Molecular Biology of Meniscal Healing: A Narrative Review
Abstract
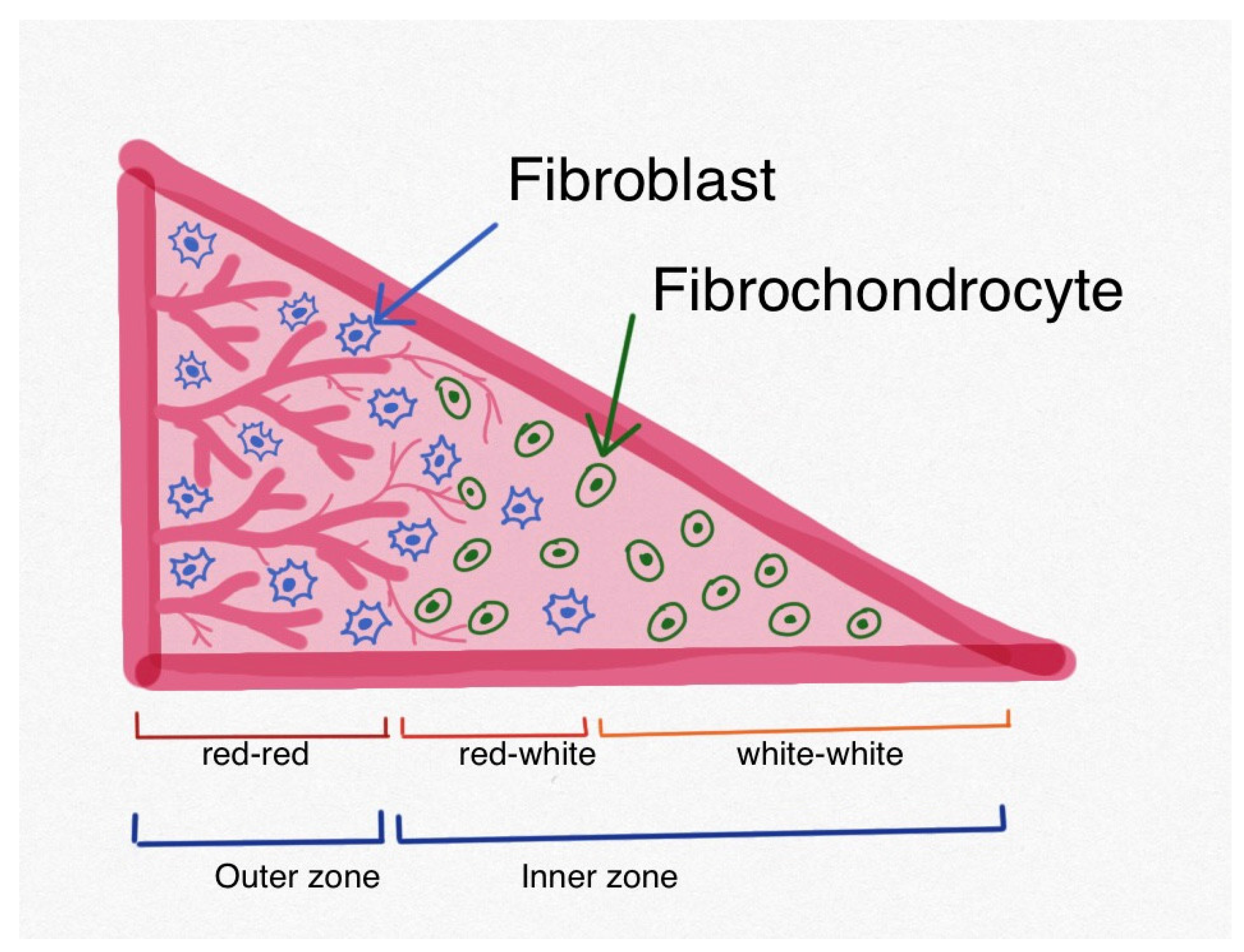
1. Introduction
2. Methods
3. Biomarkers
3.1. Catabolic Growth Factors (GF)
3.2. Anabolic Growth Factors
4. miRNA
5. Hyaluronic Acid
6. Avascular Meniscal Healing
7. Mechanical Loading and Hypoxia
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paxton, E.S.; Stock, M.V.; Brophy, R.H. Meniscal Repair Versus Partial Meniscectomy: A Systematic Review Comparing Reoperation Rates and Clinical Outcomes. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Hulet, C.; Pereira, H.; Peretti, G.; Denti, M. (Eds.) Surgery of the Meniscus, 1st ed.; ESSKA: Luxembourg, 2016; ISBN 978-3-662-49186-7. [Google Scholar]
- Tarafder, S.; Park, G.; Lee, C.H. Explant Models for Meniscus Metabolism, Injury, Repair, and Healing. Connect. Tissue Res. 2020, 61, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A.; Monibi, F.; Dehghani, B.; Maher, S. Biological and Mechanical Predictors of Meniscus Function: Basic Science to Clinical Translation. J. Orthop. Res. 2020, 38, 937–945. [Google Scholar] [CrossRef]
- Rangger, C.; Kathrein, A.; Klestil, T.; Glötzer, W. Partial Meniscectomy and Osteoarthritis: Implications for Treatment of Athletes. Sports Med. 1997, 23, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Atik, O.Ş.; Erdoğan, D.; Seymen, C.M.; Bozkurt, H.H.; Kaplanoğlu, G.T. Is There Crosstalk between Subchondral Bone, Cartilage, and Meniscus in the Pathogenesis of Osteoarthritis? Jt. Dis. Relat. Surg. 2016, 27, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Markes, A.R.; Hodax, J.D.; Ma, C.B. Meniscus Form and Function. Clin. Sports Med. 2020, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Seil, R.; Krych, A.J.; Koga, H. Surgical Treatment of Complex Meniscus Tear and Disease: State of the Art. J. ISAKOS 2021, 6, 35–45. [Google Scholar] [CrossRef]
- Hasan, J.; Fisher, J.; Ingham, E. Current Strategies in Meniscal Regeneration. J. Biomed. Mater. Res. 2014, 102, 619–634. [Google Scholar] [CrossRef]
- Raj, M.A.; Bubnis, M.A. Knee Meniscal Tears. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- De Albornoz, P.M.; Forriol, F. The Meniscal Healing Process. Muscles Ligaments Tendons J. 2012, 2, 10–18. [Google Scholar]
- Wenger, A.; Wirth, W.; Hudelmaier, M.; Noebauer-Huhmann, I.; Trattnig, S.; Bloecker, K.; Frobell, R.B.; Kwoh, C.K.; Eckstein, F.; Englund, M. Meniscus Body Position, Size, and Shape in Persons With and Persons Without Radiographic Knee Osteoarthritis: Quantitative Analyses of Knee Magnetic Resonance Images from the Osteoarthritis Initiative. Arthritis Rheum. 2013, 65, 1804–1811. [Google Scholar] [CrossRef]
