Preparation and In Vitro Characterization of Magnetic CS/PVA/HA/pSPIONs Scaffolds for Magnetic Hyperthermia and Bone Regeneration
Abstract
:1. Introduction
2. Results and Discussion
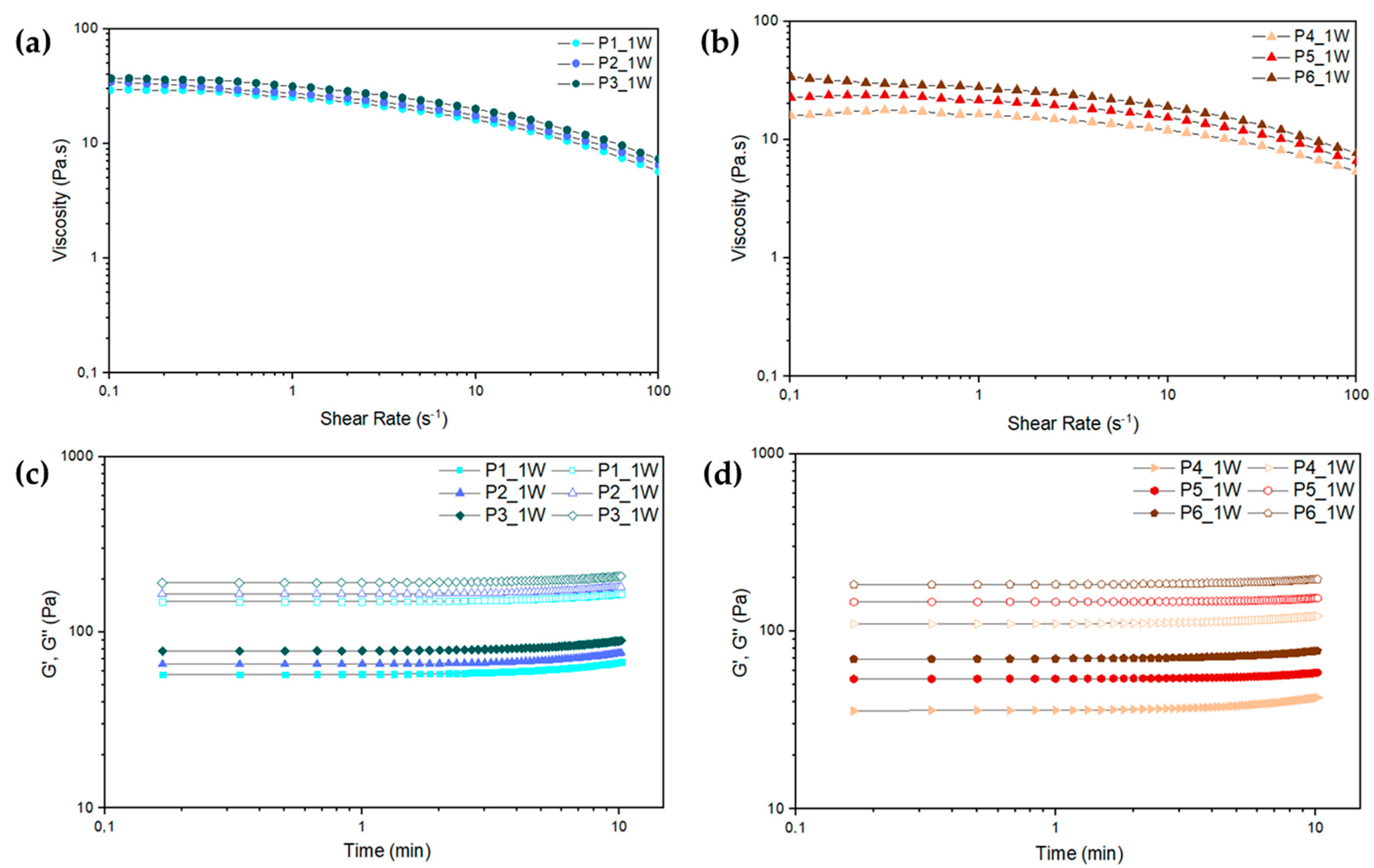
2.1. Paste Characterization
Paste Rheological Behavior
2.2. Paste Formulations Containing pSPIONs
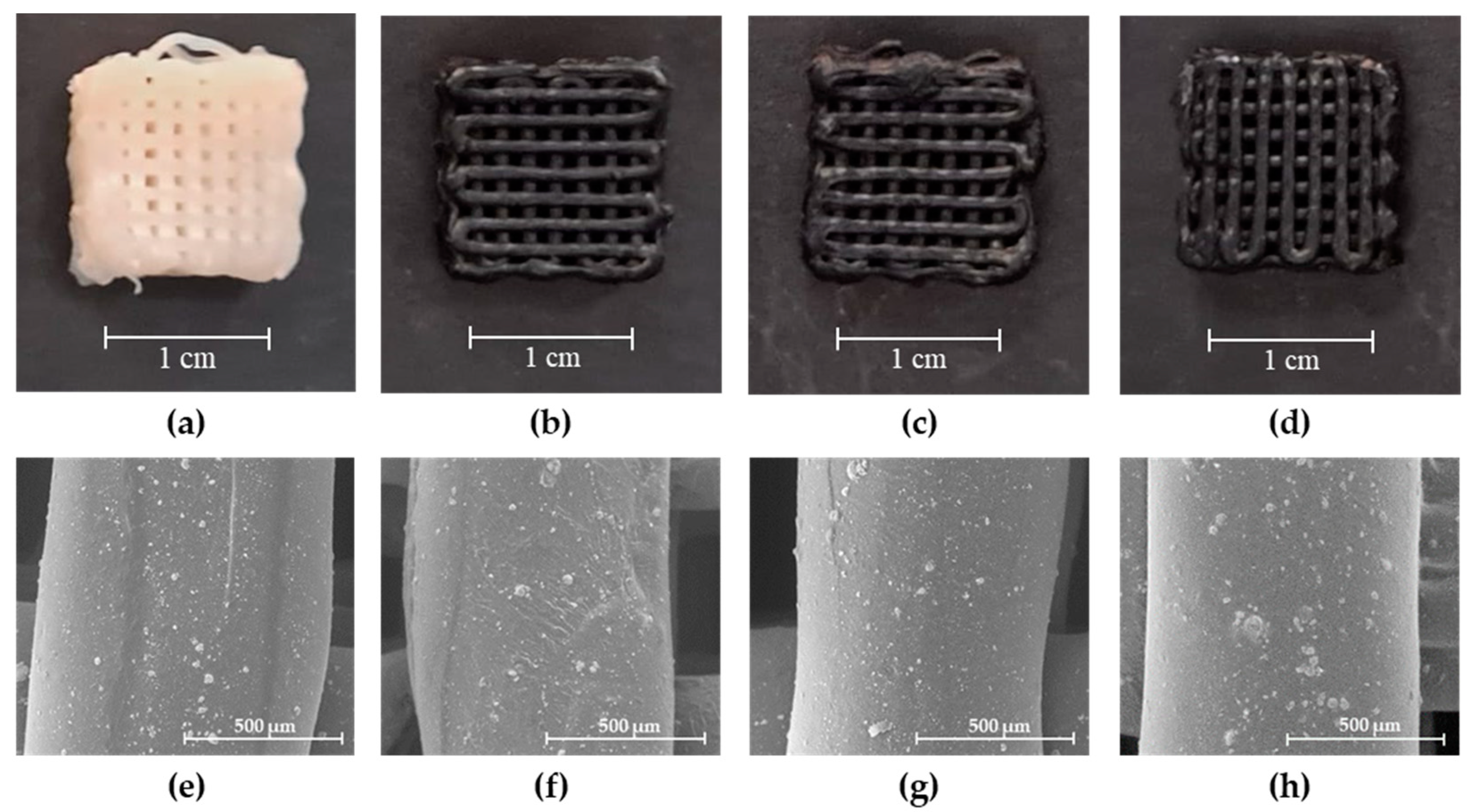
2.3. Scaffold Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR)
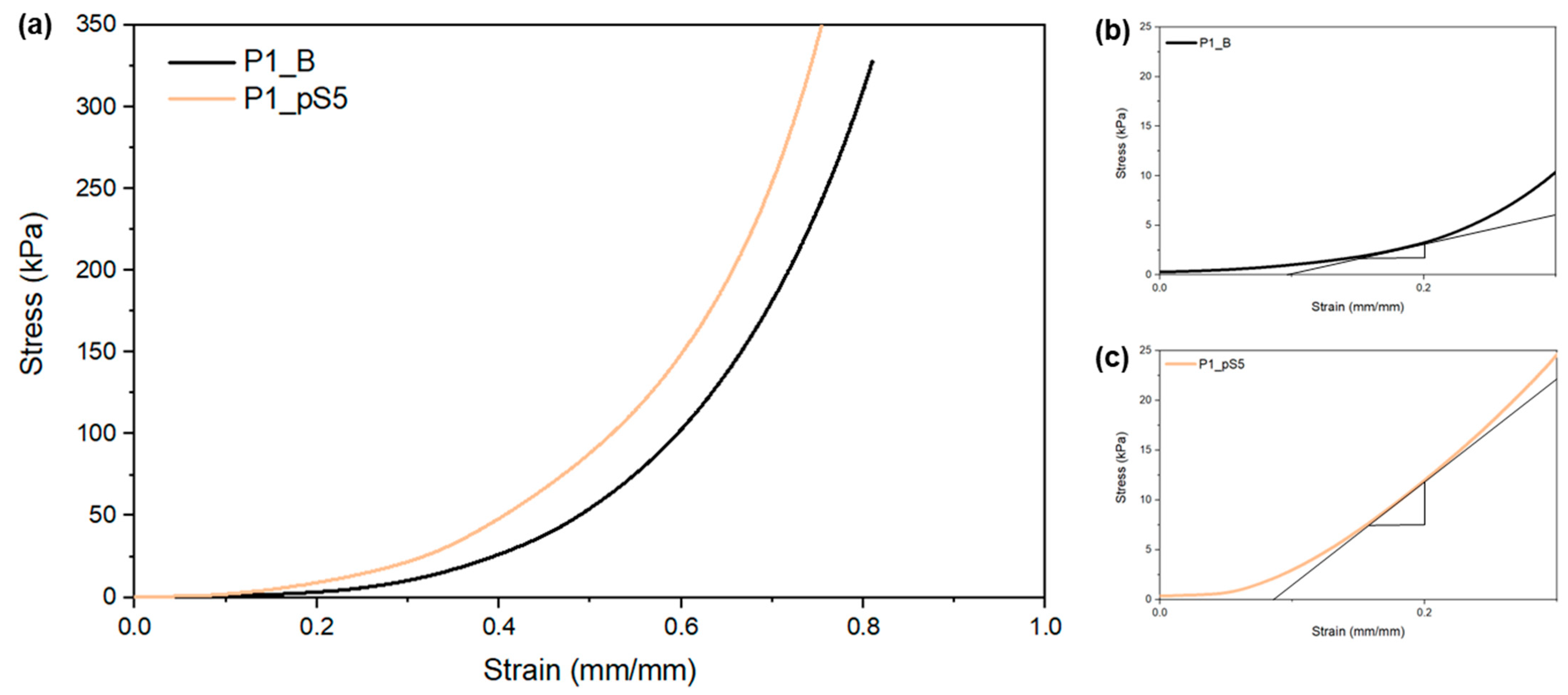
2.3.3. Mechanical Characterization
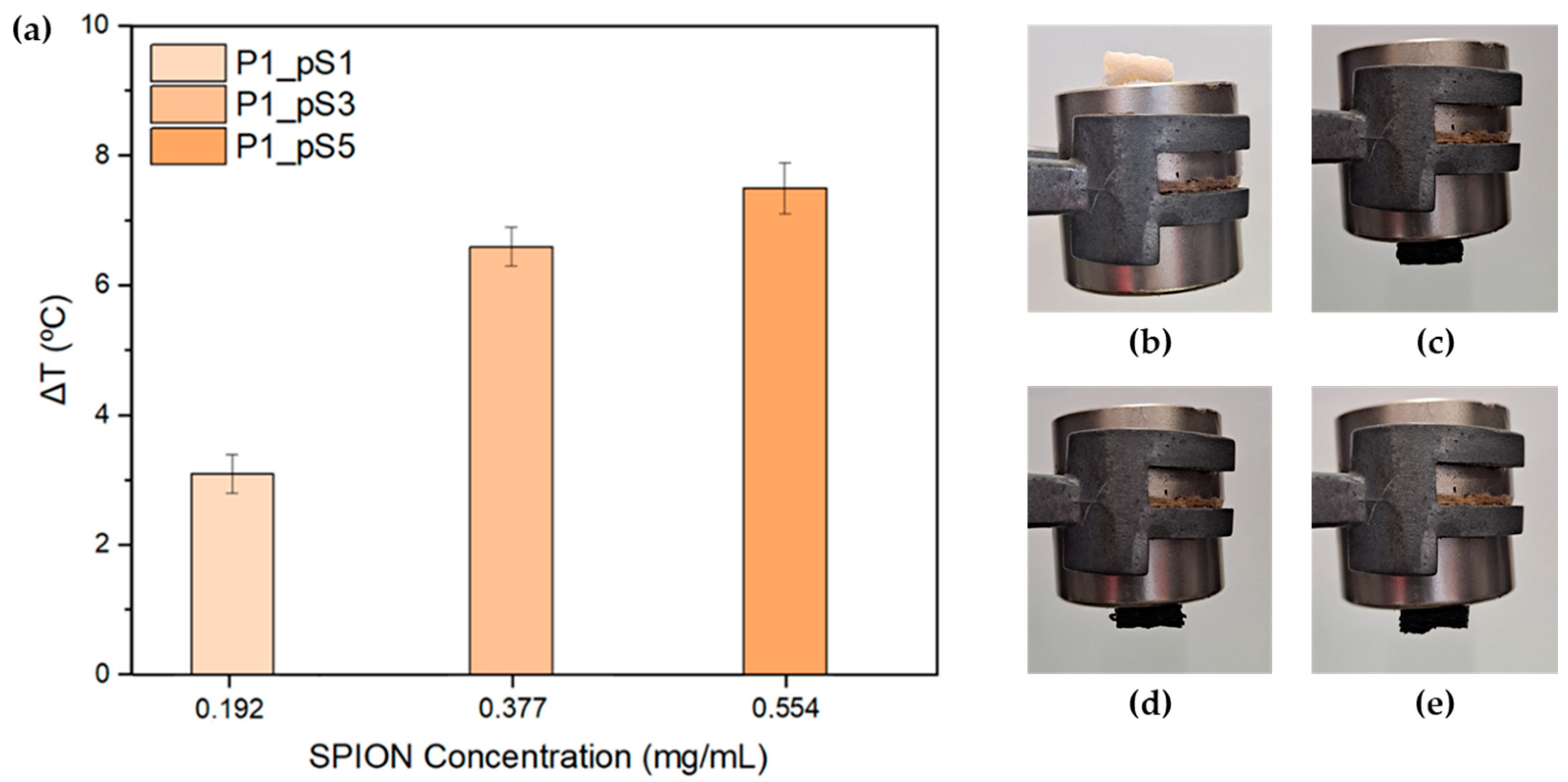
2.3.4. Magnetic Hyperthermia (MHT)
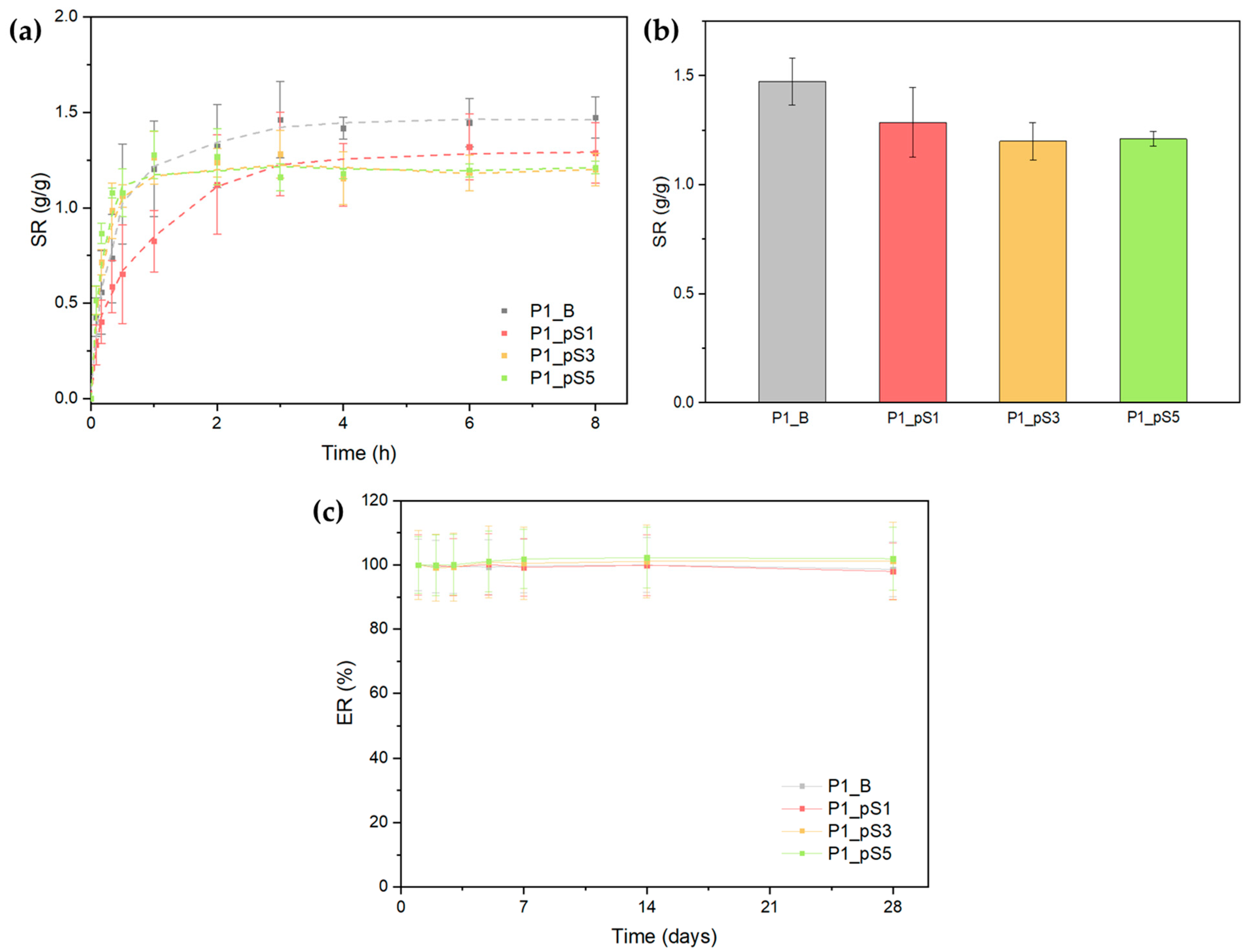
2.3.5. Swelling Ratio and Scaffold Erosion
2.4. In Vitro Studies
2.4.1. Cell Viability Assays
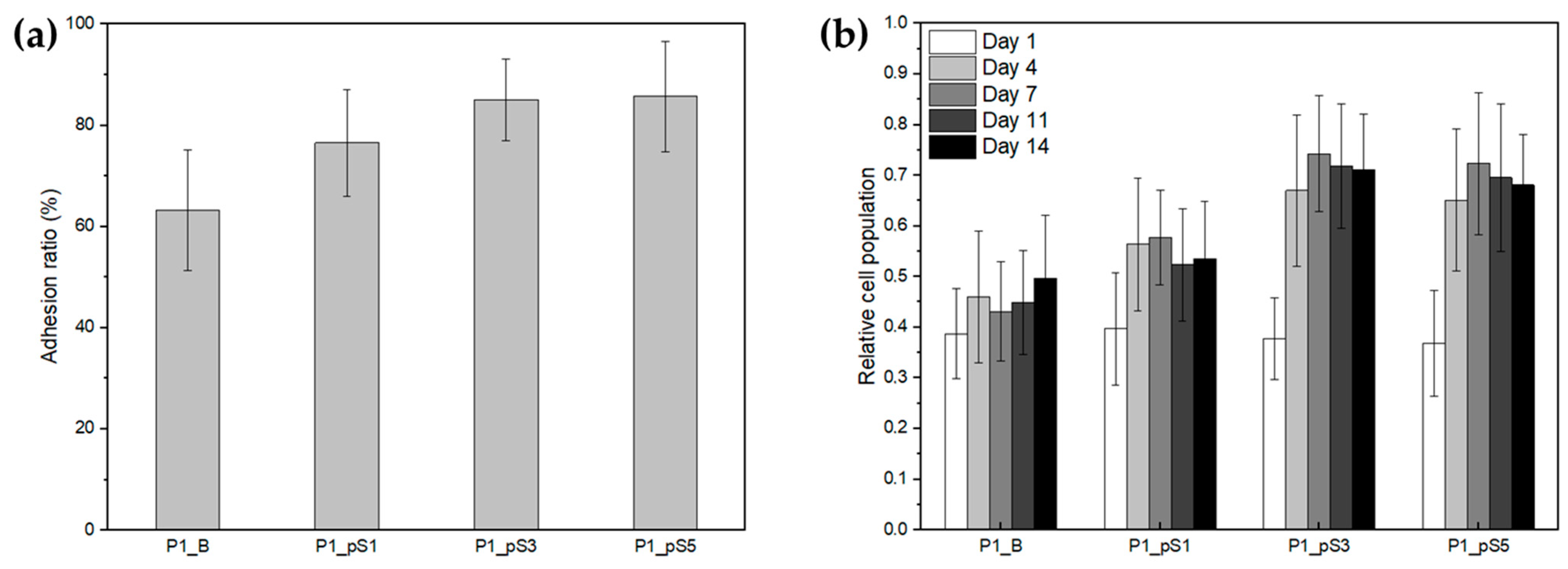
2.4.2. Cell Adhesion and Proliferation
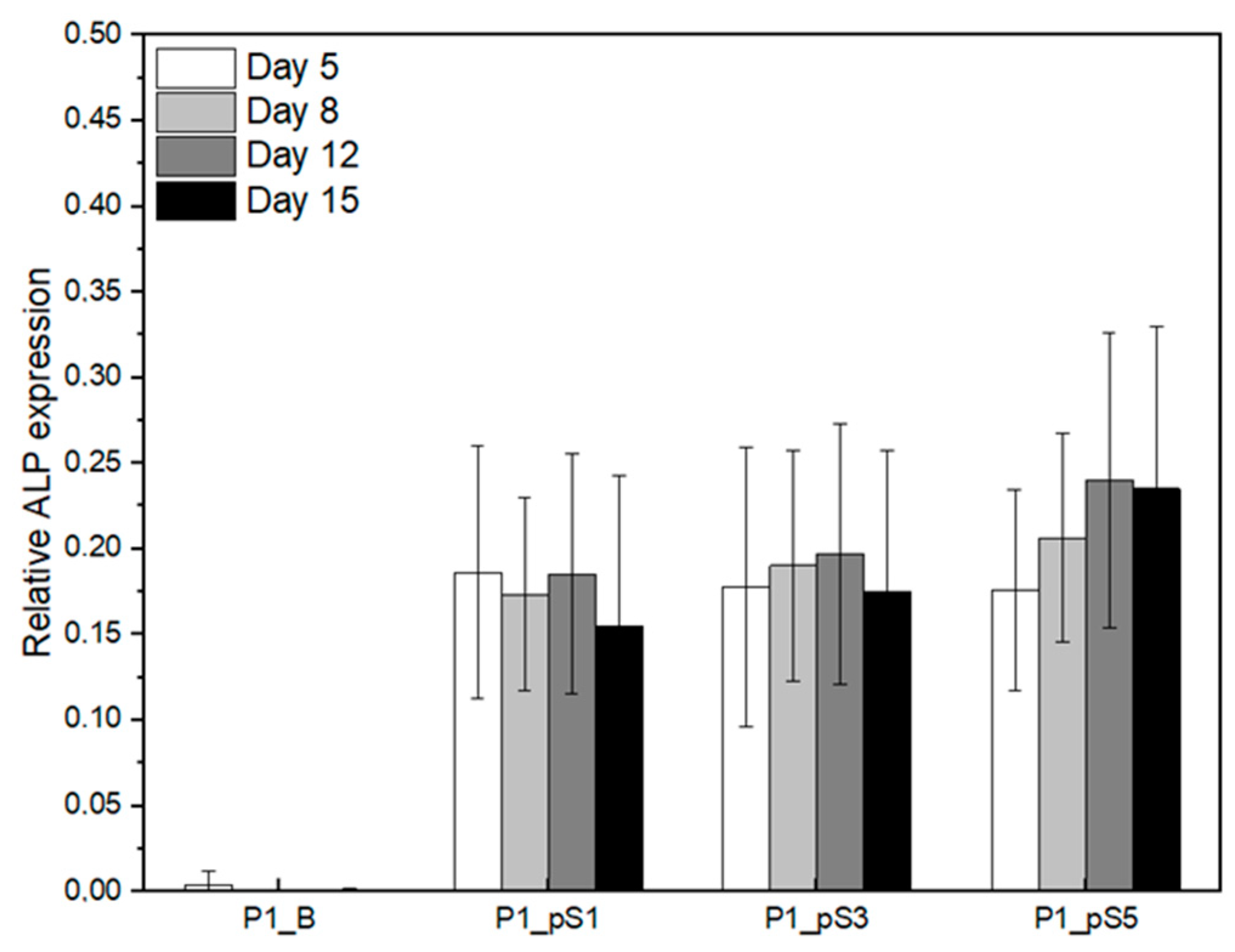
2.4.3. Relative ALP Expression of Saos-2 Cells on CS/PVA/HA/pSPIONs Scaffolds
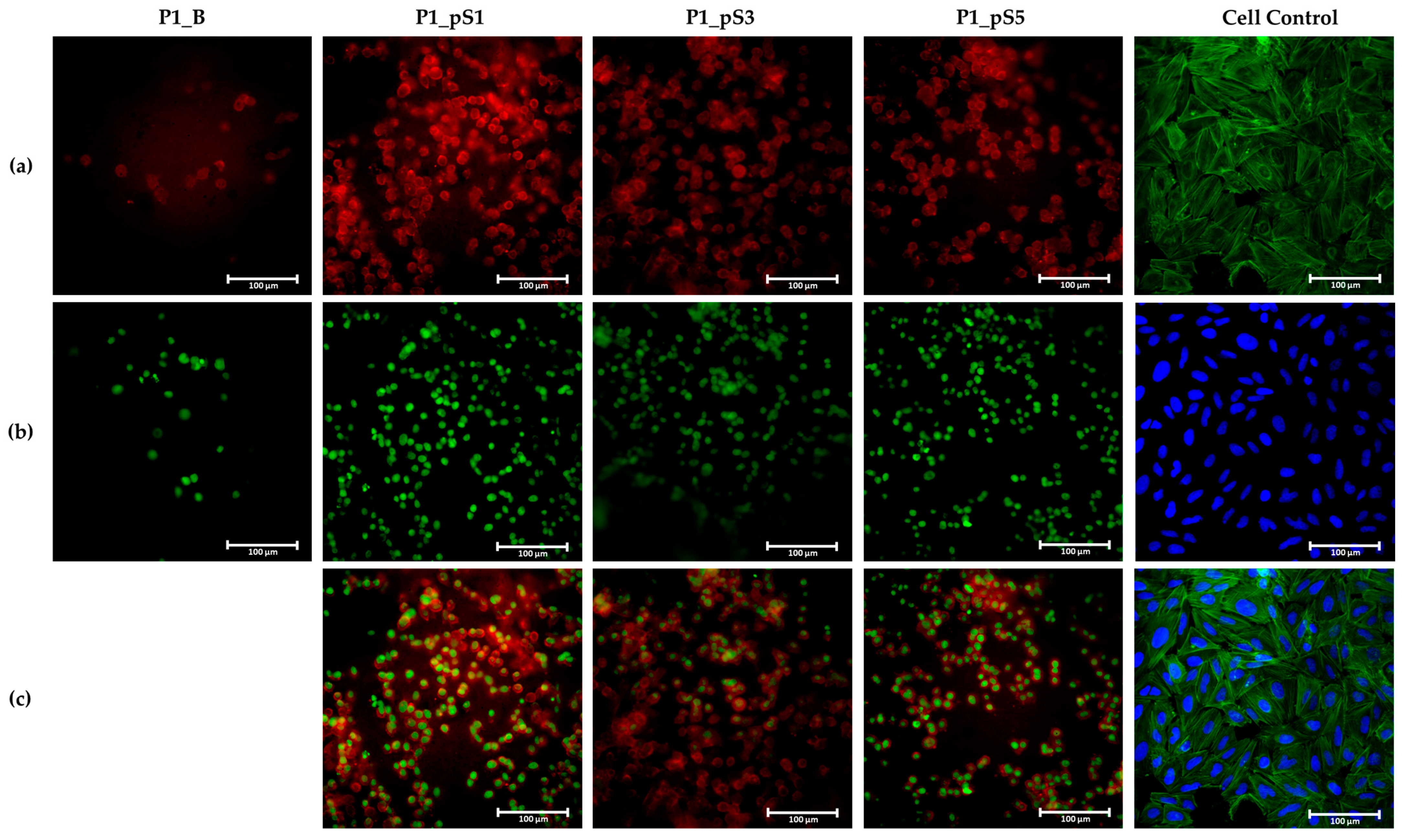
2.4.4. Cell Morphology
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Pristine Superparamagnetic Iron Oxide Nanoparticles (pSPIONs) Synthesis
3.2.2. Paste Preparation
3.2.3. Three-Dimensional (3D) Printing and Post-Printing Treatments
3.2.4. Paste Characterization
Rheological Behavior
3.2.5. Physicochemical, Morphological, and Magnetic Characterization of the Scaffolds
Scanning Electron Microscopy (SEM)
Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR)
Magnetic Hyperthermia (MHT)
Mechanical Characterization
Porosity
Swelling Ratio and Scaffold Erosion
3.2.6. In Vitro Studies
Cell Viability Assays
Cell Adhesion and Proliferation
Alkaline Phosphatase Assay
Immunofluorescence Study
3.2.7. Statistical Analysis
