinsky, A.J.; Aicher, W.K. Onset of preclinical osteoarthritis: The angular spatial organization permits early diagnosis. Arthritis Rheum. 2011, 63, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Giannoudis, P.V. Modulation of cartilage’s response to injury: Can chondrocyte apoptosis be reversed? Injury 2017, 48, 2657–2669. [Google Scholar] [CrossRef]
- Duan, R.; Xie, H.; Liu, Z.Z. The Role of Autophagy in Osteoarthritis. Front. Cell Dev. Biol. 2020, 8, 608388. [Google Scholar] [CrossRef] [PubMed]
- Kurz, B.; Lemke, A.; Kehn, M.; Domm, C.; Patwari, P.; Frank, E.H.; Grodzinsky, A.J.; Schunke, M. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004, 50, 123–130. [Google Scholar] [CrossRef]
- Lotz, M.K.; Otsuki, S.; Grogan, S.P.; Sah, R.; Terkeltaub, R.; D’Lima, D. Cartilage cell clusters. Arthritis Rheum. 2010, 62, 2206–2218. [Google Scholar] [CrossRef]
- Felka, T.; Rothdiener, M.; Bast, S.; Uynuk-Ool, T.; Zouhair, S.; Ochs, B.G.; De Zwart, P.; Stoeckle, U.; Aicher, W.K.; Hart, M.L.; et al. Loss of spatial organization and destruction of the pericellular matrix in early osteoarthritis in vivo and in a novel in vitro methodology. Osteoarthr. Cartil. 2016, 24, 1200–1209. [Google Scholar] [CrossRef]
- Meinhardt, M.; Luck, S.; Martin, P.; Felka, T.; Aicher, W.; Rolauffs, B.; Schmidt, V. Modeling chondrocyte patterns by elliptical cluster processes. J. Struct. Biol. 2012, 177, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Eyre, D.R.; Koide, S.; Glimcher, M.J. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J. Bone Jt. Surg. Am. 1980, 62, 79–89. [Google Scholar] [CrossRef]
- Brittberg, M.; Peterson, L.; Sjogren-Jansson, E.; Tallheden, T.; Lindahl, A. Articular cartilage engineering with autologous chondrocyte transplantation: A review of recent developments. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. 3), 109–115. [Google Scholar] [CrossRef]
- Mankin, H.J. The response of articular cartilage to mechanical injury. J. Bone Jt. Surg. Am. 1982, 64, 460–466. [Google Scholar] [CrossRef]
- Nehrer, S.; Spector, M.; Minas, T. Histologic analysis of tissue after failed cartilage repair procedures. Clin. Orthop. Relat. Res. 1999, 365, 149–162. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.A. Articular cartilage chondrons: Form, function and failure. J. Anat. 1997, 191 Pt 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Youn, I.; Choi, J.B.; Cao, L.; Setton, L.A.; Guilak, F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthr. Cartil. 2006, 14, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Rothdiener, M.; Uynuk-Ool, T.; Sudkamp, N.; Aurich, M.; Grodzinsky, A.J.; Kurz, B.; Rolauffs, B. Human osteoarthritic chondrons outnumber patient- and joint-matched chondrocytes in hydrogel culture-Future application in autologous cell-based OA cartilage repair? J. Tissue Eng. Regen. Med. 2018, 12, e1206–e1220. [Google Scholar] [CrossRef] [PubMed]
- Chery, D.R.; Han, B.; Li, Q.; Zhou, Y.; Heo, S.J.; Kwok, B.; Chandrasekaran, P.; Wang, C.; Qin, L.; Lu, X.L.; et al. Early changes in cartilage pericellular matrix micromechanobiology portend the onset of post-traumatic osteoarthritis. Acta Biomater. 2020, 111, 267–278. [Google Scholar] [CrossRef]
- Kestila, I.; Thevenot, J.; Finnila, M.A.; Karhula, S.S.; Hadjab, I.; Kauppinen, S.; Garon, M.; Quenneville, E.; Haapea, M.; Rieppo, L.; et al. In vitro method for 3D morphometry of human articular cartilage chondrons based on micro-computed tomography. Osteoarthr. Cartil. 2018, 26, 1118–1126. [Google Scholar] [CrossRef]
