A Visible Light-Induced and ROS-Dependent Method for the Rapid Formation of a MOF Composite Membrane with Antibacterial Properties
Abstract
:1. Introduction
2. Results and Discussion
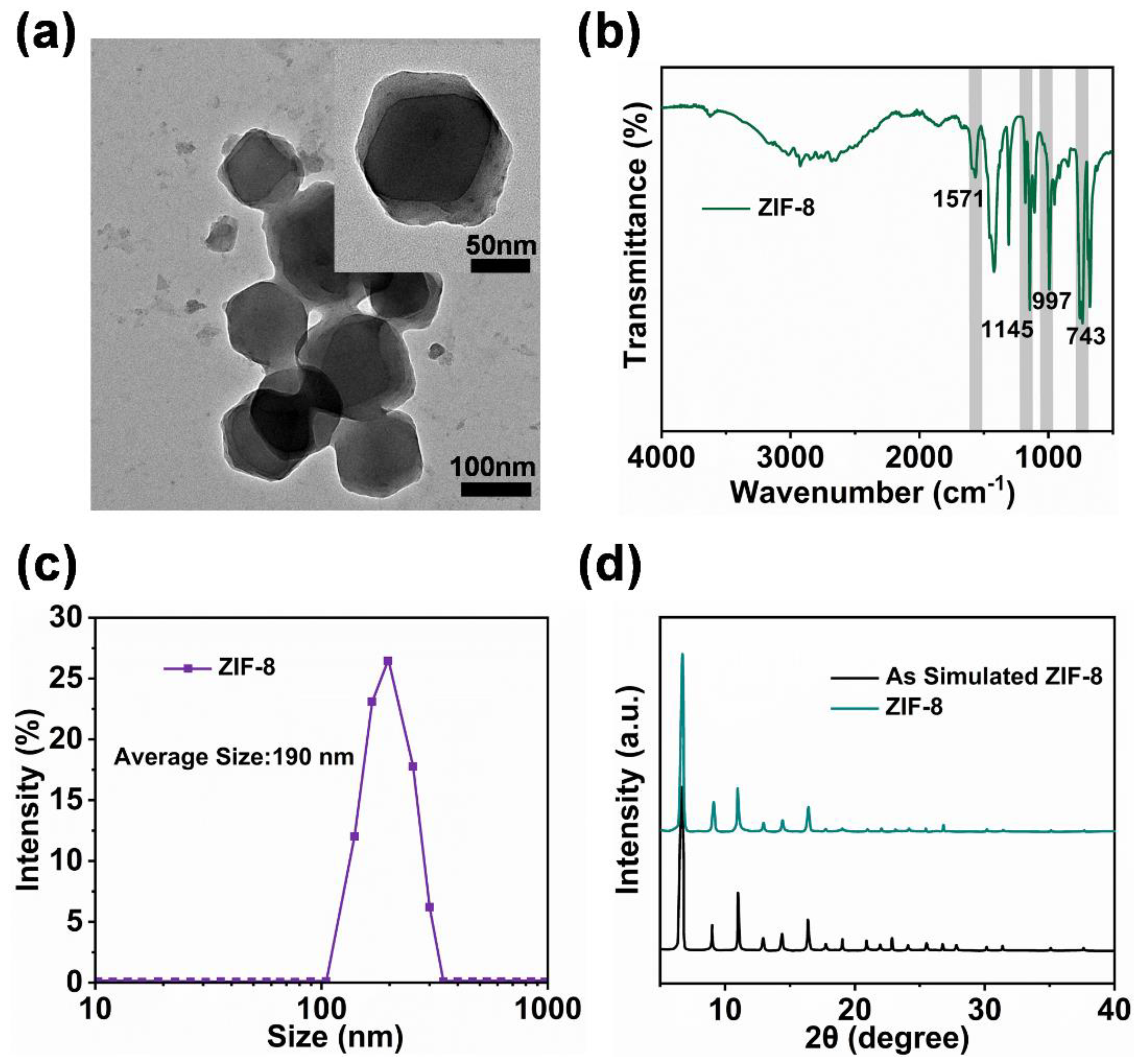
2.1. Characterization of Physical and Chemical Properties of ZIF-8 Particles
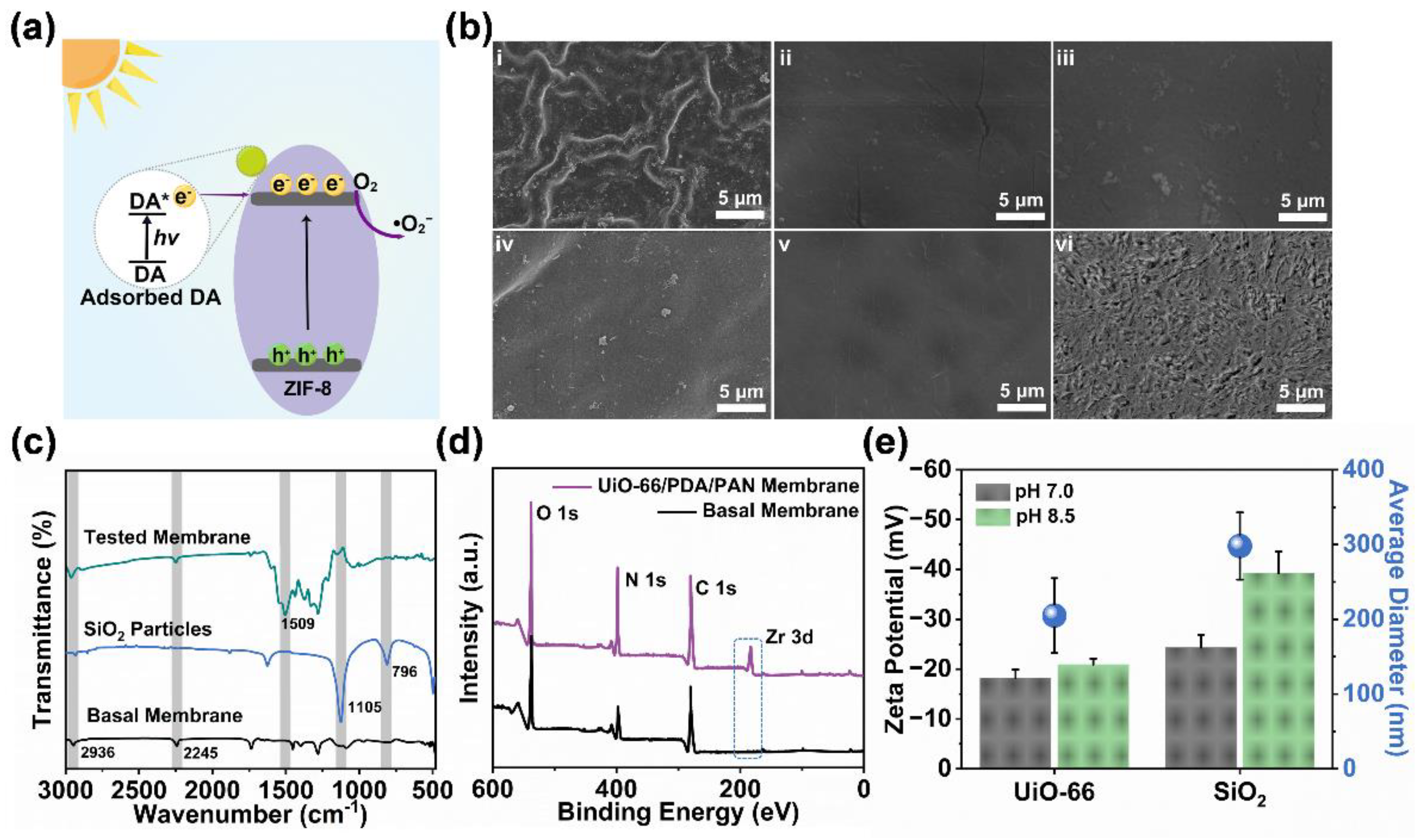
2.2. Investigation and Analysis of Physical and Chemical Properties of ZIF-8/PDA/PAN Composite Membrane
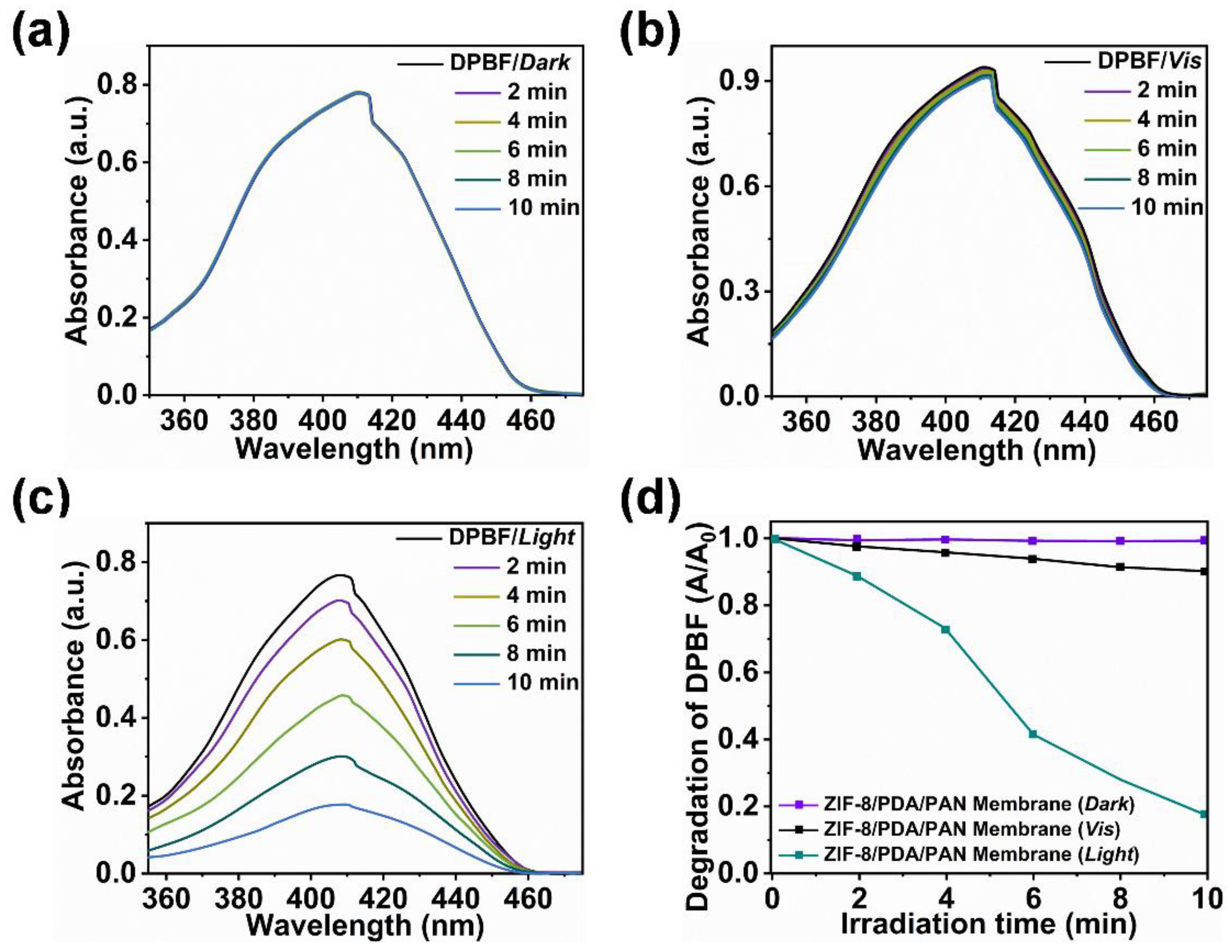
2.3. Mechanism Analysis and Verification of ZIF-8/PDA/PAN Composite Membrane Constructed by ZIF-8 Particle Assembly and DA Codeposition
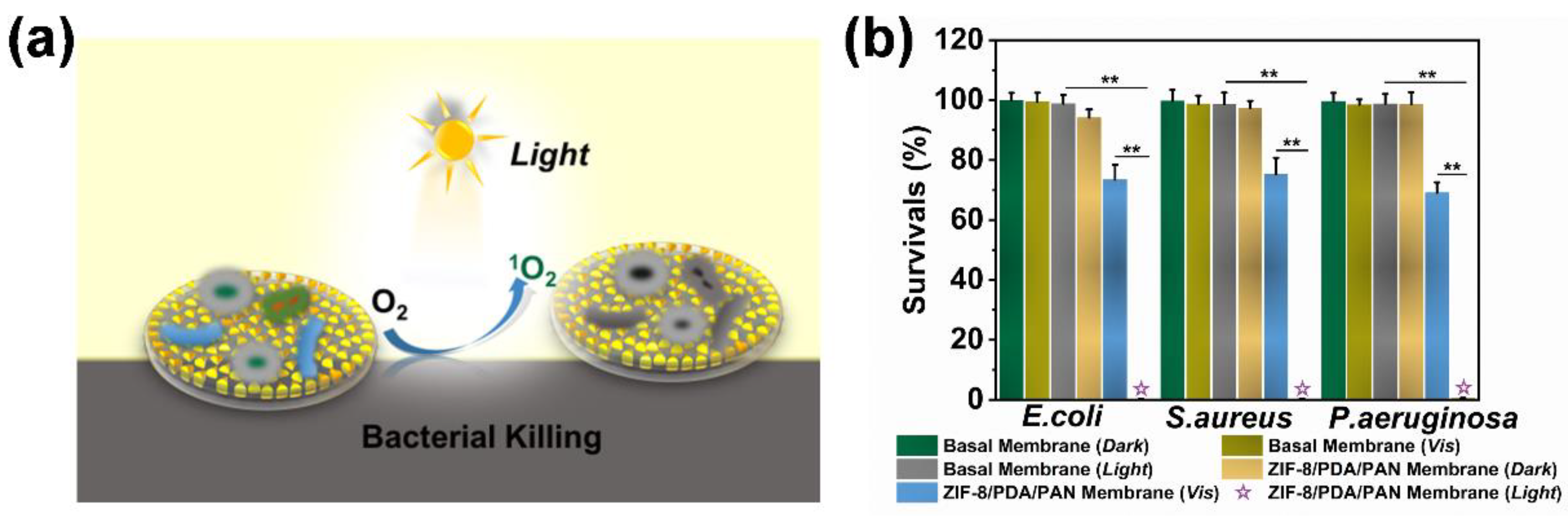
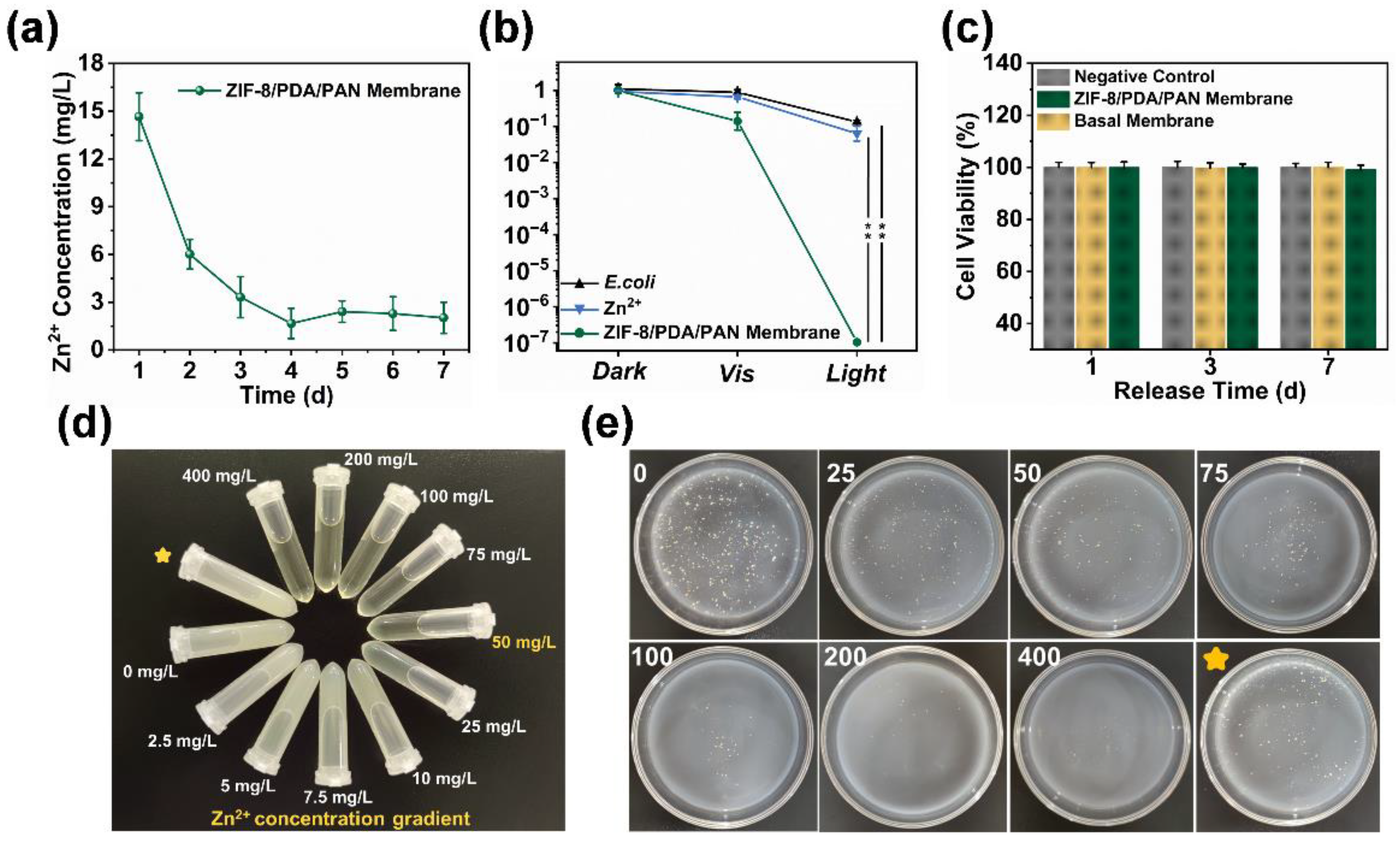
2.4. Antibacterial Mechanism and Application of ZIF-8/PDA/PAN Composite Membrane
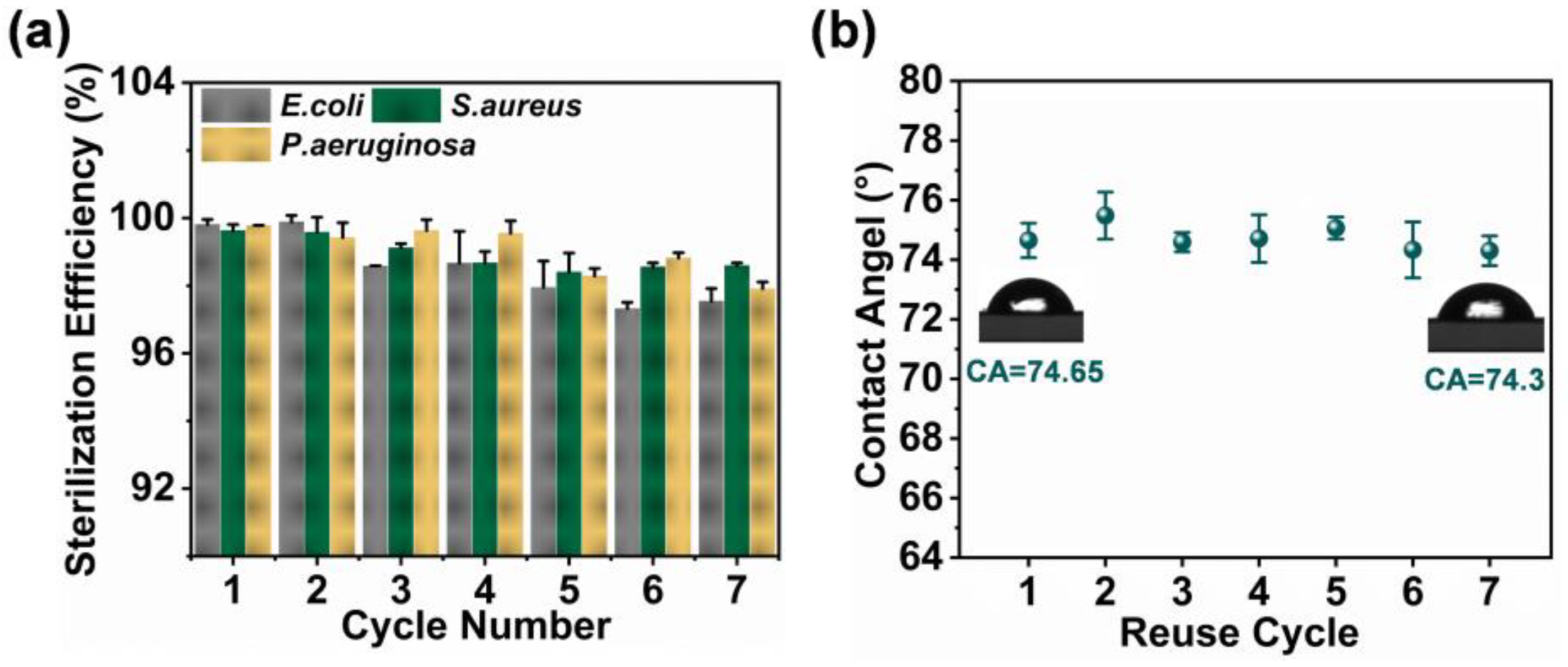
2.5. Characterization of ZIF-8/PDA/PAN Composite Membrane Stability
3. Materials and Methods
3.1. Experimental Materials
3.2. Preparation of ZIF-8/PDA/PAN Composite Membrane
3.3. Characterization of ZIF-8 Nanoparticles and ZIF-8/PDA/PAN Composite Membrane Physicochemical Properties
3.4. ROS Yield Analysis of ZIF-8/PDA/PAN Composite Membrane
3.5. Zn2+ Release Test of the ZIF-8/PDA/PAN Composite Membrane
3.6. Bacterial Culture
3.7. Assessment of Antibacterial Properties of the ZIF-8/PDA/PAN Composite Membrane under Simulated Sunlight
3.8. Cytotoxicity Test
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.J.; Li, B.; He, H.J.; Zhou, W.; Chen, B.L.; Qian, G.D. Metal-Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Hassan, Z.; Brase, S.; Woll, C.; Tsotsalas, M. Metal-Organic Framework-Templated Biomaterials: Recent Progress in Synthesis, Functionalization, and