Comparative Analysis of Volatile Compounds from Four Radish Microgreen Cultivars Based on Ultrasonic Cell Disruption and HS-SPME/GC–MS
Abstract
:1. Introduction
2. Results and Discussion
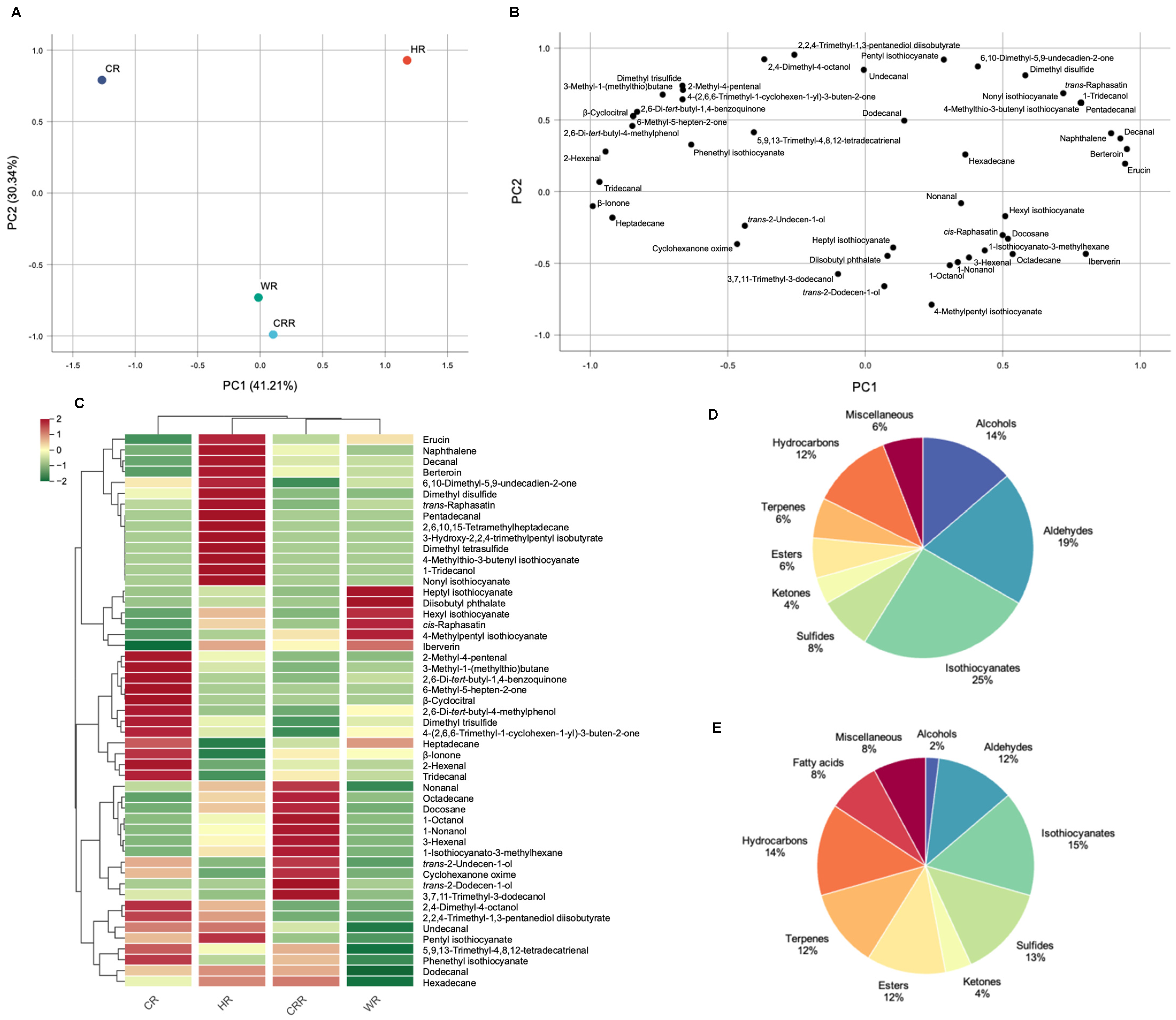
2.1. Detection of Volatile Compounds among Four Radish Microgreen Cultivars
2.2. Analysis of Volatile Compounds and Aroma Profile
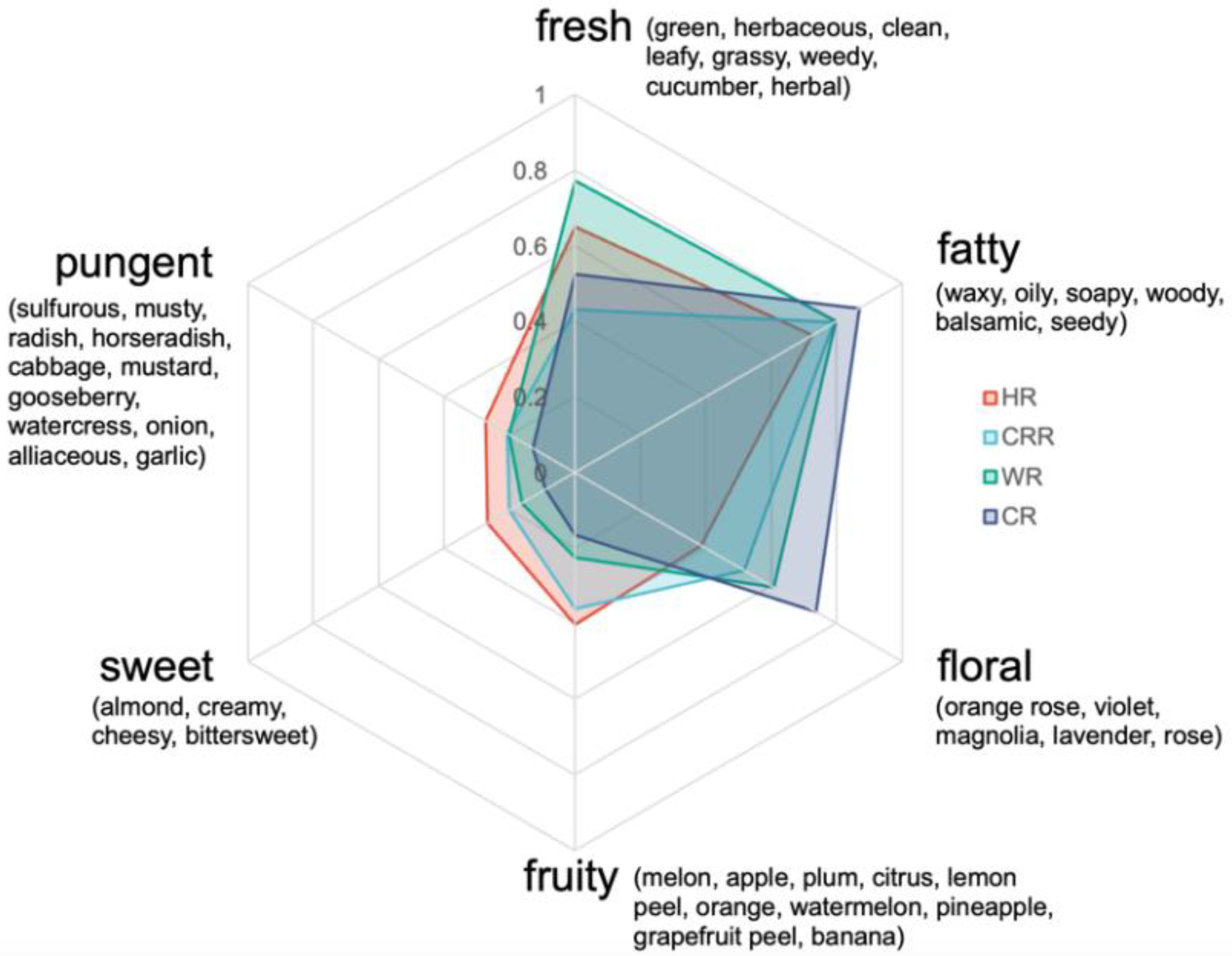
2.3. Odor Characteristics of Radish Microgreens Based on Odor Activity Value (OAV), Odor Contribution Rate (OCR), and Radar Fingerprint Chart (RFC)
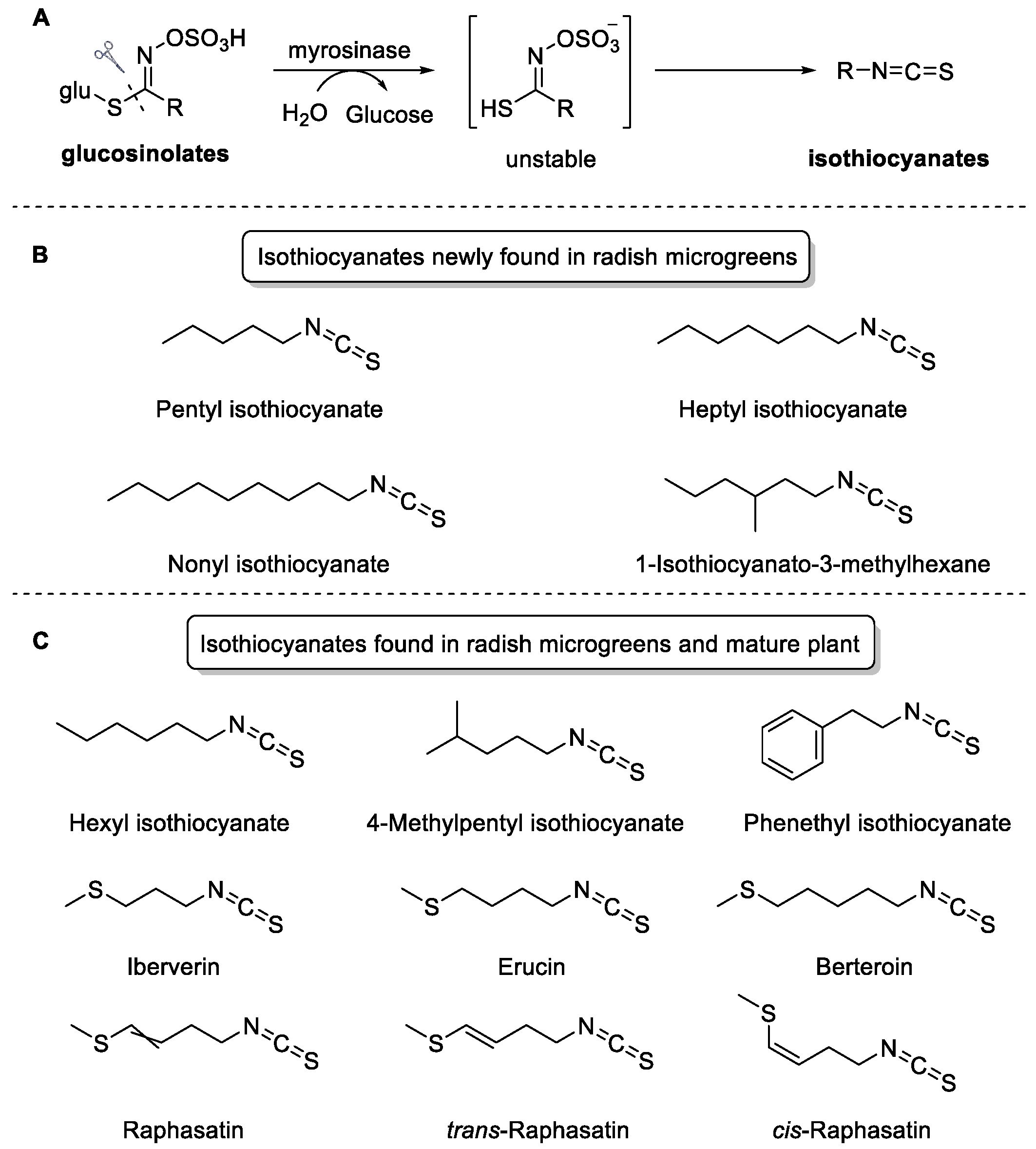
2.4. Characteristic Volatile Isothiocyanates
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
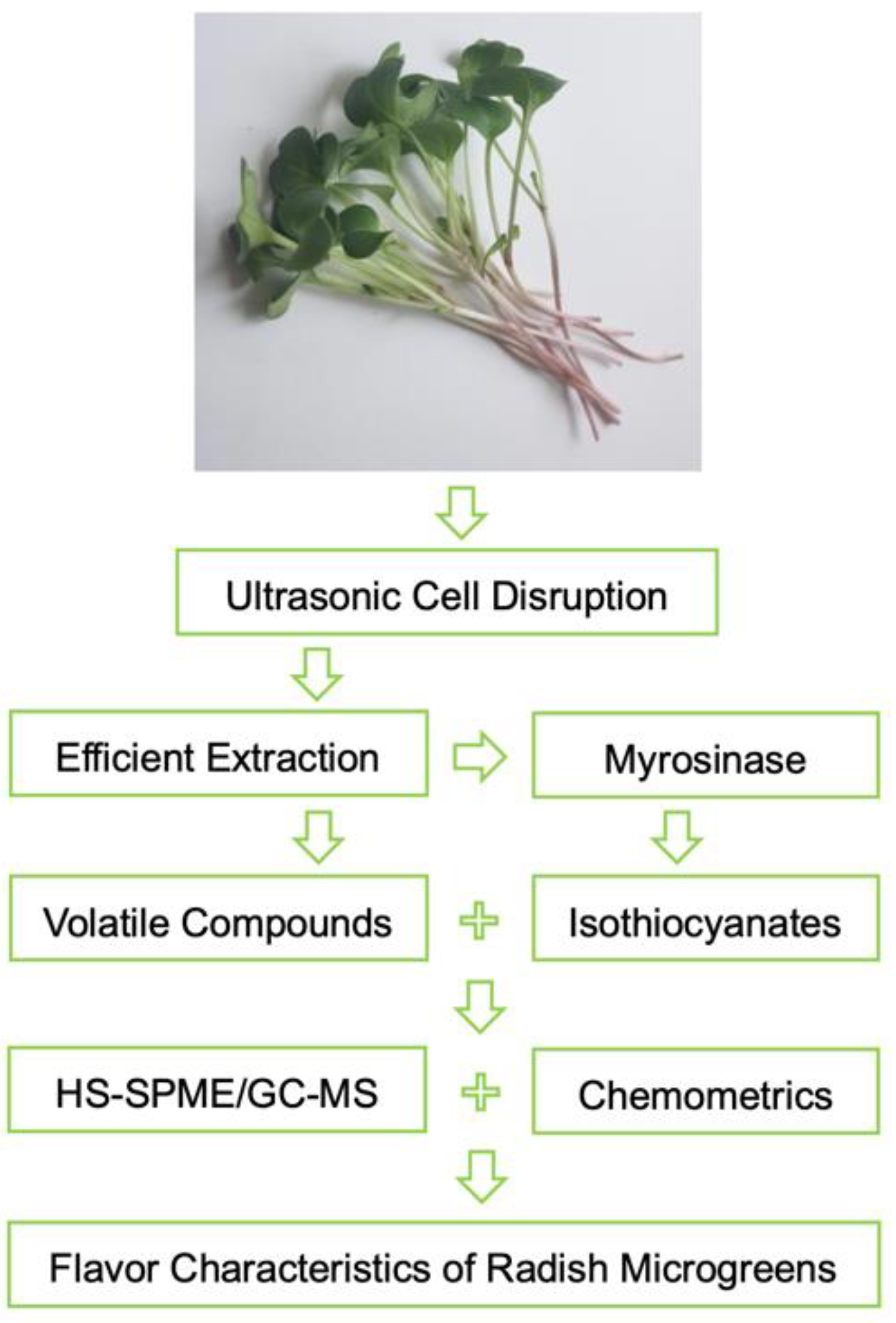
3.3. Sample Preparation with Ultrasonic Cell Disruption Treatment
3.4. HS-SPME and GC–MS Analysis
3.5. Qualitative and Quantitative Analysis of Volatile Compounds
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef]
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The Science behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Sharma, S.; Shree, B.; Sharma, D.; Kumar, S.; Kumar, V.; Sharma, R.; Saini, R. Vegetable microgreens: The gleam of next generation super foods, their genetic enhancement, health benefits and processing approaches. Food Res. Int. 2022, 155, 111038. [Google Scholar]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M.P.L.V.O. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Curtis, I.S. The noble radish: Past, present and future. Trends Plant Sci. 2003, 8, 305–307. [Google Scholar] [CrossRef]
- Demir, K.; Sarıkamış, G.; Çakırer Seyrek, G. Effect of LED lights on the growth, nutritional quality and glucosinolate content of broccoli, cabbage and radish microgreens. Food Chem. 2023, 401, 134088. [Google Scholar] [CrossRef]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral composition and content of 30 varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.-U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Xu, B.; Azam, S.M.R.; Feng, M.; Wu, B.; Yan, W.; Zhou, C.; Ma, H. Application of multi-frequency power ultrasound in selected food processing using large-scale reactors: A review. Ultrason. Sonochem. 2021, 81, 105855. [Google Scholar]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of Extraction Methods for Recovery of Astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010, 46, 64–70. [Google Scholar] [CrossRef]
