Involvement of the Membrane Nanodomain Protein, AtFlot1, in Vesicular Transport of Plasma Membrane H+-ATPase in Arabidopsis thaliana under Salt Stress
Abstract
:1. Introduction
2. Results
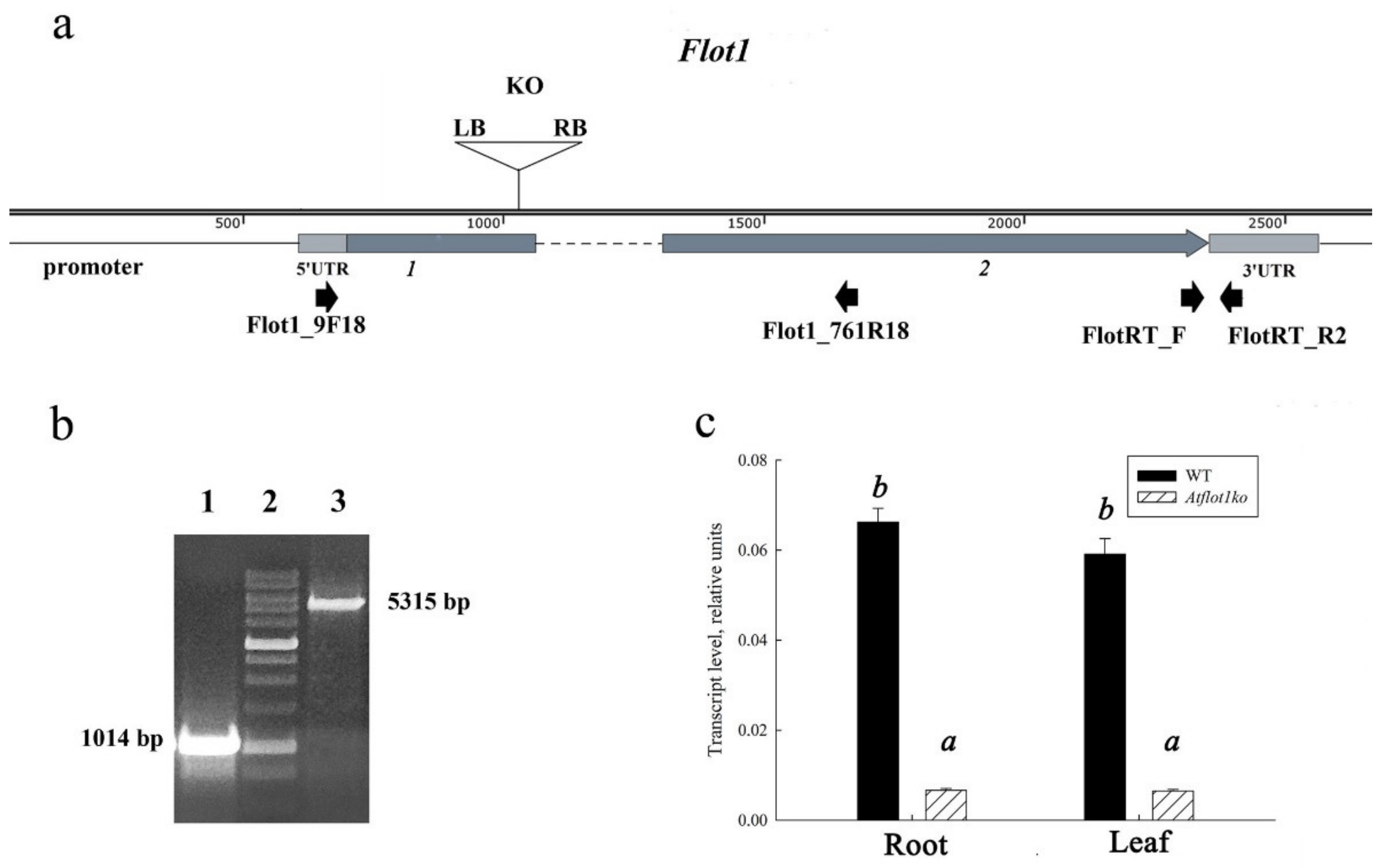
2.1. Characterization of SALK_205125C Mutant Plants
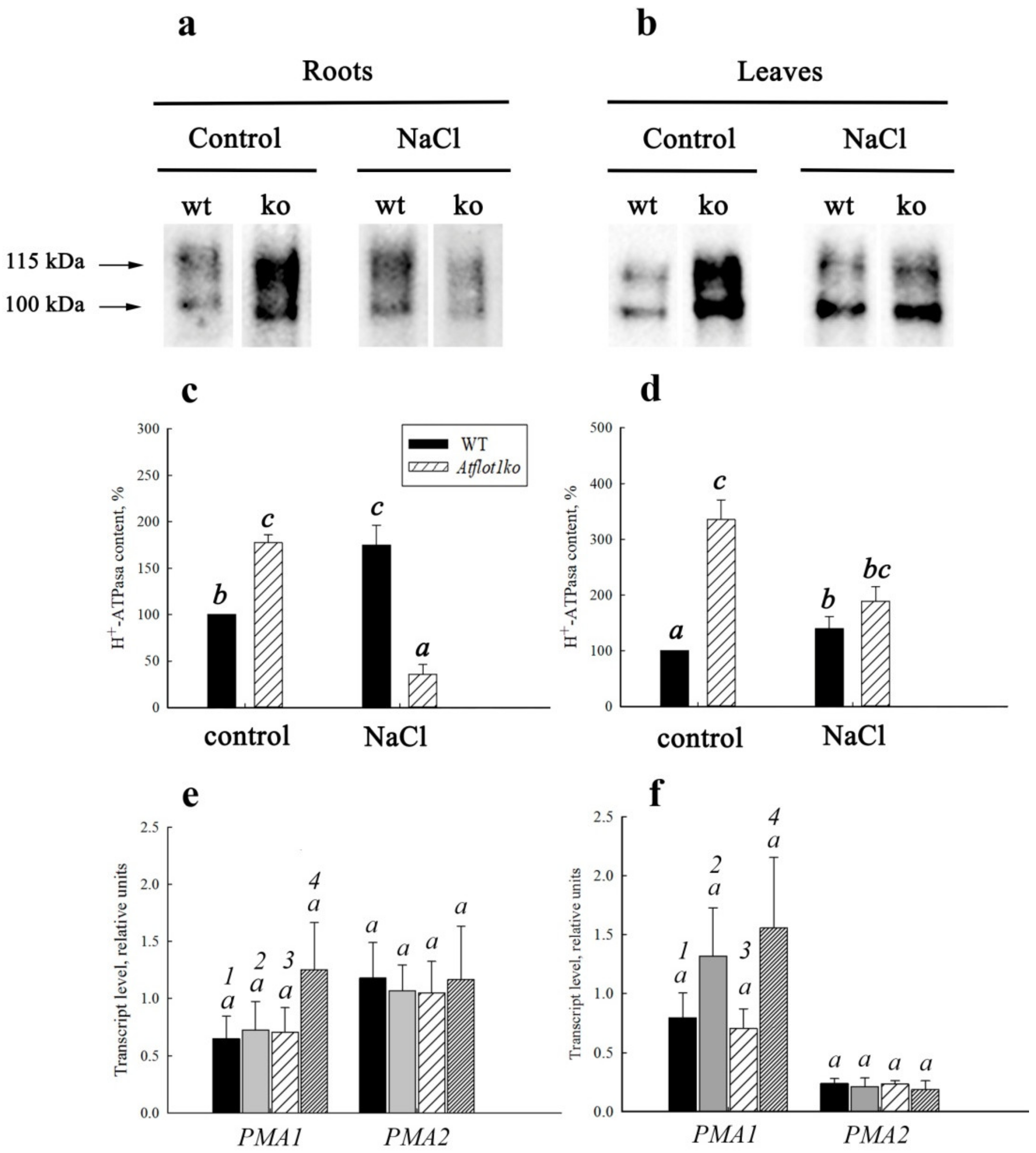
2.2. Immunoblot Analysis of Plasma Membrane H+-ATPase in Atflot1ko Mutants
2.3. Expression of Plasma Membrane H+-ATPase Genes in Atflot1ko Mutant
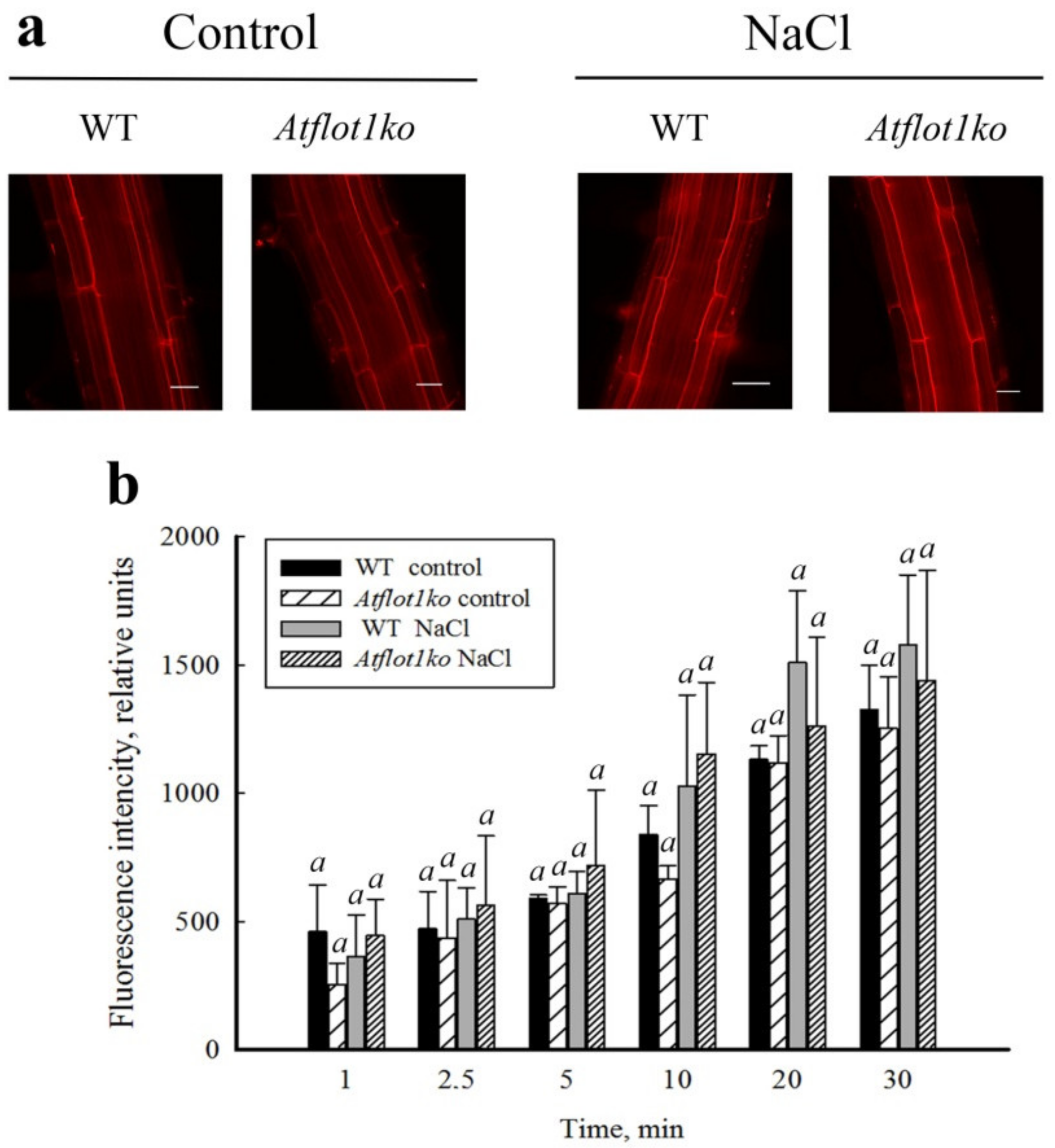
2.4. Endocytic Probe FM4-64 Uptake by Roots of Atflot1ko Mutant Seedlings
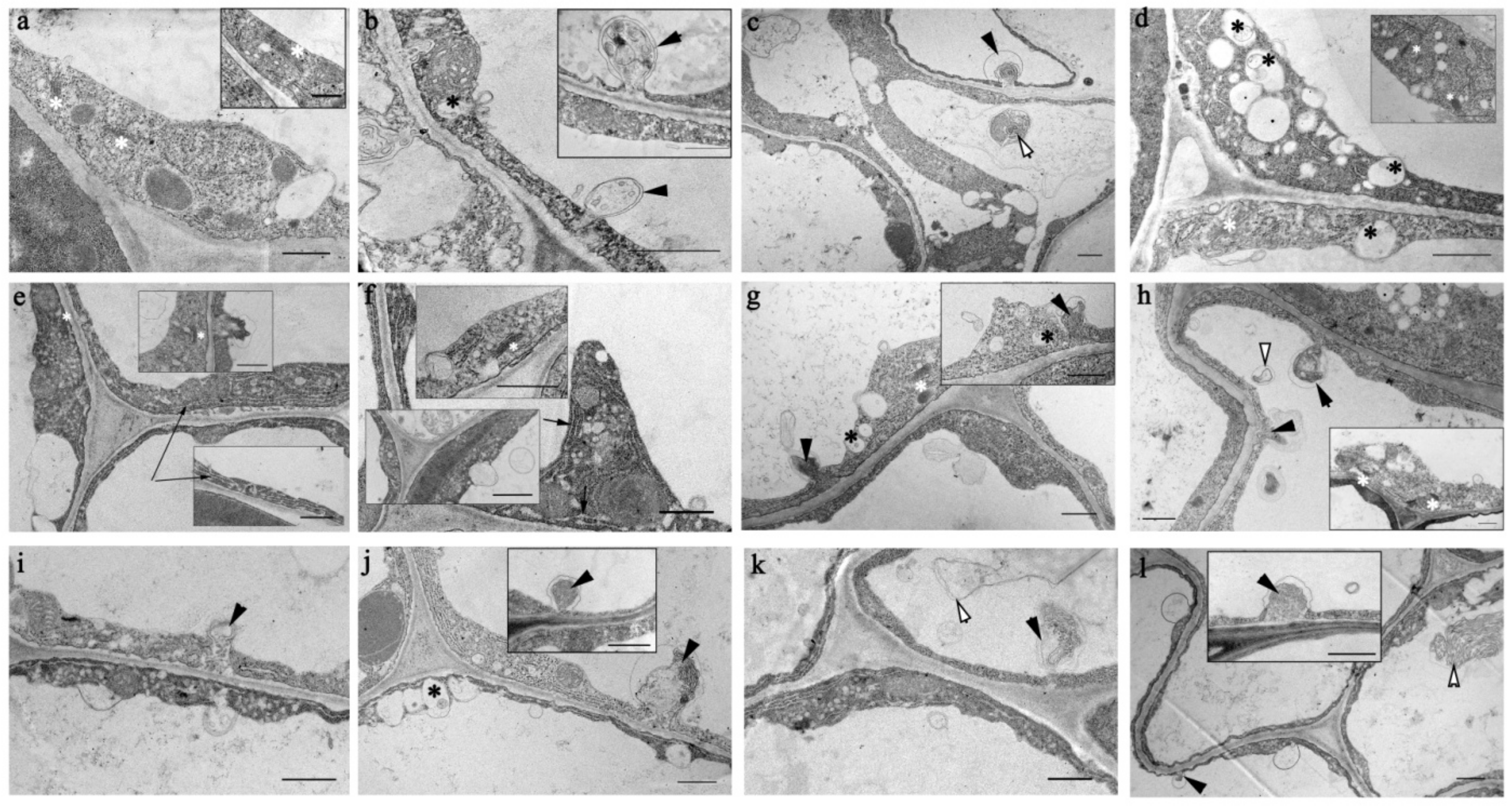
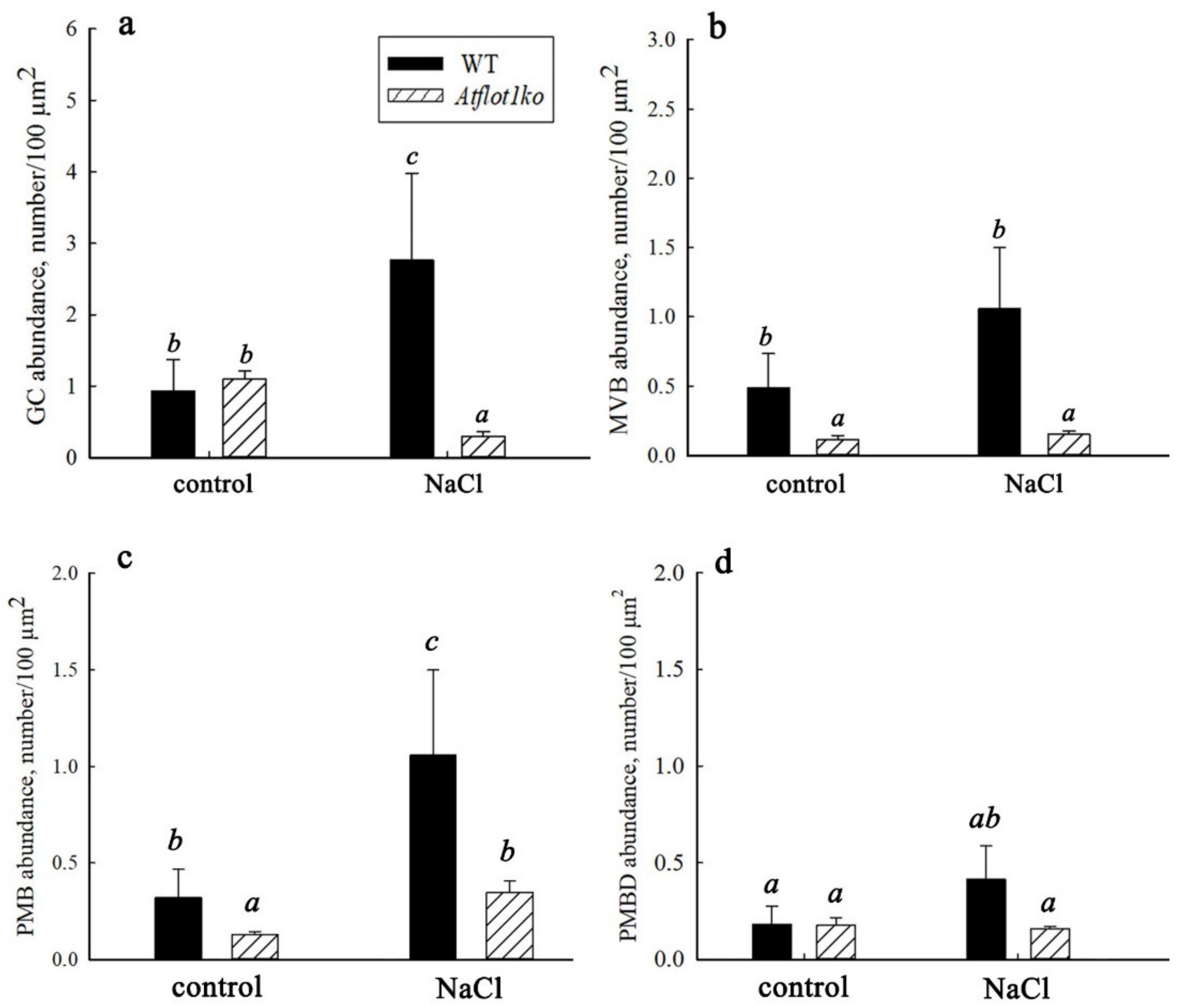
2.5. Ultrastructural Peculiarities of Root Cells in the Knockout Mutant Atflot1ko Plants
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Isolation of Plant Genomic DNA
4.3. Determination of Homozygosity of SALK_205125C Mutants
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. FM4-64 Endocytic Probe Uptake Assay
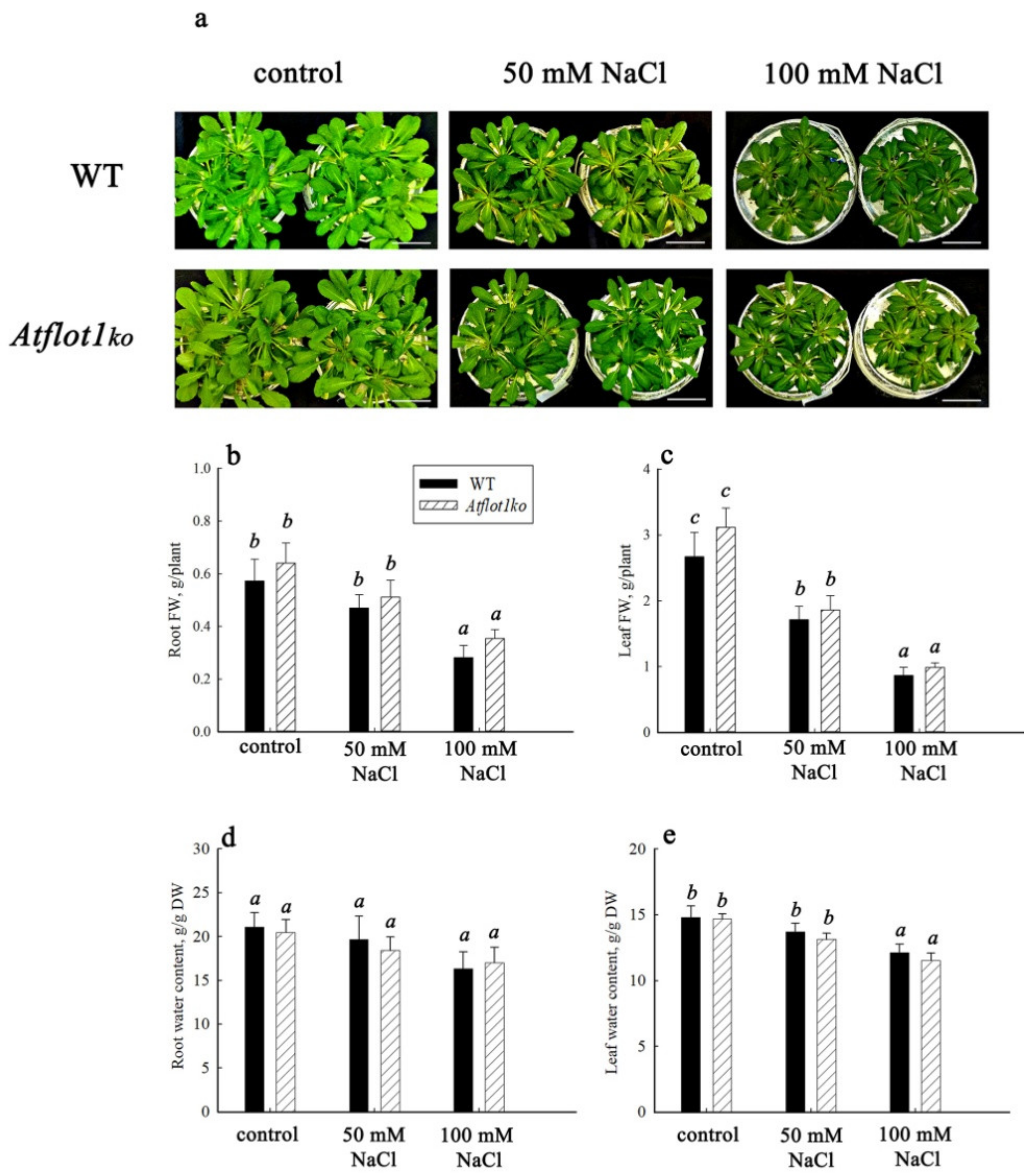
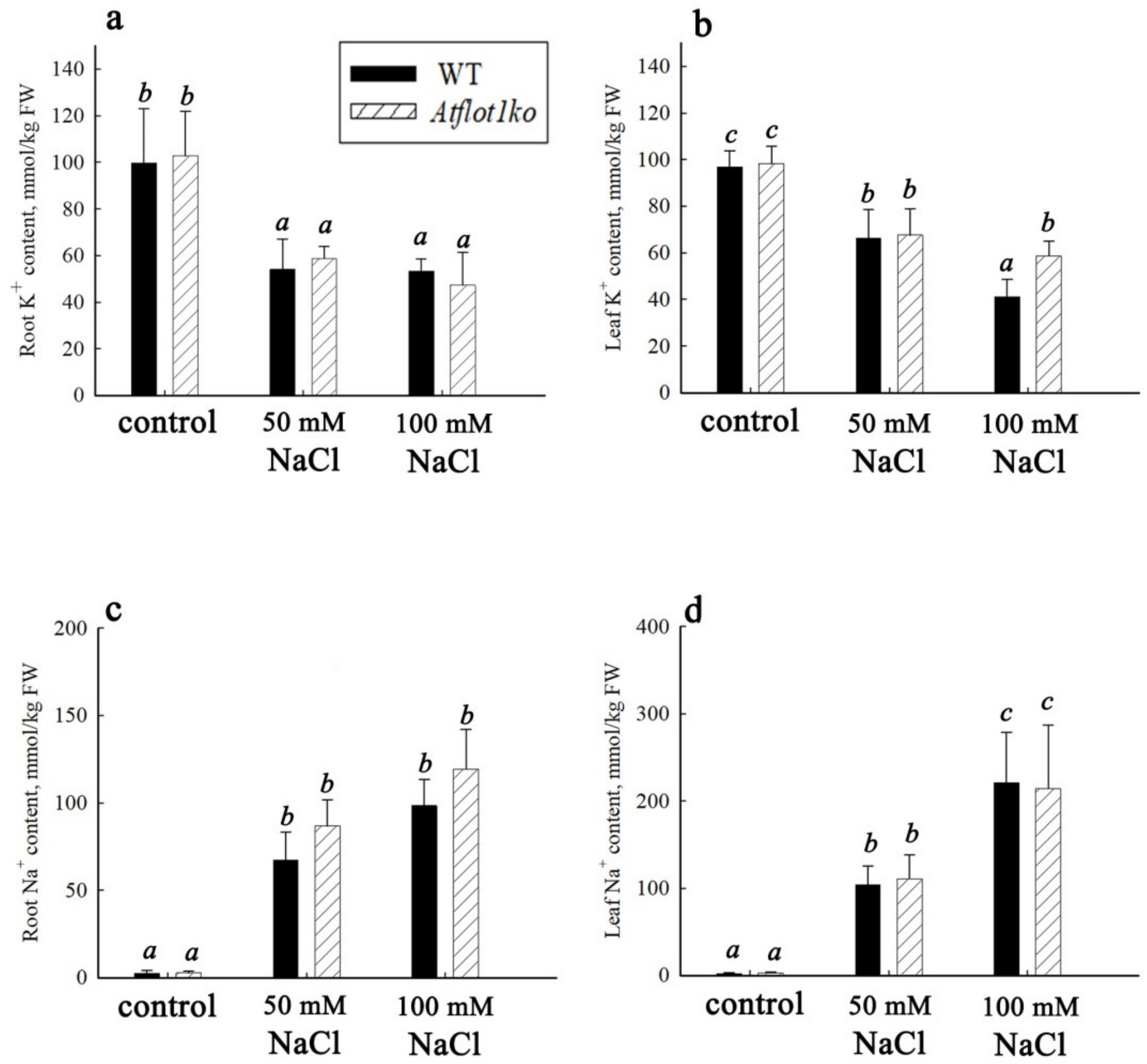
4.6. Growth Characteristics, Determination of Water, Na+, and K+ Content in Plant Organs
4.7. Ultrastructure Studies of A. thaliana Seedling Root Cells
4.8. Isolation of the PM-Enriched Membrane Fraction from Roots of A. thaliana
4.9. Immunoblotting
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, L.G.; Hamid, E.; Shin, W.; Chiang, H.C. Exocytosis and endocytosis: Modes, functions, and coupling mechanisms. Annu. Rev. Physiol. 2014, 76, 301–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Li, R.; Pan, J.; Ding, Z.; Lin, J. Endocytosis and its regulation in plants. Trends Plant Sci. 2015, 20, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Valencia, J.P.; Goodman, K.; Otegui, M.S. Endocytosis and endosomal trafficking in plants. Annu. Rev. Plant Biol. 2016, 67, 309–335. [Google Scholar] [CrossRef]