- Burr, D.B. Anatomy and physiology of the mineralized tissues: Role in the pathogenesis of osteoarthrosis. Osteoarthr. Cartil. 2004, 12 (Suppl. A), S20–S30. [Google Scholar] [CrossRef]
- Boushell, M.K.; Hung, C.T.; Hunziker, E.B.; Strauss, E.J.; Lu, H.H. Current strategies for integrative cartilage repair. Connect. Tissue Res. 2017, 58, 393–406. [Google Scholar] [CrossRef]
- Mente, P.L.; Lewis, J.L. Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J. Orthop. Res. 1994, 12, 637–647. [Google Scholar] [CrossRef]
- Hargrave-Thomas, E.; van Sloun, F.; Dickinson, M.; Broom, N.; Thambyah, A. Multi-scalar mechanical testing of the calcified cartilage and subchondral bone comparing healthy vs early degenerative states. Osteoarthr. Cartil. 2015, 23, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Doube, M.; Firth, E.C.; Boyde, A. Variations in articular calcified cartilage by site and exercise in the 18-month-old equine distal metacarpal condyle. Osteoarthr. Cartil. 2007, 15, 1283–1292. [Google Scholar] [CrossRef][Green Version]
- Madi, K.; Staines, K.A.; Bay, B.K.; Javaheri, B.; Geng, H.; Bodey, A.J.; Cartmell, S.; Pitsillides, A.A.; Lee, P.D. In situ characterization of nanoscale strains in loaded whole joints via synchrotron X-ray tomography. Nat. Biomed. Eng. 2020, 4, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.J.; McClure, S.F.; Stoddart, R.W.; McClure, J. The normal human chondro-osseous junctional region: Evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet. Disord. 2006, 7, 52. [Google Scholar] [CrossRef]
- Havelka, S.; Horn, V.; Spohrová, D.; Valouch, P. The calcified-noncalcified cartilage interface: The tidemark. Acta Biol. Hung. 1984, 35, 271–279. [Google Scholar] [PubMed]
- Laverty, S.; Lacourt, M.; Gao, C.; Henderson, J.E.; Boyde, A. High density infill in cracks and protrusions from the articular calcified cartilage in osteoarthritis in standardbred horse carpal bones. Int. J. Mol. Sci. 2015, 16, 9600–9611. [Google Scholar] [CrossRef]
- Sun, M.M.; Beier, F. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 74–82. [Google Scholar] [CrossRef]
- van der Kraan, P.M.; van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef]
- Neefjes, M.; van Caam, A.P.M.; van der Kraan, P.M. Transcription Factors in Cartilage Homeostasis and Osteoarthritis. Biology 2020, 9, 290. [Google Scholar] [CrossRef]
- Donell, S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019, 4, 221–229. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, X.; Li, W.; Novotny, J.E.; Doty, S.B.; Wang, L. In situ measurement of transport between subchondral bone and articular cartilage. J. Orthop. Res. 2009, 27, 1347–1352. [Google Scholar] [CrossRef]
- Beckwee, D.; Vaes, P.; Shahabpour, M.; Muyldermans, R.; Rommers, N.; Bautmans, I. The Influence of Joint Loading on Bone Marrow Lesions in the Knee: A Systematic Review with Meta-analysis. Am. J. Sports Med. 2015, 43, 3093–3107. [Google Scholar] [CrossRef]
- Buckland-Wright, J.C.; Lynch, J.A.; Dave, B. Early radiographic features in patients with anterior cruciate ligament rupture. Ann. Rheum. Dis. 2000, 59, 641–646. [Google Scholar] [CrossRef]
- Muratovic, D.; Findlay, D.M.; Cicuttini, F.M.; Wluka, A.E.; Lee, Y.R.; Edwards, S.; Kuliwaba, J.S. Bone marrow lesions in knee osteoarthritis: Regional differences in tibial subchondral bone microstructure and their association with cartilage degeneration. Osteoarthr. Cartil. 2019, 27, 1653–1662. [Google Scholar] [CrossRef]