Applications. Acc. Chem. Res. 2019, 52, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Hanikel, N.; Pei, X.K.; Chheda, S.; Lyu, H.; Jeong, W.; Sauer, J.; Gagliardi, L.; Yaghi, O.M. Evolution of water structures in metal-organic frameworks for improved atmospheric water harvesting. Science 2021, 374, 454–459. [Google Scholar] [CrossRef]
- Cacho-Bailo, F.; Seoane, B.; Tellez, C.; Coronas, J. ZIF-8 continuous membrane on porous polysulfone for hydrogen separation. J. Membr. Sci. 2014, 464, 119–126. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Wang, H.L.; Wang, L.; Zhao, B.; Qian, Y.R.; Zhang, H.W. Polyamide thin-film nanocomposite membrane containing star-shaped ZIF-8 with enhanced water permeance and PPCPs removal. Sep. Purif. Technol. 2022, 292, 120886. [Google Scholar] [CrossRef]
- Rao, Z.; Feng, K.; Tang, B.B.; Wu, P.Y. Surface Decoration of Amino-Functionalized Metal-Organic Framework/Graphene Oxide Composite onto Polydopamine-Coated Membrane Substrate for Highly Efficient Heavy Metal Removal. ACS Appl. Mater. Interfaces 2017, 9, 2594–2605. [Google Scholar] [CrossRef]
- Yang, F.Y.; Du, M.; Yin, K.L.; Qiu, Z.M.; Zhao, J.W.; Liu, C.L.; Zhang, G.X.; Gao, Y.J.; Pang, H. Applications of Metal-Organic Frameworks in Water Treatment: A Review. Small 2022, 18, 2105715. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Qi, J.Y.; Lu, X.H.; Jiang, H.C.; Wang, P.P.; He, M.R.; Ma, J. Epitaxially grown MOF membranes with photocatalytic bactericidal activity for biofouling mitigation in desalination. J. Membr. Sci. 2021, 630, 119327. [Google Scholar] [CrossRef]