- Haque, F.; Thimmanagari, M.; Chiang, Y.W. Ultrasound assisted cyanotoxin extraction for nematode inhibition in soil. Ultrason. Sonochem. 2022, 89, 106120. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Radman, S.; Opačić, N.; Rašić, A.; Dujmović, M.; Brnčić, M.; Barba, F.J.; Castagnini, J.M.; Voća, S. Application of Ultrasound as Clean Technology for Extraction of Specialized Metabolites from Stinging Nettle (Urtica dioica L.). Front. Nutr. 2022, 9, 870923. [Google Scholar] [CrossRef]
- Friis, P.; Kjaer, A. 4-(Methylthio)-3-butenyl isothiocyanate, the pungent principle of radish root. Acta Chem. Scand. 1966, 20, 698–705. [Google Scholar] [CrossRef]
- Coogan, R.C.; Wills, R.B.H.; Nguyen, V.Q. Pungency levels of white radish (Raphanus sativus L.) grown in different seasons in Australia. Food Chem. 2001, 72, 1–3. [Google Scholar] [CrossRef]
- Nakamura, Y.; Iwahashi, T.; Tanaka, A.; Koutani, J.; Matsuo, T.; Okamoto, S.; Sato, K.; Ohtsuki, K. 4-(Methylthio)-3-butenyl Isothiocyanate, a Principal Antimutagen in Daikon (Raphanus sativus; Japanese White Radish). J. Agric. Food Chem. 2001, 49, 5755–5760. [Google Scholar] [CrossRef]
- Visentin, M.; Tava, A.; Iori, R.; Palmieri, S. Isolation and identification for trans-4-(methylthio)-3-butenyl glucosinolate from radish roots (Raphanus sativus L.). J. Agric. Food Chem. 1992, 40, 1687–1691. [Google Scholar] [CrossRef]
- Khalil, M.N.A.; Fekry, M.I.; Farag, M.A. Metabolome based volatiles profiling in 13 date palm fruit varieties from Egypt via SPME GC–MS and chemometrics. Food Chem. 2017, 217, 171–181. [Google Scholar] [CrossRef]
- Wei, S.; Xiao, X.; Wei, L.; Li, L.; Li, G.; Liu, F.; Xie, J.; Yu, J.; Zhong, Y. Development and comprehensive HS-SPME/GC–MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. var. capitata L.) volatile components. Food Chem. 2021, 340, 128166. [Google Scholar] [CrossRef]
- Blažević, I.; Mastelić, J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem. 2009, 113, 96–102. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar]
- Zhu, M.; Sun, J.; Zhao, H.; Wu, F.; Xue, X.; Wu, L.; Cao, W. Volatile compounds of five types of unifloral honey in Northwest China: Correlation with aroma and floral origin based on HS-SPME/GC–MS combined with chemometrics. Food Chem. 2022, 384, 132461. [Google Scholar] [CrossRef]