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, E.Y.; Seeger, L.L.; Nelson, S.D.; Eckardt, J.J. Primary osteosarcoma of a metatarsal bone. Skeletal Radiol. 2000, 29, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Toledo, S.R.C.; Oliveira, I.D.; Okamoto, O.K.; Zago, M.A.; de Seixas Alves, M.T.; Filho, R.J.G.; Macedo, C.R.P.D.; Petrilli, A.S. Bone deposition, bone resorption, and osteosarcoma. J. Orthop. Res. 2010, 28, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Agarawal, J.P.; Swangsilpa, T.; van der Linden, Y.; Rades, D.; Jeremic, B.; Hoskin, P.J. The Role of External Beam Radiotherapy in the Management of Bone Metastases. Clin. Oncol. 2006, 18, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.C. Late effects of radiotherapy for pediatric extremity sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Zhao, N.; Wang, C.; Kamar, S.; Zhou, Y. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol. Lett. 2018, 16, 6228–6237. [Google Scholar] [CrossRef] [Green Version]
- Fidler, M. Incidence of fracture through metastases in long bones. Acta Orthop. 1981, 52, 623–627. [Google Scholar] [CrossRef]
- Roddy, E.; Debaun, M.R.; Daoud, A.; Yunzhi, G.; Gardner, M.J. Treatment of critical - sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Romão, J.; Matos, R.; Carvalho, J.; Paulo, J. Design and engineering of magneto-responsive devices for cancer theranostics: Nano to macro perspective. Prog. Mater. Sci. 2021, 116, 100742. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shekhawat, D.; Singh, A.; Banerjee, M.K.; Singh, T.; Patnaik, A. Bioceramic composites for orthopaedic applications: A comprehensive review of mechanical, biological, and microstructural properties. Ceram. Int. 2021, 47, 3013–3030. [Google Scholar] [CrossRef]
