Abstract
This review provides an overview of histopathology, clinical presentation, molecular pathways, and potential new systemic treatments of high-grade chondrosarcomas (CS), including grade 2–3 conventional, dedifferentiated, and mesenchymal CS. The diagnosis of CS combines radiological and histological data in conjunction with patient clinical presentations. Conventional CS is the most frequent subtype of CS (85%) and represents about 25% of primary bone tumors in adults; they can be categorized according to their bone location into central, peripheral, and periosteal chondrosarcomas. Central and peripheral CS differ at the molecular level with either IDH1/2 mutations or EXT1/2 mutations, respectively. CDKN2A/B deletions are also frequent in conventional CS, as well as COL2A1 mutations. Dedifferentiated CS develops when low-grade conventional CS transforms into a high-grade sarcoma and most frequently exhibits features of osteosarcoma, fibrosarcoma, or undifferentiated pleomorphic sarcoma. Their molecular characteristics are similar to conventional CS. Mesenchymal CS is a totally different pathological entity exhibiting recurrent translocations. Their clinical presentation and management are different too. The standard treatment of CSs is wide en-bloc resection. CS are relatively radiotherapy resistant; therefore, doses >60 Gy are needed in an attempt to achieve local control in unresectable tumors. Chemotherapy is possibly effective in mesenchymal chondrosarcoma and is of uncertain value in dedifferentiated chondrosarcoma. Due to resistance to standard anticancer agents, the prognosis is poor in patients with metastatic or unresectable chondrosarcomas. Recently, the refined characterization of the molecular profile, as well as the development of new treatments, allow new therapeutic options for these rare tumors. The efficiency of IDH1 inhibitors in other malignancies suggests that these inhibitors will be part of IDH1/2 mutated conventional CS management soon. Other treatment approaches, such as PIK3-AKT-mTOR inhibitors, cell cycle inhibitors, and epigenetic or immune modulators based on improving our understanding of CS molecular biology, are emerging.
1. Introduction
Chondrosarcomas (CS) are rare mesenchymal neoplasms that are defined by the production of an abnormal cartilaginous matrix. They define a diverse group of sarcomas with varied morphological features and different clinical behaviors. CSs account for 25% of primary bone tumors, with about 150 new diagnosed cases per year in France [1]. These are mostly adult tumors.
The most common locations of CS include the pelvis and the proximal femur. CS can be endo-medullary or grow on the bone surface. They can develop in a healthy bone or occur in a pre-existing bone lesion (chondroma or osteochondroma, rarely in Paget’s disease). Some radiation-induced CS have been described in old series or in rare case reports. They most likely represent chondroblastic osteosarcomas [2,3]. In the context of enchondromatosis (Ollier’s disease and Maffucci syndrome), the risk of malignant transformation of enchondromas is increased (46% and 57%, respectively) [4].
The diagnosis of CS is based on a multidisciplinary approach considering radio-pathological correlations in conjunction with patient clinical presentation. Standard radiograph and computed tomography (CT) often suggest the diagnosis of CS because of the identification of typical “ring-and-arc” chondroid matrix mineralization and aggressive features (deep endosteal scalloping and soft-tissue extensions). Additional imaging modality, including magnetic resonance imaging (MRII), is frequently employed to evaluate staging and guide surgical resection.
Conventional CS is the most common pathological subtype. Surgical excision is the primary treatment modality of CS. The treatment of conventional CS is problematic in unresectable or metastatic diseases because of its primary resistance to standard sarcoma chemotherapy regimens.
Other subtypes of CS are significantly less common. Dedifferentiated CS are biphasic tumors with a low-grade conventional CS component juxtaposed to a non-cartilaginous high-grade sarcoma, most frequently exhibiting features of osteosarcoma, fibrosarcoma, or undifferentiated pleomorphic sarcoma. Mesenchymal CS (MCS) is a different pathological entity. It is a highly malignant tumor exhibiting a bimorphic histological pattern, with an undifferentiated small round cell component admixed with islands of well-differentiated cartilage. Chemotherapy is possibly effective in mesenchymal chondrosarcoma and is of uncertain value in grade 3 conventional chondrosarcoma and in dedifferentiated chondrosarcoma; these subtypes are rare and bear a poor prognosis.
Understanding the different histological subtypes of CS is helpful in predicting biological behavior. In this review, a summary is given about CS clinical behavior, pathological characteristics, and current treatment modalities. In the following, we will also discuss the molecular pathways in high-grade CS, including conventional CS, dedifferentiated CS, and mesenchymal CS, and a schematic illustration of key signaling pathways underpinning CS genesis. Furthermore, published and ongoing clinical trials for CS patients will also be presented.
2. Clinical, Pathological and Molecular Characteristics
There are several CS subtypes (Table 1). We will describe all the subtypes according to the 2020 WHO classification of tumors on bone and soft tissues, which notably identifies conventional chondrosarcoma, dedifferentiated chondrosarcoma, and mesenchymal chondrosarcoma. We will highlight the main clinical and molecular characteristics of these subtypes. We will not describe in this review clear cell chondrosarcoma, which is commonly considered a low-grade tumor, is less aggressive and usually treated with surgery alone, as well as extraskeletal myxoid chondrosarcoma, which is a soft tissue sarcoma (Table 1).

Table 1.
Chondrosarcoma histological subtypes. We excluded clear cell chondrosarcomas (CS) which represent around 2.5% of all CS.
2.1. Conventional Chondrosarcoma
Conventional chondrosarcoma is the most frequent subtype of chondrosarcoma (85%) and represents about 25% of primary bone tumors. Conventional CSs can be categorized according to their bone location in central and peripheral CSs [5] (Figure 1). The vast majority (±85%) are central CSs, designated as such based on their central location within the medullar cavity. Peripheral chondrosarcomas are intermediate-grade to the high-grade malignant cartilaginous matrix–producing neoplasms that originate from the bone surface of a pre-existing osteochondroma. These tumors most commonly arise in osteochondromas of the pelvis, trunk, and proximal femur. Finally, a minority (<1%) occurs on the surface of the bone, in between the cortical bone and the periosteum, and invades the underlying cortex or is >5 cm. This latter entity is named periosteal CS [6].