- Buettner, A. Springer Handbook of Odor; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners B.V.: Zeist, The Netherlands, 2003. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; Taylor & Francis Group: Abingdon-on-Thames, UK, 2010. [Google Scholar]
- Qiu, D.; Duan, R.; Wang, Y.; He, Y.; Li, C.; Shen, X.; Li, Y. Effects of different drying temperatures on the profile and sources of flavor in semi-dried golden pompano (Trachinotus ovatus). Food Chem. 2023, 401, 134112. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and sensory studies on tomato paste volatiles. J. Agric. Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Buttery, R.G.; Guadagni, D.G.; Ling, L.C.; Seifert, R.M.; Lipton, W. Additional volatile components of cabbage, broccoli, and cauliflower. J. Agric. Food Chem. 1976, 24, 829–832. [Google Scholar] [CrossRef]
- McAusland, L.; Lim, M.-T.; Morris, D.E.; Smith-Herman, H.L.; Mohammed, U.; Hayes-Gill, B.R.; Crowe, J.A.; Fisk, I.D.; Murchie, E.H. Growth Spectrum Complexity Dictates Aromatic Intensity in Coriander (Coriandrum sativum L.). Front. Plant Sci. 2020, 11, 462. [Google Scholar] [CrossRef]
- Alrifai, O.; Mats, L.; Liu, R.; Hao, X.; Marcone, M.F.; Tsao, R. Effect of combined light-emitting diodes on the accumulation of glucosinolates in Brassica microgreens. Food Prod. Process. Nutr. 2021, 3, 30. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Amaro, A.L.; Pereira, M.J.; Müller, C.T.; Pintado, M.; Rogers, H.J. Multi-trait analysis of post-harvest storage in rocket salad (Diplotaxis tenuifolia) links sensorial, volatile and nutritional data. Food Chem. 2016, 211, 114–123. [Google Scholar] [CrossRef]
- Raffo, A.; Masci, M.; Moneta, E.; Nicoli, S.; Sánchez del Pulgar, J.; Paoletti, F. Characterization of volatiles and identification of odor-active compounds of rocket leaves. Food Chem. 2018, 240, 1161–1170. [Google Scholar] [CrossRef]
- French, R.C.; Leather, G.R. Screening of nonanal and related volatile flavor compounds on the germination of 18 species of weed seed. J. Agric. Food Chem. 1979, 27, 828–832. [Google Scholar] [CrossRef]
- Xue, Y.-L.; Han, H.-T.; Liu, C.-J.; Gao, Q.; Li, J.-H.; Zhang, J.-H.; Li, D.-J.; Liu, C.-Q. Multivariate analyses of the volatile components in fresh and dried turnip (Brassica rapa L.) chips via HS-SPME–GC–MS. J. Food Sci. Technol. 2020, 57, 3390–3399. [Google Scholar] [CrossRef]