- Hwang, S.H.; Jung, K.A.; Lee, W.J.; Yang, K.H.; Lee, D.W.; Carter, A.; Park, C.H.; Hunter, D.J. Morphological Changes of the Lateral Meniscus in End-Stage Lateral Compartment Osteoarthritis of the Knee. Osteoarthr. Cartil. 2012, 20, 110–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Battistelli, M.; Favero, M.; Burini, D.; Trisolino, G.; Dallari, D.; De Franceschi, L.; Goldring, S.R.; Goldring, M.B.; Belluzzi, E.; Filardo, G.; et al. Morphological and Ultrastructural Analysis of Normal, Injured and Osteoarthritic Human Knee Menisci. Eur. J. Histochem. 2019, 63, 2998. [Google Scholar] [CrossRef] [PubMed]
- Englund, M.; Roos, E.M.; Lohmander, L.S. Impact of Type of Meniscal Tear on Radiographic and Symptomatic Knee Osteoarthritis: A Sixteen-year Followup of Meniscectomy with Matched Controls. Arthritis Rheum. 2003, 48, 2178–2187. [Google Scholar] [CrossRef]
- Piontek, T.; Ciemniewska-Gorzela, K.; Naczk, J.; Jakob, R.; Szulc, A.; Grygorowicz, M.; Slomczykowski, M. Complex Meniscus Tears Treated with Collagen Matrix Wrapping and Bone Marrow Blood Injection: A 2-Year Clinical Follow-Up. Cartilage 2016, 7, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.; Maksymowicz-Wleklik, M.; Kulinski, K.; Kozar-Kaminska, K.; Dabrowska-Thing, A.; Pomianowski, S. Short-Term Outcomes of Percutaneous Trephination with a Platelet Rich Plasma Intrameniscal Injection for the Repair of Degenerative Meniscal Lesions. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study. Int. J. Mol. Sci. 2019, 20, 856. [Google Scholar] [CrossRef]
- Cook, J.L.; Kuroki, K.; Stoker, A.M.; Monibi, F.A.; Roller, B.L. Meniscal Biology in Health and Disease. Connect. Tissue Res. 2017, 58, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Campi, S.; Romeo, G.; Spiezia, F.; Maffulli, N.; Denaro, V. Biological Strategies to Enhance Healing of the Avascular Area of the Meniscus. Stem Cells Int. 2012, 2012, 528359. [Google Scholar] [CrossRef]
- Lemmon, E.A.; Bonnevie, E.D.; Patel, J.M.; Miller, L.M.; Mauck, R.L. Transient Inhibition of Meniscus Cell Migration Following Acute Inflammatory Challenge. J. Orthop. Res. 2023, 41, 2055–2064. [Google Scholar] [CrossRef]
- Cuellar, J.M.; Scuderi, G.J.; Cuellar, V.G.; Golish, S.R.; Yeomans, D.C. Diagnostic Utility of Cytokine Biomarkers in the Evaluation of Acute Knee Pain. J. Bone Jt. Surg.-Am. Vol. 2009, 91, 2313–2320. [Google Scholar] [CrossRef][Green Version]
- Fujii, M.; Furumatsu, T.; Yokoyama, Y.; Kanazawa, T.; Kajiki, Y.; Abe, N.; Ozaki, T. Chondromodulin-I Derived from the Inner Meniscus Prevents Endothelial Cell Proliferation. J. Orthop. Res. 2013, 31, 538–543. [Google Scholar] [CrossRef]
- Fox, D.B.; Warnock, J.J.; Stoker, A.M.; Luther, J.K.; Cockrell, M. Effects of Growth Factors on Equine Synovial Fibroblasts Seeded on Synthetic Scaffolds for Avascular Meniscal Tissue Engineering. Res. Vet. Sci. 2010, 88, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Narita, A.; Takahara, M.; Ogino, T.; Fukushima, S.; Kimura, Y.; Tabata, Y. Effect of Gelatin Hydrogel Incorporating Fibroblast Growth Factor 2 on Human Meniscal Cells in an Organ Culture Model. Knee 2009, 16, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Izal, I.; Ripalda, P.; Acosta, C.A.; Forriol, F. In Vitro Healing of Avascular Meniscal Injuries with Fresh and Frozen Plugs Treated with TGF-Beta1 and IGF-1 in Sheep. Int. J. Clin. Exp. Pathol. 2008, 1, 426–434. [Google Scholar] [PubMed]
