Serosal Adhesion Ex Vivo of Hydrogels Prepared from Apple Pectin Cross-Linked with Fe3+ Ions
Abstract
:1. Introduction
2. Results
2.1. Effect of the Type and Concentration of a Cross-Linking Cation on the Adhesivity of Pectin Hydrogel
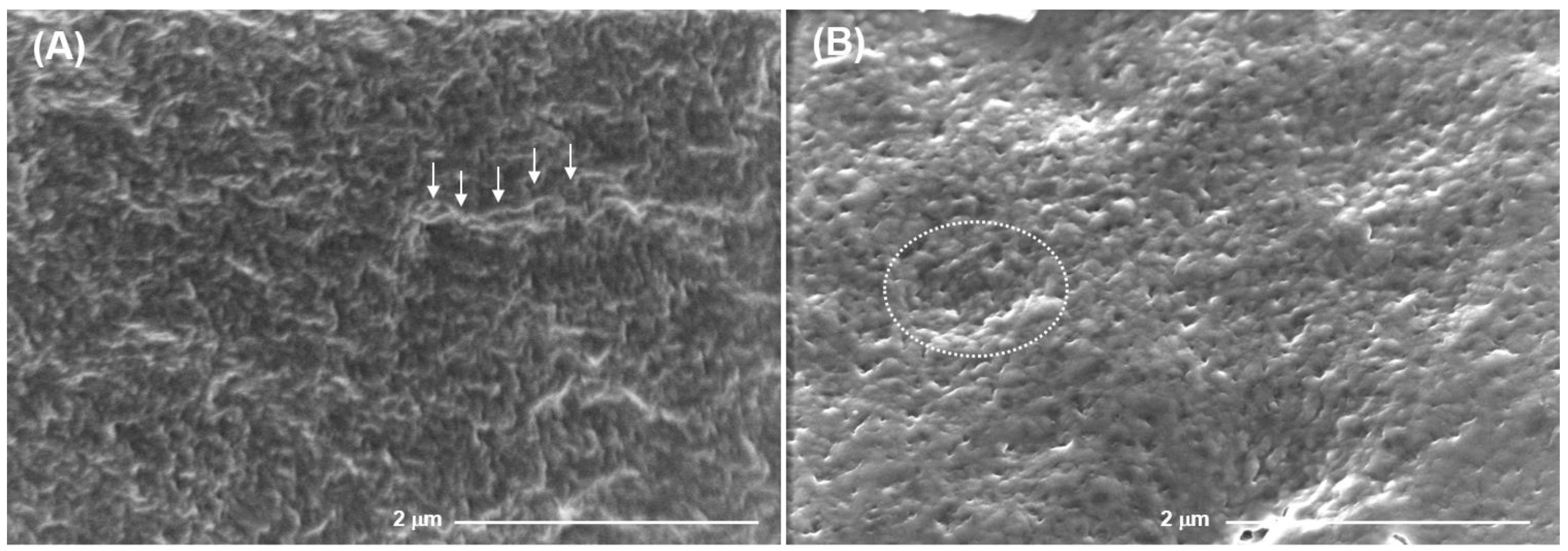