- Xu, B.-g.; Zhang, M.; Bhandari, B.; Cheng, X.-f.; Islam, M.N. Effect of ultrasound-assisted freezing on the physico-chemical properties and volatile compounds of red radish. Ultrason. Sonochem. 2015, 27, 316–324. [Google Scholar] [CrossRef]
- Li, J.; Xie, B.; Yan, S.; Li, H.; Wang, Q. Extraction and determination of 4-methylthio-3-butenyl isothiocyanate in Chinese radish (Raphanus sativus L.) roots. LWT Food Sci. Technol. 2015, 60, 1080–1087. [Google Scholar] [CrossRef]
- Papi, A.; Orlandi, M.; Bartolini, G.; Barillari, J.; Iori, R.; Paolini, M.; Ferroni, F.; Grazia Fumo, M.; Pedulli, G.F.; Valgimigli, L. Cytotoxic and Antioxidant Activity of 4-Methylthio-3-butenyl Isothiocyanate from Raphanus sativus L. (Kaiware Daikon) Sprouts. J. Agric. Food Chem. 2008, 56, 875–883. [Google Scholar] [CrossRef]
- Ho, C.W.; Wan Aida, W.M.; Maskat, M.Y.; Osman, H. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC-MS) analysis of aroma compound in palm sugar (Arenga pinnata). J. Food Compos. Anal. 2006, 19, 822–830. [Google Scholar] [CrossRef]
| No. | Compounds a | CAS | RT b (min) | The Content of Volatile Compound in Different Cultivars c of Radish Microgreens (µg/kg) d | Characteristic Ions (m/z) e | |||
|---|---|---|---|---|---|---|---|---|
| HR | CRR | WR | CR | |||||
| Alcohols | ||||||||
| 1 | 1-Octanol | 111-87-5 | 20.30 | 13.30 | 47.18 | - | - | 56, 70, 84, 112 |
| 2 | trans-2-Dodecen-1-ol | 69064-37-5 | 22.35 | - | 18.01 | - | - | 57, 82, 95, 109, 138, 166, 184 |
| 3 | 1-Nonanol | 143-08-8 | 23.85 | 25.04 | 79.18 | - | - | 56, 70, 83, 126 |
| 4 | trans-2-Undecen-1-ol | 75039-84-8 | 26.97 | 17.90 | 26.01 | 16.79 | 23.09 | 57, 68, 82, 109, 152, 170 |
| 5 | 3,7,11-Trimethyl-3-dodecanol | 7278-65-1 | 29.94 | 24.78 | 232.04 | 22.02 | 65.10 | 43, 73, 83, 111, 139, 199, 213 |
| 6 | 2,4-Dimethyl-4-octanol | 33933-79-8 | 34.54 | 19.36 | - | - | 31.48 | 57, 59, 101, 111, 143 |
| 7 | 1-Tridecanol | 112-70-9 | 36.11 | 14.03 | - | - | - | 55, 69, 83, 111, 139, 154, 182 |
| Aldehydes | ||||||||
| 8 | 2-Methyl-4-pentenal | 5187-71-3 | 9.19 | 14.26 | - | - | 51.77 | 41, 69, 83 |
| 9 | 3-Hexenal | 4440-65-7 | 9.73 | 16.82 | 46.03 | - | - | 41, 69, 98 |
| 10 | 2-Hexenal | 505-57-7 | 11.96 | 160.91 | 374.90 | 299.24 | 897.95 | 41, 69, 83 |
| 11 | Nonanal | 124-19-6 | 21.58 | 453.96 | 548.23 | 269.86 | 365.36 | 57, 70, 98, 114, 142 |
| 12 | Decanal | 112-31-2 | 25.30 | 490.84 | 322.46 | 311.43 | 256.83 | 57, 70, 83, 112, 138, 156 |