- Datta, S.J.; Mayoral, A.; Bettahalli, N.M.S.; Bhatt, P.M.; Karunakaran, M.; Carja, I.D.; Fan, D.; Mileo, P.G.M.; Semino, R.; Maurin, G.; et al. Rational design of mixed-matrix metal-organic framework membranes for molecular separations. Science 2022, 376, 1080–1087. [Google Scholar] [CrossRef]
- Lyu, D.P.; Xu, W.; Payong, J.E.L.; Zhang, T.R.; Wang, Y.F. Low-dimensional assemblies of metal-organic framework particles and mutually coordinated anisotropy. Nat. Commun. 2022, 13, 3980. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Rassu, P.; Wang, X.; Li, H.W.; Wang, X.R.; Wang, X.Q.; Feng, X.; Yin, A.X.; Li, P.F.; Jin, X.; et al. An Iron-Containing Metal-Organic Framework as a Highly Efficient Catalyst for Ozone Decomposition. Angew. Chem. Int. Ed. 2018, 57, 16416–16420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Yuan, S.; Feng, X.; Li, H.W.; Zhou, J.W.; Wang, B. Preparation of Nanofibrous Metal-Organic Framework Filters for Efficient Air Pollution Control. J. Am. Chem. Soc. 2016, 138, 5785–5788. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Zhang, S.H.; Cao, S.J.; Li, S.Q.; Chen, F.; Yuan, S.; Xu, C.; Zhou, J.W.; Feng, X.; Ma, X.J.; et al. Roll-to-Roll Production of Metal-Organic Framework Coatings for Particulate Matter Removal. Adv. Mater. 2017, 29, 1606221. [Google Scholar] [CrossRef]
- Zhou, S.; Shekhah, O.; Jia, J.T.; Czaban-Jozwiak, J.; Bhatt, P.M.; Ramirez, A.; Gascon, J.; Eddaoudi, M. Electrochemical synthesis of continuous metal-organic framework membranes for separation of hydrocarbons. Nat. Energy 2021, 6, 882–891. [Google Scholar] [CrossRef]
- Chen, Y.F.; Li, S.Q.; Pei, X.K.; Zhou, J.W.; Feng, X.; Zhang, S.H.; Cheng, Y.Y.; Li, H.W.; Han, R.D.; Wang, B. A Solvent-Free Hot-Pressing Method for Preparing Metal-Organic-Framework Coatings. Angew. Chem. Int. Ed. 2016, 55, 3419–3423. [Google Scholar] [CrossRef]