- Huey, D.J.; Athanasiou, K.A. Maturational Growth of Self-Assembled, Functional Menisci as a Result of TGF-Β1 and Enzymatic Chondroitinase-ABC Stimulation. Biomaterials 2011, 32, 2052–2058. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Ishiguro, T.; Okumura, M.; Iwanaga, T.; Kadosawa, T.; Fujinaga, T. Chondrogenic Differentiation of Bovine Bone Marrow Mesenchymal Stem Cells in Pellet Cultural System. Exp. Hematol. 2004, 32, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.; Ghosh, P. Effects of Transforming Growth Factor Beta on Proteoglycan Synthesis by Cell and Explant Cultures Derived from the Knee Joint Meniscus. Osteoarthr. Cartil. 1995, 3, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Tumia, N.S.; Johnstone, A.J. Platelet Derived Growth Factor-AB Enhances Knee Meniscal Cell Activity in Vitro. Knee 2009, 16, 73–76. [Google Scholar] [CrossRef]
- Lee, K.I.; Olmer, M.; Baek, J.; D’Lima, D.D.; Lotz, M.K. Platelet-Derived Growth Factor-Coated Decellularized Meniscus Scaffold for Integrative Healing of Meniscus Tears. Acta Biomater. 2018, 76, 126–134. [Google Scholar] [CrossRef]
- Chen, C.; Song, J.; Qiu, J.; Zhao, J. Repair of a Meniscal Defect in a Rabbit Model Through Use of a Thermosensitive, Injectable, In Situ Crosslinked Hydrogel With Encapsulated Bone Mesenchymal Stromal Cells and Transforming Growth Factor Β1. Am. J. Sports Med. 2020, 48, 884–894. [Google Scholar] [CrossRef]
- Cui, P.; Sun, B.; Dai, Y.; Cui, T.; Sun, J.; Shen, K.; Zhang, L.; Shi, C.; Wang, X. Healing of the Torn Anterior Horn of Rabbit Medial Meniscus to Bone after Transtibial Pull-Out Repair and Autologous Platelet-Rich Plasma Gel Injection. Orthop. Surg. 2023, 15, 617–627. [Google Scholar] [CrossRef]
- Lu, Z.; Furumatsu, T.; Fujii, M.; Maehara, A.; Ozaki, T. The Distribution of Vascular Endothelial Growth Factor in Human Meniscus and a Meniscal Injury Model. J. Orthop. Sci. 2017, 22, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Hashimoto, Y.; Orita, K.; Nishino, K.; Kinoshita, T.; Nakamura, H. Intra-Articular Injection of Stromal Cell-Derived Factor 1α Promotes Meniscal Healing via Macrophage and Mesenchymal Stem Cell Accumulation in a Rat Meniscal Defect Model. Int. J. Mol. Sci. 2020, 21, 5454. [Google Scholar] [CrossRef] [PubMed]