- Molino, G.; Palmieri, M.C.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C. Biomimetic and mesoporous nano-hydroxyapatite for bone tissue application: A short review. Biomed. Mater. 2020, 15, 022001. [Google Scholar] [CrossRef]
- dos Santos, V.; Brandalise, R.N.; Savaris, M. Engineering of Biomaterials; Springer: Berlin/Heidelberg, Germany, 2017; Volume X, ISBN 978-3-319-58606-9. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef]
- Valente, T.; Ferreira, J.L.; Henriques, C.; Borges, J.P.; Silva, J.C. Polymer blending or fiber blending: A comparative study using chitosan and poly(ε-caprolactone) electrospun fibers. J. Appl. Polym. Sci. 2019, 136, 47191. [Google Scholar] [CrossRef]
- Filipe, C.; Jo, C.; Silva, J.C. Eco-Friendly Polymer Nanocomposites; Thakur, V.K., Thakur, M.K., Eds.; Advanced Structured Materials; Springer: New Delhi, India, 2015; Volume 74, ISBN 978-81-322-2472-3. [Google Scholar]
- Pineda-Castillo, S.; Bernal-Ballén, A.; Bernal-López, C.; Segura-Puello, H.; Nieto-Mosquera, D.; Villamil-Ballesteros, A.; Muñoz-Forero, D.; Munster, L. Synthesis and Characterization of Poly(Vinyl Alcohol)-Chitosan-Hydroxyapatite Scaffolds: A Promising Alternative for Bone Tissue Regeneration. Molecules 2018, 23, 2414. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Li, W.; Liu, H.; Yuan, T.; Yang, Y.; Zhou, W.; Hu, Y.; Yang, Z. Preparation of 3D Printed Chitosan/Polyvinyl Alcohol Double Network Hydrogel Scaffolds. Macromol. Biosci. 2021, 21, 2000398. [Google Scholar] [CrossRef]
- Ergul, N.M.; Unal, S.; Kartal, I.; Kalkandelen, C.; Ekren, N.; Kilic, O.; Chi-Chang, L.; Gunduz, O. 3D printing of chitosan/ poly(vinyl alcohol) hydrogel containing synthesized hydroxyapatite scaffolds for hard-tissue engineering. Polym. Test. 2019, 79, 106006. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Matsumine, A.; Takegami, K.; Asanuma, K.; Matsubara, T. A novel hyperthermia treatment for bone metastases using magnetic materials. Int. J. Clin. Oncol. 2011, 16, 101–108. [Google Scholar] [CrossRef]