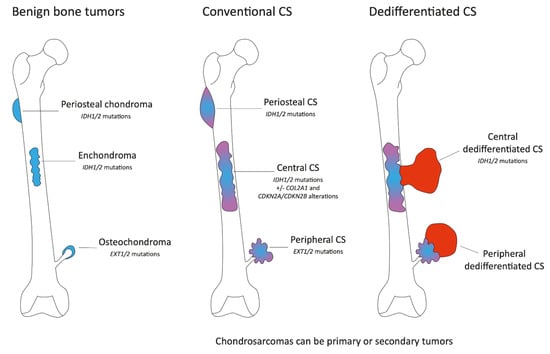
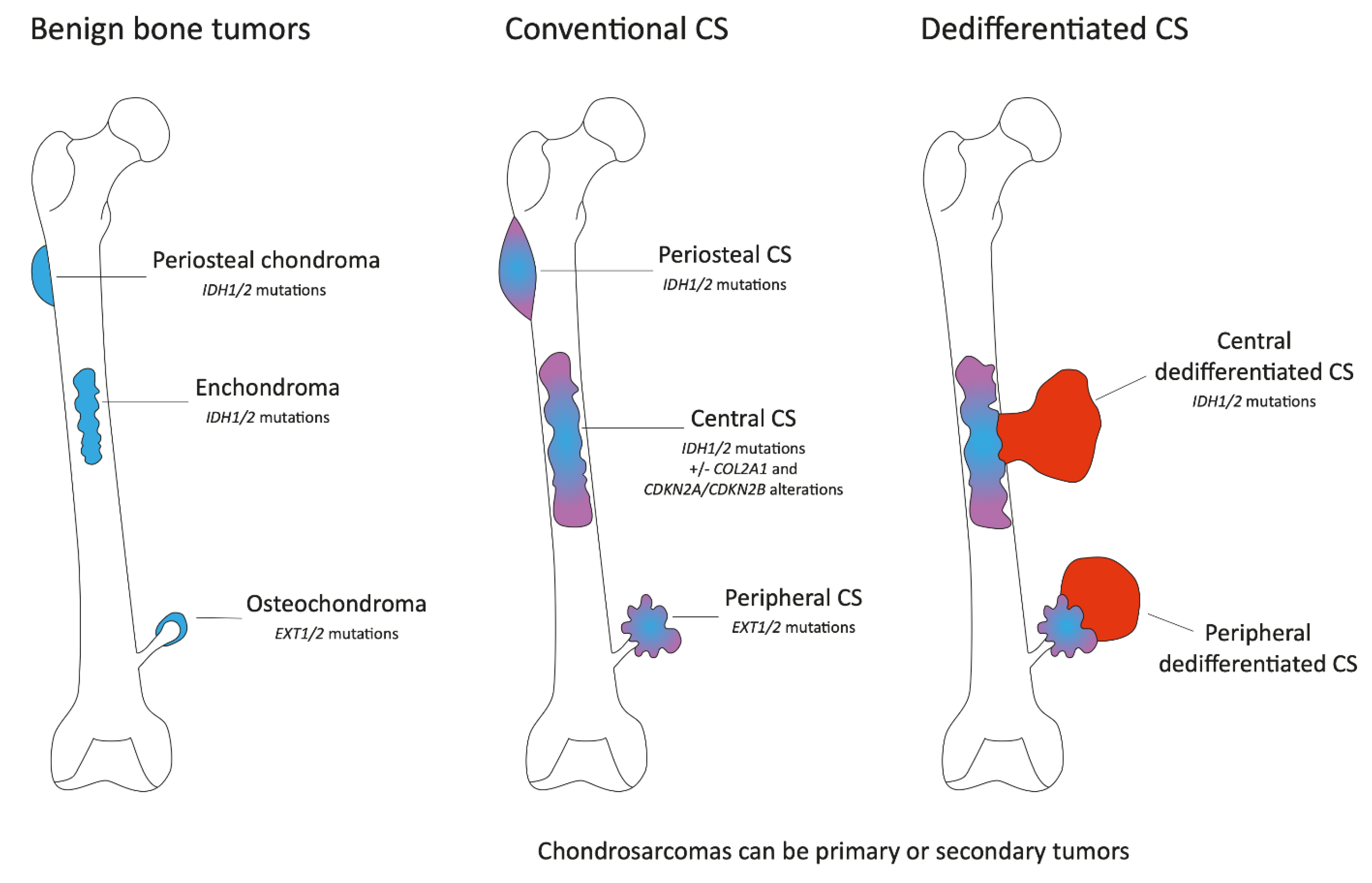
Figure 1.
Schematic representation of conventional cartilaginous tumors of bone. The figure represents the benign bone lesions, osteochondromas (sessile osteochondroma and pedunculated osteochondroma), chondromas (enchondroma and periosteal chondroma), their location, and the frequency of somatic tumors present in these lesions (left part). These lesions may progress to malignant conventional chondrosarcomas (middle part). To note, conventional chondrosarcomas may be primary or secondary tumors. In some cases, central and peripheral conventional chondrosarcomas may progress to dedifferentiated chondrosarcomas (right part).
Chondrosarcomas can be primary or secondary tumors. Indeed, enchondromas and osteochondromas can evolve towards central or peripheral CSs, respectively (Figure 1). Central and peripheral CSs differ at the molecular genetic level. Isocitrate dehydrogenase 1/2 (IDH1 or IDH2) mutations are present in 85% of hereditary enchondromatosis-associated disorders, Ollier disease (enchondromatosis only), Maffuci syndrome (enchondromatosis and hemangiomas) and 50% of solitary enchondromas, whereas the biallelic inactivation of the exostosin glycosyltransferase (EXT1 or EXT2) genes is observed in the majority of both sporadic and hereditary osteochondromas (Figure 1). These mutations are early events, and similar alterations are found in chondrosarcomas with the presence of IDH1/2 mutations in central CS and EXT1/2 mutations in peripheral CS. IDH1/2 mutations are found in primary or secondary tumors. Interestingly, EXT1/2 mutations seem to be less frequently detected in sporadic secondary peripheral chondrosarcomas than expected based on the assumption that they originate in sporadic osteochondromas, in which the homozygous inactivation of EXT1 is found in about 80% of our cases. De Andrea and colleagues suggest that wild-type cells with functional EXT are predisposed to acquire other mutations, giving rise to secondary peripheral chondrosarcoma and indicating that EXT-independent mechanisms are involved in the pathogenesis of secondary peripheral chondrosarcoma [7]. The genetic background of periostal CS is less known. A cohort of 38 samples identified no EXT1/2 mutations and IDH1/2 mutations in only 15% of cases suggesting a supposed relationship with central CS [8].
The mutations of IDH1/2 are present in 30–90% of central CS. In addition to IDH1/2 mutations, the collagen type II alpha-1 gene (COL2A1) mutations are found in about 40% of central CS. COL2A1 encodes the α-chain of type II collagen fibers: the major collagen constituent of articular cartilage. Interestingly, COL2A1 mutations are specific to chondrosarcomas. No COL2A1 mutations have been found in other bone sarcomas. Cyclin-dependent kinase inhibitor 2A/B (CDKN2A and CDKN2B) deletions are also recurrent in CS chondrosarcomas and occur in 75% of high-grade central CS. They are not present in low-grade cartilaginous tumors. Moreover, the CDKN2A/B copy number variation is seen in both IDH1/2 wild-type and mutant central CS with enrichment in IDH1/2 mutated tumors [9].
Chondrosarcomas can progress from low grade to high grade, which is reflected by their increased cellularity, nuclear atypia, myxoid changes, and increased vascularization. Central and peripheral CS are histologically subdivided into atypical cartilaginous tumors (ACT), grade 1, grade 2, and grade 3 CS. In the 2020 classification, it was proposed that ACT should be restricted to tumors arising in the appendicular skeleton, which are more amenable to complete surgical resection than tumors located in the axial skeleton. Conversely, « chondrosarcoma, grade 1 » should be used for axial tumors [10]. The reported 5-year overall survival rate is 74% and 31% for grade 2 CS and grade 3 CS, respectively. The 10-year overall survival rate is 62% for grade 2 and 26% for grade 3 tumors [11]. A high grade (2 or 3) was highly related to the increased risk of metastatic disease (29.3% at 5 years vs. 4.6% for Grade 1 tumors; p = 0.0001, log-rank test) in a Mayo Clinic descriptive cohort of 344 primary conventional CS patients [12]. Chondrosarcomas in an axial location have significantly lower survival than chondrosarcomas of the extremities. It is unclear whether the IDH1/2 mutations are associated with the outcome [13,14].
2.2. Dedifferentiated Chondrosarcoma
Dedifferentiated chondrosarcomas represent approximately 10% of all reported cases of chondrosarcomas. Dedifferentiated chondrosarcoma is a high-grade subtype of chondrosarcoma with the bimorphic histological appearance of a conventional chondrosarcoma component with an abrupt transition to a high-grade, non-cartilaginous sarcoma (Figure 1). Central dedifferentiated CS and central conventional CS showed similar molecular characteristics and identical tumor suppressor p53 (TP53) and IDH1/2 mutations in their conventional and dedifferentiated components, indicating a common origin. PD-L1 expression has been reported in approximately 50% of dedifferentiated chondrosarcomas [15].
Most patients are older than 50 years at diagnosis. The majority of these tumors are symptomatic at diagnosis, with a pathological fracture in about 20% of patients [16]. The prognosis of patients with dedifferentiated chondrosarcoma is uniformly poor, whatever the treatments used. Most patients die of distant metastases within 2 years of the initial diagnosis [17,18].
2.3. Mesenchymal Chondrosarcoma
In 1959, Liechtenstein and Berstein described two « frankly malignant mesenchymal tumors showing focal chondroid differentiation ». They named this tumor mesenchymal chondrosarcoma. Mesenchymal chondrosarcoma is a rare, high-grade, bimorphic sarcoma composed of undifferentiated small round cells and variable amounts of well-differentiated hyaline cartilage. Immunohistochemically, the small round tumor cells stain positive for NKX2.2, CD99, and the chondrocytes for S100 and SOX9. The immunohistochemistry profile of the undifferentiated component is non-specific and overlaps with other round blue cell tumors. NKX3.1, though widely used as a diagnostic biomarker for prostatic adenocarcinoma, has been recently proposed by Yoshida et al. as a unique marker of mesenchymal chondrosarcoma [19]. The reciprocal translocation (11;22)(q24;q12) was first described in 1993, suggesting that, because of the presence of a similar cytogenetic abnormality, mesenchymal chondrosarcoma may belong to the wide group of small round cell tumors [20]. More recently, recurrent hairy/enhancer-of-split related with YRPW motif 1—nuclear receptor coactivator 2 (HEY1-NCOA2) and interferon regulatory factor 2 binding protein 2 gene—caudal type homeobox 1 (IRF2BP2-CDX1) fusions have been identified in mesenchymal chondrosarcomas [21,22]. There is no recurrent translocation in conventional and dedifferentiated CS, highlighting that mesenchymal chondrosarcomas have a totally different genetic background.
