The Accumulation of Volatile Compounds and the Change in the Morphology of the Leaf Wax Cover Accompanied the “Anti-Aging” Effect in Anethum graveolens L. Plants Sprayed with 6-Benzylaminopurine
Abstract
:1. Introduction
2. Results
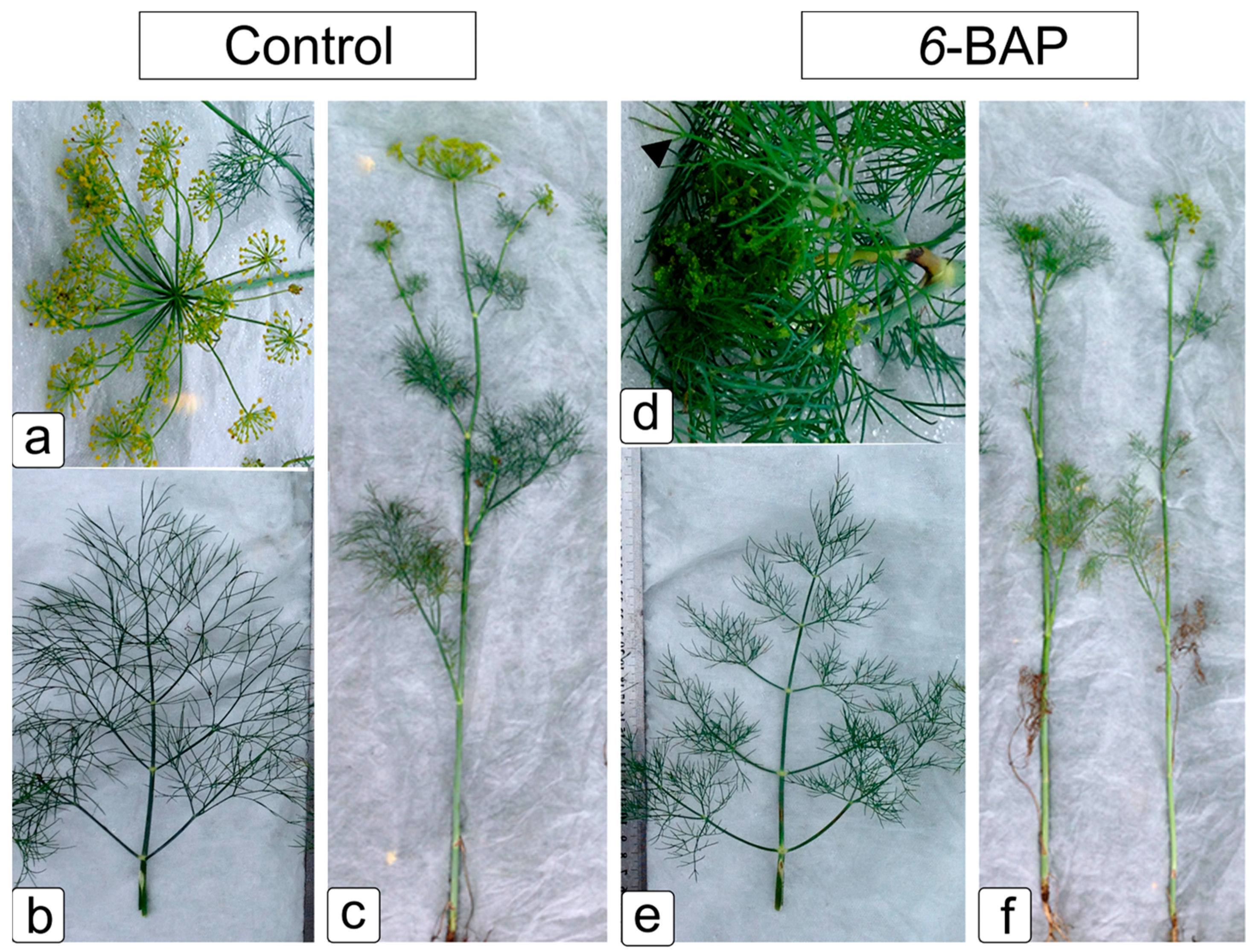
2.1. Effect of 6-BAP on Development and Morphological Traits of Dill Plants
2.2. Yield of EO and Its Component Composition
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Spraying of Dill Plants with 6-BAP
4.3. Essential Oil Extraction
4.4. Essential Oil Component Identification by Gas Chromatography
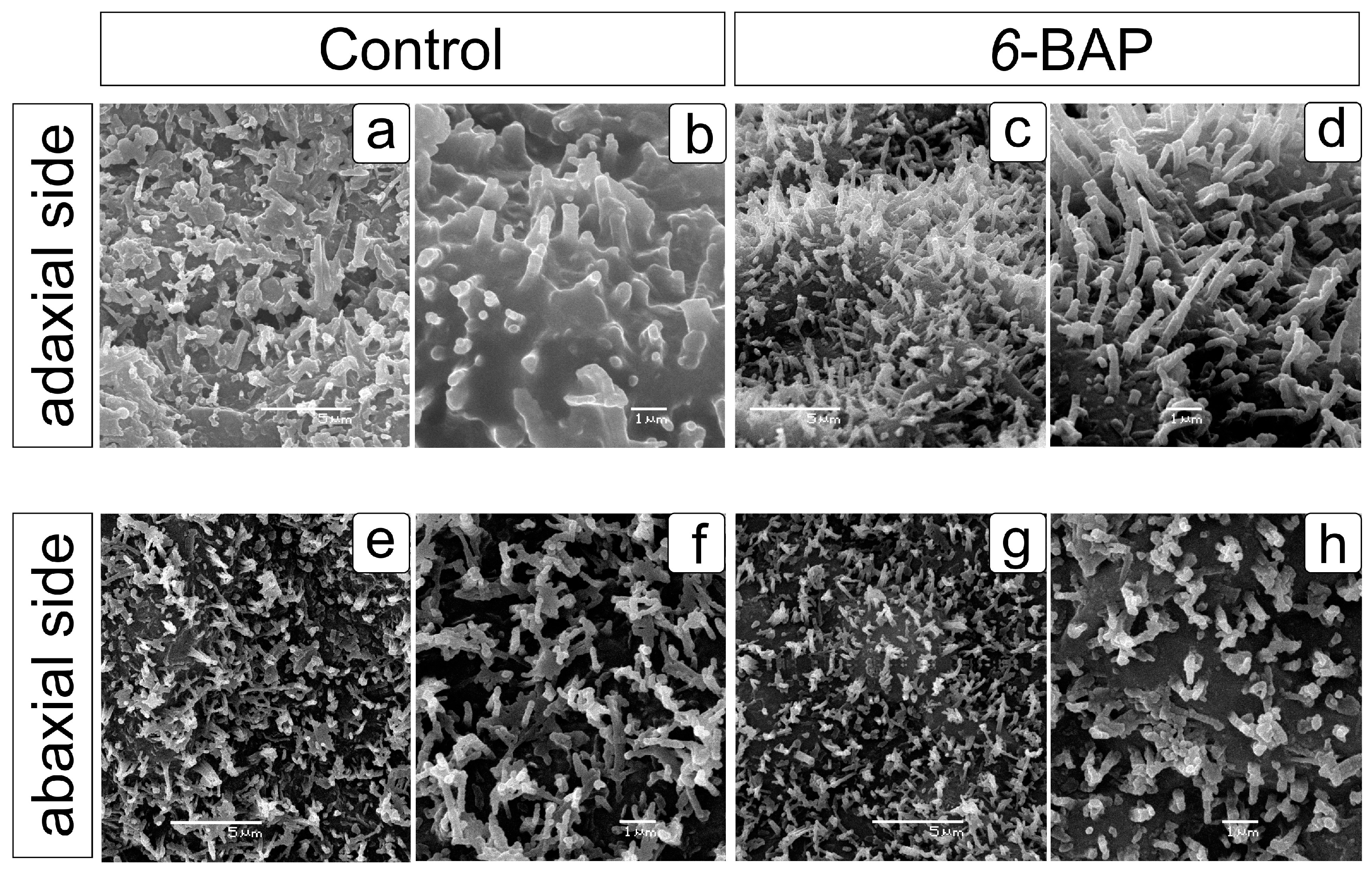
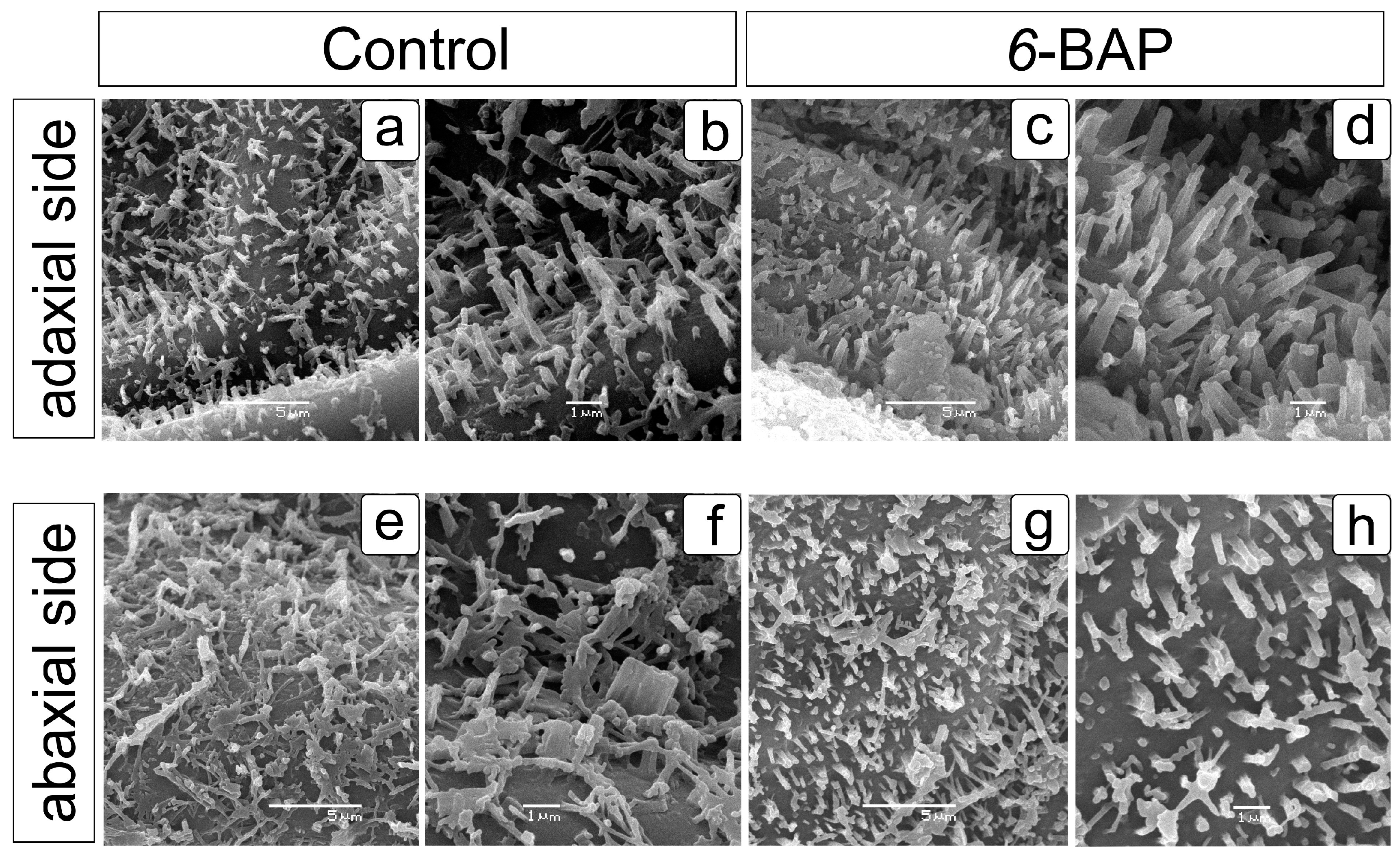
4.5. Scanning Electron Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prins, C.L.; Vieira, I.J.C.; Freitas, S.P. Growth regulators and essential oil production. Brazil. Soc. Plant Physiol. 2010, 22, 91–102. [Google Scholar] [CrossRef]
- Stoeva, T.; Iliev, L. Influence of some phenylureina cytokinins on spearmint essential oil composition. Bulg. J. Plant Physiol. 1997, 23, 66–71. [Google Scholar]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- El-Keltawi, N.E.; Crotaeu, R. Influence of foliar applied cytokinins on growth and essential oil content of several members of Lamiaceae. Phytochemistry 1987, 26, 891–895. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Nikpour-Rashidabad, N.; Samea-Andabjadid, S. Application of growth promoting hormones alters the composition and antioxidant potential of dill essential oil under salt stress. Sci. Rep. 2022, 12, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, J. The impact of auxin and cytokinin on the growth. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Binns, A.N. Cytokinin accumulation and action: Biochemical, genetic, and molecular approaches. Ann. Rev. Plant Physiol. 1994, 45, 173–196. [Google Scholar] [CrossRef]
- Bano, U.; Khan, A.; Mujeeb, F.; Maurya, N.; Tabassum, H.; Siddiqui, M.; Haneef, M.; Osama, K.; Farooqui, A. Effect of plant growth regulators on essential oil yield in aromatic plants. J. Chem. Pharma. Res. 2016, 8, 733–739. [Google Scholar]