- Wang, X.; Chung, K.P.; Lin, W.; Jiang, L. Protein secretion in plants: Conventional and unconventional pathways and new techniques. J. Exp. Bot. 2018, 69, 21–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xing, J.; Lin, J. At the intersection of exocytosis and endocytosis in plants. New Phytol. 2019, 224, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, Y.; Shen, J. A Diverse membrane interaction network for plant multivesicular bodies: Roles in proteins vacuolar delivery and unconventional secretion. Front. Plant Sci. 2020, 11, 425. [Google Scholar] [CrossRef]
- Sutter, J.-U.; Sieben, C.; Hartel, A.; Eisenach, C.; Thiel, G.; Blatt, M.R. Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 2007, 17, 1396–1402. [Google Scholar] [CrossRef] [Green Version]
- Li, X.J.; Wang, X.H.; Yang, Y.; Li, R.L.; He, Q.H.; Fang, X.H.; Luu, D.T.; Maurel, C.; Lin, J.X. Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 2011, 23, 3780–3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.Q.; Fan, L.S.; Chen, T.; Li, R.L.; Li, X.J.; He, Q.H.; Botella, M.A.; Lin, J.X. Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 2014, 26, 1729–1745. [Google Scholar] [CrossRef] [Green Version]
- Takano, J.; Tanaka, M.; Toyoda, A.; Miwa, K.; Kasai, K.; Fuji, K.; Onouchi, H.; Naito, S.; Fujiwara, T. Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 5220–5225. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Wabnik, K.; Martiniere, A.; Langowski, L.; Willig, K.; Naramoto, S.; Leitner, J.; Tanaka, H.; Jakobs, S.; Robert, S.; et al. Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol. Syst. Biol. 2008, 7, e540. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M.; Zelaznya, E.; Robert, S.; Conéjéroa, G.; Curiea, C.; Friml, J.; Vert, G. Monoubiquitin-dependent endocytosis of the IRON-REGULATED TRANSPORTER 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. USA 2011, 108, E450–E458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, G.P.; Nichols, B.J. The roles of flotillin microdomains—Endocytosis and beyond. J. Cell Sci. 2011, 124, 3933–3940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Zhang, J.; Liu, Y.S.; Li, L.; He, Y.L. Research advances on flotillins. Virol. J. 2011, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Gauthier-Rouvière, C.; Bodin, S.; Comunale, F.; Planchon, D. Flotillin membrane domains in cancer. Cancer Metastasis Rev. 2020, 39, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Junková, P.; Daněk, M.; Brouzdová, J.; Kroumanová, K.; Zelazny, E.; Janda, M.; Hynek, R.; Martinec, J.; Valentová, O. Mapping of plasma membrane proteins interacting with Arabidopsis thaliana flotillin 2. Front. Plant Sci. 2018, 9, 991. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.-S.; Barkla, B.J.; Vera-Estrella, R.; Zhu, J.-K.; Schumaker, K.S. Na+/H+ exchange activity in the plasma membrane of Arabidopsis. Plant Physiol. 2003, 132, 1041–1052. [Google Scholar] [CrossRef] [Green Version]
- Kroumanova, K.; Kocourkova, D.; Daněk, M.; Lamparova, L.; Pospichalova, R.; Malinska, K.; Krĉkova, Z.; Burketova, L.; Valentová, O.; Martinec, J.; et al. Characterisation of Arabidopsis flotillins in response to stresses. Biol. Plant 2019, 63, 144–152. [Google Scholar] [CrossRef]
- Rosas-Santiago, P.; Lagunas-Gomez, D.; Barkla, B.J.; Vera-Estrella, R.; Lalonde, S.; Jones, A.; Frommer, W.B.; Zimmermannova, O.; Sychrova, H.; Pantoja, O. Identification of rice cornichon as a possible cargo receptor for the Golgi-localized sodium transporter OsHKT1;3. J. Exp. Bot. 2015, 66, 2733–2748. [Google Scholar] [CrossRef]