- Repasky, E.A.; Evans, S.S.; Dewhirst, M.W. Temperature Matters! And Why it Should Matter to Tumor Immunologists. Cancer Immunol. Res. 2014, 23, 1–13. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-lópez, M.; Martins, P.; Lanceros-mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, S.; Son, S.; Kim, S.H. Theranostic nanoparticles for future personalized medicine. J. Control. Release 2014, 190, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Hu, S.; Cho, M.H.; Hong, S.H.; Choi, Y.; Choi, H.S. Theranostic nanosystems for targeted cancer therapy. Nano Today 2018, 23, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.E.; Rosenzweig, D.H. The rheology of direct and suspended extrusion bioprinting. APL Bioeng. 2021, 5, 011502. [Google Scholar] [CrossRef]
- Chopin-Doroteo, M.; Mandujano-Tinoco, E.A.; Krötzsch, E. Tailoring of the rheological properties of bioinks to improve bioprinting and bioassembly for tissue replacement. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129782. [Google Scholar] [CrossRef]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Kargozar Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomedicine 2012, 7, 25–34. [Google Scholar]
- Kharazmi, A.; Faraji, N.; Mat Hussin, R.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, optical, opto-thermal and thermal properties of ZnS–PVA nanofluids synthesized through a radiolytic approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite–Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Alves, A.M.R.; Pereira, L.C.J.; Coutinho, J.T.