- Montesinos, J.C.; Abuzeineh, A.; Mok, M.C. Cytokinins and plant development—An overview. In Cytokinins: Chemistry and Function; Mok, D.W.S., Mok, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Najaran, Z.T.; Hassanzadeh, M.K.; Nasery, M.; Emami, S.A. Dill (Anethum graveolens L.) Oils; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 405–412. [Google Scholar]
- Zacchino, S.A. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 37, 27–48. [Google Scholar] [CrossRef]
- Ahmad, A.; Elisha, I.L.; van Vuuren, S.; Viljoen, A. Volatile phenolics: A comprehensive review of the anti-infective properties of an important class of essential oil constituents. Phytochemistry 2021, 190, 112864. [Google Scholar] [CrossRef] [PubMed]
- Bayala, B.; Bassole, I.H.; Scifo, R.; Gnoula, C.; Morel, L.; Lobaccaro, J.M.; Simpore, J. Anticancer activity of essential oils and their chemical components—A review. Am. J. Cancer Res. 2014, 4, 591–607. [Google Scholar] [PubMed]
- Abbas, M.M.; Kandil, Y.I.; Abbas, M.A. R-(-)-carvone attenuated doxorubicin induced cardiotoxicity in vivo and potentiated its anticancer toxicity in vitro. Balkan Med. J. 2020, 37, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential oils as multicomponent mixtures and their potential for human health and wellbeing. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Padilla-Gonzalez, G.F.; Phumthum, M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Gupta, C.D.J.; Simon, J.E.; Widrlechner, M.P. Characterization of essential oil of dill (Anethum graveolens L.). J. Essential Oil Res. 1995, 7, 11–20. [Google Scholar]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Mochona, B.; Qi, X. Biosynthetic factories of essential oils: The aromatic plants. Nat. Prod. Chem. Res. 2016, 4, 4. [Google Scholar] [CrossRef]
- Turner, L.; Lignou, S.; Gawthrop, F.; Wagstaff, C. Investigating the factors that influence the aroma profile of Apium graveolens: A review. Food Chem. 2021, 345, 128673. [Google Scholar] [CrossRef]