- Khalilova, L.A.; Sergienko, O.V.; Orlova, Y.V.; Myasoedov, N.A.; Karpichev, I.V.; Balnokin, Y.V. Arabidopsis thaliana mutant with T-DNA insertion in the Flot1 (At5g25250) gene promotor possesses increased resistance to NaCl. Russ. J. Plant Physiol. 2020, 67, 275–284. [Google Scholar] [CrossRef]
- Apse, M.P.; Blumwald, E. Na+ transport in plants. FEBS Lett. 2007, 581, 2247–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haruta, M.; Burch, H.L.; Nelson, R.B.; Barrett-Wilt, G.; Kline, K.G.; Mohsin, S.B.; Young, J.C.; Otegui, M.S.; Sussman, M.R. Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J. Biol. Chem. 2010, 285, 17918–17929. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, R.; Vert, G. Endocytosis in plants: Peculiarities and roles in the regulated trafficking of plant metal transporters. Biol. Cell 2021, 113, 1–13. [Google Scholar] [CrossRef]
- Kunzl, F.; Fruholz, S.; Fassler, F.; Li, B.; Pimpl, P. Receptor-mediated sorting of soluble vacuolar proteins ends at the trans-Golgi network/early endosome. Nat. Plants 2016, 2, 16017. [Google Scholar] [CrossRef]
- Robinson, D.G.; Neuhaus, J.M. Receptor-mediated sorting of soluble vacuolar proteins: Myths, facts, and a new model. J. Exp. Bot. 2016, 67, 4435–4449. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, G.D.; Wang, C.; Pan, J.; Bednarek, S.Y. Inroads into internalization: Five years of endocytic exploration. Plant Physiol. 2018, 176, 208–218. [Google Scholar] [CrossRef] [Green Version]
- Nagel, M.K.; Kalinowska, K.; Vogel, K.; Reynolds, G.D.; Wu, Z.; Anzenberger, F.; Ichikawa, M.; Tsutsumi, C.; Sato, M.H.; Kuster, B.; et al. Arabidopsis SH3P2 is an ubiquitin-binding protein that functions together with ESCRT-I and the deubiquitylating enzyme AMSH3. Proc. Natl. Acad. Sci. USA 2017, 114, E7197–E7204. [Google Scholar] [CrossRef] [Green Version]
- Heucken, N.; Ivanov, R. The retromer, sorting nexins and the plant endomembrane protein trafficking. J. Cell Sci. 2018, 131, jcs203695. [Google Scholar] [CrossRef]