- Mull, C.; Wohlmuth, P.; Krause, M.; Alm, L.; Kling, H.; Schilling, A.F.; Frosch, K.-H. Hepatocyte Growth Factor and Matrix Metalloprotease 2 Levels in Synovial Fluid of the Knee Joint Are Correlated with Clinical Outcome of Meniscal Repair. Knee 2020, 27, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Goode, A.P.; Carter, T.E.; Utturkar, G.M.; Huebner, J.L.; Taylor, D.C.; Moorman, C.T.; Garrett, W.E.; Kraus, V.B.; Guilak, F.; et al. Matrix Metalloproteinase Activity and Prostaglandin E2 Are Elevated in the Synovial Fluid of Meniscus Tear Patients. Connect. Tissue Res. 2017, 58, 305–316. [Google Scholar] [CrossRef]
- Tarafder, S.; Ghataure, J.; Langford, D.; Brooke, R.; Kim, R.; Eyen, S.L.; Bensadoun, J.; Felix, J.T.; Cook, J.L.; Lee, C.H. Advanced Bioactive Glue Tethering Lubricin/PRG4 to Promote Integrated Healing of Avascular Meniscus Tears. Bioact. Mater. 2023, 28, 61–73. [Google Scholar] [CrossRef]
- Goshima, A.; Etani, Y.; Hirao, M.; Yamakawa, S.; Okamura, G.; Miyama, A.; Takami, K.; Miura, T.; Fukuda, Y.; Kurihara, T.; et al. Basic Fibroblast Growth Factor Promotes Meniscus Regeneration through the Cultivation of Synovial Mesenchymal Stem Cells via the CXCL6–CXCR2 Pathway. Osteoarthr. Cartil. 2023, 31, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Genemaras, A.A.; Ennis, H.; Kaplan, L.; Huang, C.-Y. Inflammatory Cytokines Induce Specific Time- and Concentration-Dependent MicroRNA Release by Chondrocytes, Synoviocytes, and Meniscus Cells. J. Orthop. Res. 2016, 34, 779–790. [Google Scholar] [CrossRef]
- Endisha, H.; Datta, P.; Sharma, A.; Nakamura, S.; Rossomacha, E.; Younan, C.; Ali, S.A.; Tavallaee, G.; Lively, S.; Potla, P.; et al. MicroRNA-34a-5p Promotes Joint Destruction During Osteoarthritis. Arthritis Rheumatol. 2021, 73, 426–439. [Google Scholar] [CrossRef]
- Long, Y.; Xie, J.; Zhang, Z.-Q.; Zhang, Z.; Meng, F.; He, A. Substantive Molecular and Histological Changes within the Meniscus with Tears. BMC Musculoskelet. Disord. 2019, 20, 577. [Google Scholar] [CrossRef]
- Kawanishi, Y.; Nakasa, T.; Shoji, T.; Hamanishi, M.; Shimizu, R.; Kamei, N.; Usman, M.A.; Ochi, M. Intra-Articular Injection of Synthetic microRNA-210 Accelerates Avascular Meniscal Healing in Rat Medial Meniscal Injured Model. Arthritis Res. Ther. 2014, 16, 488. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, X.; Ren, S.; Meng, C.; Yang, Z. Construction and Analysis of a lncRNA–miRNA–mRNA Competing Endogenous RNA Network from Inflamed and Normal Synovial Tissues after Anterior Cruciate Ligament and/or Meniscus Injuries. Front. Genet. 2022, 13, 983020. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The Mechanism of Action for Hyaluronic Acid Treatment in the Osteoarthritic Knee: A Systematic Review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Koga, H.; Sekiya, I. Degenerative Meniscus in Knee Osteoarthritis: From Pathology to Treatment. Life 2022, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Rabbani, S.A.; Wadhwa, T. Role and Effectiveness of Intra-Articular Injection of Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Systematic Review. Cureus 2022, 14, e24503. [Google Scholar] [CrossRef] [PubMed]