- Holopainen, J.K. Multiple functions of inducible plant volatiles. Trends Plant Sci. 2004, 9, 529–533. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Loreto, F.; Dicke, M.; Schnitzler, J.-P.; Turlings, T.C.J. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Ziv, C.; Zhao, Z.; Gao, Y.G.; Xia, Y. Multifunctional roles of plant cuticle during plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes. Biol. Plant Cuticle 2008, 23, 145–181. [Google Scholar]
- Tassone, E.E.; Lipka, A.E.; Tomasi, P.; Lohrey, G.T.; Qian, W.; Dyer, J.M.; Gore, M.A.; Jenks, M.A. Chemical variation for leaf cuticular waxes and their levels revealed in a diverse panel of Brassica napus L. Ind. Crops Prod. 2016, 79, 77–83. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Plant epicuticular waxes: Chemistry, form, self-assembly and function. Nat. Prod. Comm. 2006, 1, 1067–1072. [Google Scholar] [CrossRef]
- Shevchenko, J.P.; Kharchenko, V.A.; Shevchenko, G.S.; Soldatenko, A.V. Green and Spicy-Flavoring Crops 2019; Federal Scientific Center of Vegetable Production: Moscow, Russia, 2019. [Google Scholar]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á.A. Volatile composition of essential oils from different aromatic herbs grown in mediterranean regions of spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef]
- Said-Al, H.A.H.; Sarhan, A.M.Z.; Abou Dahab, A.D.M.; Abou-Zeid, E.S.N.; Ali, M.S.; Naguib, N.Y. Volatile oil composition of Anethum graveolens affected by harvest stage. Int. J. Plant Sci. Ecol. 2015, 1, 93–97. [Google Scholar]
- Radulescu, V.; Popescu, M.L.; Ilies, D.C. Chemical composition of the volatile oil from different plant parts of Anethum graveolens L. (Umbelliferae) cultivated in Romania. Farmacia 2010, 58, 594–600. [Google Scholar]
- Amin, W.M.A.; Sleem, A.A. Chemical and biological study of aerial parts of dill (Anethum graveolens L.). Egypt. J. Biomed. Sci. 2007, 23, 73–90. [Google Scholar] [CrossRef]
- Kazemi, M.; Abdossi, V. Chemical composition of the essential oils of Anethum graveolens (L.). Bangladesh J. Bot. 2015, 44, 159–161. [Google Scholar] [CrossRef]