| 13 | Undecanal | 112-44-7 | 28.84 | 47.44 | 33.60 | 19.62 | 46.80 | 57, 82, 96, 126, 142 |
| 14 | Tridecanal | 10486-19-8 | 31.54 | 20.27 | 26.23 | 23.85 | 32.34 | 57, 68, 82, 110, 154, 180 |
| 15 | Dodecanal | 112-54-9 | 32.16 | 35.76 | 32.85 | - | 30.34 | 41, 67, 82, 110, 140, 156, 184 |
| 16 | Pentadecanal | 2765-11-9 | 33.70 | 10.91 | - | - | - | 57, 68, 82, 110, 138, 154, 182, 208 |
| 17 | 5,9,13-Trimethyl-4,8,12-tetradecatrienal | 66408-55-7 | 42.24 | 22.97 | 32.16 | - | 40.87 | 41, 69, 93, 124, 136, 161, 179, 205, 248 |
| Isothiocyanates | ||||||||
| 18 | Pentyl isothiocyanate | 629-12-9 | 21.31 | 37.45 | 7.46 | - | 24.04 | 43, 72, 101, 129, 131 |
| 19 | 4-Methylpentyl isothiocyanate | 17608-07-0 | 23.69 | 169.02 | 202.84 | 263.39 | 140.52 | 43, 72, 101, 128, 143 |
| 20 | Nonyl isothiocyanate | 4430-43-7 | 23.78 | 15.17 | - | - | - | 41, 72, 96, 115, 152, 156, 184 |
| 21 | Hexyl isothiocyanate | 4404-45-9 | 25.06 | 399.11 | 248.27 | 508.09 | 224.60 | 43, 72, 100, 115, 143 |
| 22 | 4-Methylthio-3-butenyl isothiocyanate (Raphasatin) | 51598-96-0 | 25.73 | 12.97 | - | - | - | 45, 72, 87, 112, 159 |
| 23 | Heptyl isothiocyanate | 4426-83-9 | 27.31 | 44.33 | 36.77 | 82.31 | 38.10 | 43, 72, 100, 124, 142, 157 |
| 24 | 1-Isothiocyanato-3-methylhexane | 206761-72-0 | 27.60 | 6.22 | 14.23 | - | - | 43, 72, 100, 114, 142, 156 |
| 25 | 3-Methylthiopropyl isothiocyanate (Iberverin) | 505-79-3 | 29.08 | 130.44 | 98.71 | 151.84 | - | 41, 72, 101, 147 |
| 26 | (Z)-4-Methylthio-3-butenyl isothiocyanate (cis-Raphasatin) | 123954-93-8 | 32.54 | 2390.87 | 1959.88 | 2911.14 | 1736.70 | 45, 72, 87, 112, 159 |
| 27 | 4-(Methylthio)butyl isothiocyanate (Erucin) | 4430-36-8 | 33.05 | 8212.93 | 5049.85 | 6142.74 | 3741.82 | 55, 61, 85, 115, 146, 161 |
| 28 | (E)-4-Methylthio-3-butenyl isothiocyanate (trans-Raphasatin) | 13028-50-7 | 33.14 | 11,564.41 | 3173.18 | 4483.32 | 4453.34 | 45, 72, 87, 112, 142, 159 |
| 29 | Phenethyl isothiocyanate | 2257-09-2 | 33.96 | 12.00 | 25.17 | - | 37.61 | 51, 65, 91, 128, 135, 163 |
| 30 | 1-Isothiocyanato-5-(methylthio)pentane (Berteroin) | 4430-42-6 | 36.54 | 502.58 | 218.24 | 174.79 | 57.01 | 41, 61, 101, 129, 142, 175, 178 |
| Sulfides | ||||||||
| 31 | Dimethyl disulfide | 624-92-0 | 8.48 | 38.44 | - | - | 10.70 | 45, 79, 94 |
| 32 | Dimethyl trisulfide | 3658-80-8 | 16.58 | 16.10 | 14.10 | 15.96 | 19.40 | 45, 79, 82, 126, 130 |