Similar to skeletal osteosarcomas and Ewing sarcomas, mesenchymal CS most commonly occurs in patients between 10 and 30 years old, but it may arise at any age. Males and females appear to be equally affected. It rarely arises in extraosseous soft tissues, notably meninges. Common skeletal sites include the skull and craniofacial region (especially the mandible), ribs, spine, pelvis, and lower extremities. Mesenchymal CS is an aggressive form of cancer with a poor prognosis that can spread to other areas of the body, especially the lungs, liver, lymph nodes, meninges, and other bones.
Thus, clinical presentation and outcome are totally different between conventional, dedifferentiated CS and mesenchymal chondrosarcoma, which can be considered totally different diseases.
3. Current Treatment Management
3.1. Conventional and Dedifferentiated Chondrosarcoma
Conventional chondrosarcomas are generally considered to be resistant to conventional chemotherapy and radiotherapy. Complete surgical resection with wide margins is the cornerstone of primary management. Neoadjuvant or adjuvant chemotherapy may provide, in some studies, a survival advantage in dedifferentiated CS [17,23,24]. However, a retrospective study supplied by the European Musculo-Skeletal Oncology Society (EMSOS) with 337 dedifferentiated CS patients showed a non-significant improvement in the survival of patients receiving chemotherapy. In this study, most of the patients were under 60 years old with no metastasis and had undergone limb salvage surgery [16]. The presence of a pathological fracture was the most significant poor prognostic factor.
Most often, the chemotherapy regimen is based on a combination of doxorubicin and cisplatin or ifosfamide. In a large series of 171 patients with advanced unresectable CS from the Rizzoli Institute and Leiden University Medical Center, overall survival was observed to be 48% at 1 year and only 2% at 5 years. The use of either doxorubicin-based or non-cytotoxic chemotherapy (imatinib combined with sirolimus) in this cohort significantly prolonged the median overall survival to 20 months in contrast to 15 months for those who received no treatment [25]. In selected cases, radiotherapy may achieve encouraging results when adequate doses are delivered by conformational techniques or proton beam/carbon ion radiotherapy [26]. Clinical trial enrollment should be actively encouraged in all patients diagnosed with advanced, surgically unresectable chondrosarcoma due to the lack of consensus treatment recommendations.
3.2. Mesenchymal Chondrosarcoma
As mentioned previously, mesenchymal chondrosarcomas are a distinct entity with a different biological background and clinical behavior that compares to conventional and dedifferentiated chondrosarcoma. Many medical oncologists advocate a chemotherapy treatment plan such as Ewing’s sarcoma. Radiation therapy after surgery is also typically standard practice for large tumors. Other agents, including trabectedin, may be clinically valuable. A small phase II study reported a median progression-free survival (PFS) at 12.5 months (95% CI: 7.4-not reached) in the trabectedin group, and 1.0 months (95% CI: 0.3–1.0 months), in the best supportive care group in metastatic mesenchymal chondrosarcoma patients [27].
4. Targets and Novel Treatments Options in Chondrosarcoma
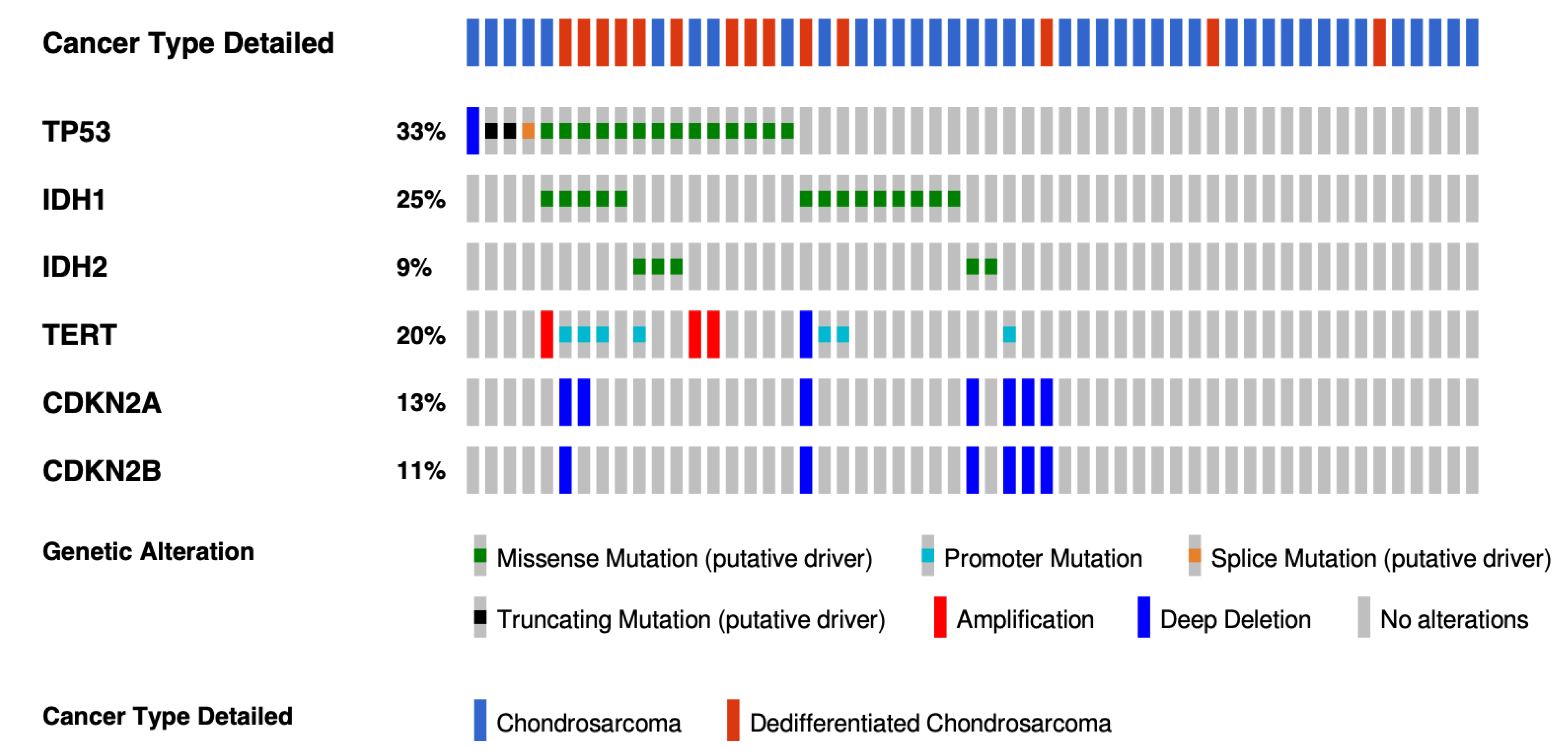
Genomic initiatives spearheaded by The Cancer Genome Atlas (TCGA) consortium have accelerated the pace of discovery for many cancers with the molecular characterization of more than 20,000 primary cancers with matched normal samples spanning 33 cancer types. Sarcomas are very heterogeneous, and no chondrosarcoma was included in the program because of a lack of readily accessible and adequate tumor tissue, as well as the rarity of this sarcoma. The most extensive cohort includes 55 conventional or dedifferentiated CS and has been performed by the MSKCC-IMPACT program [28]. As expected, the most common alterations were IDH1/2 mutations (34%), CDKN2A/B deletions (34%), TP53 mutations (33%), and Telomerase reverse transcriptase (TERT) alterations (20%) (Figure 2). No extensive genomic data in a large cohort included mesenchymal chondrosarcomas. Because mesenchymal chondrosarcomas harbor a recurrent translocation, the number of molecular alterations which could be efficient targets is probably lower.