- Maheu, E.; Bannuru, R.R.; Herrero-Beaumont, G.; Allali, F.; Bard, H.; Migliore, A. Why We Should Definitely Include Intra-Articular Hyaluronic Acid as a Therapeutic Option in the Management of Knee Osteoarthritis: Results of an Extensive Critical Literature Review. Semin. Arthritis Rheum. 2019, 48, 563–572. [Google Scholar] [CrossRef]
- Beaufils, P.; Becker, R.; Kopf, S.; Englund, M.; Verdonk, R.; Ollivier, M.; Seil, R. Surgical Management of Degenerative Meniscus Lesions: The 2016 ESSKA Meniscus Consensus. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 335–346. [Google Scholar] [CrossRef]
- Sonoda, M.; Harwood, F.L.; Amiel, M.E.; Moriya, H.; Temple, M.; Chang, D.G.; Lottman, L.M.; Sah, R.L.; Amiel, D. The Effects of Hyaluronan on Tissue Healing after Meniscus Injury and Repair in a Rabbit Model. Am. J. Sports Med. 2000, 28, 90–97. [Google Scholar] [CrossRef]
- Murakami, T.; Otsuki, S.; Okamoto, Y.; Nakagawa, K.; Wakama, H.; Okuno, N.; Neo, M. Hyaluronic Acid Promotes Proliferation and Migration of Human Meniscus Cells via a CD44-Dependent Mechanism. Connect. Tissue Res. 2019, 60, 117–127. [Google Scholar] [CrossRef]
- Tanaka, T.; Furumatsu, T.; Miyazawa, S.; Fujii, M.; Inoue, H.; Kodama, Y.; Ozaki, T. Hyaluronan Stimulates Chondrogenic Gene Expression in Human Meniscus Cells. Connect. Tissue Res. 2017, 58, 520–530. [Google Scholar] [CrossRef]
- Berton, A.; Longo, U.G.; Candela, V.; Greco, F.; Martina, F.M.; Quattrocchi, C.C.; Denaro, V. Quantitative Evaluation of Meniscal Healing Process of Degenerative Meniscus Lesions Treated with Hyaluronic Acid: A Clinical and MRI Study. J. Clin. Med. 2020, 9, 2280. [Google Scholar] [CrossRef] [PubMed]
- Abpeikar, Z.; Javdani, M.; Alizadeh, A.; Khosravian, P.; Tayebi, L.; Asadpour, S. Development of Meniscus Cartilage Using Polycaprolactone and Decellularized Meniscus Surface Modified by Gelatin, Hyaluronic Acid Biomacromolecules: A Rabbit Model. Int. J. Biol. Macromol. 2022, 213, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Beaufils, P.; Hirschmann, M.T.; Rotigliano, N.; Ollivier, M.; Pereira, H.; Verdonk, R.; Darabos, N.; Ntagiopoulos, P.; Dejour, D.; et al. Management of Traumatic Meniscus Tears: The 2019 ESSKA Meniscus Consensus. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tong, Z.-M.; Dai, Z.; Chen, Z.-W. Regeneration of Meniscal Avascular Zone Using Autogenous Meniscal Fragments in a Rabbit Model. BMC Surg. 2022, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Hammer, S.; Fuellerer, J.; Lang, S.; Pfeifer, C.; Pattappa, G.; Weber, J.; Loibl, M.; Nerlich, M.; Angele, P.; et al. Bone Marrow Aspirate Concentrate for the Treatment of Avascular Meniscus Tears in a One-Step Procedure—Evaluation of an In Vivo Model. Int. J. Mol. Sci. 2019, 20, 1120. [Google Scholar] [CrossRef]
