Environmental Endocrinology: Parabens Hazardous Effects on Hypothalamic–Pituitary–Thyroid Axis
Abstract
1. Introduction
2. Thyroid Morphophysiology: An Overview
3. Parabens
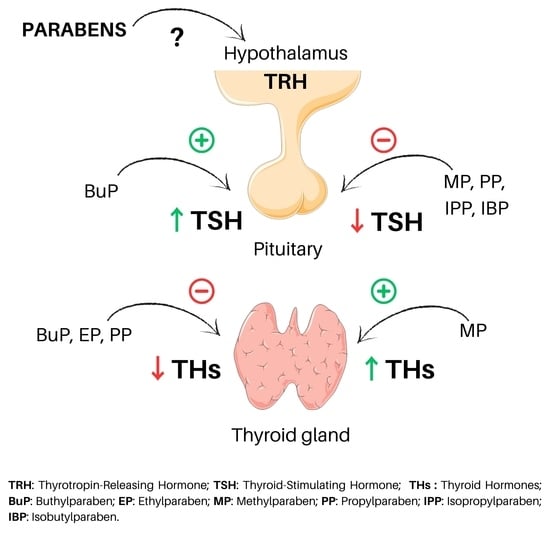
3.1. Parabens and TSH
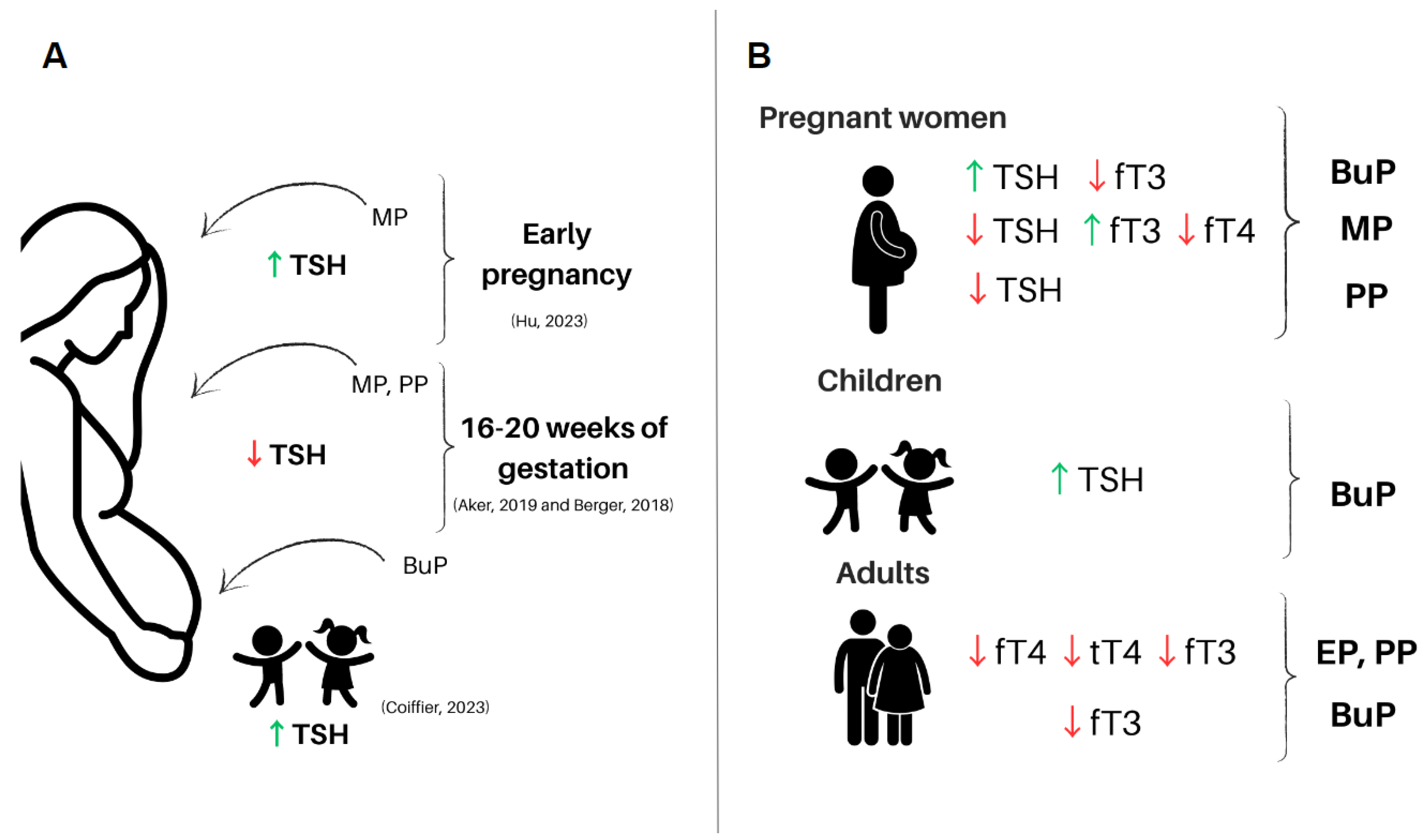
3.1.1. Parabens and TSH in Human
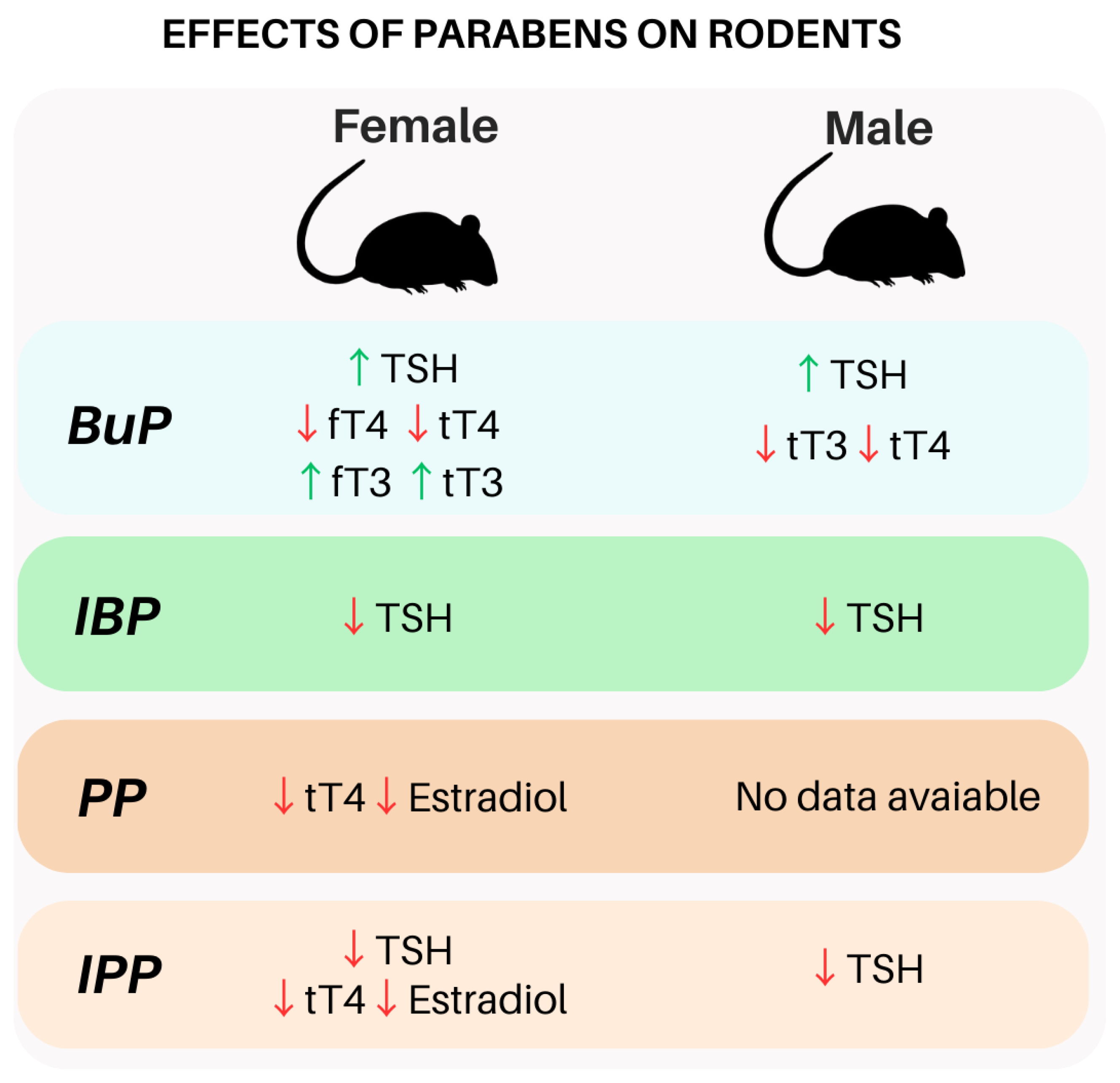
3.1.2. Parabens and TSH in Rodents
3.2. Parabens and Thyroid Hormones
3.2.1. Parabens and Thyroid Hormones in Human
3.2.2. Parabens and Thyroid Hormones in Rodents
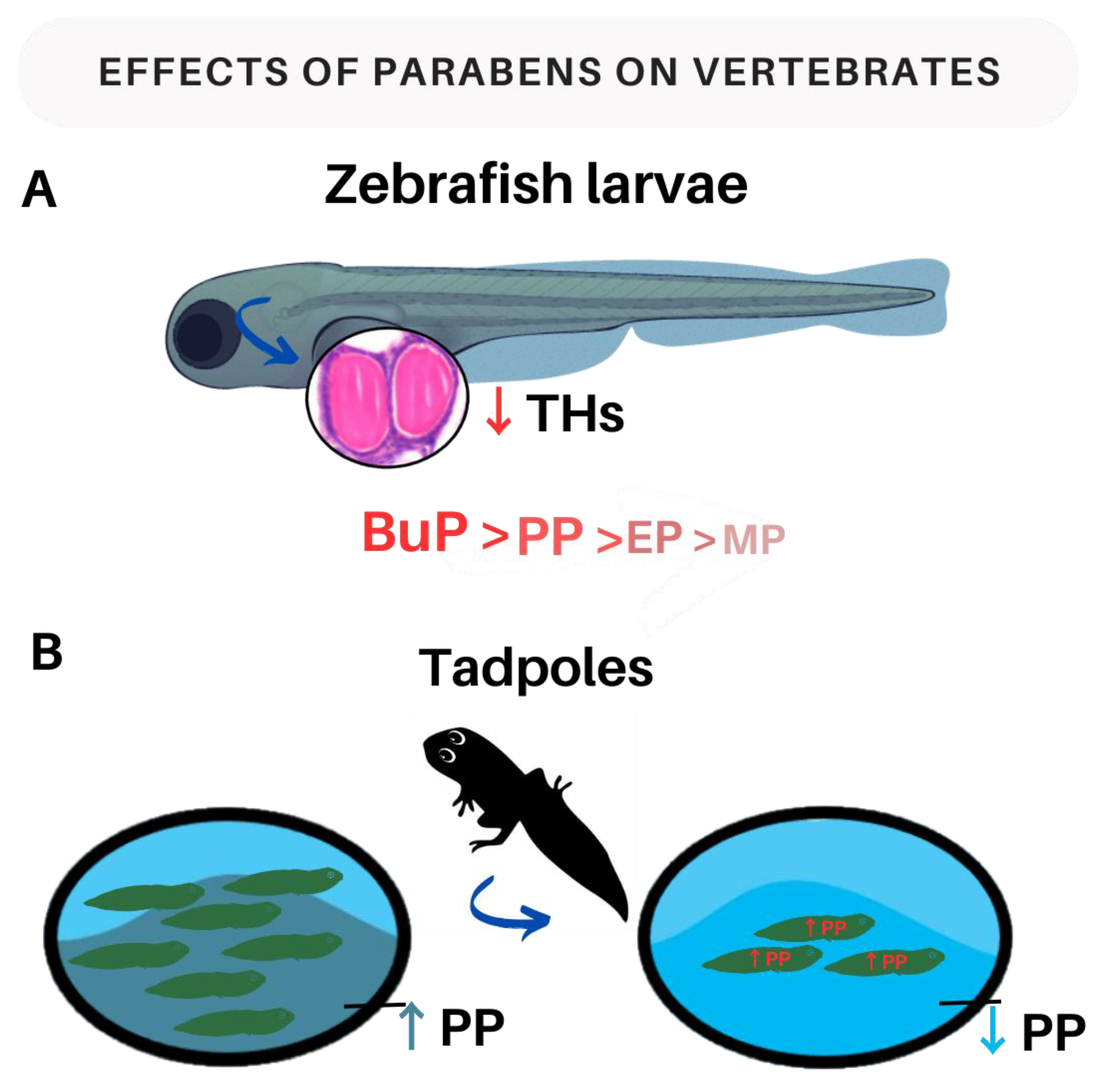
3.2.3. Parabens and Thyroid Hormones in Vertebrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-V.-T.; Kitahara, C.M.; de Vathaire, F.; Boutron-Ruault, M.-C.; Journy, N. Thyroid Dysfunction and Cancer Incidence: A Systematic Review and Meta-Analysis. Endocr.-Relat. Cancer 2020, 27, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-V.-T.; Kitahara, C.M.; Leenhardt, L.; de Vathaire, F.; Boutron-Ruault, M.-C.; Journy, N. The Effect of Thyroid Dysfunction on Breast Cancer Risk: An Updated Meta-Analysis. Endocr.-Relat. Cancer 2023, 30, e220155. [Google Scholar] [CrossRef] [PubMed]
- Kogai, T.; Endo, T.; Saito, T.; Miyazaki, A.; Kawaguchi, A.; Onaya, T. Regulation by Thyroid-Stimulating Hormone of Sodium/Iodide Symporter Gene Expression and Protein Levels in FRTL-5 Cells. Endocrinology 1997, 138, 2227–2232. [Google Scholar] [CrossRef]
- Dietrich, J.W.; Landgrafe, G.; Fotiadou, E.H. TSH and Thyrotropic Agonists: Key Actors in Thyroid Homeostasis. J. Thyroid Res. 2012, 2012, 351864. [Google Scholar] [CrossRef]
- Haisenleder, D.J.; Ortolano, G.A.; Dalkin, A.C.; Yasin, M.; Marshall, J.C. Differential Actions of Thyrotropin (TSH)-Releasing Hormone Pulses in the Expression of Prolactin and TSH Subunit Messenger Ribonucleic Acid in Rat Pituitary Cells in Vitro. Endocrinology 1992, 130, 2917–2923. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Tan, S.W.; Tyl, R.W. General Background on the Hypothalamic-Pituitary-Thyroid (HPT) Axis. Crit. Rev. Toxicol. 2007, 37, 11–53. [Google Scholar] [CrossRef]
- Fernandez, M.O.; Bourguignon, N.S.; Arocena, P.; Rosa, M.; Libertun, C.; Lux-Lantos, V. Neonatal Exposure to Bisphenol A Alters the Hypothalamic-Pituitary-Thyroid Axis in Female Rats. Toxicol. Lett. 2018, 285, 81–86. [Google Scholar] [CrossRef]
- Rodrigues-Pereira, P.; Andrade, M.N.; Santos-Silva, A.P.; Teixeira, M.P.; Soares, P.; Graceli, J.B.; de Carvalho, D.P.; Dias, G.R.M.; Ferreira, A.C.F.; Miranda-Alves, L. Subacute and Low-Dose Tributyltin Exposure Disturbs the Mammalian Hypothalamus-Pituitary-Thyroid Axis in a Sex-Dependent Manner. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109279. [Google Scholar] [CrossRef]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Ríos Morris, E.A.; Miranda-Alves, L.; Denicol, A.C. The Impact of Endocrine-Disrupting Chemical Exposure in the Mammalian Hypothalamic-Pituitary Axis. Mol. Cell. Endocrinol. 2020, 518, 110997. [Google Scholar] [CrossRef]
- Santos-Silva, A.P.; Andrade, M.N.; Pereira-Rodrigues, P.; Paiva-Melo, F.D.; Soares, P.; Graceli, J.B.; Dias, G.R.M.; Ferreira, A.C.F.; de Carvalho, D.P.; Miranda-Alves, L. Frontiers in Endocrine Disruption: Impacts of Organotin on the Hypothalamus-Pituitary-Thyroid Axis. Mol. Cell. Endocrinol. 2018, 460, 246–257. [Google Scholar] [CrossRef]
- Zhu, L.; Li, W.; Zha, J.; Wang, M.; Yuan, L.; Wang, Z. Butachlor Causes Disruption of HPG and HPT Axes in Adult Female Rare Minnow (Gobiocypris rarus). Chem.-Biol. Interact. 2014, 221, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.-P.; Ma, Y.-B.; Lu, C.-J.; Mirza, Z.; Zhang, W.; Jia, Y.-F.; Li, W.-G.; Pei, D.-S. The Effects of Disturbance on Hypothalamus-Pituitary-Thyroid (HPT) Axis in Zebrafish Larvae after Exposure to DEHP. PLoS ONE 2016, 11, e0155762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, H.; Li, J.; Lou, L.; Zhang, S.; Feng, D.; Feng, X. Exposure to Deltamethrin in Adolescent Mice Induced Thyroid Dysfunction and Behavioral Disorders. Chemosphere 2020, 241, 125118. [Google Scholar] [CrossRef] [PubMed]
- McLanahan, E.D.; Campbell, J.L., Jr.; Ferguson, D.C.; Harmon, B.; Hedge, J.M.; Crofton, K.M.; Mattie, D.R.; Braverman, L.; Keys, D.A.; Mumtaz, M.; et al. Low-Dose Effects of Ammonium Perchlorate on the Hypothalamic-Pituitary-Thyroid Axis of Adult Male Rats Pretreated with PCB126. Toxicol. Sci. 2007, 97, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global Epidemiology of Hyperthyroidism and Hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Marotta, V.; Russo, G.; Gambardella, C.; Grasso, M.; La Sala, D.; Chiofalo, M.G.; D’Anna, R.; Puzziello, A.; Docimo, G.; Masone, S.; et al. Human Exposure to Bisphenol AF and Diethylhexylphthalate Increases Susceptibility to Develop Differentiated Thyroid Cancer in Patients with Thyroid Nodules. Chemosphere 2019, 218, 885–894. [Google Scholar] [CrossRef]