- Crema, M.D.; Roemer, F.W.; Zhu, Y.; Marra, M.D.; Niu, J.; Zhang, Y.; Lynch, J.A.; Javaid, M.K.; Lewis, C.E.; El-Khoury, G.Y.; et al. Subchondral cystlike lesions develop longitudinally in areas of bone marrow edema-like lesions in patients with or at risk for knee osteoarthritis: Detection with MR imaging—The MOST study. Radiology 2010, 256, 855–862. [Google Scholar] [CrossRef]
- MacKay, J.W.; Murray, P.J.; Kasmai, B.; Johnson, G.; Donell, S.T.; Toms, A.P. Subchondral bone in osteoarthritis: Association between MRI texture analysis and histomorphometry. Osteoarthr. Cartil. 2017, 25, 700–707. [Google Scholar] [CrossRef]
- Chan, T.F.; Couchourel, D.; Abed, E.; Delalandre, A.; Duval, N.; Lajeunesse, D. Elevated Dickkopf-2 levels contribute to the abnormal phenotype of human osteoarthritic osteoblasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 1399–1410. [Google Scholar] [CrossRef]
- Couchourel, D.; Aubry, I.; Delalandre, A.; Lavigne, M.; Martel-Pelletier, J.; Pelletier, J.P.; Lajeunesse, D. Altered mineralization of human osteoarthritic osteoblasts is attributable to abnormal type I collagen production. Arthritis Rheum. 2009, 60, 1438–1450. [Google Scholar] [CrossRef]
- Guermazi, A.; Roemer, F.W.; Haugen, I.K.; Crema, M.D.; Hayashi, D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 236–251. [Google Scholar] [CrossRef]
- Zhang, M.; Min, Z.; Rana, N.; Liu, H. Accuracy of magnetic resonance imaging in grading knee chondral defects. Arthroscopy 2013, 29, 349–356. [Google Scholar] [CrossRef]
- Matzat, S.J.; van Tiel, J.; Gold, G.E.; Oei, E.H. Quantitative MRI techniques of cartilage composition. Quant. Imaging Med. Surg. 2013, 3, 162–174. [Google Scholar] [CrossRef]
- Regatte, R.R.; Akella, S.V.; Borthakur, A.; Kneeland, J.B.; Reddy, R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: Comparison of T2 and T1rho. Acad. Radiol. 2002, 9, 1388–1394. [Google Scholar] [CrossRef]
- Nishioka, H.; Hirose, J.; Nakamura, E.; Oniki, Y.; Takada, K.; Yamashita, Y.; Mizuta, H. T1rho and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J. Magn. Reson. Imaging 2012, 35, 147–155. [Google Scholar] [CrossRef]
- Lukas, V.A.; Fishbein, K.W.; Lin, P.C.; Schär, M.; Schneider, E.; Neu, C.P.; Spencer, R.G.; Reiter, D.A. Classification of histologically scored human knee osteochondral plugs by quantitative analysis of magnetic resonance images at 3T. J. Orthop. Res. 2015, 33, 640–650. [Google Scholar] [CrossRef]
- Watanabe, A.; Wada, Y.; Obata, T.; Ueda, T.; Tamura, M.; Ikehira, H.; Moriya, H. Delayed gadolinium-enhanced MR to determine glycosaminoglycan concentration in reparative cartilage after autologous chondrocyte implantation: Preliminary results. Radiology 2006, 239, 201–208. [Google Scholar] [CrossRef]
- Kurkijärvi, J.E.; Mattila, L.; Ojala, R.O.; Vasara, A.I.; Jurvelin, J.S.; Kiviranta, I.; Nieminen, M.T. Evaluation of cartilage repair in the distal femur after autologous chondrocyte transplantation using T2 relaxation time and dGEMRIC. Osteoarthr. Cartil. 2007, 15, 372–378. [Google Scholar] [CrossRef][Green Version]
- Williams, A.; Qian, Y.; Bear, D.; Chu, C.R. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthr. Cartil. 2010, 18, 539–546. [Google Scholar] [CrossRef]
- Xia, Y. Relaxation anisotropy in cartilage by NMR microscopy (muMRI) at 14-microm resolution. Magn. Reson. Med. 1998, 39, 941–949. [Google Scholar] [CrossRef]
- Garnov, N.; Gründer, W.; Thörmer, G.; Trampel, R.; Turner, R.; Kahn, T.; Busse, H. In vivo MRI analysis of depth-dependent ultrastructure in human knee cartilage at 7 T. NMR Biomed. 2013, 26, 1412–1419. [Google Scholar] [CrossRef]