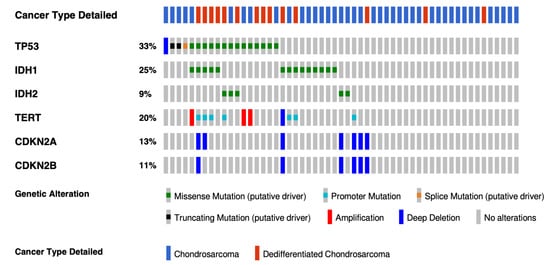
Figure 2.
Oncoprint with the most frequently altered genes in conventional and dedifferentiated chondrosarcomas from MSKCC-IMPACT cohort. The oncoprint represents the most common alterations in 44 conventional and 14 dedifferentiated chondrosarcomas from the MSKCC impact cohort (taken from cbioportal website, https://www.cbioportal.org (accessed on 17 February 2022)).
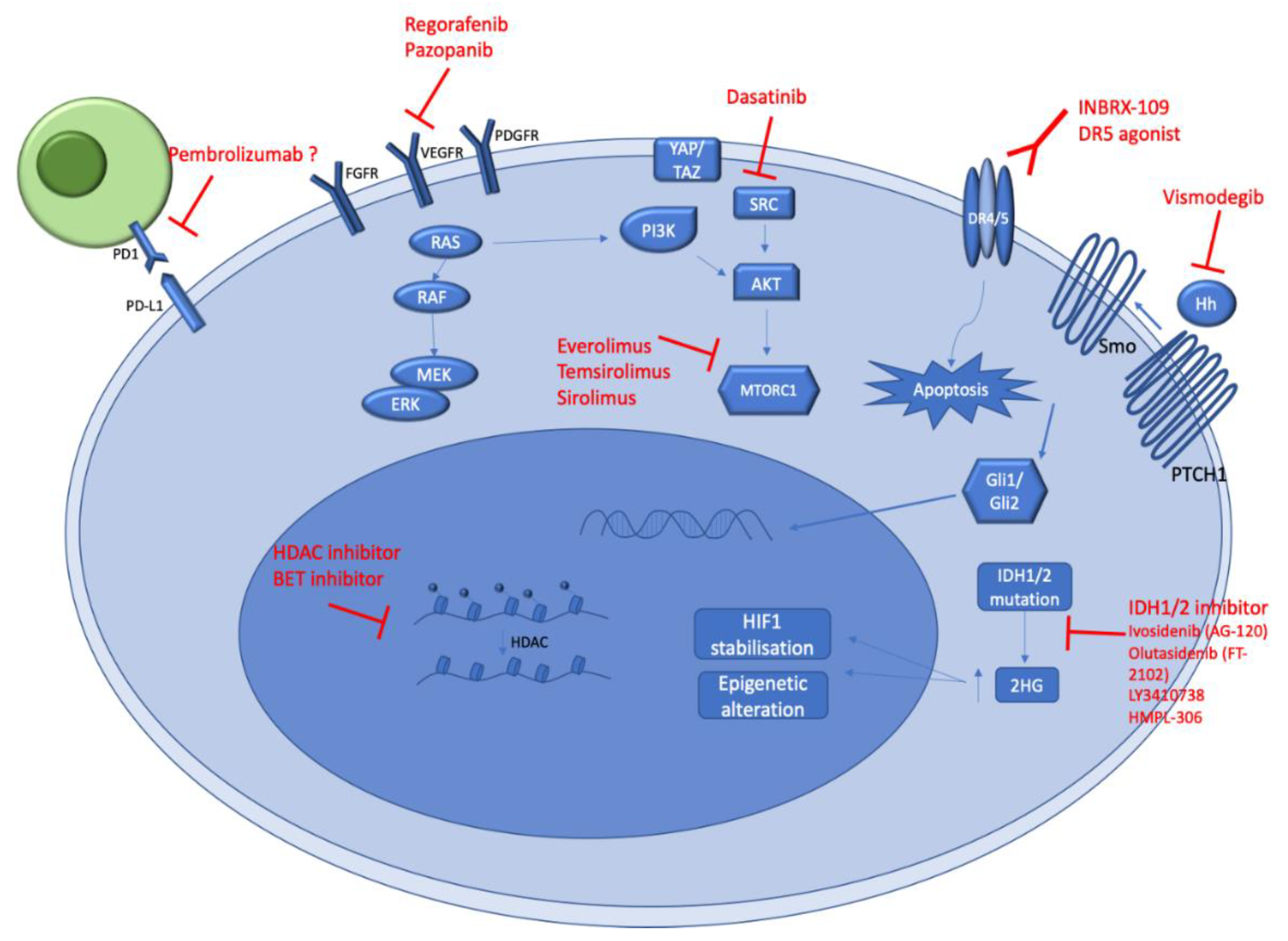
A better understanding of CS genomic alterations and biology should allow new potential therapeutic options (Figure 3). Ongoing clinical trials (Table 2), as well as future potential treatments for CS management, are summarized below.
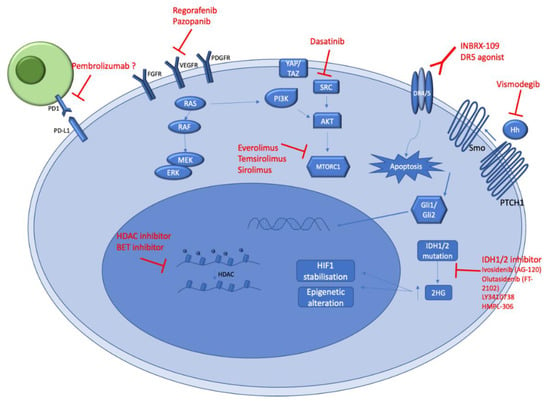
Figure 3.
Schematic illustration of key signaling pathways underpinning chondrosarcoma genesis. The figure represents the main biological pathways (in blue) activated in conventional and dedifferentiated chondrosarcomas. The potential therapeutic agents which could target these pathways are represented in red. All the mentioned pharmacological agents in this figure are under evaluation in ongoing clinical trials.

Table 2.
Ongoing clinical trials for chondrosarcomas patients.
4.1. Angiogenesis Pathway
Angiogenesis pathways are potentially effective targets for arresting the growth and spread of chondrosarcomas. It is necessary for tumors to develop a vasculature to grow. Although originating from cartilaginous tissue, which is one of a handful of avascular tissues in the human body, chondrosarcomas have been shown to exhibit a microvascularity that has been associated with aggressive clinical behavior and a higher potential for metastasis [29,30]. The vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) are essential for the neovascularization required for tumor maintenance and propagation (Figure 3). Efforts to develop antiangiogenic therapies have produced many agents, including small molecule tyrosine kinase inhibitors and fully human monoclonal antibodies which affect angiogenesis.
Pazopanib is a multitargeted tyrosine kinase inhibitor that inhibits angiogenesis pathways, namely VEGF, and has shown a significant effect on tumor vascular density, viability, and volume in mice with chondrosarcoma xenografts. Pazopanib has also been shown to provide a benefit to non-adipocytic soft tissue sarcoma patients by extending progression-free survival for several months when compared to the placebo [31].
Several studies and clinical trials in bone sarcomas are published or ongoing. The first studies focused on imatinib: a multikinase inhibitor that notably targets PDGFR. Imatinib failed to show benefits in unresectable and metastatic CS [32]. However, recent studies showed an improvement in the disease control of unresectable or metastatic conventional chondrosarcoma who received pazopanib or regorafenib: another multikinase inhibitor, which also targets VEGFRs [33,34,35,36] (Table 3). These recent results are very promising and will allow VEGFR inhibitors used in daily practice soon. Approaches based on the association of treatments are also in development, including clinical trials with chemotherapy and/or PD-L1 inhibitors associated with antiangiogenic agents (Table 2).

Table 3.
Published clinical trials.