- Chahal, K.K.; Monika, K.A.; Bhardwaj, U.; Kaur, R. Chemistry and biological activities of Anethum graveolens (L.) (dill) essential oil: A review. J. Pharmacogn. Phytochem. 2017, 6, 295–306. [Google Scholar]
- Trifan, A.; Aprotosoaie, A.C.; Cioancă, O.; Hăcianu, M.; Jităreanu, A.; Gille, E.; Miron, A. Antioxidant activity of essential oil from Carum carvi L. cultivated in North-Eastern Romania. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2016, 120, 732–736. [Google Scholar] [PubMed]
- Hojjati, M. Chemical constituents and antibacterial activity of dill (Anethum graveolens) essential oil. J. Food Sci. Technol. 2017, 15, 260–263. [Google Scholar]
- Karim, A.; Osman, A.; Satti, A.A.E.; Al-Hafez, M. GC-MS analysis and antimicrobial activity of Anethum graveolens L. (Umbelliferae) essential oil. World J. Pharma. Life Sci. 2021, 6, 16–20. [Google Scholar]
- Kaur, V.; Kaur, R.; Bhardwaj, U. A review on dill essential oil and its chief compounds as natural biocide. Flavour Fragr. J. 2021, 36, 412–431. [Google Scholar] [CrossRef]
- Mukhtar, Y.M.; Adu-Frimpong, M.; Xu, X.; Yu, J. Biochemical significance of limonene and its metabolites: Future prospects for designing and developing highly potent anticancer drugs. Biosci. Rep. 2018, 38, BSR20181253. [Google Scholar] [CrossRef]
- Alasmari, E.A.; Mehanna, A.S. Carvone on selected human cancer cell lines. J. Anal. Pharm. Res. 2019, 8, 149–158. [Google Scholar]
- Radice, M.; Durofil, A.; Buzzi, R.; Baldini, E.; Martínez, A.P.; Scalvenzi, L.; Manfredini, S. Alpha-phellandrene and alpha-phellandrene-rich essential oils: A systematic review of biological activities. Pharmaceutical and food applications. Life 2022, 12, 1602. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Tiyajamorn, T.; Bharathi, M.; Chaiyasut, C.A. Narrative review on the bioactivity and health benefits of alpha-phellandrene. Sci. Pharm. 2022, 90, 57. [Google Scholar] [CrossRef]