- Khan, I.; Gratz, R.; Denezhkin, P.; Schott-Verdugo, S.N.; Angrand, K.; Genders, L.; Basgaran, R.M.; Fink-Straube, C.; Brumbarova, T.; Gohlke, H.; et al. Calcium-promoted interaction between the C2-Domain Protein EHB1 and metal transporter IRT1 inhibits Arabidopsis iron acquisition. Plant Physiol. 2019, 180, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Moulinier-Anzola, J.; Schwihla, M.; De-Araujo, L.; Artner, C.; Jorg, L.; Konstantinova, N.; Luschnig, C.; Korbei, B. TOLs function as ubiquitin receptors in the early steps of the ESCRT pathway in higher plants. Mol. Plant. 2020, 13, 717–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Yang, C.; Liu, C.; Yang, L.; Yang, S.; Zhou, J.; Li, F.; Jiang, L.; Xiao, S.; Gao, C.; et al. SINAT E3 ligases regulate the stability of the ESCRT component FREE1 in response to iron deficiency in plants. J. Integr. Plant Biol. 2020, 62, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, E. Vesicle-mediated solute transport between the vacuole and the plasma membrane. Plant Physiol. 2000, 123, 1217–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contento, A.L.; Bassham, D.C. Structure and function of endosomes in plant cells. J. Cell Sci. 2012, 125, 3511–3518. [Google Scholar] [CrossRef] [Green Version]
- Maricchiolo, E.; Panfili, E.; Pompa, A.; De Marchis, F.; Bellucci, M.; Pallotta, M.T. Unconventional Pathways of Protein Secretion: Mammals vs. Plants. Front. Sec. Membr. Traffic. 2022, 10, 895853. [Google Scholar] [CrossRef]
- Palmgren, M.; Morsomme, P. The plasma membrane H+-ATPase, a simple polypeptide with a long history. Yeast 2019, 36, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Baumann, C.A.; Ribon, V.; Kanzaki, M.; Thurmond, D.C.; Mora, S.; Shigematsu, S.; Bickel, P.E.; Pessin, J.E.; Saltiel, A.R. CAP defines a second signaling pathway required for