4.2. Isocitrate Dehydrogenase (IDH) Mutations
Isocitrate dehydrogenase 1 (IDH1) and Isocitrate dehydrogenase 2 (IDH2) are enzymes that catalyze the reversible oxidative decarboxylation of isocitrate to yield α-ketoglutarate (α-KG) as part of the tricarboxylic acid cycle in glucose metabolism. Indeed, IDH1 and IDH2 participate in fatty and glucose metabolism and oxidative damage. IDH1/2 mutations are present in 50% of central CS and 60% of dedifferentiated CS [39] (Figure 3). The presence of IDH mutations in benign enchondromas and malignant CS supports the notion that IDH mutations are early events, and these cartilaginous neoplasms represent a spectrum of malignant potential. IDH mutations are also found in gliomas, acute myeloid leukemia (AML), and cholangiocarcinomas [40]. These mutations are considered driver events in these types of cancers too. IDH1 and IDH2 mutations are recurrent and typically involve an amino acid substitution in the active site of the enzyme in codon 132 (IDH1) and in codon 172 (IDH2). Specific inhibitors are in development. In AML, the US Food and Drug Administration (FDA) has already approved ivosidenib as the first IDH1 inhibitor for patients with relapsed or refractory AML and an IDH1 mutation. New IDH1 inhibitors, as well as dual IDH1 and IDH2 inhibitors, are in development. These drugs are currently evaluated for chondrosarcoma patients (Table 2).
4.3. Immunotherapy Strategies
In 2016, Kostine M. et al. reported an immunohistochemical analysis of PD-L1 expression in a large series of conventional, mesenchymal, and dedifferentiated chondrosarcomas using tissue microarrays. PD-L1 expression was absent in conventional and mesenchymal chondrosarcomas. Forty-one percent (9 of the 22) of dedifferentiated chondrosarcomas displayed PD-L1 positivity. In this study, PD-L1 expression was exclusively found in the dedifferentiated component and its expression positively correlated with a high number of tumor-infiltrating lymphocytes (p = 0.014) and positive HLA class I expression (p = 0.024) but not with patient overall survival (p = 0.22) [15]. Yang X et al. reported that the positivity for the PD-L1 and PD-L2 expressions in chondrosarcoma were 68% and 42%, respectively [41] (Figure 3). A 2020 study published by Iseulys and colleagues found that tumor-associated macrophages were the predominant immune cell type in the immune environment of chondrosarcoma [42]. High levels of CD68+ macrophages were associated with metastatic disease at diagnosis and a poor prognosis. The authors also reported the increased expression of the colony-stimulating factor 1 receptor (CSF1R), signal regulatory protein alpha (SIRPA), B7 superfamily member-H3 (B7H3), T cell immunoglobulin mucin (TIM3), and lymphocyte activation gene-3 (LAG3). Although low levels of the tumor mutational burden (TMB) have been reported in chondrosarcoma [43], TMB seems associated with histological grade. Grades 2 and 3 and dedifferentiated chondrosarcomas show levels of somatic mutations more than two times higher than grade 1 CS [44].
To date, only a few clinical studies that focused on immune checkpoint inhibitors in sarcoma patients are available. SARC028 was the first multicenter, open-label phase 2 clinical trial evaluating pembrolizumab monotherapy in 85 patients with advanced soft tissue and bone sarcomas. Only five patients with chondrosarcoma were enrolled in this study, and one patient with dedifferentiated CS achieved a partial response [37] (Table 3).
In a non-randomized phase 1/2 clinical trial of 37 patients with advanced sarcoma, the combination of doxorubicin and pembrolizumab was well tolerated. Three out of eight patients with chondrosarcoma had tumor regression, including one conventional chondrosarcoma with a 26% decrease in size [45]. Taken together, further evaluation of immune checkpoint blockades in chondrosarcomas is warranted. Several clinical trials are currently ongoing (Table 2).
4.4. Cell Cycle Pathway
The cyclin-dependent kinase inhibitor 2A (CDKN2A) and cyclin-dependent kinase inhibitor 2B (CDKN2B) act as tumor suppressors by regulating the cell cycle; they encode p16 and p15Ink4b, respectively. They inhibit cyclin-dependent kinases 4 and 6 (CDK4 and CDK6) and thereby activate the retinoblastoma family of proteins, which block traversal from G1 to S-phase. CDKN2A and CDKN2B deletions are recurrent in chondrosarcomas as well as in several subtypes of cancers. Palbociclib, a CDK4/CDK6 inhibitor, was approved to be used in combination with an endocrine therapy to treat advanced-stage or metastatic, hormone-receptor-positive, HER2-negative breast cancer patients regardless of CDK4N2A/B status. There is no validated predictive biomarker for CDK4/6 inhibitor response, including CDKN2A/B deletions. The first reports of CDK4/6 used in other subtypes of cancer seem to show that Palbociclib monotherapy does not have clinical activity [46]. Several studies are ongoing, including studies with new CDK4/6 inhibitors for chondrosarcoma patients (Table 2).
4.5. PI3K-AKT-mTOR Pathway
mTOR is a serine/threonine tyrosine kinase that acts as a key regulatory protein in normal cell growth, development, metabolism, as well as angiogenic pathways [47]. The (PI3K)-AKT signaling network has many direct and indirect downstream effects on cellular metabolism [48]. The activation of the downstream PI3K/AKT pathway has been demonstrated in CS cells [48] (Figure 3). Ten consecutive patients with unresectable CS were offered off-label treatment with a sirolimus/cyclophosphamide combination, which was well tolerated and had modest clinical activity [49]. One (10%) objective response was observed, and six (60%) patients experienced the stabilization of the disease for at least 6 months. Three patients had progressive disease.
Temsirolimus may also potentiate the cytotoxicity of liposomal doxorubicin [50]. Lastly, neoadjuvant everolimus has been tried in CS (NCT02008019, CHONRAD). The study was suspended due to limited activity. Preclinical data indicate that the combination of mTOR with an IGF-1R blockade results in greater AKT downregulation and enhanced antiproliferative effects. More encouraging results were achieved in combination studies, and partial responses were shown in CS cases treated with a dual IGF1R/mTOR blockade [38].
4.6. Epigenetic Strategies
Epigenetic regulation is critical to the physiological control of development, cell fate, cell proliferation, genomic integrity, and, more specifically, transcriptional regulation. Post-transcriptional gene regulation is caused by DNA methylation and histone modification. Mutations in IDH1/2 genes result in progressive increases in DNA and histone methylation and are observed in 50% of conventional chondrosarcomas, suggesting that epigenetic dysregulation represents a potential barrier for tumor progression and a target for therapeutic intervention [51]. Venneker S. et al. reported that high-grade tumors demonstrated an increased number of hypermethylated genes compared to low-grade tumors, suggesting that epigenetic mechanisms play an important role in CS progression [52]. A broad compound screen that targets different epigenetic key players (including histone deacetylases (HDACs), sirtuins (SIRTs), histone demethylases (HDMs), histone acetyltransferases (HATs), histone methyltransferases (HMTs), and DNA methyltransferases (DNMTs)) was performed on IDH wild type and mutant CS cell lines to explore whether these epigenetic changes could be used as a target for novel anti-cancer therapy. Interesting compound classes included HDAC and bromodomain, and extra-terminal motif (BET) protein inhibitors were identified (Figure 3). For example, it has been shown that the HDAC inhibitors romidepsin, trichostatin A, and sodium valproate affect cell proliferation in 2D in vitro models of CS.
The analysis of 92 central and 45 peripheral CS tumors also showed that although the CS were strongly positive for the H3K4me3, H3K9me3, and H3K27me3 histone modifications, neither of these modifications nor the variable levels of 5-hydroxymethylcytosine and 5-methylcytosine correlated with the IDH1/2 mutation status [13].
4.7. Anti DR5
TNF-related apoptosis-inducing ligand (TRAIL) receptor (DR4 or DR5) agonists are promising agents for cancer therapy because they selectively induce apoptosis in cancer cells. DR5 is expressed in a broad spectrum of hematologic malignancies and solid tumors (Figure 3). Monoclonal antibodies targeting DR5 are under development. Among them, INBRX-109 seems to be promising for chondrosarcoma patient management. The phase 1 (NCT04553692, Table 3) results were presented at a 2020 CTOS virtual meeting: disease control was observed in 11 of the 12 patients (92%), and 8 of the 12 patients (67%) achieved objective response rates. After the success of the phase 1 trial, phase 2 should open soon.