- Sur, U.; Erkekoglu, P.; Bulus, A.D.; Andiran, N.; Kocer-Gumusel, B. Oxidative Stress Markers, Trace Elements, and Endocrine Disrupting Chemicals in Children with Hashimoto’s Thyroiditis. Toxicol. Mech. Methods 2019, 29, 633–643. [Google Scholar] [CrossRef]
- Coperchini, F.; Croce, L.; Ricci, G.; Magri, F.; Rotondi, M.; Imbriani, M.; Chiovato, L. Thyroid Disrupting Effects of Old and New Generation PFAS. Front. Endocrinol. 2021, 11, 612320. [Google Scholar] [CrossRef]
- Sun, H.-J.; Li, H.-B.; Xiang, P.; Zhang, X.; Ma, L.Q. Short-Term Exposure of Arsenite Disrupted Thyroid Endocrine System and Altered Gene Transcription in the HPT Axis in Zebrafish. Environ. Pollut. 2015, 205, 145–152. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.S.; Kizys, M.M.L.; da Conceição, R.R.; Glebocki, G.; Romano, R.M.; Ortiga-Carvalho, T.M.; Giannocco, G.; da Silva, I.D.C.G.; Dias da Silva, M.R.; Romano, M.A.; et al. Perinatal Exposure to Glyphosate-Based Herbicide Alters the Thyrotrophic Axis and Causes Thyroid Hormone Homeostasis Imbalance in Male Rats. Toxicology 2017, 377, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L. EPA’s Exposure Assessment Toolbox (EPA-Expo-Box). J. Environ. Inform. 2015, 25, 81–84. [Google Scholar] [CrossRef]
- Damstra, T. Endocrine Disrupters: The Need for a Refocused Vision. Toxicol. Sci. 2003, 74, 231–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2019, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B. Endocrine Disrupting Chemicals (EDCs) and the Neuroendocrine System: Beyond Estrogen, Androgen, and Thyroid. In Endocrine-Disrupting Chemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 101–150. [Google Scholar]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A Review on Endocrine Disruptors and Their Possible Impacts on Human Health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Gogoi, P.; Kalita, J.C. Effects of Butylparaben Exposure on Thyroid Peroxidase (TPO) and Type 1 Iodothyronine Deiodinase (D1) in Female Wistar Rats. Toxicology 2020, 443, 152562. [Google Scholar] [CrossRef]
- Macedo, S.; Teixeira, E.; Gaspar, T.B.; Boaventura, P.; Soares, M.A.; Miranda-Alves, L.; Soares, P. Endocrine-Disrupting Chemicals and Endocrine Neoplasia: A Forty-Year Systematic Review. Environ. Res. 2023, 218, 114869. [Google Scholar] [CrossRef]
- Sonntag, J.; Vogel, M.; Geserick, M.; Eckelt, F.; Körner, A.; Raue, F.; Kiess, W.; Kratzsch, J. Age-Related Association of Calcitonin with Parameters of Anthropometry, Bone and Calcium Metabolism during Childhood. Horm. Res. Paediatr. 2020, 93, 361–370. [Google Scholar] [CrossRef]
- Anast, C.; Arnaud, C.D.; Rasmussen, H.; Tenenhouse, A. Thyrocalcitonin and the Response to Parathyroid Hormone. J. Clin. Investig. 1967, 46, 57–64. [Google Scholar] [CrossRef]
- Raue, F.; Scherübl, H. Extracellular Calcium Sensitivity and Voltage-Dependent Calcium Channels in C Cells. Endocr. Rev. 1995, 16, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology E-Book: Guyton and Hall Textbook of Medical Physiology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Gerard, A.; Denef, J.; Colin, I.; van den Hove, M. Evidence for Processing of Compact Insoluble Thyroglobulin Globules in Relation with Follicular Cell Functional Activity in the Human and the Mouse Thyroid. Eur. J. Endocrinol. 2004, 150, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.E.; Targovnik, H.M.; Arvan, P. The Role of Thyroglobulin in Thyroid Hormonogenesis. Nat. Rev. Endocrinol. 2019, 15, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Rousset, B.; Dupuy, C.; Miot, F.; Dumont, J. Thyroid Hormone Synthesis and Secretion; MDText.com, Inc.: South Dartmouth, MA, USA, 2015; Chapter 2. [Google Scholar]
- Deme, D.; Pommier, J.; Nunez, J. Kinetics of Thyroglobulin Iodination and of Hormone Synthesis Catalyzed by Thyroid Peroxidase. Role of Iodide in the Coupling Reaction. Eur. J. Biochem. 1976, 70, 435–440. [Google Scholar] [CrossRef]
- Maurizis, J.-C.; Marriq, C.; Rolland, M.; Lissitzky, S. Thyroid Hormone Synthesis and Reactivity of Hormone-forming Tyrosine Residues of Thyroglobulin. FEBS Lett. 1981, 132, 29–32. [Google Scholar] [CrossRef]
- Ma, Y.-A.; Sih, C.J. Mechanism of Thyroid Hormone Biosynthesis. Enzymatic Oxidative Coupling of 3,5-Diiodo-l-Tyrosine Derivatives. Tetrahedron Lett. 1999, 40, 9211–9214. [Google Scholar] [CrossRef]
- Dunn, J.T.; Dunn, A.D. The Importance of Thyroglobulin Structure for Thyroid Hormone biosynthesis. Biochimie 1999, 81, 505–509. [Google Scholar] [CrossRef]
- Carvalho, D.P.; Dupuy, C. Thyroid Hormone Biosynthesis and Release. Mol. Cell. Endocrinol. 2017, 458, 6–15. [Google Scholar] [CrossRef]
