Exosome-Laden Scaffolds for Treatment of Post-Traumatic Cartilage Injury and Osteoarthritis of the Knee: A Systematic Review
Abstract
:1. Introduction
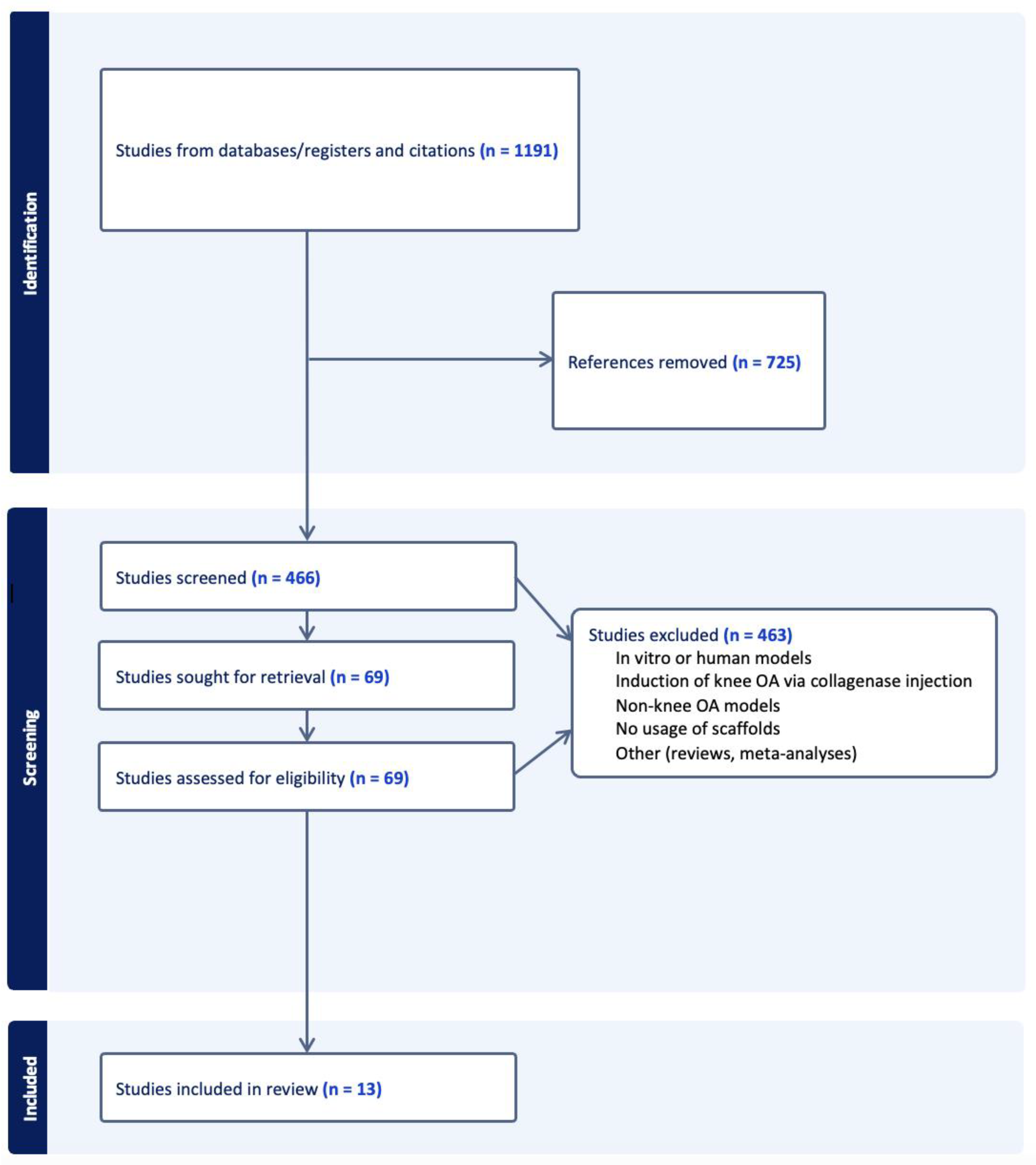
2. Results
2.1. Hydrogels
2.2. Acellular Extracelllular Matrices
2.3. Hyaluronic Acid
2.4. Risk of Bias
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Evers, B.J.; Bosch, M.H.J.V.D.; Blom, A.B.; van der Kraan, P.M.; Koëter, S.; Thurlings, R.M. Post-traumatic knee osteoarthritis; the role of inflammation and hemarthrosis on disease progression. Front. Med. 2022, 9, 973870. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Brogini, S.; Pagani, S.; Giavaresi, G.; Tschon, M. Current Trends in the Evaluation of Osteochondral Lesion Treatments: Histology, Histomorphometry, and Biomechanics in Preclinical Models. BioMed Res. Int. 2019, 2019, 4040236. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, D.H.; Lee, Y.S. Is there an optimal age for total knee arthroplasty?: A systematic review. Knee Surg. Relat. Res. 2020, 32, 60. [Google Scholar] [CrossRef]
- Stiebel, M.; Miller, L.E.; Block, J.E. Post-traumatic knee osteoarthritis in the young patient: Therapeutic dilemmas and emerging technologies. Open Access J. Sports Med. 2014, 5, 73–79. [Google Scholar] [CrossRef]
- D’arrigo, D.; Roffi, A.; Cucchiarini, M.; Moretti, M.; Candrian, C.; Filardo, G. Secretome and Extracellular Vesicles as New Biological Therapies for Knee Osteoarthritis: A Systematic Review. J. Clin. Med. 2019, 8, 1867. [Google Scholar] [CrossRef]
- Jousheghan, S.S.; Sajjadi, M.M.; Jousheghan, S.S.; Hosseininejad, S.-M.; Maleki, A. Extracellular vesicles as novel approaches for the treatment of osteoarthritis: A narrative review on potential mechanisms. Histochem. J. 2021, 52, 879–891. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Hu, H.; Dong, L.; Bu, Z.; Shen, Y.; Luo, J.; Zhang, H.; Zhao, S.; Lv, F.; Liu, Z. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J. Extracell. Vesicles 2020, 9, 1778883. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tian, G.; Yang, Z.; Gao, X.; Wang, F.; Li, J.; Tian, Z.; Huang, B.; Wei, F.; Sang, X.; et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater. 2021, 6, 2711–2728. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Yang, Z.; Zhou, Z.; Lou, Z.; Zhang, Q. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomedicine 2020, 15, 273–288. [Google Scholar] [CrossRef]
- Pang, L.; Jin, H.; Lu, Z.; Xie, F.; Shen, H.; Li, X.; Zhang, X.; Jiang, X.; Wu, L.; Zhang, M.; et al. Treatment with Mesenchymal Stem Cell-Derived Nanovesicle-Containing Gelatin Methacryloyl Hydrogels Alleviates Osteoarthritis by Modulating Chondrogenesis and Macrophage Polarization. Adv. Healthc. Mater. 2023, 12, e2300315. [Google Scholar] [CrossRef]
- Shen, K.; Duan, A.; Cheng, J.; Yuan, T.; Zhou, J.; Song, H.; Chen, Z.; Wan, B.; Liu, J.; Zhang, X.; et al. Exosomes derived from hypoxia preconditioned mesenchymal stem cells laden in a silk hydrogel promote cartilage regeneration via the miR-205–5p/PTEN/AKT pathway. Acta Biomater. 2022, 143, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Huang, J.-Y.; Gao, Y.; Li, Z.-X.; Wei, Z.-Y.; Dawes, H.; Guo, S.-C. Small extracellular vesicles in combination with sleep-related circRNA3503: A targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021, 6, 4455–4469. [Google Scholar] [CrossRef]