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P.M.R. Effects of surfactants on the magnetic properties of iron oxide colloids. J. Colloid Interface Sci. 2014, 419, 46–51. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Lochte, F.; Echeverria, C.; Pereira, L.C.J.; Coutinho, J.T.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P.M.R. Thermal and magnetic properties of iron oxide colloids: Influence of surfactants. Nanotechnology 2015, 26, 425704. [Google Scholar] [CrossRef]
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef]
- Sharma, A.; Brand, D.; Fairbank, J.; Ye, H.; Lavy, C.; Czernuszka, J. A self-organising biomimetic collagen/nano-hydroxyapatite-glycosaminoglycan scaffold for spinal fusion. J. Mater. Sci. 2017, 52, 12574–12592. [Google Scholar] [CrossRef] [Green Version]
- Chittajallu, S.N.S.H.; Richhariya, A.; Tse, K.M.; Chinthapenta, V. A Review on Damage and Rupture Modelling for Soft Tissues. Bioengineering 2022, 9, 26. [Google Scholar] [CrossRef]
- Silva, A.R.P.d.; Macedo, T.L.; Coletta, D.J.; Feldman, S.; Pereira, M.d.M. Synthesis, characterization and cytotoxicity of Chitosan/Polyvinyl Alcohol/Bioactive Glass hybrid scaffolds obtained by lyophilization. Matéria 2016, 21, 964–973. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Hu, K.-H.; Wei, Z.-H. Comparison of cell behavior on pva/pva-gelatin electrospun nanofibers with random and aligned configuration. Sci. Rep. 2016, 6, 37960. [Google Scholar] [CrossRef] [Green Version]
- Molyneaux, K.; Wnek, M.D.; Craig, S.E.L.; Vincent, J.; Rucker, I.; Wnek, G.E.; Brady-Kalnay, S.M. Physically-cross-linked poly(vinyl alcohol) cell culture plate coatings facilitate preservation of cell–cell interactions, spheroid formation, and stemness. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1744–1753. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, G.; Du, G.; Zhan, D.; Cong, Y.; Cheng, Y.; Fu, J. Magnetic nanohydroxyapatite/PVA composite hydrogels for promoted osteoblast adhesion and proliferation. Colloids Surf. B Biointerfaces 2013, 103, 318–325. [Google Scholar] [CrossRef]