- Coscia, F.; Taler-Verčič, A.; Chang, V.T.; Sinn, L.; O’Reilly, F.J.; Izoré, T.; Renko, M.; Berger, I.; Rappsilber, J.; Turk, D.; et al. The Structure of Human Thyroglobulin. Nature 2020, 578, 627–630. [Google Scholar] [CrossRef]
- Carvalho, D.P.; Dupuy, C. Role of the NADPH Oxidases DUOX and NOX4 in Thyroid Oxidative Stress. Eur. Thyroid J. 2013, 2, 160–167. [Google Scholar] [CrossRef]
- Deme, D.; Virion, A.; Hammou, N.A.; Pommier, J. NADPH-dependent Generation of H2O2 in a Thyroid Particulate Fraction Requires Ca2+. FEBS Lett. 1985, 186, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Ameziane-El-Hassani, R.; Morand, S.; Boucher, J.-L.; Frapart, Y.-M.; Apostolou, D.; Agnandji, D.; Gnidehou, S.; Ohayon, R.; Noël-Hudson, M.-S.; Francon, J.; et al. Dual Oxidase-2 Has an Intrinsic Ca2+-Dependent H2O2-Generating Activity. J. Biol. Chem. 2005, 280, 30046–30054. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ruf, J.; Lothaire, P.; Dequanter, D.; Andry, G.; Willemse, E.; Dumont, J.E.; Van Sande, J.; De Deken, X. Association of Duoxes with Thyroid Peroxidase and Its Regulation in Thyrocytes. J. Clin. Endocrinol. Metab. 2010, 95, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Loo, D.D.F.; Dai, G.; Levy, O.; Wright, E.M.; Carrasco, N. Thyroid Na+/I− Symporter. J. Biol. Chem. 1997, 272, 27230–27238. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Driessens, N.; Versteyhe, S.; Ghaddhab, C.; Burniat, A.; De Deken, X.; Van Sande, J.; Dumont, J.-E.; Miot, F.; Corvilain, B. Hydrogen Peroxide Induces DNA Single- and Double-Strand Breaks in Thyroid Cells and Is Therefore a Potential Mutagen for This Organ. Endocr.-Relat. Cancer 2009, 16, 845–856. [Google Scholar] [CrossRef]
- Donkó, Á.; Morand, S.; Korzeniowska, A.; Boudreau, H.E.; Zana, M.; Hunyady, L.; Geiszt, M.; Leto, T.L. Hypothyroidism-Associated Missense Mutation Impairs NADPH Oxidase Activity and Intracellular Trafficking of Duox2. Free Radic. Biol. Med. 2014, 73, 190–200. [Google Scholar] [CrossRef]
- Faria, C.C.; Peixoto, M.S.; Carvalho, D.P.; Fortunato, R.S. The Emerging Role of Estrogens in Thyroid Redox Homeostasis and Carcinogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 2514312. [Google Scholar] [CrossRef]
- Villanueva, I.; Alva-Sánchez, C.; Pacheco-Rosado, J. The Role of Thyroid Hormones as Inductors of Oxidative Stress and Neurodegeneration. Oxidative Med. Cell. Longev. 2013, 2013, 218145. [Google Scholar] [CrossRef]
- Weyemi, U.; Caillou, B.; Talbot, M.; Ameziane-El-Hassani, R.; Lacroix, L.; Lagent-Chevallier, O.; Al Ghuzlan, A.; Roos, D.; Bidart, J.-M.; Virion, A.; et al. Intracellular Expression of Reactive Oxygen Species-Generating NADPH Oxidase NOX4 in Normal and Cancer Thyroid Tissues. Endocr.-Relat. Cancer 2010, 17, 27–37. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Sola, E.; Moyano, P.; Flores, A.; García, J.M.; García, J.; Anadon, M.J.; Frejo, M.T.; Pelayo, A.; de la Cabeza Fernandez, M.; del Pino, J. Cadmium-Promoted Thyroid Hormones Disruption Mediates ROS, Inflammation, Aβ and Tau Proteins Production, Gliosis, Spongiosis and Neurodegeneration in Rat Basal Forebrain. Chem.-Biol. Interact. 2023, 375, 110428. [Google Scholar] [CrossRef]
- Macvanin, M.T.; Gluvic, Z.; Zafirovic, S.; Gao, X.; Essack, M.; Isenovic, E.R. The Protective Role of Nutritional Antioxidants against Oxidative Stress in Thyroid Disorders. Front. Endocrinol. 2023, 13, 1092837. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative Stress in Health and Disease: The Therapeutic Potential of Nrf2 Activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Pop, A.; Kiss, B.; Vlase, L.; Popa, D.S.; Paltinean, R.; Iepure, R.; Loghin, F. Study of Oxidative Stress Induction after Exposure to Bisphenol a and Methylparaben in Rats. Toxicol. Lett. 2011, 205, S224. [Google Scholar] [CrossRef]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety Assessment of Esters of P-Hydroxybenzoic Acid (Parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef]
- Andersen, F.A. Final Amended Report on the Safety Assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as Used in Cosmetic Products. Int. J. Toxicol. 2008, 27 (Suppl. S4), 1–82. [Google Scholar] [CrossRef]
- Haman, C.; Dauchy, X.; Rosin, C.; Munoz, J.-F. Occurrence, Fate and Behavior of Parabens in Aquatic Environments: A Review. Water Res. 2015, 68, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Liao, C.; Liu, F.; Wu, Q.; Guo, Y.; Moon, H.-B.; Nakata, H.; Kannan, K. Occurrence and Human Exposure of P-Hydroxybenzoic Acid Esters (Parabens), Bisphenol A Diglycidyl Ether (BADGE), and Their Hydrolysis Products in Indoor Dust from the United States and Three East Asian Countries. Environ. Sci. Technol. 2012, 46, 11584–11593. [Google Scholar] [CrossRef]