- Raya, J.G. Techniques and applications of in vivo diffusion imaging of articular cartilage. J. Magn. Reson. Imaging 2015, 41, 1487–1504. [Google Scholar] [CrossRef]
- Raya, J.G.; Melkus, G.; Adam-Neumair, S.; Dietrich, O.; Mützel, E.; Reiser, M.F.; Putz, R.; Kirsch, T.; Jakob, P.M.; Glaser, C. Diffusion-tensor imaging of human articular cartilage specimens with early signs of cartilage damage. Radiology 2013, 266, 831–841. [Google Scholar] [CrossRef]
- Apprich, S.; Trattnig, S.; Welsch, G.H.; Noebauer-Huhmann, I.M.; Sokolowski, M.; Hirschfeld, C.; Stelzeneder, D.; Domayer, S. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 Tesla. Osteoarthr. Cartil. 2012, 20, 703–711. [Google Scholar] [CrossRef][Green Version]
- Ling, W.; Regatte, R.R.; Navon, G.; Jerschow, A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc. Natl. Acad. Sci. USA 2008, 105, 2266–2270. [Google Scholar] [CrossRef]
- Abrar, D.B.; Schleich, C.; Radke, K.L.; Frenken, M.; Stabinska, J.; Ljimani, A.; Wittsack, H.J.; Antoch, G.; Bittersohl, B.; Hesper, T.; et al. Detection of early cartilage degeneration in the tibiotalar joint using 3 T gagCEST imaging: A feasibility study. Magma 2021, 34, 249–260. [Google Scholar] [CrossRef]
- Shapiro, E.M.; Borthakur, A.; Gougoutas, A.; Reddy, R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn. Reson. Med. 2002, 47, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Zbýň, S.; Stelzeneder, D.; Welsch, G.H.; Negrin, L.L.; Juras, V.; Mayerhoefer, M.E.; Szomolanyi, P.; Bogner, W.; Domayer, S.E.; Weber, M.; et al. Evaluation of native hyaline cartilage and repair tissue after two cartilage repair surgery techniques with 23Na MR imaging at 7 T: Initial experience. Osteoarthr. Cartil. 2012, 20, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.T.; Töyräs, J.; Laasanen, M.S.; Silvennoinen, J.; Helminen, H.J.; Jurvelin, J.S. Prediction of biomechanical properties of articular cartilage with quantitative magnetic resonance imaging. J. Biomech. 2004, 37, 321–328. [Google Scholar] [CrossRef]
- Gold, G.E.; Besier, T.F.; Draper, C.E.; Asakawa, D.S.; Delp, S.L.; Beaupre, G.S. Weight-bearing MRI of patellofemoral joint cartilage contact area. J. Magn. Reson. Imaging 2004, 20, 526–530. [Google Scholar] [CrossRef]
- Souza, R.B.; Kumar, D.; Calixto, N.; Singh, J.; Schooler, J.; Subburaj, K.; Li, X.; Link, T.M.; Majumdar, S. Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1367–1376. [Google Scholar] [CrossRef]
- Lange, T.; Maclaren, J.; Herbst, M.; Lovell-Smith, C.; Izadpanah, K.; Zaitsev, M. Knee cartilage MRI with in situ mechanical loading using prospective motion correction. Magn. Reson. Med. 2014, 71, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Knowles, B.R.; Herbst, M.; Izadpanah, K.; Zaitsev, M. Comparative T(2) and T(1ρ) mapping of patellofemoral cartilage under in situ mechanical loading with prospective motion correction. J. Magn. Reson. Imaging 2017, 46, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Watkins, B.; Saini, N.; Gannon, S.; Nadeau, E.; Reeves, R.; Gao, B.; Pelligrini, V.; Yao, H.; et al. Nonlabeling and quantitative assessment of chondrocyte viability in articular cartilage with intrinsic nonlinear optical signatures. Exp. Biol. Med. 2020, 245, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; Akkus, O.; Sun, J.; Cai, L.; Erol, U.L.; Sabri, L.; Neu, C.P. Raman spectroscopy-based water content is a negative predictor of articular human cartilage mechanical function. Osteoarthr. Cartil. 2019, 27, 304–313. [Google Scholar] [CrossRef]