- McNamara, A.; Jenne, B.M.; Dean, M.F. Fibroblasts acquire β-glucuronidase by direct and indirect transfer during co-culture with macrophages. Exp. Cell Res. 1985, 160, 150–157. [Google Scholar] [CrossRef]
- Murray, E.; Provvedini, D.; Curran, D.; Catherwood, B.; Sussman, H.; Manolagas, S. Characterization of a human osteoblastic osteosarcoma cell line (SAOS-2) with high bone alkaline phosphatase activity. J. Bone Miner. Res. 2009, 2, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Tamburaci, S.; Tihminlioglu, F. Development of Si doped nano hydroxyapatite reinforced bilayer chitosan nanocomposite barrier membranes for guided bone regeneration. Mater. Sci. Eng. C 2021, 128, 112298. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Shirzadi, B.; Azizi, Z.; Maedler, K.; Volkmann, E.; Hildebrand, N.; Maas, M.; Treccani, L.; Rezwan, K. Enhanced cell adhesion on bioinert ceramics mediated by the osteogenic cell membrane enzyme alkaline phosphatase. Mater. Sci. Eng. C 2016, 69, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar]
- Bassi, G.; Panseri, S.; Dozio, S.M.; Sandri, M.; Campodoni, E.; Dapporto, M.; Sprio, S.; Tampieri, A.; Montesi, M. Scaffold-based 3D cellular models mimicking the heterogeneity of osteosarcoma stem cell niche. Sci. Rep. 2020, 10, 22294. [Google Scholar] [CrossRef]