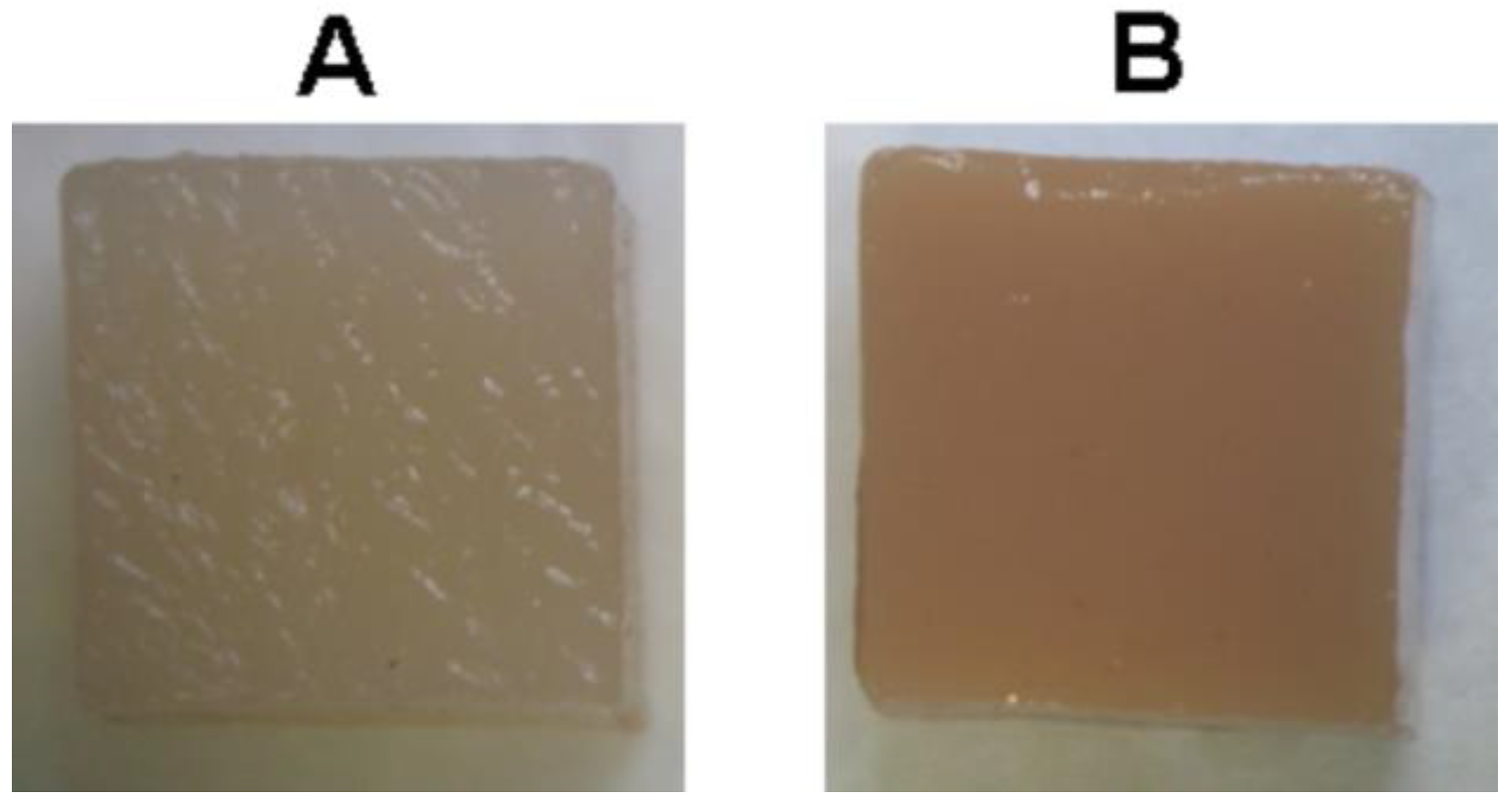
2.2. Mechanical Properties and Surface Microrelief of FeP and CaP Hydrogels
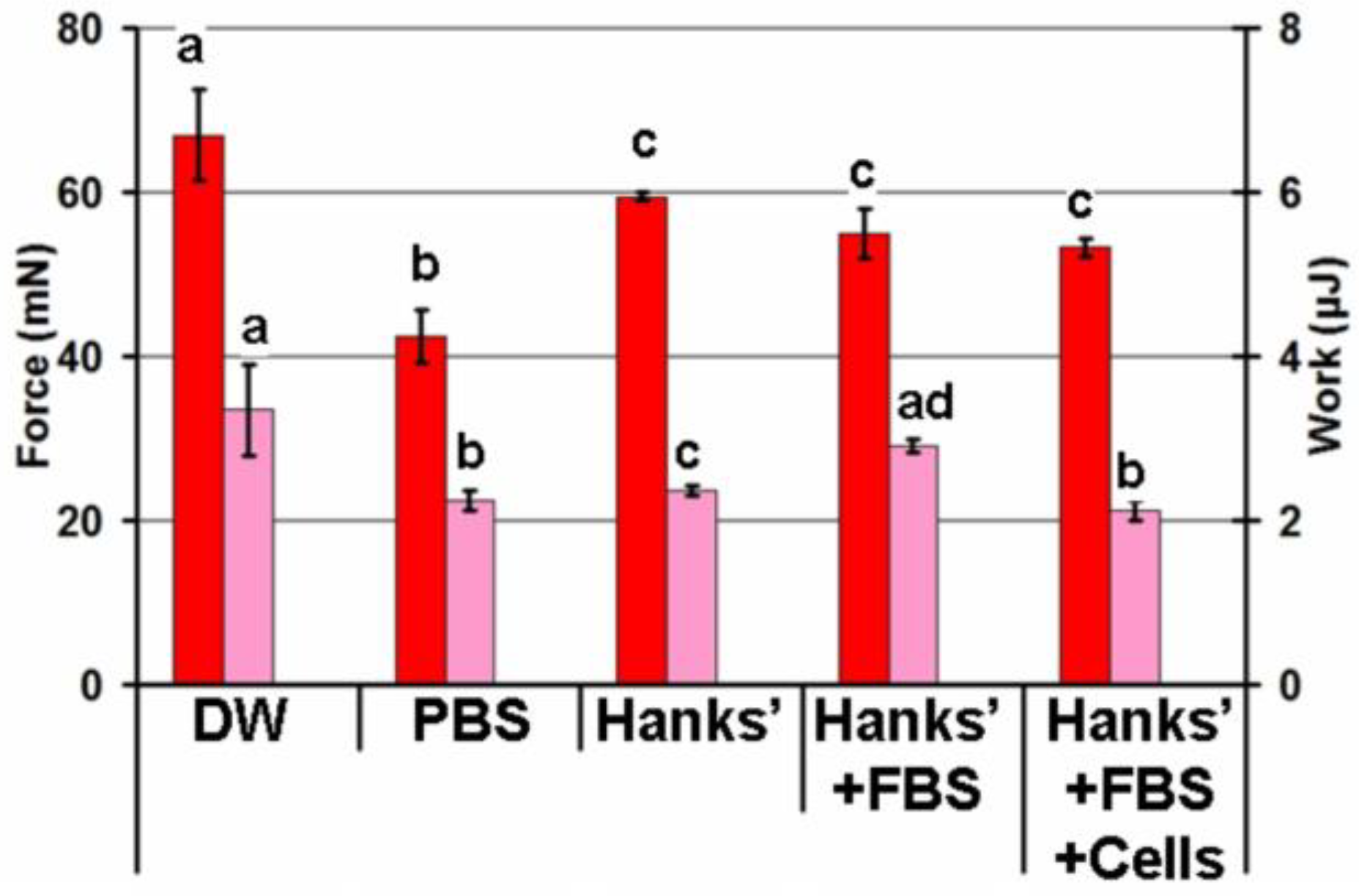
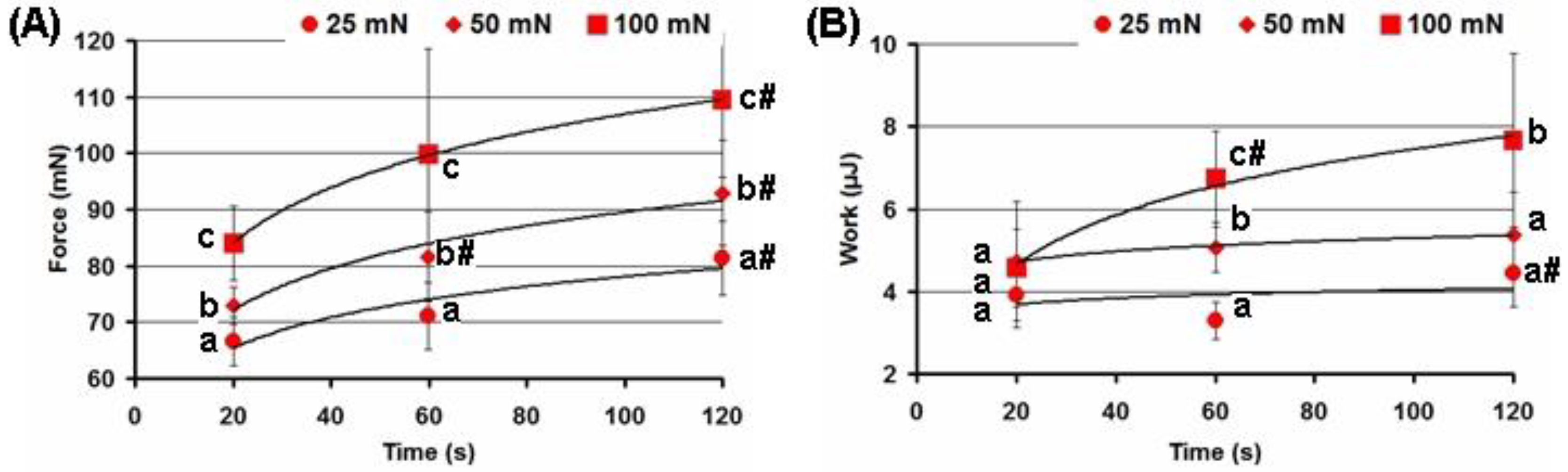
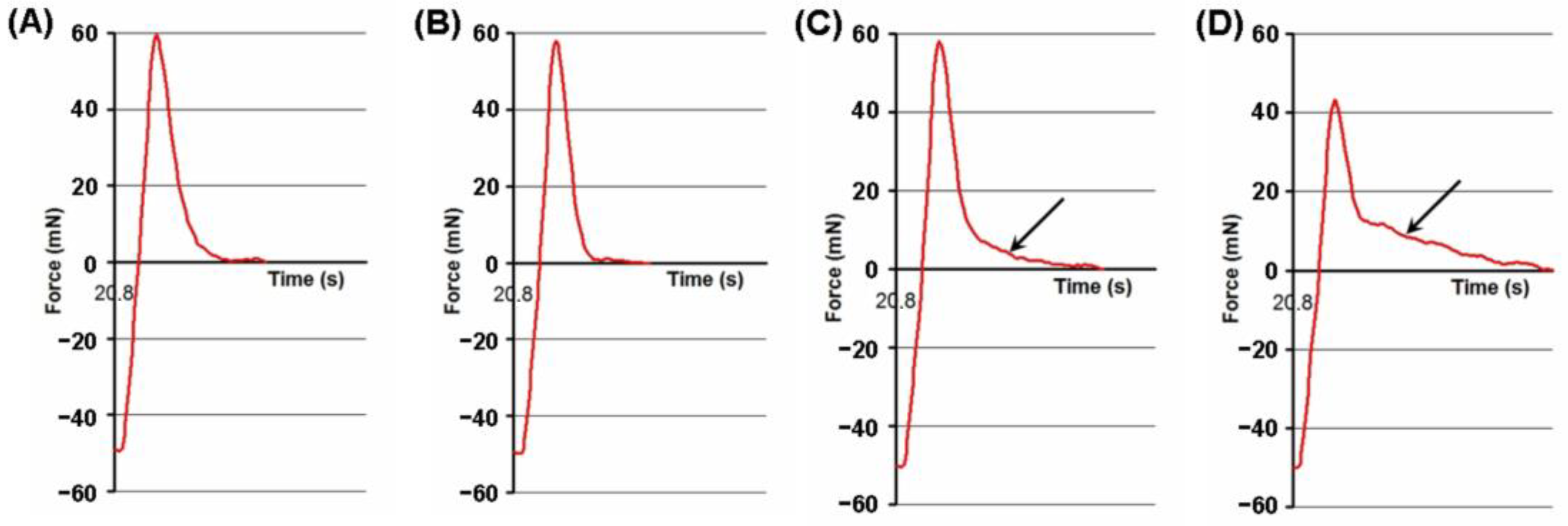
2.3. Effect of the Adhesion Conditions on the Adhesivity of the FeP Hydrogel