- Liao, C.; Chen, L.; Kannan, K. Occurrence of Parabens in Foodstuffs from China and Its Implications for Human Dietary Exposure. Environ. Int. 2013, 57–58, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Bishop, A.M.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Parabens as Urinary Biomarkers of Exposure in Humans. Environ. Health Perspect. 2006, 114, 1843–1846. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.R.; Frederiksen, H.; Skakkebæk, N.E.; Wulf, H.C.; Andersson, A.-M. Urinary Excretion of Phthalates and Paraben after Repeated Whole-Body Topical Application in Humans. Int. J. Androl. 2008, 31, 118–130. [Google Scholar] [CrossRef]
- Abbas, S.; Greige-Gerges, H.; Karam, N.; Piet, M.-H.; Netter, P.; Magdalou, J. Metabolism of Parabens (4-Hydroxybenzoic Acid Esters) by Hepatic Esterases and UDP-Glucuronosyltransferases in Man. Drug Metab. Pharmacokinet. 2010, 25, 568–577. [Google Scholar] [CrossRef]
- Moos, R.K.; Angerer, J.; Dierkes, G.; Brüning, T.; Koch, H.M. Metabolism and Elimination of Methyl, Iso- and n-Butyl Paraben in Human Urine after Single Oral Dosage. Arch. Toxicol. 2015, 90, 2699–2709. [Google Scholar] [CrossRef]
- Frederiksen, H.; Nielsen, J.K.S.; Mørck, T.A.; Hansen, P.W.; Jensen, J.F.; Nielsen, O.; Andersson, A.-M.; Knudsen, L.E. Urinary Excretion of Phthalate Metabolites, Phenols and Parabens in Rural and Urban Danish Mother–Child Pairs. Int. J. Hyg. Environ. Health 2013, 216, 772–783. [Google Scholar] [CrossRef]
- Shirai, S.; Suzuki, Y.; Yoshinaga, J.; Shiraishi, H.; Mizumoto, Y. Urinary Excretion of Parabens in Pregnant Japanese Women. Reprod. Toxicol. 2013, 35, 96–101. [Google Scholar] [CrossRef]
- Fransway, A.F.; Fransway, P.J.; Belsito, D.V.; Yiannias, J.A. Paraben Toxicology. Dermatitis 2019, 30, 32–45. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Wu, C.; Zhang, J.; Zhang, L.; Lv, S.; Lu, D.; Qi, X.; Feng, C.; Liang, W.; et al. Effects of Prenatal Exposure to Five Parabens on Neonatal Thyroid Function and Birth Weight: Evidence from SMBCS Study. Environ. Res. 2020, 188, 109710. [Google Scholar] [CrossRef]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible Endocrine Disrupting Effects of Parabens and Their Metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Ratajczak–Wrona, W.; Górska, M.; Jabłońska, E. Parabens and Their Effects on the Endocrine System. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef] [PubMed]
- 2021—Agência Nacional de Vigilância Sanitária. Anvisa. Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/novas-normas-tratam-de-produtos-de-higiene-pessoal-cosmeticos-e-perfumes (accessed on 25 September 2023).
- Scientific Committees. Public Health. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/opinions/index_en.htm (accessed on 25 September 2023).
- Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to Para Hydroxybenzoates (E 214-219). EFSA J. 2004, 2, 83. [CrossRef]
- Hoberman, A.M.; Schreur, D.K.; Leazer, T.; Daston, G.P.; Carthew, P.; Re, T.; Loretz, L.; Mann, P. Lack of Effect of Butylparaben and Methylparaben on the Reproductive System in Male Rats. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2008, 83, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Núñez, Z.; Ashrap, P.; Barrett, E.S.; Llanos, A.A.M.; Watkins, D.J.; Cathey, A.L.; Vélez-Vega, C.M.; Rosario, Z.; Cordero, J.F.; Alshawabkeh, A.; et al. Personal Care Products: Demographic Characteristics and Maternal Hormones in Pregnant Women from Puerto Rico. Environ. Res. 2022, 206, 112376. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.H.; Wu, H.; Laue, H.E.; Boivin, A.; Gillet, V.; Langlois, M.-F.; Bellenger, J.-P.; Baccarelli, A.A.; Takser, L. Methylparaben in Meconium and Risk of Maternal Thyroid Dysfunction, Adverse Birth Outcomes, and Attention-Deficit Hyperactivity Disorder (ADHD). Environ. Int. 2020, 139, 105716. [Google Scholar] [CrossRef]
- Park, N.-Y.; Cho, Y.H.; Choi, K.; Lee, E.-H.; Kim, Y.J.; Kim, J.H.; Kho, Y. Parabens in Breast Milk and Possible Sources of Exposure among Lactating Women in Korea. Environ. Pollut. 2019, 255, 113142. [Google Scholar] [CrossRef]
- Valle-Sistac, J.; Molins-Delgado, D.; Díaz, M.; Ibáñez, L.; Barceló, D.; Silvia Díaz-Cruz, M. Determination of Parabens and Benzophenone-Type UV Filters in Human Placenta. First Description of the Existence of Benzyl Paraben and Benzophenone-4. Environ. Int. 2016, 88, 243–249. [Google Scholar] [CrossRef]
- Philippat, C.; Wolff, M.S.; Calafat, A.M.; Ye, X.; Bausell, R.; Meadows, M.; Stone, J.; Slama, R.; Engel, S.M. Prenatal Exposure to Environmental Phenols: Concentrations in Amniotic Fluid and Variability in Urinary Concentrations during Pregnancy. Environ. Health Perspect. 2013, 121, 1225–1231. [Google Scholar] [CrossRef]
- Geer, L.A.; Pycke, B.F.G.; Waxenbaum, J.; Sherer, D.M.; Abulafia, O.; Halden, R.U. Association of Birth Outcomes with Fetal Exposure to Parabens, Triclosan and Triclocarban in an Immigrant Population in Brooklyn, New York. J. Hazard. Mater. 2017, 323, 177–183. [Google Scholar] [CrossRef]