| 33 | 3-Methyl-1-(methylthio)butane | 13286-90-3 | 22.71 | 62.74 | 34.73 | 48.23 | 159.19 | 55, 70, 103, 118 |
| 34 | Dimethyl tetrasulfide | 5756-24-1 | 26.49 | 8.32 | - | - | - | 45, 79, 94, 111, 143, 158 |
| Ketones | ||||||||
| 35 | 6-Methyl-5-hepten-2-one | 110-93-0 | 16.18 | - | - | - | 20.35 | 43, 69, 83, 108 |
| 36 | 6,10-Dimethyl-5,9-undecadien-2-one | 689-67-8 | 33.35 | 576.27 | 206.14 | 333.40 | 399.23 | 43, 69, 93, 107, 136, 161, 194 |
| Esters | ||||||||
| 37 | 3-Hydroxy-2,2,4-trimethylpentyl isobutyrate | 77-68-9 | 31.07 | 58.03 | - | - | - | 56, 71, 89, 113, 143, 173 |
| 38 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 6864-50-0 | 37.66 | 37.72 | 27.42 | 26.76 | 41.53 | 43, 71, 83, 111, 143, 159, 185, 215, 243 |
| 39 | Diisobutyl phthalate | 84-69-5 | 44.30 | 35.34 | 25.09 | 135.12 | 23.05 | 57, 76, 104, 121, 149, 167, 195, 223, 278 |
| Terpenes | ||||||||
| 40 | β-Cyclocitral | 432-25-7 | 24.29 | - | - | - | 23.98 | 41, 67, 91, 123, 137, 154 |
| 41 | 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one | 14901-07-6 | 33.55 | 50.34 | 17.82 | 58.62 | 102.52 | 43, 77, 91, 121, 135, 177, 192 |
| 42 | β-Ionone | 79-77-6 | 33.68 | - | 41.73 | 37.93 | 75.42 | 43, 77, 91, 107, 135, 177, 192 |
| Hydrocarbons | ||||||||
| 43 | Naphthalene | 91-20-3 | 24.93 | 149.27 | 105.57 | 92.73 | 86.23 | 51, 64, 102, 128 |
| 44 | Hexadecane | 544-76-3 | 38.37 | 38.98 | 40.48 | - | 23.34 | 57, 71, 85, 113, 141, 155, 183, 226 |
| 45 | Heptadecane | 629-78-7 | 40.45 | - | 13.15 | 25.59 | 30.64 | 57, 71, 85, 113, 141, 169, 183, 211, 240 |
| 46 | 2,6,10,15-Tetramethylheptadecane | 54833-48-6 | 40.94 | 44.29 | - | - | - | 57, 71, 85, 113, 141, 155, 183, 211, 239, 267, 296 |
| 47 | Octadecane | 593-45-3 | 42.75 | 57.23 | 78.92 | 38.57 | 32.75 | 57, 71, 85, 113, 141, 155, 183, 211, 226, 254 |
| 48 | Docosane | 629-97-0 | 43.09 | 43.02 | 77.93 | - | - | 57, 71, 85, 113, 141, 155, 183, 211, 239, 253, 281, 310 |
| Miscellaneous | ||||||||
| 49 | Cyclohexanone oxime | 100-64-1 | 24.81 | 20.99 | 58.73 | 22.30 | 44.17 | 41, 59, 98, 113 |
| 50 | 2,6-Di-tert-butyl-1,4-benzoquinone | 719-22-2 | 33.93 | 15.43 | 12.70 | 16.91 | 39.41 | 41, 67, 91, 121, 135, 177, 178, 220 |
| 51 | 2,6-Di-tert-butyl-4-methylphenol | 128-37-0 | 35.16 | 232.94 | 193.57 | 325.71 | 531.82 | 57, 81, 105, 119, 145, 177, 189, 205 |
| The total number of volatile compounds | 46 | 39 | 28 | 37 | ||||