- Bergholt, M.; Serio, A.; Albro, M. Raman Spectroscopy: Guiding Light for the Extracellular Matrix. Front. Bioeng. Biotechnol. 2019, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.C.; Bourdakos, K.N.; Tare, R.S.; Oreffo, R.O.C.; Mahajan, S. Live-imaging of Bioengineered Cartilage Tissue using Multimodal Non-linear Molecular Imaging. Sci. Rep. 2019, 9, 5561. [Google Scholar] [CrossRef]
- Kumar, R.; Grønhaug, K.M.; Romijn, E.I.; Finnøy, A.; Davies, C.L.; Drogset, J.O.; Lilledahl, M.B. Polarization second harmonic generation microscopy provides quantitative enhanced molecular specificity for tissue diagnostics. J. Biophotonics 2015, 8, 730–739. [Google Scholar] [CrossRef]
- Bonifacio, A.; Beleites, C.; Vittur, F.; Marsich, E.; Semeraro, S.; Paoletti, S.; Sergo, V. Chemical imaging of articular cartilage sections with Raman mapping, employing uni- and multi-variate methods for data analysis. Analyst 2010, 135, 3193–3204. [Google Scholar] [CrossRef] [PubMed]
- Gąsior-Głogowska, M.; Komorowska, M.; Hanuza, J.; Ptak, M.; Kobielarz, M. Structural alteration of collagen fibres—Spectroscopic and mechanical studies. Strain 2010, 12, 55–62. [Google Scholar]
- Boyanich, R.; Becker, T.; Chen, F.; Kirk, T.B.; Allison, G.; Wu, J.P. Application of confocal, SHG and atomic force microscopy for characterizing the structure of the most superficial layer of articular cartilage. J. Microsc. 2019, 275, 159–171. [Google Scholar] [CrossRef]
- Camp Jr, C.H.; Cicerone, M.T. Chemically sensitive bioimaging with coherent Raman scattering. Nat. Photonics 2015, 9, 295–305. [Google Scholar] [CrossRef]
- Chen, X.; Nadiarynkh, O.; Plotnikov, S.; Campagnola, P.J. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc. 2012, 7, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Hall, G.; Messerschmidt, B.; Li, M.-J.; Li, X. Nonlinear optical endomicroscopy for label-free functional histology in vivo. Light Sci. Appl. 2017, 6, e17082. [Google Scholar] [CrossRef] [PubMed]
- Baskey, S.J.; Andreana, M.; Lanteigne, E.; Ridsdale, A.; Stolow, A.; Schweitzer, M.E. Pre-Clinical Translation of Second Harmonic Microscopy of Meniscal and Articular Cartilage Using a Prototype Nonlinear Microendoscope. IEEE J. Transl. Eng. Health Med. 2018, 7, 1800211. [Google Scholar] [CrossRef] [PubMed]
- Centonze, V.E.; White, J.G. Multiphoton Excitation Provides Optical Sections from Deeper within Scattering Specimens than Confocal Imaging. Biophys. J. 1998, 75, 2015–2024. [Google Scholar] [CrossRef]
- Campagnola, P. Second harmonic generation imaging microscopy: Applications to diseases diagnostics. Anal. Chem. 2011, 83, 3224–3231. [Google Scholar] [CrossRef]
- Kiyomatsu, H.; Oshima, Y.; Saitou, T.; Miyazaki, T.; Hikita, A.; Miura, H.; Iimura, T.; Imamura, T. Quantitative SHG imaging in osteoarthritis model mice, implying a diagnostic application. Biomed. Opt. Express 2015, 6, 405–420. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A. Assessment of Articular Cartilage by Second Harmonic Microscopy: Challenges and Opportunities. Front. Phys. 2019, 7, 137. [Google Scholar] [CrossRef]
- Novakofski, K.D.; Williams, R.M.; Fortier, L.A.; Mohammed, H.O.; Zipfel, W.R.; Bonassar, L.J. Identification of cartilage injury using quantitative multiphoton microscopy. Osteoarthr. Cartil. 2014, 22, 355–362. [Google Scholar] [CrossRef]
- Mansfield, J.; Yu, J.; Attenburrow, D.; Moger, J.; Tirlapur, U.; Urban, J.; Cui, Z.; Winlove, P. The elastin network: Its relationship with collagen and cells in articular cartilage as visualized by multiphoton microscopy. J. Anat. 2009, 215, 682–691. [Google Scholar] [CrossRef]