- Taxvig, C.; Vinggaard, A.M.; Hass, U.; Axelstad, M.; Boberg, J.; Hansen, P.R.; Frederiksen, H.; Nellemann, C. Do Parabens Have the Ability to Interfere with Steroidogenesis? Toxicol. Sci. 2008, 106, 206–213. [Google Scholar] [CrossRef]
- Carlsson, G.; Pohl, J.; Athanassiadis, I.; Norrgren, L.; Weiss, J. Thyroid Disruption Properties of Three Indoor Dust Chemicals Tested in Silurana Tropicalis Tadpoles. J. Appl. Toxicol. 2019, 39, 1248–1256. [Google Scholar] [CrossRef]
- Aker, A.M.; Johns, L.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Associations between Maternal Phenol and Paraben Urinary Biomarkers and Maternal Hormones during Pregnancy: A Repeated Measures Study. Environ. Int. 2018, 113, 341–349. [Google Scholar] [CrossRef]
- Koeppe, E.S.; Ferguson, K.K.; Colacino, J.A.; Meeker, J.D. Relationship between Urinary Triclosan and Paraben Concentrations and Serum Thyroid Measures in NHANES 2007–2008. Sci. Total Environ. 2013, 445–446, 299–305. [Google Scholar] [CrossRef]
- Aker, A.M.; Ferguson, K.K.; Rosario, Z.Y.; Mukherjee, B.; Alshawabkeh, A.N.; Calafat, A.M.; Cordero, J.F.; Meeker, J.D. A Repeated Measures Study of Phenol, Paraben and Triclocarban Urinary Biomarkers and Circulating Maternal Hormones during Gestation in the Puerto Rico PROTECT Cohort. Environ. Health 2019, 18, 28. [Google Scholar] [CrossRef]
- Liang, J.; Yang, X.; Liu, Q.S.; Sun, Z.; Ren, Z.; Wang, X.; Zhang, Q.; Ren, X.; Liu, X.; Zhou, Q.; et al. Assessment of Thyroid Endocrine Disruption Effects of Parabens Using In Vivo, In Vitro, and In Silico Approaches. Environ. Sci. Technol. 2021, 56, 460–469. [Google Scholar] [CrossRef]
- Wu, N.-X.; Deng, L.-J.; Xiong, F.; Xie, J.-Y.; Li, X.-J.; Zeng, Q.; Sun, J.-C.; Chen, D.; Yang, P. Risk of Thyroid Cancer and Benign Nodules Associated with Exposure to Parabens among Chinese Adults in Wuhan, China. Environ. Sci. Pollut. Res. 2022, 29, 70125–70134. [Google Scholar] [CrossRef]
- Vo, T.T.B.; Yoo, Y.-M.; Choi, K.-C.; Jeung, E.-B. Potential Estrogenic Effect(s) of Parabens at the Prepubertal Stage of a Postnatal Female Rat Model. Reprod. Toxicol. 2010, 29, 306–316. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwack, S.J.; Lim, S.K.; Kim, Y.J.; Roh, T.H.; Choi, S.M.; Kim, H.S.; Lee, B.M. Toxicological Evaluation of Isopropylparaben and Isobutylparaben Mixture in Sprague–Dawley Rats Following 28 Days of Dermal Exposure. Regul. Toxicol. Pharmacol. 2015, 73, 544–551. [Google Scholar] [CrossRef]
- Berger, K.; Gunier, R.B.; Chevrier, J.; Calafat, A.M.; Ye, X.; Eskenazi, B.; Harley, K.G. Associations of Maternal Exposure to Triclosan, Parabens, and Other Phenols with Prenatal Maternal and Neonatal Thyroid Hormone Levels. Environ. Res. 2018, 165, 379–386. [Google Scholar] [CrossRef]
- Choi, S.; Kim, M.J.; Park, Y.J.; Kim, S.; Choi, K.; Cheon, G.J.; Cho, Y.H.; Jeon, H.L.; Yoo, J.; Park, J. Thyroxine-Binding Globulin, Peripheral Deiodinase Activity, and Thyroid Autoantibody Status in Association of Phthalates and Phenolic Compounds with Thyroid Hormones in Adult Population. Environ. Int. 2020, 140, 105783. [Google Scholar] [CrossRef]
- Coiffier, O.; Nakiwala, D.; Rolland, M.; Malatesta, A.; Lyon-Caen, S.; Chovelon, B.; Faure, P.; Sophie Gauchez, A.; Guergour, D.; Sakhi, A.K.; et al. Exposure to a Mixture of Non-Persistent Environmental Chemicals and Neonatal Thyroid Function in a Cohort with Improved Exposure Assessment. Environ. Int. 2023, 173, 107840. [Google Scholar] [CrossRef]
- Aker, A.M.; Watkins, D.J.; Johns, L.E.; Ferguson, K.K.; Soldin, O.P.; Anzalota Del Toro, L.V.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Phenols and Parabens in Relation to Reproductive and Thyroid Hormones in Pregnant Women. Environ. Res. 2016, 151, 30–37. [Google Scholar] [CrossRef]
- Genuis, S.J.; Birkholz, D.; Curtis, L.; Sandau, C. Paraben Levels in an Urban Community of Western Canada. ISRN Toxicol. 2013, 2013, 507897. [Google Scholar] [CrossRef]
- Hu, L.; Mei, H.; Cai, X.; Hu, X.; Duan, Z.; Liu, J.; Tan, Y.; Yang, P.; Xiao, H.; Zhou, A. Maternal Paraben Exposure and Intra-Pair Thyroid-Stimulating Hormone Difference in Twin Neonates. Ecotoxicol. Environ. Saf. 2023, 250, 114502. [Google Scholar] [CrossRef]
- Fortunato, R.S.; Braga, W.M.O.; Ortenzi, V.H.; Rodrigues, D.C.; Andrade, B.M.; Miranda-Alves, L.; Rondinelli, E.; Dupuy, C.; Ferreira, A.C.F.; Carvalho, D.P. Sexual Dimorphism of Thyroid Reactive Oxygen Species Production Due to Higher NADPH Oxidase 4 Expression in Female Thyroid Glands. Thyroid 2013, 23, 111–119. [Google Scholar] [CrossRef]
- Bosch i Ara, L.; Katugampola, H.; Dattani, M.T. Congenital Hypopituitarism during the Neonatal Period: Epidemiology, Pathogenesis, Therapeutic Options, and Outcome. Front. Pediatr. 2021, 8, 600962. [Google Scholar] [CrossRef]