- WWong, K.L.; Zhang, S.; Wang, M.; Ren, X.; Afizah, H.; Lai, R.C.; Lim, S.K.; Lee, E.H.; Hui, J.H.P.; Toh, W.S. Intra-Articular Injections of Mesenchymal Stem Cell Exosomes and Hyaluronic Acid Improve Structural and Mechanical Properties of Repaired Cartilage in a Rabbit Model. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2020, 36, 2215–2228.e2. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, H.; Wu, J.; Tian, G.; Li, M.; Liao, Z.; He, S.; Deng, H.; Ning, C.; Ding, Z.; et al. Engineering exosomes by three-dimensional porous scaffold culture of human umbilical cord mesenchymal stem cells promote osteochondral repair. Mater. Today Bio 2023, 19, 100549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-X.; Liu, P.; Ding, W.; Meng, Q.-B.; Su, D.-H.; Zhang, Q.-C.; Lian, R.-X.; Yu, B.-Q.; Zhao, M.-D.; Dong, J.; et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]
- Zhang, S.; Wong, K.L.; Ren, X.; Teo, K.Y.W.; Afizah, H.; Choo, A.B.H.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Mesenchymal Stem Cell Exosomes Promote Functional Osteochondral Repair in a Clinically Relevant Porcine Model. Am. J. Sports Med. 2022, 50, 788–800. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Riau, A.K.; Ong, H.S.; Yam, G.H.F.; Mehta, J.S. Sustained Delivery System for Stem Cell-Derived Exosomes. Front. Pharmacol. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Osteoarthritis and articular cartilage use, disuse, and abuse: Experimental studies. J. Rheumatol. Suppl. 1995, 43, 13–15. [Google Scholar]
- Buckwalter, J.A.; Lane, N.E. Athletics and Osteoarthritis. Am. J. Sports Med. 1997, 25, 873–881. [Google Scholar] [CrossRef]
- Englund, M.; Roos, E.M.; Lohmander, L.S. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: A sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003, 48, 2178–2187. [Google Scholar] [CrossRef]
- Gelber, A.C.; Hochberg, M.C.; Mead, L.A.; Wang, N.-Y.; Wigley, F.M.; Klag, M.J. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann. Intern. Med. 2000, 133, 321–328. [Google Scholar] [CrossRef]
- Sahin, V.; Karakaş, E.S.; Aksu, S.; Atlihan, D.; Turk, C.Y.; Halici, M. Traumatic Dislocation and fracture-dislocation of the hip: A long-term follow-up study. J. Trauma: Inj. Infect. Crit. Care 2003, 54, 520–529. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Si, H.-B.; Tang, L.; Xie, H.-Q.; Shen, B. Exosomes Derived from Human Urine–Derived Stem Cells Overexpressing miR-140-5p Alleviate Knee Osteoarthritis through Downregulation of VEGFA in a Rat Model. Am. J. Sports Med. 2022, 50, 1088–1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.-N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef]
- Fan, W.-J.; Liu, D.; Pan, L.-Y.; Wang, W.-Y.; Ding, Y.-L.; Zhang, Y.-Y.; Ye, R.-X.; Zhou, Y.; An, S.-B.; Xiao, W.-F. Exosomes in osteoarthritis: Updated insights on pathogenesis, diagnosis, and treatment. Front. Cell Dev. Biol. 2022, 10, 949690. [Google Scholar] [CrossRef]