- Costa, M.D.S.; Rocha, J.E.; Campina, F.F.; Silva, A.R.P.; Da Cruz, R.P.; Pereira, R.L.S.; Quintans-Júnior, L.J.; De Menezes, I.R.A.; De S Araújo, A.A.; De Freitas, T.S.; et al. Comparative analysis of the antibacterial and drug-modulatory effect of d-limonene alone and complexed with β-cyclodextrin. Eur. J. Pharm. Sci. 2019, 128, 158–161. [Google Scholar]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; Omari, N.E. Health benefits and pharmacological properties of carvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Mesquita, R.F.; de Araújo Ribeiro, L.A.; de Lima, J.T. Spasmolytic activity of carvone and limonene enantiomers. Nat. Prod. Commun. 2015, 10, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Seidler-Lozykowska, K.; Baranska, M.; Baranski, R.; Krol, D.J. Raman analysis of caraway (Carum carvi L.) single fruits. Evaluation of essential oil content and its composition. Agric. Food Chem. 2010, 58, 5271–5275. [Google Scholar] [CrossRef]
- Martín-Luengo, M.A.; Yates, M.; Martínez, D.M.J.; Casal, B.; Iglesias, M.; Steban, M.; Ruiz-Hitzky, E. Synthesis of p-cymene from limonene, a renewable feedstock. Appl. Catal. B Environ. 2008, 81, 218–224. [Google Scholar] [CrossRef]
- Babri, R.A.; Khokhar, I.; Mahmood, Z.; Mahmud, S. Chemical composition and insecticidal activity of the essential oil of Anethum graveolens. Sci Int. 2012, 24, 453–455. [Google Scholar]
- Jana, S.; Shekhawat, G.S. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacogn. Rev. 2010, 4, 179–184. [Google Scholar]
- Corot, A.; Roman, H.; Douillet, O.; Autret, H.; Perez-Garcia, M.D.; Citerne, S.; Bertheloot, J.; Sakr, S.; Leduc, N.; Demotes-Mainard, S. Cytokinins and abscisic acid act antagonistically in the regulation of the bud outgrowth pattern by light intensity. Front. Plant Sci. 2017, 8, 1724. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, J.; Bat’ková, P. Effects of pre-treatments with abscisic acid and/or benzyladenine on gas exchange of French bean, sugar beet, and maize leaves during water stress and after rehydration. Biol. Plant. 2004, 48, 395–399. [Google Scholar] [CrossRef]