- Chan, Y.-H.M.; Marshall, W.F. Scaling properties of cell and organelle size. Organogenesis 2010, 6, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Ang, T.; Sultana, F.S.; Hutmacher, D.; Wong, Y.; Fuh, J.Y.; Mo, X.; Loh, H.; Burdet, E.; Teoh, S. Fabrication of 3D chitosan–hydroxyapatite scaffolds using a robotic dispensing system. Mater. Sci. Eng. C 2002, 20, 35–42. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Vieira, T.; Carvalho Silva, J.; Botelho do Rego, A.M.; Borges, J.P.; Henriques, C. Electrospun biodegradable chitosan based-poly(urethane urea) scaffolds for soft tissue engineering. Mater. Sci. Eng. C 2019, 103, 109819. [Google Scholar] [CrossRef]


| Paste Designation | Paste Formulation (wt.%) | Paste Solids Content (wt.%) | |||||
|---|---|---|---|---|---|---|---|
| CS [a] | PVA/HA | CS:PVA/HA Ratio | CS | PVA | HA | ||
| PVA [a] | HA [b] | ||||||
| P1 | 6 | 12.5 | 15 | 3:1 | 49.83 | 37.13 | 13.04 |
| P2 | 6 | 15.0 | 15 | 3:1 | 45.28 | 41.68 | 13.04 |
| P3 | 6 | 17.5 | 15 | 3:1 | 41.28 | 45.68 | 13.04 |
| P4 | 6 | 12.5 | 15 | 3:2 | 34.89 | 52.07 | 13.04 |
| P5 | 6 | 15.0 | 15 | 3:2 | 30.58 | 56.38 | 13.04 |
| P6 | 6 | 17.5 | 15 | 3:2 | 27.04 | 59.92 | 13.04 |
| Samples Designation | Samples | CS (wt.%) | PVA (wt.%) | HA (wt.%) | pSPIONs (wt.%) |
|---|---|---|---|---|---|
| P1_B | CS/PVA/HA | 49.83 | 37.13 | 13.04 | - |
| P1_pS1 | CS/PVA/HA/15%pSPIONs | 48.87 | 36.42 | 12.79 | 1.92 |
| P1_pS3 | CS/PVA/HA/30%pSPIONs | 47.95 | 35.73 | 12.55 | 3.77 |
| P1_pS5 | CS/PVA/HA/45%pSPIONs | 47.07 | 35.07 | 12.32 | 5.54 |
| Samples | 18G Needle | P1_B | P1_pS1 | P1_pS3 | P1_pS5 |
|---|---|---|---|---|---|
| Filament Diameter (µm) | 840 ± 2 | 918 ± 37 | 839 ± 17 | 832 ± 32 | 876 ± 8 |
| Samples | Estimated Porosity [a] (%) | Toe Modulus (kPa) | pSPIONs Content (wt. %) |
|---|---|---|---|
| P1_B | 70 ± 3 | 27 ± 8 | 0.00 |
| P1_pS5 | 73 ± 2 | 92 ± 4 | 5.54 |
| Samples | Adhesion Ratio (%) | P1_B | P1_pS1 | P1_pS3 | P1_pS5 |
|---|---|---|---|---|---|