- Song, J.-S.; Hong, K.-T.; Kim, N.-M.; Jung, J.-Y.; Park, H.-S.; Chun, Y.S.; Kim, S.J. Cartilage regeneration in osteoarthritic knees treated with distal femoral osteotomy and intra-lesional implantation of allogenic human umbilical cord blood-derived mesenchymal stem cells: A report of two cases. Knee 2019, 26, 1445–1450. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.-H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef]
- Yuana, Y.; Levels, J.; Grootemaat, A.; Sturk, A.; Nieuwland, R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J. Extracell. Vesicles 2014, 3, 23262. [Google Scholar] [CrossRef]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of Exo-somes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatog-raphy Methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Kowal, J.; Théry, C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos. Trans. R. Soc. B: Biol. Sci. 2017, 373, 20160479. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Morishita, M.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: Yield, physicochemical properties, and pharmacokinetics. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 96, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Carvalho, J.; Oliveira, P.; Voglstaetter, M.; Schvartz, D.; Thomsen, A.R.; Walter, N.; Khanduri, R.; Sanchez, J.; Keller, A.; et al. 3D Cellular Architecture Affects MicroRNA and Protein Cargo of Extracellular Vesicles. Adv. Sci. 2018, 6, 1800948. [Google Scholar] [CrossRef]
- Sohrabi, B.; Dayeri, B.; Zahedi, E.; Khoshbakht, S.; Pour, N.N.; Ranjbar, H.; Nejad, A.D.; Noureddini, M.; Alani, B. Mesenchymal stem cell (MSC)-derived exosomes as novel vehicles for delivery of miRNAs in cancer therapy. Cancer Gene Ther. 2022, 29, 1105–1116. [Google Scholar] [CrossRef]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure–function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef]
- Hasany, M.; Thakur, A.; Taebnia, N.; Kadumudi, F.B.; Shahbazi, M.-A.; Pierchala, M.K.; Mohanty, S.; Orive, G.; Andresen, T.L.; Foldager, C.B.; et al. Combinatorial Screening of Nanoclay-Reinforced Hydrogels: A Glimpse of the “Holy Grail” in Orthopedic Stem Cell Therapy? ACS Appl. Mater. Interfaces 2018, 10, 34924–34941. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Li, Y.; Feng, L.; Wang, B.; Wang, M.; Wang, H.; Zhu, M.; Yang, Y.; Waldorff, E.I.; et al. Enhancing cartilage repair with optimized supramolecular hydrogel-based scaffold and pulsed electromagnetic field. Bioact. Mater. 2022, 22, 312–324. [Google Scholar] [CrossRef]
- Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.Z.; Duan, Z.; Wang, Y.; Feng, R.R. A Novel Human-Like Collagen Hydrogel Scaffold with Porous Structure and Sponge-Like Properties. Polymers 2017, 9, 638. [Google Scholar] [CrossRef]
- Lee, J.H.; Badar, F.; Kahn, D.; Matyas, J.; Qu, X.; Xia, Y. Loading-induced changes on topographical distributions of the zonal properties of osteoarthritic tibial cartilage—A study by magnetic resonance imaging at microscopic resolution. J. Biomech. 2015, 48, 3625–3633. [Google Scholar] [CrossRef] [PubMed]