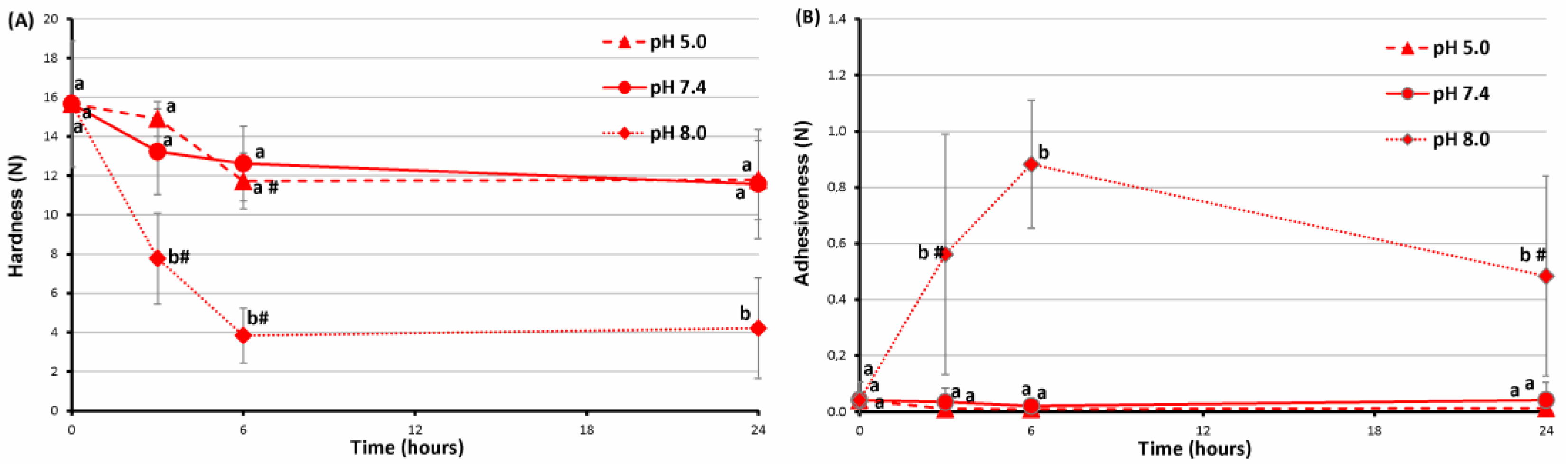
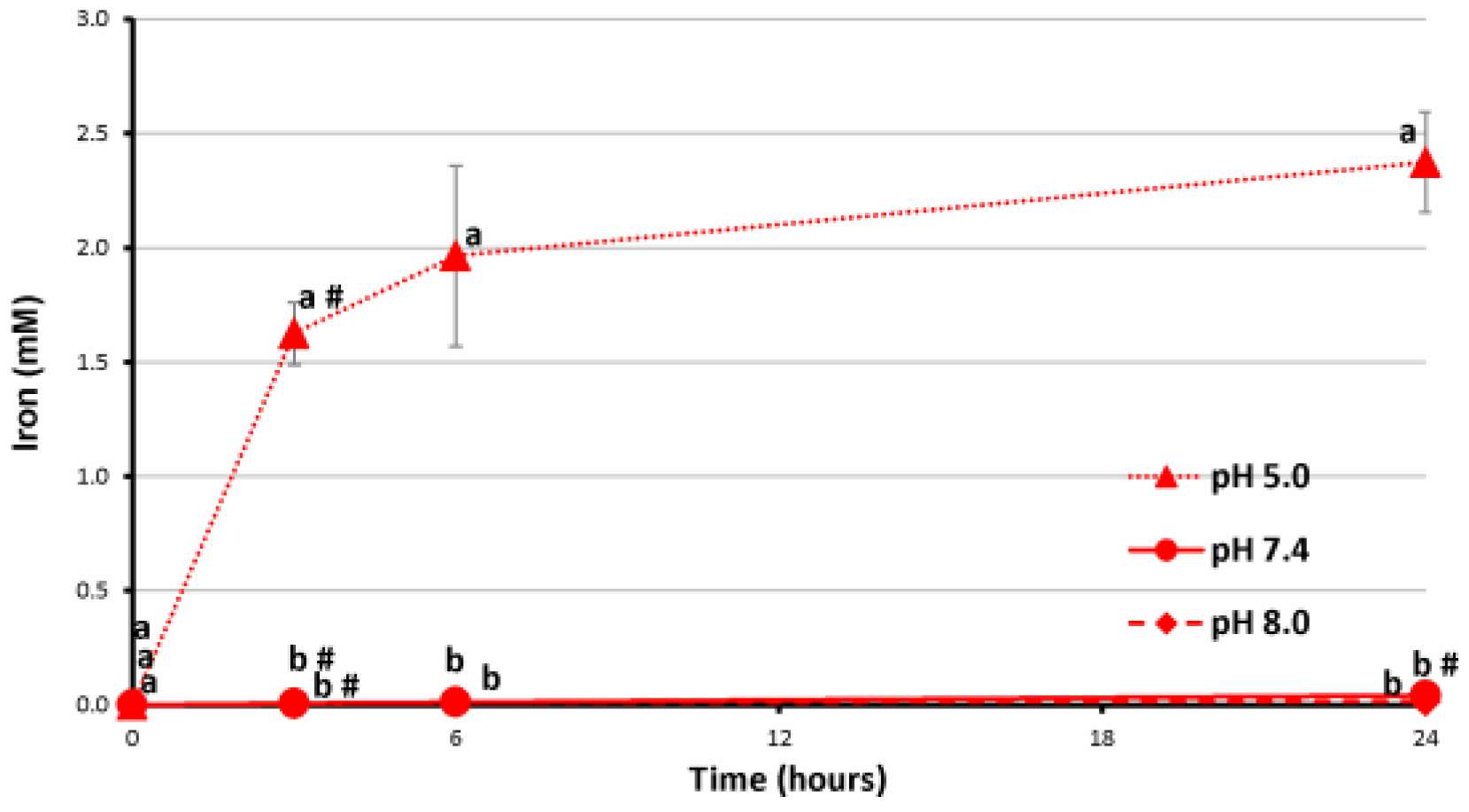
2.4. Stability of FeP Hydrogels Incubated at Different pH
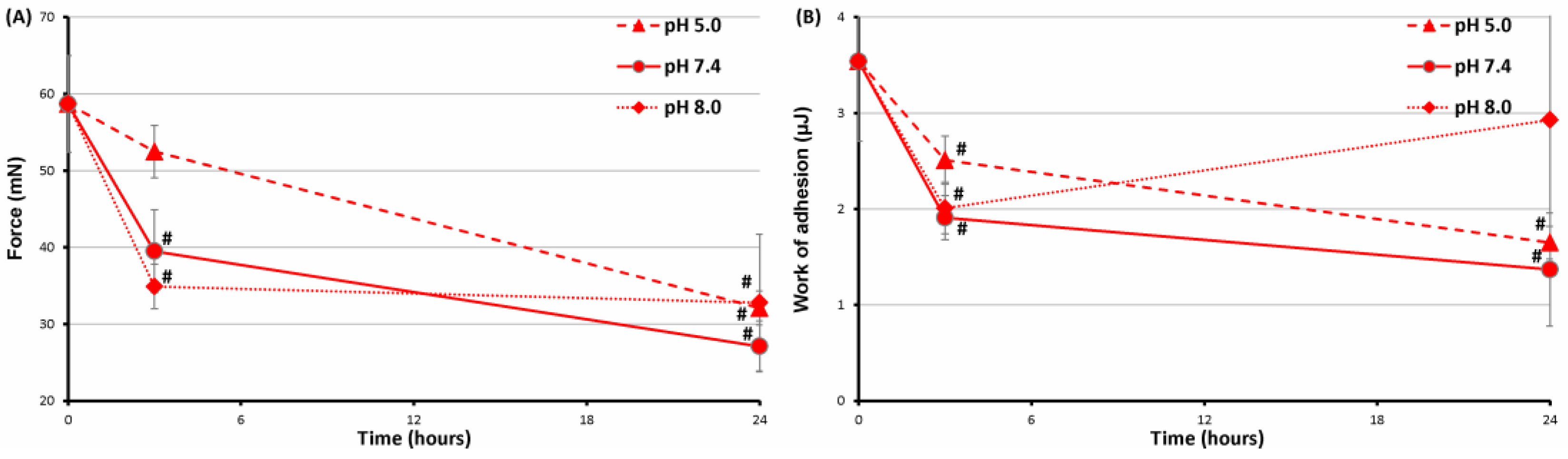
2.5. Serosal Adhesion of FeP Hydrogels Pre-Incubated at Different pH
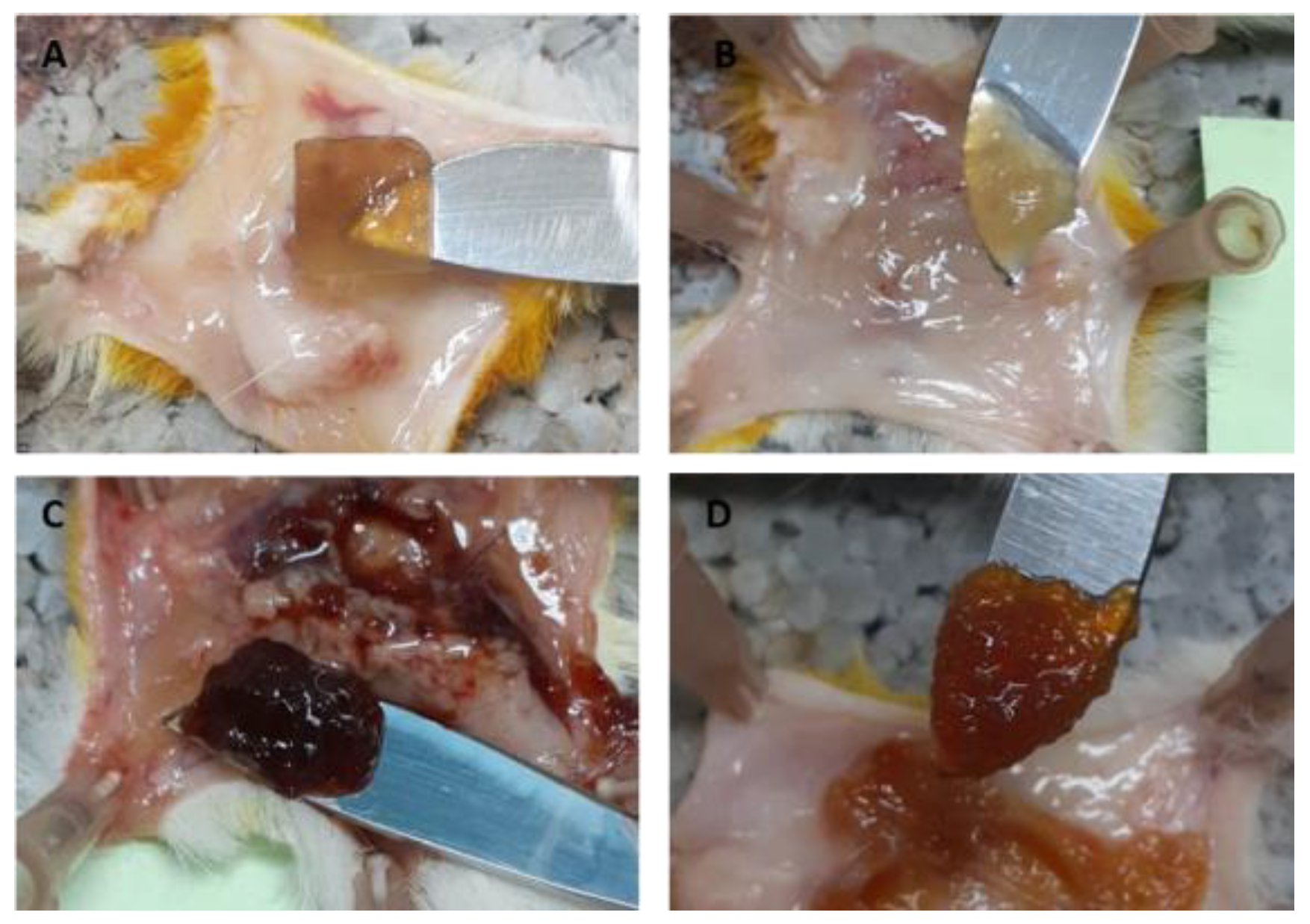