4.8. Others
YAP (encoded by Yes-associated protein 1) and TAZ (encoded by WWTR1) [53,54] are transcriptional activators that regulate cell proliferation, survival, and differentiation [55]. The Hippo signaling pathway is a conserved cascade that regulates the growth and size of tissues, involving the translocation of Yorkie, the YAP/TAZ homolog, between the cytoplasm and nucleus. YAP/TAZ is also known to play a vital role in osteogenesis [56] and chondrogenesis [57]. A study showed that less than half of CS specimens harbored activated YAP/TAZ [58]. The downregulation of YAP/TAZ and LATS1 in chondrosarcoma cells treated with the BRD4 inhibitor, JQ1, led to cell cycle arrest, senescence, and apoptosis [59]. A multi-institutional, open-label, single-arm trial of dasatinib, which inhibits the nuclear localization and target gene expression of YAP and TAZ, was performed in patients with advanced sarcoma, including grade 1 or 2 CS (n = 33) [60]. The median PFS was 5.5 months for CS. Three patients with CS remained on treatment for more than 2 years. Six patients with CS had an objective tumor response according to the Choi criteria. Specific targeted therapies against Hippo signaling pathways should be developed in the future and could be good candidates for CS treatments.
The Hedgehog pathway (Hh) is a signaling cascade that plays a crucial role in many fundamental processes, including stem cell maintenance, cell differentiation, tissue polarity, and cell proliferation. The components of the Hh signaling pathway, involved in the signaling transfer to the Gli transcription factors, include Hedgehog ligands (Sonic Hh [SHh], Indian Hh [IHh], and Desert Hh [DHh]), Patched receptor (Ptch1, Ptch2), Smoothened receptor (Smo), Suppressor of fused homolog (Sufu), kinesin protein Kif7, protein kinase A (PKA), and cyclic adenosine monophosphate (cAMP). The misregulation of the Hh pathway has been found to lead to carcinogenesis in many cancers, including chondrosarcomas [61]. Preclinical studies showed encouraging results when using saridegib (IPI-926), an oral Hh inhibitor, in primary mice CHS xenografts [62]. Unfortunately, the clinical trials had to be stopped due to disappointing clinical data. Vismodegib is an FDA-approved Hh inhibitor for basocellular carcinoma patients. The results of clinical trials conducted with vismodegib have also been discouraging. In a single-arm phase II trial, vismodegib showed some activity in patients with grade 1 or 2 conventional chondrosarcomas. However, the primary endpoint (6 months clinical benefit rate) was met [63] (Figure 3). A selection of patients more likely to benefit from this kind of targeted therapy with surrogate markers appears to be needed for future studies.
Behind TP53 and IDH1/2 mutations, COL2A1 molecular alterations (insertions, deletions, and rearrangements) have been shown to be present in 37% of chondrosarcomas [42]. COL2A1 encodes the α-chain of type II collagen fibers: the major collagen constituent of articular cartilage. The disruption of the collagen maturation process through the production of aberrant pro-collagen α-chain is the likely result. Mutations in exostosin glycosyltransferase 1/2 (EXT1/2) genes, which participate in the differentiation of chondrocytes, have been observed in peripheral chondrosarcomas. To date, there is no targeted therapy developed against these genes.
5. Conclusions
Conventional, dedifferentiated, and mesenchymal CS are rare bone sarcomas with a very poor prognosis. Mesenchymal chondrosarcomas harbor recurrent translocations and are distinct pathological, biological, and clinical entities. The management is multimodal and based on polychemotherapy, radiotherapy, and surgery, as performed in Ewing sarcoma patients. Conventional chemotherapies fail to improve the survival of conventional and dedifferentiated CS patients. Conventional and dedifferentiated CS remains a primarily surgical disease at present which may respond to doxorubicin-containing chemotherapy in only rare cases. Recently, genomic programs identified recurrent alterations, especially in IDH1/2 genes. The efficiency of IDH1 inhibitors in other malignancies suggests that these inhibitors will be part of IDH-mutated CS management soon. Other treatment approaches based on a better understanding of CS biology are emerging, such as PIK3-AKT-mTOR inhibitors, cell cycle inhibitors, and epigenetic or immune modulators.
This review highlights how a comprehensive and multimodal understanding of CS is crucial for patient management and improvement. We showed that CS patient care is based on radiologic, pathological, and clinical expertise. Recently, the understanding of the genomic background and biology of CS has opened new therapeutic options and will probably change the CS standard of care in the future.
Author Contributions
Conceptualization, C.T. and P.B.-R. methodology, C.T. and P.B.-R.; Formal Analysis, C.T., P.B.-R. and S.D.P., Supervision, C.T., E.P., F.G. and P.B.-R., validation, C.T., P.B.-R.and E.P.; investigation, C.T.,P.B.-R., F.L. and S.D.P.; writing—original draft preparation, C.T., F.L. and P.B.-R.; writing—review and editing, C.T., F.L., S.D.P., P.B.-R., E.P., V.A., D.B., F.G., D.H., J.C., P.A. and B.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available within the article.
Acknowledgments
We would like to thank Claus Daublebsky von Sterneck for the Figure 1 design.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Pinieux, G.; Karanian, M.; Le Loarer, F.; Le Guellec, S.; Chabaud, S.; Terrier, P.; Bouvier, C.; Batistella, M.; Neuville, A.; Robin, Y.M.; et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS ONE 2021, 16, e0246958. [Google Scholar] [CrossRef]
- Brandolini, F.; Bacchini, P.; Moscato, M.; Bertoni, F. Chondrosarcoma as a complicating factor in Paget’s disease of bone. Skelet. Radiol. 1997, 26, 497–500. [Google Scholar] [CrossRef]
- Wu, L.C.; Kleinerman, R.A.; Curtis, R.E.; Savage, S.A.; Berrington de Gonzalez, A. Patterns of bone sarcomas as a second malignancy in relation to radiotherapy in adulthood and histologic type. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1993–1999. [Google Scholar] [CrossRef]
- Herget, G.W.; Strohm, P.; Rottenburger, C.; Kontny, U.; Krauss, T.; Bohm, J.; Sudkamp, N.; Uhl, M. Insights into Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma 2014, 61, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Hogendoorn, P.C.; Dijkstra, S.D.; van Rijswijk, C.S.; Krol, A.D.; Taminiau, A.H.; Bovée, J.V. The clinical approach towards chondrosarcoma. Oncologist 2008, 13, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Arjen, H.G.; Cleven, J.L.B.R.T. Periosteal Chondrosarcoma; WHO Classification of Tumours; © International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- de Andrea, C.E.; Reijnders, C.M.; Kroon, H.M.; de Jong, D.; Hogendoorn, P.C.; Szuhai, K.; Bovee, J.V. Secondary peripheral chondrosarcoma evolving from osteochondroma as a result of outgrowth of cells with functional EXT. Oncogene 2012, 31, 1095–1104. [Google Scholar] [CrossRef]