| The total content of volatile compounds (μg/kg) | 26,371.50 | 13,801.56 | 16,858.26 | 13,959.40 | ||||
| Group (No.) a | Volatile Compound | CAS | OTS b μg/kg | HR | CRR | WR | CR | Flavor Description e | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OAV c | OCR d | OAV | OCR | OAV | OCR | OAV | OCR | |||||
| 1 | 1-Octanol | 111-87-5 | 42 | 0.32 | 0.001 | 1.12 | 0.006 | - | 0 | - | 0 | green, herbaceous, waxy, oily, sweet |
| 3 | 1-Nonanol | 143-08-8 | 34 | 0.74 | 0.003 | 2.33 | 0.012 | - | 0 | - | 0 | green, clean, oily, orange rose |
| 9 | 3-Hexenal | 4440-65-7 | 0.12 | 140.17 | 0.609 | 383.58 | 1.899 | - | 0 | - | 0 | leafy, grassy, weedy, melon, apple, fatty |
| 10 | 2-Hexenal | 505-57-7 | 17 | 9.47 | 0.041 | 22.05 | 0.109 | 17.60 | 0.073 | 52.82 | 0.146 | sweet, almond, apple, plum, green, leafy |
| 11 | Nonanal | 124-19-6 | 0.32 | 1418.63 | 6.163 | 1713.22 | 8.480 | 843.31 | 3.508 | 1141.75 | 3.147 | waxy, orange rose, citrus, green, lemon peel, cucumber |
| 12 | Decanal | 112-31-2 | 0.08 | 6135.50 | 26.654 | 4030.75 | 19.952 | 3892.88 | 16.193 | 3210.38 | 8.850 | sweet, citrus, orange, waxy, green |
| 13 | Undecanal | 112-44-7 | 0.03 | 1581.33 | 6.870 | 1120.00 | 5.544 | 654.00 | 2.720 | 1560.00 | 4.300 | soapy, waxy, watermelon, pineapple |
| 14 | Tridecanal | 10486-19-8 | 8 | 2.53 | 0.011 | 3.28 | 0.016 | 2.98 | 0.012 | 4.04 | 0.011 | clean, soapy, waxy, citrus, grapefruit peel |
| 15 | Dodecanal | 112-54-9 | 0.13 | 275.08 | 1.195 | 252.69 | 1.251 | - | 0 | 233.38 | 0.643 | soapy, waxy, woody, violet |
| 16 | Pentadecanal | 2765-11-9 | 430 | <1 | 0 | - | 0 | - | 0 | - | 0 | fresh, waxy |
| 25 | 3-Methylthiopropyl isothiocyanate | 505-79-3 | 5 | 26.09 | 0.113 | 19.74 | 0.098 | 30.37 | 0.126 | - | 0 | sulfurous, radish, horseradish, cabbage, mustard |
| 27 | 4-(Methylthio)butyl isothiocyanate (Erucin) | 4430-36-8 | 3 | 2737.64 | 11.893 | 1683.28 | 8.332 | 2047.58 | 8.517 | 1247.27 | 3.438 | cabbage, radish |
| 29 | Phenethyl isothiocyanate | 2257-09-2 | 6 | 2.00 | 0.009 | 4.20 | 0.021 | - | 0 | 6.27 | 0.017 | horseradish, gooseberry, watercress |
| 30 | 1-Isothiocyanato-5-(methylthio)pentane (Berteroin) | 4430-42-6 | 800 | <1 | 0 | <1 | 0 | <1 | 0 | <1 | 0 | cabbage, radish |
| 31 | Dimethyl disulfide | 624-92-0 | 0.16 | 240.25 | 1.044 | - | 0 | - | 0 | 66.88 | 0.184 | sulfurous, onion, cabbage |