2.6. Subcutaneous Implantation of Pectin Hydrogels in Rats
3. Discussion
4. Materials and Methods
4.1. Preparation of Pectin Hydrogels
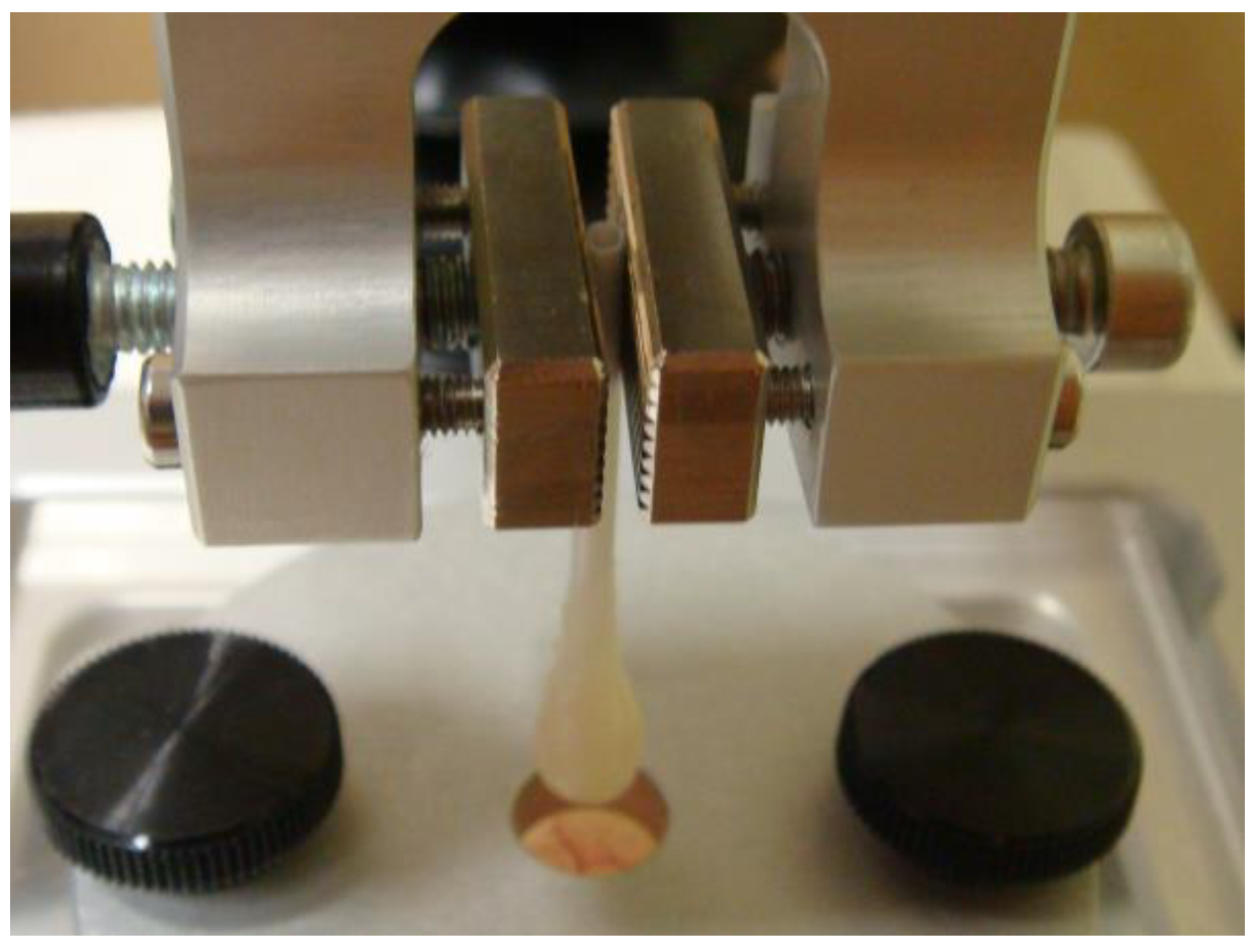
4.2. Mechanical Properties
4.3. Scanning Electron Microscopy
4.4. Stability of FeP Hydrogel
4.5. Serosal Adhesion
4.6. Subcutaneous Implantation of Hydrogels
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandran-Quintana, R.; Lopez-Franco, Y.; Rascon-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [Green Version]
- Moslemi, M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr. Polym. 2021, 254, 117324. [Google Scholar] [CrossRef] [PubMed]
- Ropartz, D.; Ralet, M.-C. Pectin structure. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer: Cham, Switzerland, 2020; pp. 17–36. Available online: https://link.springer.com/chapter/10.1007/978-3-030-53421-9_2 (accessed on 28 November 2022).