- Cleven, A.H.; Zwartkruis, E.; Hogendoorn, P.C.; Kroon, H.M.; Briaire-de Bruijn, I.; Bovee, J.V. Periosteal chondrosarcoma: A histopathological and molecular analysis of a rare chondrosarcoma subtype. Histopathology 2015, 67, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Amary, M.F.; Ye, H.; Forbes, G.; Damato, S.; Maggiani, F.; Pollock, R.; Tirabosco, R.; Flanagan, A.M. Isocitrate dehydrogenase 1 mutations (IDH1) and p16/CDKN2A copy number change in conventional chondrosarcomas. Virchows Arch. 2015, 466, 217–222. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. World Health Organization Classification of Soft Tissue and Bone Tumours, 5th ed.; IARC Press: Lyon, France, 2020. [Google Scholar]
- Van Praag Veroniek, V.M.; Rueten-Budde, A.J.; Ho, V.; Dijkstra, P.D.S.; Study group Bone and Soft tissue tumours, (WeBot); Fiocco, M.; van de Sande, M.A.J. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg. Oncol. 2018, 27, 402–408. [Google Scholar] [CrossRef]
- Bjornsson, J.; McLeod, R.A.; Unni, K.K.; Ilstrup, D.M.; Pritchard, D.J. Primary chondrosarcoma of long bones and limb girdles. Cancer 1998, 83, 2105–2119. [Google Scholar] [CrossRef]
- Cleven, A.H.G.; Suijker, J.; Agrogiannis, G.; Briaire-de Bruijn, I.H.; Frizzell, N.; Hoekstra, A.S.; Wijers-Koster, P.M.; Cleton-Jansen, A.M.; Bovee, J. IDH1 or -2 mutations do not predict outcome and do not cause loss of 5-hydroxymethylcytosine or altered histone modifications in central chondrosarcomas. Clin. Sarcoma Res. 2017, 7, 8. [Google Scholar] [CrossRef]
- Lugowska, I.; Teterycz, P.; Mikula, M.; Kulecka, M.; Kluska, A.; Balabas, A.; Piatkowska, M.; Wagrodzki, M.; Pienkowski, A.; Rutkowski, P.; et al. IDH1/2 Mutations Predict Shorter Survival in Chondrosarcoma. J. Cancer 2018, 9, 998–1005. [Google Scholar] [CrossRef]
- Kostine, M.; Cleven, A.H.; de Miranda, N.F.; Italiano, A.; Cleton-Jansen, A.M.; Bovee, J.V. Analysis of PD-L1, T-cell infiltrate and HLA expression in chondrosarcoma indicates potential for response to immunotherapy specifically in the dedifferentiated subtype. Mod. Pathol. 2016, 29, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.J.; Gosheger, G.; Taminiau, A.; Biau, D.; Matejovsky, Z.; Kollender, Y.; San-Julian, M.; Gherlinzoni, F.; Ferrari, C. Dedifferentiated chondrosarcoma: Prognostic factors and outcome from a European group. Eur. J. Cancer 2007, 43, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Mir, O.; Cioffi, A.; Palmerini, E.; Piperno-Neumann, S.; Perrin, C.; Chaigneau, L.; Penel, N.; Duffaud, F.; Kurtz, J.E.; et al. Advanced chondrosarcomas: Role of chemotherapy and survival. Ann. Oncol. 2013, 24, 2916–2922. [Google Scholar] [CrossRef]
- Van Maldegem, A.M.; Bovee, J.V.; Gelderblom, H. Comprehensive analysis of published studies involving systemic treatment for chondrosarcoma of bone between 2000 and 2013. Clin. Sarcoma Res. 2014, 4, 11. [Google Scholar] [CrossRef]
- Yoshida, K.I.; Machado, I.; Motoi, T.; Parafioriti, A.; Lacambra, M.; Ichikawa, H.; Kawai, A.; Antonescu, C.R.; Yoshida, A. NKX3-1 Is a Useful Immunohistochemical Marker of EWSR1-NFATC2 Sarcoma and Mesenchymal Chondrosarcoma. Am. J. Surg. Pathol. 2020, 44, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Sainati, L.; Scapinello, A.; Montaldi, A.; Bolcato, S.; Ninfo, V.; Carli, M.; Basso, G. A mesenchymal chondrosarcoma of a child with the reciprocal translocation (11;22)(q24;q12). Cancer Genet. Cytogenet. 1993, 71, 144–147. [Google Scholar] [CrossRef]
- Nyquist, K.B.; Panagopoulos, I.; Thorsen, J.; Haugom, L.; Gorunova, L.; Bjerkehagen, B.; Fossa, A.; Guriby, M.; Nome, T.; Lothe, R.A.; et al. Whole-transcriptome sequencing identifies novel IRF2BP2-CDX1 fusion gene brought about by translocation t(1;5)(q42;q32) in mesenchymal chondrosarcoma. PLoS ONE 2012, 7, e49705. [Google Scholar] [CrossRef]
- Wang, L.; Motoi, T.; Khanin, R.; Olshen, A.; Mertens, F.; Bridge, J.; Dal Cin, P.; Antonescu, C.R.; Singer, S.; Hameed, M.; et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 2012, 51, 127–139. [Google Scholar] [CrossRef]
- Mitchell, A.D.; Ayoub, K.; Mangham, D.C.; Grimer, R.J.; Carter, S.R.; Tillman, R.M. Experience in the treatment of dedifferentiated chondrosarcoma. J. Bone Joint Surg. Br. 2000, 82, 55–61. [Google Scholar] [CrossRef]
- Dickey, I.D.; Rose, P.S.; Fuchs, B.; Wold, L.E.; Okuno, S.H.; Sim, F.H.; Scully, S.P. Dedifferentiated chondrosarcoma: The role of chemotherapy with updated outcomes. J. Bone Joint Surg. Am. 2004, 86, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- van Maldegem, A.M.; Gelderblom, H.; Palmerini, E.; Dijkstra, S.D.; Gambarotti, M.; Ruggieri, P.; Nout, R.A.; van de Sande, M.A.; Ferrari, C.; Ferrari, S.; et al. Outcome of advanced, unresectable conventional central chondrosarcoma. Cancer 2014, 120, 3159–3164. [Google Scholar] [CrossRef] [PubMed]
- DeLaney, T.F.; Liebsch, N.J.; Pedlow, F.X.; Adams, J.; Weyman, E.A.; Yeap, B.Y.; Depauw, N.; Nielsen, G.P.; Harmon, D.C.; Yoon, S.S.; et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J. Surg. Oncol. 2014, 110, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Morioka, H.; Takahashi, S.; Araki, N.; Sugiura, H.; Ueda, T.; Takahashi, M.; Yonemoto, T.; Hiraga, H.; Hiruma, T.; Kunisada, T.; et al. Results of sub-analysis of a phase 2 study on trabectedin treatment for extraskeletal myxoid chondrosarcoma and mesenchymal chondrosarcoma. BMC Cancer 2016, 16, 479. [Google Scholar] [CrossRef]
- Nacev, B.A.; Smith, S.A.; Antonescu, C.R.; Rosenbaum, E.; Shi, H.; Tang, C.; Socci, N.D.; Rana, S.; Gularte-Mérida, R.; Zehir, A.; et al. Clinical sequencing of soft tissue and bone sarcomas delineates diverse genomic landscapes and potential therapeutic targets. medRxiv 2022. [Google Scholar] [CrossRef]
- McGough, R.L.; Aswad, B.I.; Terek, R.M. Pathologic neovascularization in cartilage tumors. Clin. Orthop. Relat. Res. 2002, 397, 76–82. [Google Scholar] [CrossRef]
- Ayala, G.; Liu, C.; Nicosia, R.; Horowitz, S.; Lackman, R. Microvasculature and VEGF expression in cartilaginous tumors. Hum. Pathol. 2000, 31, 341–346. [Google Scholar] [CrossRef]
- van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schoffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Grignani, G.; Palmerini, E.; Stacchiotti, S.; Boglione, A.; Ferraresi, V.; Frustaci, S.; Comandone, A.; Casali, P.G.; Ferrari, S.; Aglietta, M. A phase 2 trial of imatinib mesylate in patients with recurrent nonresectable chondrosarcomas expressing platelet-derived growth factor receptor-alpha or -beta: An Italian Sarcoma Group study. Cancer 2011, 117, 826–831. [Google Scholar] [CrossRef]
- Chow, W.; Frankel, P.; Ruel, C.; Araujo, D.M.; Milhem, M.; Okuno, S.; Hartner, L.; Undevia, S.; Staddon, A. Results of a prospective phase 2 study of pazopanib in patients with surgically unresectable or metastatic chondrosarcoma. Cancer 2020, 126, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Villalobos, V.M.; Cote, G.M.; Burris, H.; Janku, F.; Mir, O.; Beeram, M.; Wagner, A.J.; Jiang, L.; Wu, B.; et al. Phase I Study of the Mutant IDH1 Inhibitor Ivosidenib: Safety and Clinical Activity in Patients With Advanced Chondrosarcoma. J. Clin. Oncol. 2020, 38, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, S.; Zhu, L.; Wang, J.; Zhang, P.; Li, P.; Zhang, F.; Yao, W. Efficacy and safety of anlotinib in patients with unresectable or metastatic bone sarcoma: A retrospective multiple institution study. Cancer Med. 2021, 10, 7593–7600. [Google Scholar] [CrossRef]