| P1_B | 63 ± 12 | - | * | ** | ** |
| P1_pS1 | 77 ± 11 | * | - | ns | ns |
| P1_pS3 | 85 ± 8 | ** | ns | - | ns |
| P1_pS5 | 86 ± 11 | ** | ns | ns | - |
| Samples | Day 1 | Day 4 | Day 7 | PR 1–4 | PR 1–7 |
|---|---|---|---|---|---|
| P1_B | 0.39 ± 0.09 | 0.46 ± 0.13 | 0.43 ± 0.10 | 1.21 ± 0.10 | 1.11 ± 0.06 |
| P1_pS1 | 0.40 ± 0.11 | 0.56 ± 0.13 | 0.58 ± 0.09 | 1.51 ± 0.13 | 1.23 ± 0.08 |
| P1_pS3 | 0.38 ± 0.08 | 0.67 ± 0.15 | 0.74 ± 0.12 | 1.96 ± 0.08 | 2.10 ± 0.14 |
| P1_pS5 | 0.37 ± 0.10 | 0.65 ± 0.14 | 0.72 ± 0.14 | 1.97 ± 0.20 | 1.87 ± 0.20 |
| Cell Control | 1.000 ± 0.004 | 1.87 ± 0.19 | 2.42 ± 0.28 | 1.87 ± 0.19 | 2.42 ± 0.28 |
| Samples | P1_B | P1_pS1 | P1_pS3 | P1_pS5 |
|---|---|---|---|---|
| P1_B | - | **** | **** | **** |
| P1_pS1 | **** | - | ns | ns |
| P1_pS3 | **** | ns | - | ns |
| P1_pS5 | **** | ns | ns | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, F.J.T.M.; Soares, P.I.P.; Silva, J.C.; Borges, J.P. Preparation and In Vitro Characterization of Magnetic CS/PVA/HA/pSPIONs Scaffolds for Magnetic Hyperthermia and Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 1128. https://doi.org/10.3390/ijms24021128
Tavares FJTM, Soares PIP, Silva JC, Borges JP. Preparation and In Vitro Characterization of Magnetic CS/PVA/HA/pSPIONs Scaffolds for Magnetic Hyperthermia and Bone Regeneration. International Journal of Molecular Sciences. 2023; 24(2):1128. https://doi.org/10.3390/ijms24021128
Chicago/Turabian StyleTavares, Francisco J. T. M., Paula I. P. Soares, Jorge Carvalho Silva, and João Paulo Borges. 2023. "Preparation and In Vitro Characterization of Magnetic CS/PVA/HA/pSPIONs Scaffolds for Magnetic Hyperthermia and Bone Regeneration" International Journal of Molecular Sciences 24, no. 2: 1128. https://doi.org/10.3390/ijms24021128