- Mansfield, J.C.; Winlove, C.P.; Moger, J.; Matcher, S.J. Collagen fiber arrangement in normal and diseased cartilage studied by polarization sensitive nonlinear microscopy. J. Biomed. Opt. 2008, 13, 044020. [Google Scholar] [CrossRef]
- Mansfield, J.C.; Mandalia, V.; Toms, A.; Winlove, C.P.; Brasselet, S. Collagen reorganization in cartilage under strain probed by polarization sensitive second harmonic generation microscopy. J. R. Soc. Interface 2019, 16, 20180611. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ye, C.; Sun, Q.; Leung, C.K.; Qu, J.Y. Label-free nonlinear optical imaging of mouse retina. Biomed. Opt. Express 2015, 6, 1055–1066. [Google Scholar] [CrossRef]
- He, S.; Xue, W.; Duan, Z.; Sun, Q.; Li, X.; Gan, H.; Huang, J.; Qu, J.Y. Multimodal nonlinear optical microscopy reveals critical role of kinesin-1 in cartilage development. Biomed. Opt. Express 2017, 8, 1771–1782. [Google Scholar] [CrossRef]
- Mahbub, S.B.; Guller, A.; Campbell, J.M.; Anwer, A.G.; Gosnell, M.E.; Vesey, G.; Goldys, E.M. Non-Invasive Monitoring of Functional State of Articular Cartilage Tissue with Label-Free Unsupervised Hyperspectral Imaging. Sci. Rep. 2019, 9, 4398. [Google Scholar] [CrossRef]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 59–85. [Google Scholar]
- Ugryumova, N.; Attenburrow, D.P.; Winlove, C.P.; Matcher, S.J. The collagen structure of equine articular cartilage, characterized using polarization-sensitive optical coherence tomography. J. Phys. D Appl. Phys. 2005, 38, 2612–2619. [Google Scholar] [CrossRef]
- Zhou, X.; Eltit, F.; Yang, X.; Maloufi, S.; Alousaimi, H.; Liu, Q.; Huang, L.; Wang, R.; Tang, S. Detecting human articular cartilage degeneration in its early stage with polarization-sensitive optical coherence tomography. Biomed. Opt. Express 2020, 11, 2745–2760. [Google Scholar] [CrossRef]
- Li, X.; Martin, S.; Pitris, C.; Ghanta, R.; Stamper, D.L.; Harman, M.; Fujimoto, J.G.; Brezinski, M.E. High-resolution optical coherence tomographic imaging of osteoarthritic cartilage during open knee surgery. Arthritis Res. Ther. 2005, 7, R318. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Williams, A.; Tolliver, D.; Kwoh, C.K.; Bruno III, S.; Irrgang, J.J. Clinical optical coherence tomography of early articular cartilage degeneration in patients with degenerative meniscal tears. Arthritis Rheum. 2010, 62, 1412–1420. [Google Scholar] [CrossRef]
- Chu, C.; Izzo, N.; Irrgang, J.; Ferretti, M.; Studer, R. Clinical diagnosis of potentially treatable early articular cartilage degeneration using optical coherence tomography. J. Biomed. Opt. 2007, 12, 051703. [Google Scholar] [CrossRef] [PubMed]
- Michalik, R.; Pauer, T.; Brill, N.; Knobe, M.; Tingart, M.; Jahr, H.; Truhn, D.; Nebelung, S. Quantitative articular cartilage sub-surface defect assessment using optical coherence tomography: An in-vitro study. Ann. Anat.-Anat. Anz. 2019, 221, 125–134. [Google Scholar] [CrossRef]
- O’Malley, M.J.; Chu, C.R. Arthroscopic optical coherence tomography in diagnosis of early arthritis. Minim. Invasive Surg. 2011, 2011, 671308. [Google Scholar] [CrossRef]
- Huynh, R.; Nehmetallah, G.; Raub, C. Noninvasive assessment of articular cartilage surface damage using reflected polarized light microscopy. J. Biomed. Opt. 2017, 22, 065001. [Google Scholar] [CrossRef]