- Duffaud, F.; Italiano, A.; Bompas, E.; Rios, M.; Penel, N.; Mir, O.; Piperno-Neumann, S.; Chevreau, C.; Delcambre, C.; Bertucci, F.; et al. Efficacy and safety of regorafenib in patients with metastatic or locally advanced chondrosarcoma: Results of a non-comparative, randomised, double-blind, placebo controlled, multicentre phase II study. Eur. J. Cancer 2021, 150, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Tap, W.D.; Qin, L.X.; Livingston, M.B.; Undevia, S.D.; Chmielowski, B.; Agulnik, M.; Schuetze, S.M.; Reed, D.R.; Okuno, S.H.; et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: A multicentre, open-label, phase 2 trial. Lancet Oncol. 2013, 14, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Amary, M.F.; Bacsi, K.; Maggiani, F.; Damato, S.; Halai, D.; Berisha, F.; Pollock, R.; O’Donnell, P.; Grigoriadis, A.; Diss, T.; et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011, 224, 334–343. [Google Scholar] [CrossRef]
- Schaap, F.G.; French, P.J.; Bovee, J.V. Mutations in the isocitrate dehydrogenase genes IDH1 and IDH2 in tumors. Adv. Anat. Pathol. 2013, 20, 32–38. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, G.; Yang, Z.; Zeng, K.; Liu, F.; Sun, J. Expression of PD-L1/PD-L2 is associated with high proliferation index of Ki-67 but not with TP53 overexpression in chondrosarcoma. Int. J. Biol. Markers 2018, 33, 507–513. [Google Scholar] [CrossRef]
- Iseulys, R.; Anne, G.B.; Corinne, B.; Gonzague, D.B.P.; Marie, K.; Jean-Yves, B.; Aurelie, D. The immune landscape of chondrosarcoma reveals an immunosuppressive environment in the dedifferentiated subtypes and exposes CSFR1+ macrophages as a promising therapeutic target. J. Bone Oncol. 2020, 20, 100271. [Google Scholar]
- Cote, G.M.; He, J.; Choy, E. Next-Generation Sequencing for Patients with Sarcoma: A Single Center Experience. Oncologist 2018, 23, 234–242. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Behjati, S.; Cooke, S.L.; Van Loo, P.; Wedge, D.C.; Pillay, N.; Marshall, J.; O’Meara, S.; Davies, H.; Nik-Zainal, S.; et al. Frequent mutation of the major cartilage collagen gene COL2A1 in chondrosarcoma. Nat. Genet. 2013, 45, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.M.; Redman, M.W.; Baker, K.K.; Wagner, M.J.; Schroeder, B.A.; Loggers, E.T.; Trieselmann, K.; Copeland, V.C.; Zhang, S.; Black, G.; et al. Assessment of Doxorubicin and Pembrolizumab in Patients with Advanced Anthracycline-Naive Sarcoma: A Phase 1/2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1778–1782. [Google Scholar] [CrossRef]
- Al Baghdadi, T.; Halabi, S.; Garrett-Mayer, E.; Mangat, P.K.; Ahn, E.R.; Sahai, V.; Alvarez, R.H.; Kim, E.S.; Yost, K.J.; Rygiel, A.L.; et al. Palbociclib in Patients with Pancreatic and Biliary Cancer with CDKN2A Alterations: Results from the Targeted Agent and Profiling Utilization Registry Study. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Pilotto, S.; Bria, E.; Cognetti, F.; Milella, M.; Ciuffreda, L. Role of mTOR Signaling in Tumor Microenvironment: An Overview. Int. J. Mol. Sci. 2018, 19, 2453. [Google Scholar] [CrossRef]
- Zhang, Y.X.; van Oosterwijk, J.G.; Sicinska, E.; Moss, S.; Remillard, S.P.; van Wezel, T.; Buhnemann, C.; Hassan, A.B.; Demetri, G.D.; Bovee, J.V.; et al. Functional profiling of receptor tyrosine kinases and downstream signaling in human chondrosarcomas identifies pathways for rational targeted therapy. Clin. Cancer Res. 2013, 19, 3796–3807. [Google Scholar] [CrossRef]
- Bernstein-Molho, R.; Kollender, Y.; Issakov, J.; Bickels, J.; Dadia, S.; Flusser, G.; Meller, I.; Sagi-Eisenberg, R.; Merimsky, O. Clinical activity of mTOR inhibition in combination with cyclophosphamide in the treatment of recurrent unresectable chondrosarcomas. Cancer Chemother. Pharm. 2012, 70, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.A.; Chen, A.R.; Trucco, M.M.; Shah, P.; Wilky, B.A.; Gul, N.; Carrera-Haro, M.A.; Ferreira, M.F.; Shafique, U.; Powell, J.D.; et al. A dose-finding study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcoma. Int. J. Cancer 2013, 133, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Gagné, L.M.; Boulay, K.; Topisirovic, I.; Huot, M.E.; Mallette, F.A. Oncogenic Activities of IDH1/2 Mutations: From Epigenetics to Cellular Signaling. Trends Cell Biol. 2017, 27, 738–752. [Google Scholar] [CrossRef]
- Venneker, S.; Kruisselbrink, A.B.; Baranski, Z.; Palubeckaite, I.; Briaire-de Bruijn, I.H.; Oosting, J.; French, P.J.; Danen, E.H.J.; Bovee, J. Beyond the Influence of IDH Mutations: Exploring Epigenetic Vulnerabilities in Chondrosarcoma. Cancers 2020, 12, 3589. [Google Scholar] [CrossRef]
- Moroishi, T.; Hansen, C.G.; Guan, K.L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79. [Google Scholar] [CrossRef]
- Moya, I.M.; Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef]
- Fervienza, A.; Moya, I.; Martinez-Camacho, A.; Sala-Blanch, X. Median nerve block in the forearm and persistent median artery. Rev. Esp. Anestesiol. Reanim. 2019, 66, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Hwang, E.S.; McManus, M.T.; Amsterdam, A.; Tian, Y.; Kalmukova, R.; Mueller, E.; Benjamin, T.; Spiegelman, B.M.; Sharp, P.A.; et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 2005, 309, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wu, A.; Li, P.; Li, G.; Qin, L.; Song, H.; Mak, K.K. Yap1 Regulates Multiple Steps of Chondrocyte Differentiation during Skeletal Development and Bone Repair. Cell Rep. 2016, 14, 2224–2237. [Google Scholar] [CrossRef]
- Fullenkamp, C.A.; Hall, S.L.; Jaber, O.I.; Pakalniskis, B.L.; Savage, E.C.; Savage, J.M.; Ofori-Amanfo, G.K.; Lambertz, A.M.; Ivins, S.D.; Stipp, C.S.; et al. TAZ and YAP are frequently activated oncoproteins in sarcomas. Oncotarget 2016, 7, 30094–30108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Gui, T.; Sang, Y.; Yang, J.; Li, Y.H.; Liang, G.H.; Li, T.; He, Q.Y.; Zha, Z.G. The BET Bromodomain Inhibitor JQ1 Suppresses Chondrosarcoma Cell Growth via Regulation of YAP/p21/c-Myc Signaling. J. Cell Biochem. 2017, 118, 2182–2192. [Google Scholar] [CrossRef]
- Schuetze, S.M.; Bolejack, V.; Choy, E.; Ganjoo, K.N.; Staddon, A.P.; Chow, W.A.; Tawbi, H.A.; Samuels, B.L.; Patel, S.R.; von Mehren, M.; et al. Phase 2 study of dasatinib in patients with alveolar soft part sarcoma, chondrosarcoma, chordoma, epithelioid sarcoma, or solitary fibrous tumor. Cancer 2017, 123, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Tiet, T.D.; Hopyan, S.; Nadesan, P.; Gokgoz, N.; Poon, R.; Lin, A.C.; Yan, T.; Andrulis, I.L.; Alman, B.A.; Wunder, J.S. Constitutive hedgehog signaling in chondrosarcoma up-regulates tumor cell proliferation. Am. J. Pathol. 2006, 168, 321–330. [Google Scholar] [CrossRef]
- Campbell, V.T.; Nadesan, P.; Ali, S.A.; Wang, C.Y.; Whetstone, H.; Poon, R.; Wei, Q.; Keilty, J.; Proctor, J.; Wang, L.W.; et al. Hedgehog pathway inhibition in chondrosarcoma using the smoothened inhibitor IPI-926 directly inhibits sarcoma cell growth. Mol. Cancer Ther. 2014, 13, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Le Cesne, A.; Bellera, C.; Piperno-Neumann, S.; Duffaud, F.; Penel, N.; Cassier, P.; Domont, J.; Takebe, N.; Kind, M.; et al. GDC-0449 in patients with advanced chondrosarcomas: A French Sarcoma Group/US and French National Cancer Institute Single-Arm Phase II Collaborative Study. Ann. Oncol. 2013, 24, 2922–2926. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).