- Tarafder, S.; Gulko, J.; Kim, D.; Sim, K.H.; Gutman, S.; Yang, J.; Cook, J.L.; Lee, C.H. Effect of Dose and Release Rate of CTGF and TGFβ3 on Avascular Meniscus Healing. J. Orthop. Res. 2019, 37, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Sovani, S.; Glembotski, N.E.; Du, J.; Jin, S.; Grogan, S.P.; D’Lima, D.D. Repair of Avascular Meniscus Tears with Electrospun Collagen Scaffolds Seeded with Human Cells. Tissue Eng. Part A 2016, 22, 436–448. [Google Scholar] [CrossRef]
- Kawamura, S.; Lotito, K.; Rodeo, S.A. Biomechanics and Healing Response of the Meniscus. Oper. Tech. Sports Med. 2003, 11, 68–76. [Google Scholar] [CrossRef]
- Logerstedt, D.S.; Ebert, J.R.; MacLeod, T.D.; Heiderscheit, B.C.; Gabbett, T.J.; Eckenrode, B.J. Effects of and Response to Mechanical Loading on the Knee. Sports Med. 2022, 52, 201–235. [Google Scholar] [CrossRef]
- McNulty, A.L.; Estes, B.T.; Wilusz, R.E.; Weinberg, J.B.; Guilak, F. Dynamic Loading Enhances Integrative Meniscal Repair in the Presence of Interleukin-1. Osteoarthr. Cartil. 2010, 18, 830–838. [Google Scholar] [CrossRef]
- Irwin, R.M.; Puranam, I.; Hoffman, B.D.; McNulty, A.L. Differential Response of Inner and Outer Zone Meniscal Cells to Tensile Load under Non-Inflammatory and Inflammatory Conditions. Osteoarthr. Cartil. 2021, 29, S3–S4. [Google Scholar] [CrossRef]
- Szojka, A.R.A.; Li, D.X.; Sopcak, M.E.J.; Ma, Z.; Kunze, M.; Mulet-Sierra, A.; Adeeb, S.M.; Westover, L.; Jomha, N.M.; Adesida, A.B. Mechano-Hypoxia Conditioning of Engineered Human Meniscus. Front. Bioeng. Biotechnol. 2021, 9, 739438. [Google Scholar] [CrossRef] [PubMed]
- Herrera Millar, V.R.; Mangiavini, L.; Polito, U.; Canciani, B.; Nguyen, V.T.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Modina, S.C.; Di Giancamillo, A. Hypoxia as a Stimulus for the Maturation of Meniscal Cells: Highway to Novel Tissue Engineering Strategies? Int. J. Mol. Sci. 2021, 22, 6905. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, E.; Trisolino, G.; Belluzzi, E.; Lazzaro, A.; Strazzari, A.; Pozzuoli, A.; Cigolotti, A.; Ruggieri, P.; Evangelista, A.; Ometto, F.; et al. Macroscopic Synovial Inflammation Correlates with Symptoms and Cartilage Lesions in Patients Undergoing Arthroscopic Partial Meniscectomy: A Clinical Study. J. Clin. Med. 2022, 11, 4330. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, E.; Belluzzi, E.; Pozzuoli, A.; Cigolotti, A.; Scioni, M.; Goldring, S.R.; Goldring, M.B.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; et al. Do Synovial Inflammation and Meniscal Degeneration Impact Clinical Outcomes of Patients Undergoing Arthroscopic Partial Meniscectomy? A Histological Study. Int. J. Mol. Sci. 2022, 23, 3903. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, G.; Jiang, H.; Liu, A.; Wu, R.; Li, J.; Sun, Z.; Lv, Z.; Sun, W.; Shi, D. Molecular Crosstalk between Articular Cartilage, Meniscus, Synovium, and Subchondral Bone in Osteoarthritis. Bone Jt. Res. 2022, 11, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A Short Overview of Certain Bioactive Components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Li, Z.; Weng, X. Platelet-Rich Plasma Use in Meniscus Repair Treatment: A Systematic Review and Meta-Analysis of Clinical Studies. J. Orthop. Surg. Res. 2022, 17, 446. [Google Scholar] [CrossRef]