- Shu-Qing, C.; Rong-Xian, Z.; Wei, L.; Zhi-Rui, D.; Qi-Ming, Z. The involvement of cytokinin and abscisic acid levels in roots in the regulation of photosynthesis function in flag leaves during grain filling in super high-yielding rice (Oryza sativa). J. Agron. Crop Sci. 2004, 190, 73–80. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Zhao, S.; Liu, Z.; Feng, Y.Q.; Wu, Y. Cytokinin antagonizes ABA suppression to seed germination of Arabidopsis by downregulating ABI5 expression. Plant J. 2011, 68, 249–261. [Google Scholar] [CrossRef]
- Lee, S.B.; Suh, M.C. Cuticular wax biosynthesis is up-regulated by the MYB94 transcription factor in Arabidopsis. Plant Cell Physiol. 2015, 56, 48–60. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Sabagh, A.E.; Mbarki, S.; Hossain, A.; Iqbal, M.A.; Islam, M.S.; Raza, A.; Llanes, A.; Reginato, M.; Rahman, M.A.; Mahboob, W.; et al. Potential role of plant growth regulators against abiotic stresses. Front. Agron. 2021, 3, 648694. [Google Scholar] [CrossRef]
- Mátyás, K.K.; Hegedűs, G.; Taller, J.; Farkas, E.; Decsi, K.; Kutasy, B.; Kálmán, N.; Nagy, E.; Kolics, B.; Virág, E. Different expression pattern of flowering pathway genes contribute to male or female organ development during floral transition in the monoecious weed Ambrosia artemisiifolia L. (Asteraceae). PeerJ 2019, 7, e7421. [Google Scholar] [CrossRef] [PubMed]
- Grünhofer, P.; Herzig, L.; Schreiber, L. Leaf morphology, wax composition, and residual (cuticular) transpiration of four poplar clones. Trees 2022, 36, 645–658. [Google Scholar] [CrossRef]
- Shepherd, T.; Wynne Griffiths, D. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Koch, K.; Hartmann, K.D.; Schreiber, L.; Barthlott, W.; Neinhuis, C. Influences of air humidity during the cultivation of plants on wax chemical composition, morphology and leaf surface wettability. Environ. Exp. Bot. 2006, 56, 1–9. [Google Scholar] [CrossRef]
- Arya, G.C.; Sarkar, S.; Manasherova, E.; Aharoni, A.; Cohen, H. The plant cuticle: An ancient guardian barrier set against long-standing rivals. Front. Plant Sci. 2021, 12, 663165. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Nikolau, B.J.; Schnable, P.S. Developmental and hormonal regulation of the Arabidopsis CER2 gene that codes for a nuclear-localised protein required for the normal accumulation of cuticular waxes. Plant Physiol. 