- Egalini, F.; Marinelli, L.; Rossi, M.; Motta, G.; Prencipe, N.; Rossetto Giaccherino, R.; Pagano, L.; Grottoli, S.; Giordano, R. Endocrine Disrupting Chemicals: Effects on Pituitary, Thyroid and Adrenal Glands. Endocrine 2022, 78, 395–405. [Google Scholar] [CrossRef]
- de Cock, M.; Maas, Y.G.H.; van de Bor, M. Does Perinatal Exposure to Endocrine Disruptors Induce Autism Spectrum and Attention Deficit Hyperactivity Disorders? Review. Acta Paediatr. 2012, 101, 811–818. [Google Scholar] [CrossRef]
- Cowell, W.J.; Wright, R.J. Sex-Specific Effects of Combined Exposure to Chemical and Non-Chemical Stressors on Neuroendocrine Development: A Review of Recent Findings and Putative Mechanisms. Curr. Environ. Health Rep. 2017, 4, 415–425. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Paraben Esters: Review of Recent Studies of Endocrine Toxicity, Absorption, Esterase and Human Exposure, and Discussion of Potential Human Health Risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Pitto, L.; Gorini, F.; Bianchi, F.; Guzzolino, E. New Insights into Mechanisms of Endocrine-Disrupting Chemicals in Thyroid Diseases: The Epigenetic Way. Int. J. Environ. Res. Public Health 2020, 17, 7787. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J. Thyroid Hormone Receptors in Brain Development and Function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Yang, T.; Ye, X.; Calafat, A.M.; Hauser, R. Urinary Concentrations of Parabens and Serum Hormone Levels, Semen Quality Parameters, and Sperm DNA Damage. Environ. Health Perspect. 2011, 119, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Moeller, L.C.; Führer, D. Thyroid Hormone, Thyroid Hormone Receptors, and Cancer: A Clinical Perspective. Endocr.-Relat. Cancer 2013, 20, R19–R29. [Google Scholar] [CrossRef]
- Lin, H.; Chin, Y.; Yang, Y.S.H.; Lai, H.; Whang-Peng, J.; Liu, L.F.; Tang, H.; Davis, P.J. Thyroid Hormone, Cancer, and Apoptosis. Compr. Physiol. 2016, 6, 1221–1237. [Google Scholar] [CrossRef]
- Taha, M.; Marie, A.M.; Ahmed-Farid, O.A. Combined Approaches for Evaluation of Xenoestrogen Neural Toxicity and Thyroid Dysfunction: Screening of Oxido-nitrosative Markers, DNA Fragmentation, and Biogenic Amine Degradation. J. Biochem. Mol. Toxicol. 2020, 34, e22521. [Google Scholar] [CrossRef]
- Miranda, R.A.; de Moura, E.G.; Soares, P.N.; Peixoto, T.C.; Lopes, B.P.; de Andrade, C.B.V.; de Oliveira, E.; Manhães, A.C.; de Faria, C.C.; Fortunato, R.S.; et al. Thyroid Redox Imbalance in Adult Wistar Rats That Were Exposed to Nicotine during Breastfeeding. Sci. Rep. 2020, 10, 15646. [Google Scholar] [CrossRef]
- Terasaka, S.; Inoue, A.; Tanji, M.; Kiyama, R. Expression Profiling of Estrogen-Responsive Genes in Breast Cancer Cells Treated with Alkylphenols, Chlorinated Phenols, Parabens, or Bis- and Benzoylphenols for Evaluation of Estrogenic Activity. Toxicol. Lett. 2006, 163, 130–141. [Google Scholar] [CrossRef]
- Costa, J.R.; Campos, M.S.; Lima, R.F.; Gomes, L.S.; Marques, M.R.; Taboga, S.R.; Biancardi, M.F.; Brito, P.V.A.; Santos, F.C.A. Endocrine-Disrupting Effects of Methylparaben on the Adult Gerbil Prostate. Environ. Toxicol. 2017, 32, 1801–1812. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Garley, M.; Radziwon, P.; Ratajczak-Wrona, W. Methylparaben-Induced Regulation of Estrogenic Signaling in Human Neutrophils. Mol. Cell. Endocrinol. 2021, 538, 111470. [Google Scholar] [CrossRef] [PubMed]
| Class | No. CAS | Molecular Weight (g/mol) | Chemical Formula | Chemical Structure |
|---|---|---|---|---|
| Benzylparaben | 94-18-8 | 228.2433 | C14H12O3 | 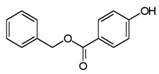 |
| Butylparaben | 94-26-8 | 194.2271 | C11H14O3 | 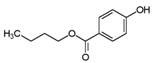 |
| Ethylparaben | 120-47-8 | 166.1739 | C9H10O3 | 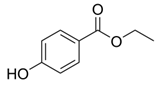 |
| Methylparaben | 99-76-3 | 152.1473 | C8H8O3 |  |
| Propylparaben | 94-13-3 | 180.2005 | C10H12O3 |  |
| Model | Exposure/Dose/Analyses | Main Results | References |
|---|---|---|---|
| Human (men) | Serum hormone analysis of Inhibin, FSH, LH, testosterone, estradiol, TSH, T3, and T4. | BuP was associated with ↑ TSH, T4, fT4 after 96 h of exposure. | [66] |
| Pregnant women (12–14 weeks) | Urine collection at 3 different gestational moments (16–20, 20–24, 24–28 gestation weeks) and hormone analyses. | BuP was associated with ↑ estradiol and progesterone with ↓ fT3 and fT4 at visit 3. tT3 and TSH levels did not change between visits. | [88] |
| Pregnant women—Boston (>15 weeks) | Collection of urine and blood at 4 different gestational moments (9, 17, 26, and 35 weeks of gestation). | BuP was associated with ↓ T3, ↓ T3/T4 ratio, and ↑ TSH. | [89] |
| Pregnant women—Puerto Rico | Urine collection at 3 different gestational moments (16–20, 20–24, 24–28 weeks of gestation). | Exposure to BuP has been associated with ↓ SHBG. | [90] |