- Mariani, C.; Meneghetti, E.; Zambon, D.; Elena, N.; Agueci, A.; Melchior, C. Use of Bone Marrow Derived Mesenchymal Stem Cells for the Treatment of Osteoarthritis: A Retrospective Long-Term Follow-up Study. J. Clin. Orthop. Trauma 2023, 36, 102084. [Google Scholar] [CrossRef]
- Massey, P.A.; Zhang, A.; Stairs, C.B.; Hoge, S.; Carroll, T.; Hamby, A.M. Meniscus Repair Outcomes with and without Bone Marrow Aspiration Concentrate. Orthop. J. Sports Med. 2019, 7, 2325967119S0028. [Google Scholar] [CrossRef]
- Fokter, S.K.; Kuhta, M.; Hojnik, M.; Ledinek, Ž.; Kostanjšek, R. Tissue Integration of Calcium Phosphate Compound after Subchondroplasty: 4-Year Follow-Up in a 76-Year-Old Female Patient. Bioengineering 2023, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, G.A.; Grassi, A.; Agostinone, P.; Di Paolo, S.; Dal Fabbro, G.; D’Alberton, C.; Pizza, N.; Zaffagnini, S. Risk Factors Affecting the Survival Rate of Collagen Meniscal Implant for Partial Meniscal Deficiency: An Analysis of 156 Consecutive Cases at a Mean 10 Years of Follow-Up. Am. J. Sports Med. 2022, 50, 2900–2908. [Google Scholar] [CrossRef]
- Kohli, S.; Schwenck, J.; Barlow, I. Failure Rates and Clinical Outcomes of Synthetic Meniscal Implants Following Partial Meniscectomy: A Systematic Review. Knee Surg. Relat. Res. 2022, 34, 27. [Google Scholar] [CrossRef] [PubMed]
- Si, H.-B.; Zeng, Y.; Liu, S.-Y.; Zhou, Z.-K.; Chen, Y.-N.; Cheng, J.-Q.; Lu, Y.-R.; Shen, B. Intra-Articular Injection of microRNA-140 (miRNA-140) Alleviates Osteoarthritis (OA) Progression by Modulating Extracellular Matrix (ECM) Homeostasis in Rats. Osteoarthr. Cartil. 2017, 25, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zhou, Y.; Zeng, B.; Yang, X.; Su, M. MicroRNA-183 Attenuates Osteoarthritic Pain by Inhibiting the TGFα -Mediated CCL2/CCR2 Signalling Axis. Bone Jt. Res. 2021, 10, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Costales, M.G.; Haga, C.L.; Velagapudi, S.P.; Childs-Disney, J.L.; Phinney, D.G.; Disney, M.D. Small Molecule Inhibition of microRNA-210 Reprograms an Oncogenic Hypoxic Circuit. J. Am. Chem. Soc. 2017, 139, 3446–3455. [Google Scholar] [CrossRef]
- Gao, X.; Gao, Y.; Du, D.; Du, H.; Wang, S.; Zhang, H. Long Non-Coding RNA HCG11 Silencing Protects against Cerebral Ischemia/Reperfusion Injury through microRNA miR-381-3p to Regulate Tumour Protein P53. Folia Neuropathol. 2022, 60, 436–448. [Google Scholar] [CrossRef]
- Rno-Mir-455-3p-Inhibitor. miRNA Inhibitor, MedChemExpress. Available online: https://www.medchemexpress.com/rno-mir-455-3p-inhibitor.html (accessed on 1 October 2023).