insulin-stimulated glucose transport. Nature 2000, 407, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Daněk, M.; Valentová, O.; Martinec, J. Flotillins, erlins, and HIRs: From animal base camp to plant new horizons. Crit. Rev. Plant Sci. 2016, 35, 191–214. [Google Scholar] [CrossRef]
- Mazel, A.; Leshem, Y.; Tiwari, B.S.; Levine, A. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol. 2004, 134, 118–128. [Google Scholar] [CrossRef]
- Leshem, Y.; Melamed˗Book, N.; Cagnac, O.; Ronen, G.; Nishri, Y.; Solomon, M.; Cohen, G.; Levine, A. Suppression of Arabidopsis vesicle—SNARE expression inhibited fusion of H2O2 containing vesicles with tonoplast and increased salt tolerance. Proc. Natl. Acad. Sci. USA 2006, 103, 18008–18013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamaji, K.; Nagira, M.; Yoshida, K.; Ohnishi, M.; Oda, Y.; Uemura, T.; Goh, T.; Sato, M.H.; Morita, M.T.; Tasaka, M.; et al. Dynamic aspects of ion accumulation by vesicle traffic under salt stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2023–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebine, K.; Fujimoto, M.; Okatani, Y.; Nishiyama, T.; Goh, T.; Ito, E.; Dainobu, T.; Nishitani, A.; Uemura, T.; Sato, M.H.; et al. A membrane trafficking pathway regulated by the plant—Specific RAB GTPase ARA6. Nat. Cell Biol. 2011, 13, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Baral, A.; Irani, N.G.; Fujimoto, M.; Nakano, A.; Mayor, S.; Mathew, M.K. Salt–Induced remodeling of spatially restricted clathrin–independent endocytic pathways in Arabidopsis root. Plant Cell 2015, 27, 1297–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Liu, P.; Wan, Y.; Chen, T.; Wang, Q.; Mettbach, U.; Baluska, F.; Samaj, J.; Fang, X.; Lucas, W.J.; et al. A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell 2012, 24, 2105–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prelich, G. Gene