1997, 115, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Yuan, Z.; Jiang, Y.; Liu, Y.; Xu, Y.; Li, S.; Guo, Y.; Jetter, R.; Ni, Y. Exogenous hormones influence Brassica napus leaf cuticular wax deposition and cuticle function. Peer J. 2020, 8, e9264. [Google Scholar] [CrossRef]
- Özel, A.; Çınar, O. Essential oil composition of dry and fresh aerial parts of the dill (Anethum graveolens L.). Bursa Uludağ Üniversitesi Ziraat Fakültesi Derg. 2021, 35, 355–363. [Google Scholar]
- Asekun, T.O.; Grierson, D.S.; Afolayan, A.J. Effects of drying methods on the quality and quantity of the essential oil of Mentha longifolia L. subsp. capensis. Food Chem. 2007, 101, 995–998. [Google Scholar] [CrossRef]
- Voirin, B.; Bayet, C. Developmental changes in the monoterpene composition of Mentha × piperita leaves from individual peltate trichomes. Phytochemistry 1996, 43, 573–580. [Google Scholar] [CrossRef]
- Gershenzon, J.; Maffei, M.; Croteau, R. Biochemical and histochemical localization of monoterpene biosynthesis in the glandular trichomes of spearmint (Mentha spicata). Plant Physiol. 1989, 89, 1351–1357. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Gershenzon, J.; Konings, M.C.J.M.; Croteau, R. Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway: I. Demonstration of enzyme activities and their changes with development. Plant Physiol. 1998, 117, 901–912. [Google Scholar] [CrossRef]


| “Uzory” | “Rusich” | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EO Components | Leaves | Umbel | Fruit | Leaves | Umbel | Fruit | ||||||||||
| Control | BAP | Control | BAP | Control | BAP | Control | BAP | Control | BAP | Control | BAP | Control | BAP | Control | BAP | |
| DAT | 23 | 42 | 23 | 42 | 23 | 43 | 23 | 43 | ||||||||
| α-Phellandrene | 20.1 c | 32.5 b | 28.3 b | 37.3 a | 26.4 b | 38.2 a | t | 0.1 | 53.7 b | 68.5 a | 54.1 b | 69.2 a | 18.0 b | 25.1 a | 1.4 b | 2.4 a |
| D-Limonene | 5.4 b | 7.3 a | 9.3 a | 8.4 a | 34.9 a | 22.1 b | 49.7 b | 65.2 a | 6. 8 a | 5.6 b | 7.1 a | 6.2 b | 57.6 a | 46.8 b | 44.4 b | 67.1 a |