- Badar, F.; Lee, J.; Qu, X.; Xia, Y. Topographical and zonal patterns of T2 relaxation in osteoarthritic tibial cartilage by low- and high-resolution MRI. Magn. Reson. Imaging 2021, 78, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage—Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2018, 146, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
| Author | Exosome Source | Exosome Dosage | Animal | Cartilage Injury/Osteoarthritis Model |
|---|---|---|---|---|
| Chen et al. [14]. | Bone marrow MSCs | 200 μg/mL | Rabbit | Drilled cylindrical defect in patellar groove |
| Hu et al. [15]. | Human umbilical cord MSCs | 44 μg/mL | Rat | Drilled cylindrical defects in articular cartilage |
| Jiang et al. [16]. | Human Wharton’s jelly MSCs | 125 μg/mL (25 μg/mL × 5 injections) | Rabbit | Drilled osteochondral defect in femoral trochlea |
| Liu et al. [17]. | Immortalized clonal MSCs | 44 μg/mL | Rabbit | Drilled cylindrical osteochondral defect in patellar groove |
| Liu et al. [18]. | Bone marrow MSCs | 50 μg/mL, 100 μg/mL | Rat | Drilled chondral defect in patellar sulcus |
| Pang et al. [19]. | Bone marrow MSCs | 80 μg/mL | Mouse | Surgical destabilization of medial meniscus |
| Shen et al. [20]. | Bone marrow MSCs | 44 μg/mL | Rat | Drilled osteochondral defects in center of patellar groove |
| Tao et al. [21]. | Synovial MSCs | 100 μg/mL | Rat | Transection of ACL, MCL, medial meniscus |
| Wong et al. [22]. | Immortalized clonal MSCs | 600 μg/mL (200 μg/mL × 3 injections) | Rabbit | Bilateral drilled osteochondral defects in femoral trochlear grooves |
| Yan et al. [23]. | Human umbilical cord MSCs | 25 μg/mL | Rat | Drilled osteochondral defect in femoral trochlea |
| Zhang et al. [24]. | Bone marrow MSCs | 200 μg/mL | Rat | Drilled osteochondral defect in femoral trochlear groove |
| Zhang et al. [25]. | Immortalized clonal MSCs | 3000 μg/mL (1000 μg/mL × 3 injections) | Micropig | Drilled osteochondral defects in medial femoral condyles |
| Author | Scaffold Type | Exosome Release | Gross Findings | ICRS Score | Biomechanical Testing | Histology | Gene Expression |
|---|---|---|---|---|---|---|---|
| Chen et al. [14]. | Hydrogel | 7 days | Smooth neo-cartilage and enhanced defect filling at 6 and 12 weeks post-op | Histological: 16.1 ± 0.83 at 12 weeks post-op | N/A | Fibrocartilage + hyaline-like cartilage at 6 and 12 weeks post-op | Upregulation of M2 macrophages, COL2A1; Downregulation of M1 macrophages, MMP13 |
| Hu et al. [15]. | Hydrogel | N/A | Intact, smooth regenerated neo-tissue with complete integration with surrounding cartilage at 12 weeks post-op | Macroscopic: 10.7 ± 1.5 Histological: 11.2 ± 0.7 at 12 weeks post-op | Ultimate strength: 48.2 ± 8.1 kPa to 259.8 ± 35.6 kPa with nanoclay addition | Intense staining similar to native cartilage | Increased COL2, P-AKT, miR-23a-3p; Reduced PTEN |
| Jiang et al. [16]. | Acellular extracellular matrix | N/A | Regenerated tissue at depth matching surrounding cartilage, somewhat visible boundary at 3 months post-op; smooth surface, no visible boundary at 6 months post-op | Highest ICRS score when compared to other groups at 3, 6 months post-op | No significant difference in Young’s modulus between regenerated and native tissue at 6 months post-op | Gap between repaired tissue and normal cartilage at 3 months post-op; completely fused with normal cartilage at 6 months post-op | Increased M2 macrophage activity; significant increase in IL-10 |