| 32 | Dimethyl trisulfide | 3658-80-8 | 0.006 | 2683.33 | 11.657 | 2350.00 | 11.632 | 2660.00 | 11.065 | 3233.33 | 8.913 | sulfurous, alliaceous, onion |
| 34 | Dimethyl tetrasulfide | 5756-24-1 | 0.02 | 416.00 | 1.807 | - | 0 | - | 0 | - | 0 | garlic, sulfurous |
| 35 | 6-Methyl-5-hepten-2-one | 110-93-0 | 50 | - | 0 | - | 0 | - | 0 | <1 | 0 | apple, creamy, cheesy, banana, bittersweet |
| 36 | 6,10-Dimethyl-5,9-undecadien-2-one | 689-67-8 | 60 | 9.60 | 0.042 | 3.44 | 0.017 | 5.56 | 0.023 | 6.65 | 0.018 | magnolia, lavender, rose, leafy, green, apple, banana |
| 40 | β-Cyclocitral | 432-25-7 | 3 | - | 0 | - | 0 | - | 0 | 7.99 | 0.022 | green, herbal, sweet |
| 41 | 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one | 14901-07-6 | 0.007 | 7191.43 | 31.241 | 2545.71 | 12.601 | 8374.29 | 34.835 | 14,645.71 | 40.372 | violet, woody, green |
| 42 | β-Ionone | 79-77-6 | 0.007 | - | 0 | 5961.43 | 29.509 | 5418.57 | 22.540 | 10,774.29 | 29.700 | balsamic, rose, violet, woody, seedy |
| 43 | Naphthalene | 91-20-3 | 1 | 149.27 | 0.648 | 105.57 | 0.523 | 92.73 | 0.386 | 86.23 | 0.238 | pungent |
| 51 | 2,6-Di-tert-butyl-4-methylphenol | 128-37-0 | 1000 | <1 | 0 | <1 | 0 | <1 | 0 | <1 | 0 | musty |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Jia, Z.; Zhou, H.; Zhang, D.; Li, G.; Yu, J. Comparative Analysis of Volatile Compounds from Four Radish Microgreen Cultivars Based on Ultrasonic Cell Disruption and HS-SPME/GC–MS. Int. J. Mol. Sci. 2023, 24, 14988. https://doi.org/10.3390/ijms241914988
Zhong Y, Jia Z, Zhou H, Zhang D, Li G, Yu J. Comparative Analysis of Volatile Compounds from Four Radish Microgreen Cultivars Based on Ultrasonic Cell Disruption and HS-SPME/GC–MS. International Journal of Molecular Sciences. 2023; 24(19):14988. https://doi.org/10.3390/ijms241914988
Chicago/Turabian StyleZhong, Yuan, Zhilong Jia, Hailong Zhou, Dan Zhang, Guichen Li, and Jihua Yu. 2023. "Comparative Analysis of Volatile Compounds from Four Radish Microgreen Cultivars Based on Ultrasonic Cell Disruption and HS-SPME/GC–MS" International Journal of Molecular Sciences 24, no. 19: 14988. https://doi.org/10.3390/ijms241914988
APA StyleZhong, Y., Jia, Z., Zhou, H., Zhang, D., Li, G., & Yu, J. (2023). Comparative Analysis of Volatile Compounds from Four Radish Microgreen Cultivars Based on Ultrasonic Cell Disruption and HS-SPME/GC–MS. International Journal of Molecular Sciences, 24(19), 14988. https://doi.org/10.3390/ijms241914988