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Assifaoui, A.; Chambin, O. Pectin as drug-release vehicle. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer: Cham, Switzerland, 2020; pp. 189–207. Available online: https://link.springer.com/chapter/10.1007/978-3-030-53421-9_10 (accessed on 28 November 2022).
- Sriamornsak, P.; Wattanakorn, N.; Takeuchi, H. Study on the mucoadhesion mechanism of pectin by atomic force microscopy and mucin-particle method. Carbohydr. Polym. 2010, 79, 54–59. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Aliabadi, H.A.M.; Sheikhaleslami, S.; Akbarzadeh, A.R.; Hashemi, S.M.; Gorab, M.G.; Maleki, A.; Cohan, R.A.; Mahdavi, M.; et al. Recent advances on biomedical applications of pectin-containing biomaterials. Int. J. Biol. Macromol. 2022, 217, 1–18. [Google Scholar] [CrossRef]
- Servais, A.B.; Kienzle, A.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharide adhesion to the glycocalyx of visceral mesothelium. Tissue Eng. Part A 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Servais, A.B.; Kienzle, A.; Ysasi, A.B.; Valenzuela, C.D.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharides as air-tight sealants of the human pleura. J. Biol. Mater. Res. 2019, 107, 799–806. [Google Scholar] [CrossRef]
- Kuckelman, J.; Conner, J.; Zheng, Y.; Pierce, A.; Jones, I.; Lammers, D.; Cuadrado, D.; Eckert, M.; Mentzer, S. Improved Outcomes Utilizing a Novel Pectin-Based Pleural Sealant Following Acute Lung Injury. J. Trauma Acute Care Surg. 2020, 89, 915–919. [Google Scholar] [CrossRef]
- Zheng, Y.; Pierce, A.F.; Wagner, W.L.; Khalil, H.A.; Chen, Z.; Servais, A.B.; Ackermann, M.; Mentzer, S.J. Functional Adhesion of Pectin Biopolymers to the Lung Visceral Pleura. Polymers 2021, 13, 2976. [Google Scholar] [CrossRef]
- Zheng, Y.; Pierce, A.F.; Wagner, W.L.; Khalil, H.A.; Chen, Z.; Funaya, C.; Ackermann, M.; Mentzer, S.J. Biomaterial-Assisted Anastomotic Healing: Serosal Adhesion of Pectin Films. Polymers 2021, 13, 2811. [Google Scholar] [CrossRef]
- Pierce, A.; Zheng, Y.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Ackermann, M.; Mentzer, S.J. Visualizing pectin polymer-polymer entanglement produced by interfacial water movement. Carbohydr. Polym. 2020, 246, 116618. [Google Scholar] [CrossRef]
- Rajput, K.; Tawade, S.; Nangare, S.; Shirsath, N.; Bari, S.; Zawar, L. Formulation, optimization, and in-vitro-ex-vivo evaluation of dual-crosslinked zinc pectinate-neem gum-interpenetrating polymer network mediated lansoprazole loaded floating microbeads. Int. J. Biol. Macromol. 2022, 222, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Sarioglu, E.; Kocaaga, B.A.; Turan, D.; Batirel, S.; Guner, F.S. Theophylline-loaded pectin-based hydrogels. II. Effect of concentration of initial pectin solution, crosslinker type and cation concentration of external solution on drug release profile. J. Appl. Polym. Sci. 2019, 136, 48155. [Google Scholar] [CrossRef]
- Assifaoui, A.; Lerbret, A.; Uyen, H.T.D.; Neiers, F.; Chambin, O.; Loupiac, C.; Cousin, F. Structural behaviour differences in low methoxy pectin solutions in the presence of divalent cations (Ca2+ and Zn2+): A process driven by the binding mechanism of the cation with the galacturonate unit. Soft Matter. 2015, 11, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Kulikouskaya, V.; Kraskouski, A.; Hileuskaya, K.; Zhura, A.; Tratsyak, S.; Agabekov, V. Fabrication and characterization of pectin-based three-dimensional porous scaffolds suitable for treatment of peritoneal adhesions. J. Biomed. Mater. Res. 2019, 107, 1814–1823. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Cury, B.S.F.; Evangelista, R.C. Mucoadhesive beads of gellan gum/pectin intended to controlled delivery of drugs. Carbohydr. Polym. 2014, 113, 286–295. [Google Scholar] [CrossRef]
- Günter, E.A.; Popeyko, O.V.; Melekhin, A.K.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G. Preparation and properties of the pectic gel microparticles based on the Zn2+, Fe3+ and Al3+ cross-linking cations. Int. J. Biol. Macromol. 2019, 138, 629–635. [Google Scholar] [CrossRef]
- El-Zahaby, S.A.; Kassem, A.A.; El-Kamel, A.H. Formulation and in vitro evaluation of size expanding gastro-retentive systems of levofloxacin hemihydrate. Int. J. Pharm. 2014, 464, 10–18. [Google Scholar] [CrossRef]
- Gadalla, H.H.; El-Gibaly, I.; Soliman, G.M.; Mohamed, F.A.; El-Sayed, A.M. Amidated pectin/sodium carboxymethylcellulose microspheres as a new carrier for colonic drug targeting: Development and optimization by factorial design. Carbohydr. Polym. 2016, 153, 526–534. [Google Scholar] [CrossRef]