- He, M.L.; Wang, L.; Lv, Y.T.; Wang, X.D.; Zhu, J.N.; Zhang, Y.; Liu, T.T. Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal. Chem. Eng. J. 2020, 389, 124452. [Google Scholar] [CrossRef]
- Huski, I.; Arhangelskis, M.; Friscic, T. Solvent-free ageing reactions of rare earth element oxides: From geomimetic synthesis of new metal-organic materials towards a simple, environmentally friendly separation of scandium. Green Chem. 2020, 22, 4364–4375. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Wang, X.P.; Lin, R.J.; Liu, Y.; Chen, F.S.; Cui, L.S.; Meng, X.M.; Hou, J.W. Improving the hydrostability of ZIF-8 membrane by biomolecule towards enhanced nanofiltration performance for dye removal. J. Membr. Sci. 2021, 61, 118630. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.Z.; Cao, R.; Ren, L.T.; Chen, M.X.; Feng, X.; Zhou, J.W.; Wang, B. Fe/Ni Metal-Organic Frameworks and Their Binder-Free Thin Films for Efficient Oxygen Evolution with Low Overpotential. ACS Appl. Mater. Interfaces 2016, 8, 16736–16743. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ai, K.L.; Lu, L.H. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 18, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Priemel, T.; Palia, G.; Forste, F.; Jehle, F.; Sviben, S.; Mantouvalou, I.; Zaslansky, P.; Bertinetti, L.; Harrington, M.J. Microfluidic-like fabrication of metal ion-cured bioadhesives by mussels. Science 2021, 374, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.H.; Messersmith, P.B. Universal Surface-Initiated Polymerization of Antifouling Zwitterionic Brushes Using a Mussel-Mimetic Peptide Initiator. Langmuir 2012, 28, 7258–7266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priemel, T.; Palia, R.; Babych, M.; Thibodeaux, C.J.; Bourgault, S.; Harrington, M.J. Compartmentalized processing of catechols during mussel byssus fabrication determines the destiny of DOPA. Proc. Natl. Acad. Sci. USA 2020, 117, 7613–7621. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.L.; Huang, Y.J.; You, S.Y.; Xiang, Y.J.; Cai, E.Y.; Mao, R.T.; Pan, W.H.; Tong, X.Q.; Dong, W.; Ye, F.F.; et al. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Adv. Sci. 2022, 9, 2106015. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Tian, E.Z.; Mo, J.H. Electrostatic Polydopamine-Interface-Mediated (e-PIM) filters with tuned surface topography and electrical properties for efficient particle capture and ozone removal. J. Hazard. Mater. 2023, 441, 129821. [Google Scholar] [CrossRef] [PubMed]
- D’Alvise, T.M.; Harvey, S.; Hueske, L.; Szelwicka, J.; Veith, L.; Knowles, T.P.J.; Kubiczek, D.; Flaig, C.; Port, F.; Gottschalk, K.E.; et al. Ultrathin Polydopamine Films with Phospholipid Nanodiscs Containing a Glycophorin A Domain. Adv. Funct. Mater. 2020, 30, 2000378. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ou, Y.; Lei, W.X.; Wan, L.S.; Ji, J.; Xu, Z.K. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angew. Chem. Int. Ed. 2016, 128, 3106–3109. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Lee, J.S.; Kim, J.; Lee, Y.B.; Shin, H.; Um, S.H.; Kim, J.B.; Park, K.I.; Lee, H.; Cho, S.W. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials 2012, 33, 6952–6964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, H.C.; Wan, L.S.; Liang, H.Q.; Li, H.Y.; Xu, Z.K. Polydopamine-Coated Porous Substrates as a Platform for Mineralized beta-FeOOH Nanorods with Photocatalysis under Sunlight. ACS Appl. Mater. Interfaces 2015, 7, 11567–11574. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Du, X.; Hou, W.; Liu, X.J.; Zhu, C.; Gao, B.B.; Sun, L.D.; Li, Q.W.; Liao, J.L.; Levkin, P.A.; et al. UV-Triggered Polydopamine Secondary Modification: Fast Deposition and Removal of Metal Nanoparticles. Adv. Funct. Mater. 2019, 29, 1901875. [Google Scholar] [CrossRef]
- Du, X.; Li, L.X.; Li, J.S.; Yang, C.W.; Frenkel, N.; Welle, A.; Heissler, S.; Nefedov, A.; Grunze, M.; Levkin, P.A. UV-Triggered Dopamine Polymerization: Control of Polymerization, Surface Coating, and Photopatterning. Adv. Mater. 2014, 26, 8029–8033. [Google Scholar] [CrossRef]
- Behboodi-Sadabad, F.; Zhang, H.J.; Trouillet, V.; Welle, A.; Plumere, N.; Levkin, P.A. UV-Triggered Polymerization, Deposition, and Patterning of Plant Phenolic Compounds. Adv. Funct. Mater. 2017, 27, 1700127. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.H.; Xu, Q.; Yu, S.H.; Jiang, H.L. Pd Nanocubes@ZIF-8: Integration of Plasmon-Driven Photothermal Conversion with a Metal-Organic Framework for Efficient and Selective Catalysis. Angew. Chem. Int. Ed. 2016, 55, 3685–3689. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Datta, K.K.R.; Rosler, C.; Petr, M.; Otyepka, M.; Zboril, R.; Fischer, R.A. Biomimetic Superhydrophobic/Superoleophilic Highly Fluorinated Graphene Oxide and ZIF-8 Composites for Oil-Water Separation. Angew. Chem. Int. Ed. 2016, 55, 1178–1182. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef]
- Sun, Z.K.; Zhou, Y.X.; Jiao, Y.; Cheng, X.Q.; Zhang, Y.J.; Wang, P.; Liang, H.; Yang, X.B.; Drioli, E.; Figoli, A.; et al. Multi-hydrophilic functional network enables porous membranes excellent anti-fouling performance for highly efficient water remediation. J. Membr. Sci. 2020, 608, 118191. [Google Scholar] [CrossRef]