| β-Phellandrene | 4.1 c | 8.8 a | 10.1 a | 8.6 a | 4.4 b | 6.6 a | t | 0.1 | 8.3 b | 9.1 a | 9.3 a | 9.6 a | 2.7 b | 3.7 a | 0.3 b | 0.4 a |
| p-Cymene | 6.2 c | 17.7 b | 22.6 a | 16.2 b | 5.77 b | 12.0 a | 0.1 b | 0.3 a | 5.1 b | 2.2 c | 12.8 a | 8.4 b | 2.1 b | 4.1 a | 0.2 b | 0.4 a |
| 3,9-Epoxy-p-menth-1-ene | 14.5 b | 8.2 c | 17.8 a | 9.9 b | 13.0 a | 11.9 a | t | t | 17.0 a | 10.7 b | 11.0 b | 4.6 c | 5.9 b | 9.9 a | t | t |
| Carvone | 0.6 a | 0.3 c | 0.8 a | 0.3 c | 9.3 c | 0.4 d | 46.3 a | 27.5 b | 0.7 a | 0.4 c | 0.2 c | 0.1 c | 9.8 a | 4.4 b | 48.0 a | 26.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirokova, A.V.; Dmitriev, L.B.; Belopukhov, S.L.; Dmitrieva, V.L.; Danilova, I.L.; Kharchenko, V.A.; Pekhova, O.A.; Myagkih, E.F.; Tsitsilin, A.N.; Gulevich, A.A.; et al. The Accumulation of Volatile Compounds and the Change in the Morphology of the Leaf Wax Cover Accompanied the “Anti-Aging” Effect in Anethum graveolens L. Plants Sprayed with 6-Benzylaminopurine. Int. J. Mol. Sci. 2023, 24, 15137. https://doi.org/10.3390/ijms242015137
Shirokova AV, Dmitriev LB, Belopukhov SL, Dmitrieva VL, Danilova IL, Kharchenko VA, Pekhova OA, Myagkih EF, Tsitsilin AN, Gulevich AA, et al. The Accumulation of Volatile Compounds and the Change in the Morphology of the Leaf Wax Cover Accompanied the “Anti-Aging” Effect in Anethum graveolens L. Plants Sprayed with 6-Benzylaminopurine. International Journal of Molecular Sciences. 2023; 24(20):15137. https://doi.org/10.3390/ijms242015137
Chicago/Turabian StyleShirokova, Anna V., Lev B. Dmitriev, Sergey L. Belopukhov, Valeria L. Dmitrieva, Irina L. Danilova, Viktor A. Kharchenko, Olga A. Pekhova, Elena F. Myagkih, Andrey N. Tsitsilin, Alexander A. Gulevich, and et al. 2023. "The Accumulation of Volatile Compounds and the Change in the Morphology of the Leaf Wax Cover Accompanied the “Anti-Aging” Effect in Anethum graveolens L. Plants Sprayed with 6-Benzylaminopurine" International Journal of Molecular Sciences 24, no. 20: 15137. https://doi.org/10.3390/ijms242015137