| Pregnant women | Collection of maternal urine on the day of delivery and collection of umbilical cord blood for hormone measurement. | BuP is associated with ↑ boys’ body weight at birth. | [72] |
| Male Wistar rats | Oral exposure BuP (10 mg/kg/day), BuP (50 mg/kg/day), and BuP+TCS (triclosan) (50 mg+10 mg/kg/day) for 60 days. | BuP (50 mg/kg/d) was associated with ↑ TSH and ↓ T3 and T4. | [91] |
| Female Wistar rats | Subcutaneous administration of BuP at doses of 1, 5, and 10 mg/kg/day for 7 and 21 days. | ↑ TSH in BuP1 at 7 and 21 days; ↓ fT4 and tT4 at all concentrations (7 and 21 days); ↑ fT3, tT3, and TPO in SP1 and SP5 at 7 and 21 days. | [28] |
| Zebrafish larvae | Larvae were exposed to the following concentrations: 0, 2, 5, and 10 μM of BuP. | ↓ T4 levels at most concentrations tested (BuP 5 and 10 μM) and ↓ T3 levels at all concentrations tested. That exposure also led to an increase in TSH gene expression at all concentrations of BuP. | [92] |
| Model | Exposure/Dose/Analyses | Main Results | References |
|---|---|---|---|
| Female Sprague Dawley rats | Oral exposure to MP, EP, PP, isopropylparaben (IPP), BuP, and isobutylparaben (IBP) (62.5; 250 and 1000 mg/kg/day) from the 21st to the 40th postnatal day. | PP and IPP were associated with ↓ T4 and estradiol and changes in thyroid weight. | [93] |
| Male and female Sprague Dawley rats | Injections of IPP, IBP, or mixture of IPP and IBP at 50, 100, 300, and 600 mg/kg bw dissolved in 100 mL of ethanol (99%), 5 days per week for 28 days. | The mixture of IPP and IBP induces a decrease of TSH in exposed individuals at an exposure of 600 mg/kg bw. | [94] |
| Pregnant women (PROTECT) | Blood collection at two different gestational moments for measurement of SHBG, TSH, fT3, fT4, and progesterone/estradiol ratio; urine collection for detection of phenols and parabens by high-performance liquid chromatography (HPLC). | ↑ estradiol and progesterone at the last visit; ↓ fT3 and fT4 at the last visit with no changes in TSH levels. | [88] |
| Pregnant women—Puerto Rico | Urine and blood collection at 4 time points during pregnancy. Parabens were detected in urine by chromatography. In the blood, tT4, fT4, TSH, and T3 were measured. | PP was inversely associated with fT4. | [89] |
| Pregnant women—California | Urine and blood collection in the second gestational trimester and blood collection from neonates for measurement of tT4 and TSH. | PP was inversely associated with TSH levels with no changes in tT4 levels. | [95] |
| Pregnant women—Puerto Rico | Urine collection at 3 different gestational moments (16–20, 20–24, 24–28 weeks of gestation). | Exposure to PP was associated with ↓ SHBG and T3/T4 ratio. | [90] |
| Human (population of Wuhan, China) | Urine collection and detection of MP, EP, and PP. | PP has been associated with an increased risk of thyroid cancer. | [96] |
| Newborn human | Newborn blood spots were collected as part of the neonatal screening program, TSH and tT4 were assessed using immunofluorescence. | BuP increased TSH and decreased T4 hormone levels have been demonstrated in newborns and women with less than 150 μg/L of iodine. | [97] |
| Amphibian tadpoles | Oral exposure to PP (0.05; 0.5 and 5 mg/L) for 14 days. | An increase in PP concentrations in water has been associated with an acute toxic effect. | [87] |
| Zebrafish larvae | Larvae were exposed to the following concentrations: 0, 5, 10, and 20 μM of PP. | Serum T3 and T4 concentrations decreased at all concentrations tested. In 10 and 20 μM groups, PP increases TSH gene expression. | [92] |
| Model | Exposure/Dose/Analyses | Main Results | References |
|---|---|---|---|
| Human | Urine samples were collected from patients at Wuhan Central Hospital who had thyroid disease and required surgery. Some types of parabens were detected in these samples, such as MP, EP, and PP. | MP and EP were found in urine samples in 99.06%, 95.29%, and 92%, respectively. There was a ↑ concentration of all parabens in the urine of both the nodule and cancer groups. MP and EP were associated with a benign nodule, especially when in higher concentrations. All three parabens studied were associated with an increased risk of thyroid cancer, with EP having the greatest association. | [96] |
| Mother—children | Urine samples from mothers of newborns were collected on the day of delivery. The concentrations of 5 parabens were determined by chromatography. Umbilical cord blood was collected immediately after birth, in which tT3, tT4, fT3, fT4, TSH, anti-TPO, and anti-TG were measured. | MP and EP were detected in the urine of the evaluated mothers. EP was positively related to increased tT3 in the umbilical cord and to anti-TPO. EP was correlated with increased birth weight in boys, but not in girls. | [72] |