- Huynh, R.N.; Pesante, B.; Nehmetallah, G.; Raub, C.B. Polarized reflectance from articular cartilage depends upon superficial zone collagen network microstructure. Biomed. Opt. Express 2019, 10, 5518–5534. [Google Scholar] [CrossRef]
- Elson, D.; Requejo-Isidro, J.; Munro, I.; Reavell, F.; Siegel, J.; Suhling, K.; Tadrous, P.; Benninger, R.; Lanigan, P.; McGinty, J.; et al. Time-domain fluorescence lifetime imaging applied to biological tissue. Photochem. Photobiol. Sci. 2004, 3, 795–801. [Google Scholar] [CrossRef]
- Manning, H.B.; Nickdel, M.B.; Yamamoto, K.; Lagarto, J.L.; Kelly, D.J.; Talbot, C.B.; Kennedy, G.; Dudhia, J.; Lever, J.; Dunsby, C.; et al. Detection of cartilage matrix degradation by autofluorescence lifetime. Matrix Biol. 2013, 32, 32–38. [Google Scholar] [CrossRef]
- Lagarto, J.L.; Nickdel, M.B.; Kelly, D.J.; Price, A.; Nanchahal, J.; Dunsby, C.; French, P.; Itoh, Y. Autofluorescence Lifetime Reports Cartilage Damage in Osteoarthritis. Sci. Rep. 2020, 10, 2154. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Martinez, J.P.; Lewis, W.; Ortega-Martinez, A.; Franco, W. Intrinsic fluorescence and mechanical testing of articular cartilage in human patients with osteoarthritis. J. Biophotonics 2018, 11, e201600269. [Google Scholar] [CrossRef]
- Camacho, N.P.; West, P.; Torzilli, P.A.; Mendelsohn, R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers 2001, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deveza, L.A.; Loeser, R.F. Is osteoarthritis one disease or a collection of many? Rheumatology 2017, 57, iv34–iv42. [Google Scholar] [CrossRef]
- Deveza, L.A.; Melo, L.; Yamato, T.P.; Mills, K.; Ravi, V.; Hunter, D.J. Knee osteoarthritis phenotypes and their relevance for outcomes: A systematic review. Osteoarthr. Cartil. 2017, 25, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Binvignat, M.; Pedoia, V.; Butte, A.J.; Louati, K.; Klatzmann, D.; Berenbaum, F.; Mariotti-Ferrandiz, E.; Sellam, J. Use of machine learning in osteoarthritis research: A systematic literature review. RMD Open 2022, 8, e001998. [Google Scholar] [CrossRef]
- Ashinsky, B.G.; Coletta, C.E.; Bouhrara, M.; Lukas, V.A.; Boyle, J.M.; Reiter, D.A.; Neu, C.P.; Goldberg, I.G.; Spencer, R.G. Machine learning classification of OARSI-scored human articular cartilage using magnetic resonance imaging. Osteoarthr. Cartil. 2015, 23, 1704–1712. [Google Scholar] [CrossRef]
- Shamir, L.; Ling, S.M.; Scott, W.; Hochberg, M.; Ferrucci, L.; Goldberg, I.G. Early detection of radiographic knee osteoarthritis using computer-aided analysis. Osteoarthr. Cartil. 2009, 17, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Ashinsky, B.G.; Bouhrara, M.; Dam, E.B.; Demehri, S.; Shifat, E.R.M.; Spencer, R.G.; Urish, K.L.; Rohde, G.K. Enabling early detection of osteoarthritis from presymptomatic cartilage texture maps via transport-based learning. Proc. Natl. Acad. Sci. USA 2020, 117, 24709–24719. [Google Scholar] [CrossRef] [PubMed]
- Urish, K.L.; Keffalas, M.G.; Durkin, J.R.; Miller, D.J.; Chu, C.R.; Mosher, T.J. T2 texture index of cartilage can predict early symptomatic OA progression: Data from the osteoarthritis initiative. Osteoarthr. Cartil. 2013, 21, 1550–1557. [Google Scholar] [CrossRef]
- Ashinsky, B.G.; Bouhrara, M.; Coletta, C.E.; Lehallier, B.; Urish, K.L.; Lin, P.C.; Goldberg, I.G.; Spencer, R.G. Predicting early symptomatic osteoarthritis in the human knee using machine learning classification of magnetic resonance images from the osteoarthritis initiative. J. Orthop. Res. 2017, 35, 2243–2250. [Google Scholar] [CrossRef]