- Kaminski, R.; Kulinski, K.; Kozar-Kaminska, K.; Wielgus, M.; Langner, M.; Wasko, M.K.; Kowalczewski, J.; Pomianowski, S. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study Evaluating Meniscal Healing, Clinical Outcomes, and Safety in Patients Undergoing Meniscal Repair of Unstable, Complete Vertical Meniscal Tears (Bucket Handle) Augmented with Platelet-Rich Plasma. BioMed Res. Int. 2018, 2018, 9315815. [Google Scholar] [CrossRef]
- Kaminski, R.; Kulinski, K.; Kozar-Kaminska, K.; Wasko, M.K.; Langner, M.; Pomianowski, S. Repair Augmentation of Unstable, Complete Vertical Meniscal Tears With Bone Marrow Venting Procedure: A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 1500–1508.e1. [Google Scholar] [CrossRef]

| Author | Investigation | |
|---|---|---|
| Catabolic Growth Factor | Cuellar [21] | INF- γ, IL-6, MCP1, MIP-1 β |
| Fujii [22] | Endostatin, ChM-1 | |
| Anabolic Growth Factor | Lee [30] | PDGF |
| Chen [31] | TGF- β 1 | |
| Cui [32] | PDGF, TGF- β 1 | |
| Lu [33] | VEGF, HIF-1 α | |
| Nishida [34] | SDF1 | |
| Mull [35] | HGF, MMP-2 | |
| Liu [36] | PGE2, MMP-10 | |
| Tarafader [37] | PRG4 | |
| Goshima [38] | FGF | |
| miRNA | Long [41] | miR-381-3p, miR-455-3p, miR-193b-3p, miR-92a-3p |
| Kawanishi [42] | miRNA-210 | |
| Xiao [43] | network of lncRNA-miRNA-mRNA | |
| Genemaras [39] | miR-146a, miR-27b, miR-16, miR-40 | |
| Hyaluronic Acid | Sonoda [50] | HA |
| Murakami [51] | HA | |
| Tanaka [52] | HA | |
| Berton [53] | HA | |
| Abpeikar [54] | HA | |
| Avascular healing | Deng [56] | Meniscus small pieces |
| Koch [57] | BMAC | |
| Tarafader [58] | CTGF, TGF- β 3 | |
| Baek [59] | Collagen scaffold | |
| Environmental stimuli | McNulty [62] | Mechanical compression |
| Irwin [63] | Dynamic loading, IL-1 | |
| Szojka [64] | Mechanical loading, hypoxia | |
| Millar [65] | Hypoxia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramś, E.; Kamiński, R. Molecular Biology of Meniscal Healing: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 768. https://doi.org/10.3390/ijms25020768
Tramś E, Kamiński R. Molecular Biology of Meniscal Healing: A Narrative Review. International Journal of Molecular Sciences. 2024; 25(2):768. https://doi.org/10.3390/ijms25020768
Chicago/Turabian StyleTramś, Ewa, and Rafał Kamiński. 2024. "Molecular Biology of Meniscal Healing: A Narrative Review" International Journal of Molecular Sciences 25, no. 2: 768. https://doi.org/10.3390/ijms25020768
APA StyleTramś, E., & Kamiński, R. (2024). Molecular Biology of Meniscal Healing: A Narrative Review. International Journal of Molecular Sciences, 25(2), 768. https://doi.org/10.3390/ijms25020768