| Liu et al. [17]. | Hyaluronic Acid | 14 days | Regenerated tissue with smooth surface, complete defect filling, integration with surrounding cartilage at 12 weeks post-op | Highest ICRS score when compared to other groups at 12 weeks post-op | N/A | Newly formed tissue was mainly hyaline cartilage | COL2 upregulation; COL1 downregulation |
| Liu et al. [18]. | Hydrogel | N/A | The articular surface was smoother with integration with adjacent host cartilage at 8 weeks pot-op | Highest ICRS score when compared to other groups at 4, 6, and 8 weeks post-op | N/A | Regular presence of mature chondrocyte cells indicative of predominate hyaline cartilage formation | COL2A1, Agg, Prg4, SOX-9 upregulation Col1A1, Adamts5, IL-1b, C-myc downregulation |
| Pang et al. [19]. | Hydrogel | 30 days with 100% release | Smooth surface in repaired cartilage at 4 weeks post-op | OARSI score -> significant reduction in cartilage lesion severity when compared to other groups at 4 weeks post-op | Compressive strength: 16.779 kPa (compared to 12.096 kPA in scaffold alone) High cyclic compression stability | Increased polysaccharide content in repaired cartilage at 4 weeks post-op | COL2, Agg, M2 macrophage upregulation MMP13, TNF-a, IL-1B, M1 macrophage downregulation |
| Shen et al. [20]. | Hydrogel | 30 days with 85–89% release | Neo-tissues in region adjacent to defects were smooth and complete with fusion with surrounding normal cartilage at 12 weeks post-op | Macroscopic: 11.25 ± 0.96 at 12 weeks post-op | N/A | Smooth, flat surfaces with regenerated articular chondrocytes arranged regularly | COL2 upregulation; COL1 downregulation |
| Tao et al. [21]. | Hydrogel | 35 days with 80% release | N/A | N/A | N/A | Reversal of OA cartilage damage, protective ECM | N/A |
| Wong et al. [22]. | Hyaluronic Acid | N/A | Greater neo-tissue filling, smooth surface regularity at 12 weeks post-op | Macroscopic: 8.75 ± 0.87 at 6 weeks post-op; Macroscopic: 10.33 ± 0.49 at 12 weeks post-op | Young’s Modulus: 7.34 ± 0.67 MPa at 6 weeks post-op 12.22 ± 3.67 MPa at 12 weeks post-op Stiffness: 3.85 ± 0.48 N/mm at 6 weeks post-op 6.40 ± 1.92 N/mm at 12 weeks post-op | Thicker than normal cartilage with high cellularity >40% hyaline cartilage at 6 weeks post-op; >80% hyaline cartilage at 12 weeks post-op | Greater areal deposition of GAG; Lower areal deposition of COL1 |
| Yan et al. [23]. | Acellular extracellular matrix | 5 days (via fluorescence) | Mostly filled cartilage defect area with uneven surface and slight border with surrounding tissue at 4 weeks post-op; new tissue was smooth with no obvious border with surrounding tissue at 8 weeks post-op | Highest ICRS score when compared to other groups at 4 and 8 weeks post-op | N/A | Small amount of fibrous tissue with increased chondrocytes at 4 weeks post-op; high level of chondrocytes with normal arrangement at 8 weeks post-op | Abundant expression of proteoglycans and COL II at 4 and 8 weeks post-op |