- Wagner, W.L.; Zheng, Y.; Pierce, A.; Ackermann, M.; Horstmann, H.; Kuner, T.; Ronchi, P.; Schwab, Y.; Konitzke, P.; Wunnemann, F.; et al. Mesopolysaccharides: The extracellular surface layer of visceral organs. PLoS ONE 2020, 15, e0238798. [Google Scholar] [CrossRef]
- Auriemma, G.; Cerciello, A.; Aquino, R.P.; Del Gaudio, P.; Fusco, B.M.; Russo, P. Pectin and Zinc Alginate: The Right Inner/Outer Polymer Combination for Core-Shell Drug Delivery Systems. Pharmaceutics 2020, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Popov, S.; Paderin, N.; Chistiakova, E.; Ptashkin, D.; Markov, P.A. Effect of Cross-Linking Cations on In Vitro Biocompatibility of Apple Pectin Gel Beads. Int. J. Mol. Sci. 2022, 23, 14789. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, H.; Qin, Z.; Che, P.; Yi, X.; Yu, Q.; Zhang, H.; Sun, X.; Yao, F.; Li, J. Fully physically crosslinked pectin-based hydrogel with high stretchability and toughness for biomedical application. Int. J. Biol. Macromol. 2020, 149, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, B.; Nakauma, M.; Funami, T.; Nishinari, K.; Draget, K.I.; Phillips, G.O.; Fang, Y. Modulation of calcium-induced gelation of pectin by oligoguluronate as compared to alginate. Food Res. Int. 2018, 116, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A. Mucoadhesive Polymers Basics, Strategies, and Future Trends; CRC Press: Boca Raton, FL, USA, 2013; pp. 193–220. [Google Scholar]
- Siew, C.K.; Williams, P.A.; Young, N. New insights into the mechanism of gelation of alginate and pectin: Charge annihilation and reversal mechanism. Biomacromolecules 2005, 6, 963–969. [Google Scholar] [CrossRef]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The blood compatibility challenge. Part 2: Protein adsorption phenomena governing blood reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef]
- Gorbet, M.; Sperling, C.; Maitz, M.F.; Siedlecki, C.A.; Werner, C.; Sefton, M.V. The blood compatibility challenge. Part 3: Material associated activation of blood cascades and cells. Acta Biomater. 2019, 94, 25–32. [Google Scholar] [CrossRef]
- Popov, S.; Paderin, N.; Khramova, D.; Kvashninova, E.; Melekhin, A.; Vityazev, F. Characterization and Biocompatibility Properties In Vitro of Gel Beads Based on the Pectin and κ-Carrageenan. Mar. Drugs 2022, 20, 94. [Google Scholar] [CrossRef]
- Popov, S.; Paderin, N.; Khramova, D.; Kvashninova, E.; Patova, O.; Vityazev, F. Swelling, Protein Adsorption, and Biocompatibility In Vitro of Gel Beads Prepared from Pectin of Hogweed Heracleum sosnówskyi Manden in Comparison with Gel Beads from Apple Pectin. Int. J. Mol. Sci. 2022, 23, 3388. [Google Scholar] [CrossRef]
- Popov, S.; Smirnov, V.; Paderin, N.; Khramova, D.; Chistiakova, E.; Vityazev, F.; Golovchenko, V. Enrichment of Agar Gel with Antioxidant Pectin from Fireweed: Mechanical and Rheological Properties, Simulated Digestibility, and Oral Processing. Gels 2022, 8, 708. [Google Scholar] [CrossRef] [PubMed]







| Hydrogel; Ion Concentration | Hardness (N) | Young Modulus (kPa) | Brittleness (mm) | Adhesiveness (N) |
|---|---|---|---|---|
| FeP; 21 mM | 1.97 ± 0.26 a# | 2429 ± 1537 a# | 1.6 ± 0.4 a | 0.10 ± 0.02 a# |
| FeP; 42 mM | 2.44 ± 0.18 b# | 8435 ± 4115 b# | 0.9 ± 0.3 b# | 0.19 ± 0.03 b# |
| FeP; 84 mM | 1.98 ± 0.15 a# | 5882 ± 1824 b# | 1.0 ± 0.3 b# | 0.19 ± 0.07 b# |
| CaP; 31 mM | 0.44 ± 0.02 a | 1182 ± 73 a | 1.4 ± 0.1 a | 0.12 ± 0.01 a |
| CaP; 62 mM | 0.58 ± 0.02 b | 1319 ± 173 a | 1.5 ± 0.1 a | 0.11 ± 0.03 a |
| CaP; 124 mM | 0.67 ± 0.05 c | 1395 ± 281 a | 1.6 ± 0.2 b | 0.13 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, S.; Paderin, N.; Chistiakova, E.; Ptashkin, D. Serosal Adhesion Ex Vivo of Hydrogels Prepared from Apple Pectin Cross-Linked with Fe3+ Ions. Int. J. Mol. Sci. 2023, 24, 1248. https://doi.org/10.3390/ijms24021248
Popov S, Paderin N, Chistiakova E, Ptashkin D. Serosal Adhesion Ex Vivo of Hydrogels Prepared from Apple Pectin Cross-Linked with Fe3+ Ions. International Journal of Molecular Sciences. 2023; 24(2):1248. https://doi.org/10.3390/ijms24021248
Chicago/Turabian StylePopov, Sergey, Nikita Paderin, Elizaveta Chistiakova, and Dmitry Ptashkin. 2023. "Serosal Adhesion Ex Vivo of Hydrogels Prepared from Apple Pectin Cross-Linked with Fe3+ Ions" International Journal of Molecular Sciences 24, no. 2: 1248. https://doi.org/10.3390/ijms24021248