| Human—Korea | Population study with 1254 people from Korea. Urine samples from this population were collected for analysis of the presence of EDC. Blood serum samples were also collected for measurement of tT4 and fT4, tT3 and fT3, TSH, anti-TPO, anti-thyroglobulin, thyroxine-binding globulin (TBG), and iodothyronine deiodinase (DIO) activity. | Parabens were found in most of the studied population (more than 90%). MP showed a positive association with altered levels of tT3. The increase in MP and EP parabens was correlated with an increase in TBG. | [59] |
| Pregnant women—Puerto Rico | Urine collection at 3 different gestational moments (16–20, 20–24, 24–28 weeks of gestation). | MP was associated with a decrease in SHBG. MP leads to a significant decrease in TSH and a decrease in the T3/T4 ratio particularly at weeks 24–28 of gestation. | [90] |
| Pregnant women—California | Urine and blood collection in the second gestational trimester and blood collection from neonates for measurement of tT4 and TSH. | MP was inversely associated with TSH levels with no changes in tT4 levels. | [95] |
| Pregnant women—Puerto Rico | Urine and blood collection at 4 time points during pregnancy. Parabens were detected in urine by chromatography. In the blood, tT4, fT4, TSH, and T3 were measured. | Urine samples that tested positive for the presence of MP were associated with increased T3 and negatively associated with fT4 at gestational age less than 21 weeks. | [89] |
| Pregnant women (12–14 weeks) | Urine collection at 3 different gestational moments (16–20, 20–24, 24–28 gestation weeks) and hormone analyses. | MP (293 ng/mL) was associated with a 7.70% increase in SHBG. | [98] |
| Human | Urine samples from a representative portion of the US population to assess urinary concentrations of triclosan and parabens. | Inverse associations have been found between parabens and circulating levels of thyroid hormones in adults, where women appear to be more vulnerable to exposure. | [99] |
| Mother–children | Maternal blood was collected during the first prenatal care visit for TSH measurement. MP was detected in meconium samples from newborns. | MP exposure leads to a decrease in gestational age, a significant change in newborn weight, and a decrease in maternal TSH levels. In addition, MP in meconium was associated with about a 16% decrease in tT3 and a decrease in fT4. MP may influence maternal thyroid physiology during pregnancy, and this may lead to the development of ADHD. | [81] |
| Mother–twin pairs | MP was extracted from urine samples of pregnant women using liquid-liquid extraction. Neonatal TSH levels were abstracted from medical records in China. | MP exposure in early pregnancy was associated with an increased intra-twin TSH difference. | [100] |
| Wistar rats | Oral exposure for 90 days to BPA (50 mg/kg) or BPA+MP (250 mg/kg). | A minimal thyroid receptor antagonistic effect was only observed after treatment with BPA+MP. MP demonstrated antioxidant properties by reducing lipid peroxidation and generation of hydroxyl radicals induced by exposure to BPA. | [101] |
| Zebrafish larvae | Larvae were exposed to the following concentrations: 0, 20, 50, and 100 μM of EP and 0, 20, 100, and 200 μM of MP. | Serum T3 concentrations decreased at most concentrations tested (EP at 50, 100 μM and MP at 20, 100, and 200 μM) and T4 concentrations decreased at all concentrations tested. | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeredo, D.B.C.; de Sousa Anselmo, D.; Soares, P.; Graceli, J.B.; Magliano, D.C.; Miranda-Alves, L. Environmental Endocrinology: Parabens Hazardous Effects on Hypothalamic–Pituitary–Thyroid Axis. Int. J. Mol. Sci. 2023, 24, 15246. https://doi.org/10.3390/ijms242015246
Azeredo DBC, de Sousa Anselmo D, Soares P, Graceli JB, Magliano DC, Miranda-Alves L. Environmental Endocrinology: Parabens Hazardous Effects on Hypothalamic–Pituitary–Thyroid Axis. International Journal of Molecular Sciences. 2023; 24(20):15246. https://doi.org/10.3390/ijms242015246
Chicago/Turabian StyleAzeredo, Damáris Barcelos Cunha, Denilson de Sousa Anselmo, Paula Soares, Jones Bernardes Graceli, D’Angelo Carlo Magliano, and Leandro Miranda-Alves. 2023. "Environmental Endocrinology: Parabens Hazardous Effects on Hypothalamic–Pituitary–Thyroid Axis" International Journal of Molecular Sciences 24, no. 20: 15246. https://doi.org/10.3390/ijms242015246
APA StyleAzeredo, D. B. C., de Sousa Anselmo, D., Soares, P., Graceli, J. B., Magliano, D. C., & Miranda-Alves, L. (2023). Environmental Endocrinology: Parabens Hazardous Effects on Hypothalamic–Pituitary–Thyroid Axis. International Journal of Molecular Sciences, 24(20), 15246. https://doi.org/10.3390/ijms242015246