| Zhang et al. [24]. | Hydrogel | 14 days with 87% release | Smooth, continuous cartilage surface with rarely distinct boundary with surrounding tissue at 6 and 12 weeks post-op | Highest ICRS score when compared to other groups at 6 and 12 weeks post-op | Adhesive strength: 121.7 ± 12.3 kPa | Newly formed cartilage in the defect at 6 weeks post-op; smooth surface similar to the normal at 12 weeks post-op | COL2, Agg upregulation; COL1 downregulation |
| Zhang et al. [25]. | Hyaluronic Acid | N/A | Significantly improved degree of defect repair, integration to border zone, macroscopic appearance | Macroscopic: 9.22 ± 1.94 at 4 months post-op Histological: 79.71 ± 12.3 at 4 months post-op | Young’s Modulus: 19.58 ± 6.79 MPa at 4 months post-op; Stiffness: 10.25 ± 3.56 N/mm at 4 months post-op | Newly formed cartilage with matrix staining and tissue and cell morphologies resembling adjacent native cartilage at 4 months post-op | N/A |
| Author | Random Sequence Allocation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Sources of Bias |
|---|---|---|---|---|---|---|---|
| Chen et al. [14]. | Unsure risk | Unsure risk | Unsure risk | Low risk | Low risk | Low risk | Low risk |
| Hu et al. [15]. | Low risk | Unsure risk | Unsure risk | Low risk | Low risk | Low risk | Low risk |
| Jiang et al. [16]. | Low risk | Unsure risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Liu et al. [17]. | Low risk | Unsure risk | Unsure risk | Low risk | Low risk | Low risk | Low risk |
| Liu et al. [18]. | Low risk | Unsure risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Pang et al. [19]. | Low risk | Unsure risk | Unsure risk | Low risk | Low risk | Low risk | Low risk |
| Shen et al. [20]. | Unsure risk | Unsure risk | Unsure risk | Low risk | Low risk | Low risk | Low risk |
| Tao et al. [21]. | Unsure risk | Unsure risk | Unsure risk | Low risk | Low risk | Low risk | Low risk |
| Wong et al. [22]. | Low risk | Unsure risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Yan et al. [23]. | Low risk | Unsure risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zhang et al. [24]. | Low risk | Unsure risk | Unsure risk | Low risk | Low risk | Low risk | Low risk |
| Zhang et al. [25]. | Low risk | Unsure risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, J.; Jerome, W.; Zaslav, K.; Grande, D. Exosome-Laden Scaffolds for Treatment of Post-Traumatic Cartilage Injury and Osteoarthritis of the Knee: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15178. https://doi.org/10.3390/ijms242015178
Xavier J, Jerome W, Zaslav K, Grande D. Exosome-Laden Scaffolds for Treatment of Post-Traumatic Cartilage Injury and Osteoarthritis of the Knee: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(20):15178. https://doi.org/10.3390/ijms242015178
Chicago/Turabian StyleXavier, Jorden, William Jerome, Kenneth Zaslav, and Daniel Grande. 2023. "Exosome-Laden Scaffolds for Treatment of Post-Traumatic Cartilage Injury and Osteoarthritis of the Knee: A Systematic Review" International Journal of Molecular Sciences 24, no. 20: 15178. https://doi.org/10.3390/ijms242015178