- Pan, Y.C.; Liu, Y.Y.; Zeng, G.F.; Zhao, L.; Lai, Z.P. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Jang, M.; Lee, S.; Hardy, J.G.; Lee, J.Y. Electrically Conductive Polydopamine-Polypyrrole as High Performance Biomaterials for Cell Stimulation in Vitro and Electrical Signal Recording in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 33032–33042. [Google Scholar] [CrossRef] [Green Version]
- Coskun, H.; Aljabour, A.; Uiberlacker, L.; Strobel, M.; Hild, S.; Cobet, C.; Farka, D.; Stadler, P.; Sariciftci, N.S. Chemical vapor deposition-based synthesis of conductive polydopamine thin-films. Thin Solid Film. 2018, 645, 320–325. [Google Scholar] [CrossRef]
- Li, P.; Li, J.Z.; Feng, X.; Li, J.; Hao, Y.C.; Zhang, J.W.; Wang, H.; Yin, A.X.; Zhou, J.W.; Ma, X.J.; et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10, 2177. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.F.; Han, S.Y.; Wang, Z.P.; Gao, H.; Wang, L.Y.; Deng, Y.H.; Wan, C.Q.; Tian, Y.; Wang, Q.; Wang, G.; et al. Modified UiO-66 frameworks with methylthio, thiol and sulfonic acid function groups: The structure and visible-light-driven photocatalytic property study. Appl. Catal. B Environ. 2019, 259, 118047. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.F.; Chen, Y.S. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, J.T.; Zhang, X.Q.; Li, Z.P.; Tang, Y.L. Cationic Oligo (thiophene ethynylene) with Broad-Spectrum and High Antibacterial Efficiency under White Light and Specific Biocidal Activity against S. aureus in Dark. ACS Appl. Mater. Interfaces 2016, 8, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.Z.; Cheng, B.; Yu, J.G.; Ho, W.K. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling Zeolitic Imidazolate Framework Nano- and Microcrystal Formation: Insight into Crystal Growth by Time-Resolved in Situ Static Light Scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Taheri, M.; Tsuzuki, T. Photo-accelerated Hydrolysis of Metal Organic Framework ZIF-8. ACS Mater. Lett. 2021, 3, 255–260. [Google Scholar] [CrossRef]
- Vankayala, R.; Lin, C.C.; Kalluru, P.; Chiang, C.S.; Hwang, K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef]
- Säbel, C.E.; Neureuther, J.M.; Siemann, S.A. Spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal. Biochem. 2010, 397, 218–226. [Google Scholar] [CrossRef]
- Ding, X.; Duan, S.; Ding, X.; Liu, R.; Xu, F.J. Versatile Antibacterial Materials: An Emerging Arsenal for Combatting Bacterial Pathogens. Adv. Funct. Mater. 2018, 28, 1802140–1802159. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Liu, D. A Visible Light-Induced and ROS-Dependent Method for the Rapid Formation of a MOF Composite Membrane with Antibacterial Properties. Int. J. Mol. Sci. 2023, 24, 1520. https://doi.org/10.3390/ijms24021520
Zhang S, Liu D. A Visible Light-Induced and ROS-Dependent Method for the Rapid Formation of a MOF Composite Membrane with Antibacterial Properties. International Journal of Molecular Sciences. 2023; 24(2):1520. https://doi.org/10.3390/ijms24021520
Chicago/Turabian StyleZhang, Shanshan, and Dongliang Liu. 2023. "A Visible Light-Induced and ROS-Dependent Method for the Rapid Formation of a MOF Composite Membrane with Antibacterial Properties" International Journal of Molecular Sciences 24, no. 2: 1520. https://doi.org/10.3390/ijms24021520