Overexpression: Uses, mechanisms, and interpretation genetics. Genetics 2012, 190, 841–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergienko, O.V.; Khalilova, L.A.; Orlova, Y.V.; Shuvalov, A.V.; Myasoedov, N.A.; Karpichev, I.V. Mutation of the ARA7/AtRabF2b gene, that increases the content of the Ara7 protein regulating endocytic trafficking pathways, improves salt tolerance of the Arabidopsis thaliana (L.) Heynh plants. Russ. J. Plant Physiol. 2022, 69, e11. [Google Scholar] [CrossRef]
- Balnokin, Y.V.; Kurkova, E.B.; Khalilova, L.A.; Myasoedov, N.A.; Yusufov, A.G. Pinocytosis in the cells of the root of the salt-accumulating halophyte Suaeda altissima and its possible involvement in chloride transport. Russ. J. Plant Physiol. 2007, 54, 797–805. [Google Scholar] [CrossRef]
- Larsson, C.; Widell, S.; Kjellbom, P. Preparation of high-purity plasma membranes. Methods Enzymol. 1987, 148, 558–568. [Google Scholar]

| Name | Sequence 5′–3′ | Experimental Purpose |
|---|---|---|
| AtPMA1_F1 | AGG TGT GAT TTG GAT TTA CAG T | PMA1/PMA2/AtFlot1/ACT2 gene expression studies using quantitative real-time RT-PCR (qRT-PCR) |
| AtPMA1_R1 | CTG TTG TCA AAC AAG CTG G | |
| AtPMA2_F1 | CTC AAC TTG TTT GAG AAC AAG ACG GC | |
| AtPMA2_R1 | GGC TGT AAA CCG TGA AGT GTC C | |
| ACT2F | CTTGCACCAAGCAGCATGAA | |
| ACT2R | CCGATCCAGACACTGTACTTCCTT | |
| FlotRT_F | GGA ACC GAA GCA AGT GAC TC | |
| FlotRT_R2 | TTC GCA TGG ATG TAT CTT CAA CCA C | |
| Flot1_9F18 | CTAAATCATTTGGGCGAA | Confirmation of a T-DNA insert presence in the nucleotide sequence of the AtFlot1 gene in Atflot1ko (SALK_205125C) mutant plants |
| Flot1_761R18 | TTAGCTACATCAGCCTCC |
| Genotype | WT | Atflot1ko |
|---|---|---|
| H+-ATPase content in PM | Increased | Decreased |
| Endocytosis activity (FM4-64 uptake) | No effect | No effect |