- Jamshidi, A.; Leclercq, M.; Labbe, A.; Pelletier, J.P.; Abram, F.; Droit, A.; Martel-Pelletier, J. Identification of the most important features of knee osteoarthritis structural progressors using machine learning methods. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720x20933468. [Google Scholar] [CrossRef] [PubMed]
- Stolz, M.; Gottardi, R.; Raiteri, R.; Miot, S.; Martin, I.; Imer, R.; Staufer, U.; Raducanu, A.; Düggelin, M.; Baschong, W.; et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat. Nanotechnol. 2009, 4, 186–192. [Google Scholar] [CrossRef]
- Doyran, B.; Tong, W.; Li, Q.; Jia, H.; Zhang, X.; Chen, C.; Enomoto-Iwamoto, M.; Lu, X.L.; Qin, L.; Han, L. Nanoindentation modulus of murine cartilage: A sensitive indicator of the initiation and progression of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2017, 25, 108–117. [Google Scholar] [CrossRef]
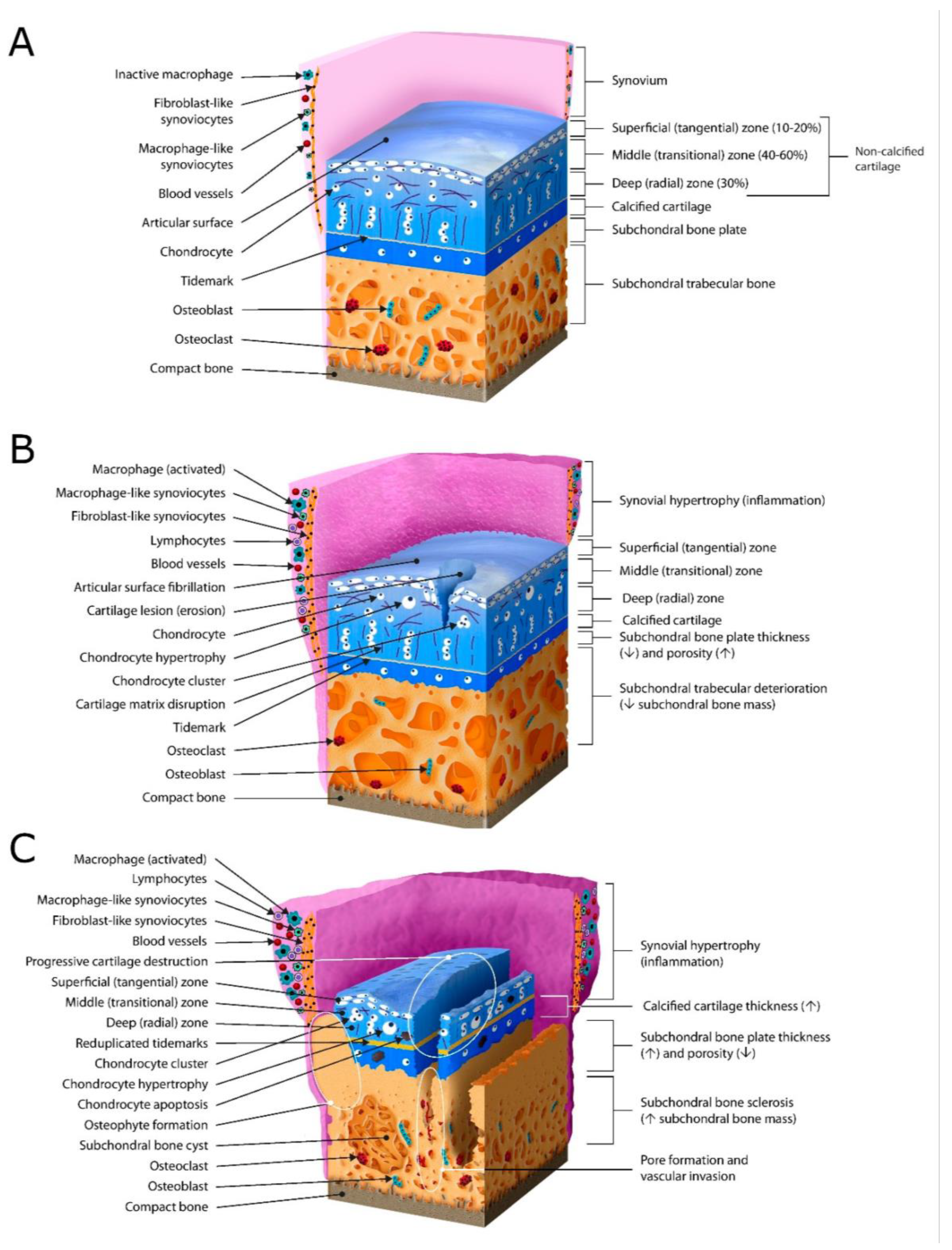


| Targets | Details | Text Sections |
|---|---|---|
| Extracellular matrix (ECM) | Collagens (types, zonal organization, disruption) Ground substance (proteoglycans and GAGs, content and zonal distribution, water content) | Text Section 2.2 |
| Non-mineralized zones of articular cartilage | Superficial (surface integrity, fibrillation, fissures, cracks) Transitional/middle Radial/deep | Text Section 2.3 and Section 2.4 |
| Cartilage thickness | Variation (joint-dependent, age, sex) Reduction (lesions, tissue loss) | Text Section 2.5 |
| Articular chondrocytes | Morphology (volume, shape) Superficial chondrocyte spatial organization (SCSO) (strings, double strings, clusters, diffuse) Zonal differences Vitality (types of cell death: necrosis, necroptosis, apoptosis, hypo- or hypercellularity) Hypertrophy | Text Section 2.6, Section 2.7 and Section 2.9 |
| Pericellular matrix (PCM), Chondron | Morphology (shape, integrity, composition) | Text Section 2.8 |
| Hypertrophic zone, tidemark, cement line | Doubling, integrity (high-density mineral infills (HDMI); cracks and high-density mineralized protrusions (HDMP) | Text Section 2.9 |
| Subchondral bone (SB) | Bone plate (microfractures, edema, vessel ingrowth) Trabecula | Text Section 2.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurz, B.; Lange, T.; Voelker, M.; Hart, M.L.; Rolauffs, B. Articular Cartilage—From Basic Science Structural Imaging to Non-Invasive Clinical Quantitative Molecular Functional Information for AI Classification and Prediction. Int. J. Mol. Sci. 2023, 24, 14974. https://doi.org/10.3390/ijms241914974
Kurz B, Lange T, Voelker M, Hart ML, Rolauffs B. Articular Cartilage—From Basic Science Structural Imaging to Non-Invasive Clinical Quantitative Molecular Functional Information for AI Classification and Prediction. International Journal of Molecular Sciences. 2023; 24(19):14974. https://doi.org/10.3390/ijms241914974
Chicago/Turabian StyleKurz, Bodo, Thomas Lange, Marita Voelker, Melanie L. Hart, and Bernd Rolauffs. 2023. "Articular Cartilage—From Basic Science Structural Imaging to Non-Invasive Clinical Quantitative Molecular Functional Information for AI Classification and Prediction" International Journal of Molecular Sciences 24, no. 19: 14974. https://doi.org/10.3390/ijms241914974
APA StyleKurz, B., Lange, T., Voelker, M., Hart, M. L., & Rolauffs, B. (2023). Articular Cartilage—From Basic Science Structural Imaging to Non-Invasive Clinical Quantitative Molecular Functional Information for AI Classification and Prediction. International Journal of Molecular Sciences, 24(19), 14974. https://doi.org/10.3390/ijms241914974