| GC and ER activity (cytoplasmic vesicles content) | Increased | Increased |
| GC content in cytoplasm | Increased | Decreased |
| MVB content in cytoplasm | Increased | No effect (in both control and saline conditions, MVB content is very low) |
| PMB content in the cells | Increased | Increased |
| PMBD content in vacuoles | Increased | No effect |
| H+-ATPase content in PM | Increased | Decreased |
| Endocytosis activity (FM4-64 uptake) | No effect | No effect |
| GC and ER activity (cytoplasmic vesicles content) | Increased | Increased |
| GC content in cytoplasm | Increased | Decreased |
| MVB content in cytoplasm | Increased | No effect (in both control and saline conditions, MVB content is very low) |
| PMB content in the cells | Increased | Increased |
| PMBD content in vacuoles | Increased | No effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalilova, L.A.; Lobreva, O.V.; Nedelyaeva, O.I.; Karpichev, I.V.; Balnokin, Y.V. Involvement of the Membrane Nanodomain Protein, AtFlot1, in Vesicular Transport of Plasma Membrane H+-ATPase in Arabidopsis thaliana under Salt Stress. Int. J. Mol. Sci. 2023, 24, 1251. https://doi.org/10.3390/ijms24021251
Khalilova LA, Lobreva OV, Nedelyaeva OI, Karpichev IV, Balnokin YV. Involvement of the Membrane Nanodomain Protein, AtFlot1, in Vesicular Transport of Plasma Membrane H+-ATPase in Arabidopsis thaliana under Salt Stress. International Journal of Molecular Sciences. 2023; 24(2):1251. https://doi.org/10.3390/ijms24021251
Chicago/Turabian StyleKhalilova, Lyudmila A., Olga V. Lobreva, Olga I. Nedelyaeva, Igor V. Karpichev, and Yurii V. Balnokin. 2023. "Involvement of the Membrane Nanodomain Protein, AtFlot1, in Vesicular Transport of Plasma Membrane H+-ATPase in Arabidopsis thaliana under Salt Stress" International Journal of Molecular Sciences 24, no. 2: 1251. https://doi.org/10.3390/ijms24021251







