Vascular Ultrasound for In Vivo Assessment of Arterial Pathologies in a Murine Model of Atherosclerosis and Aortic Aneurysm
Abstract
:1. Introduction
2. Results
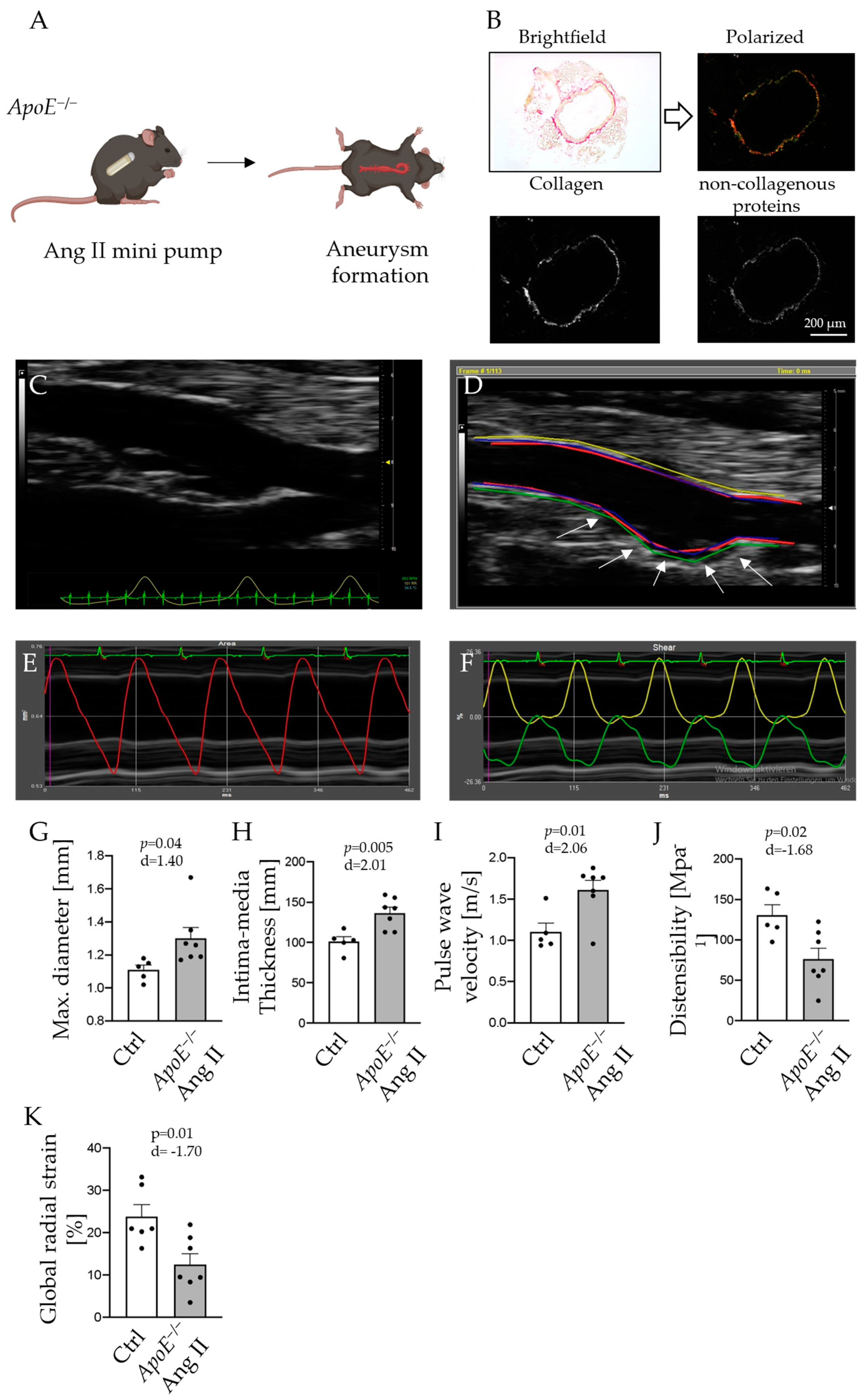
2.1. Vascular Ultrasound Analysis Traces Arterial Wall Changes in Abdominal Aortic Aneurysm
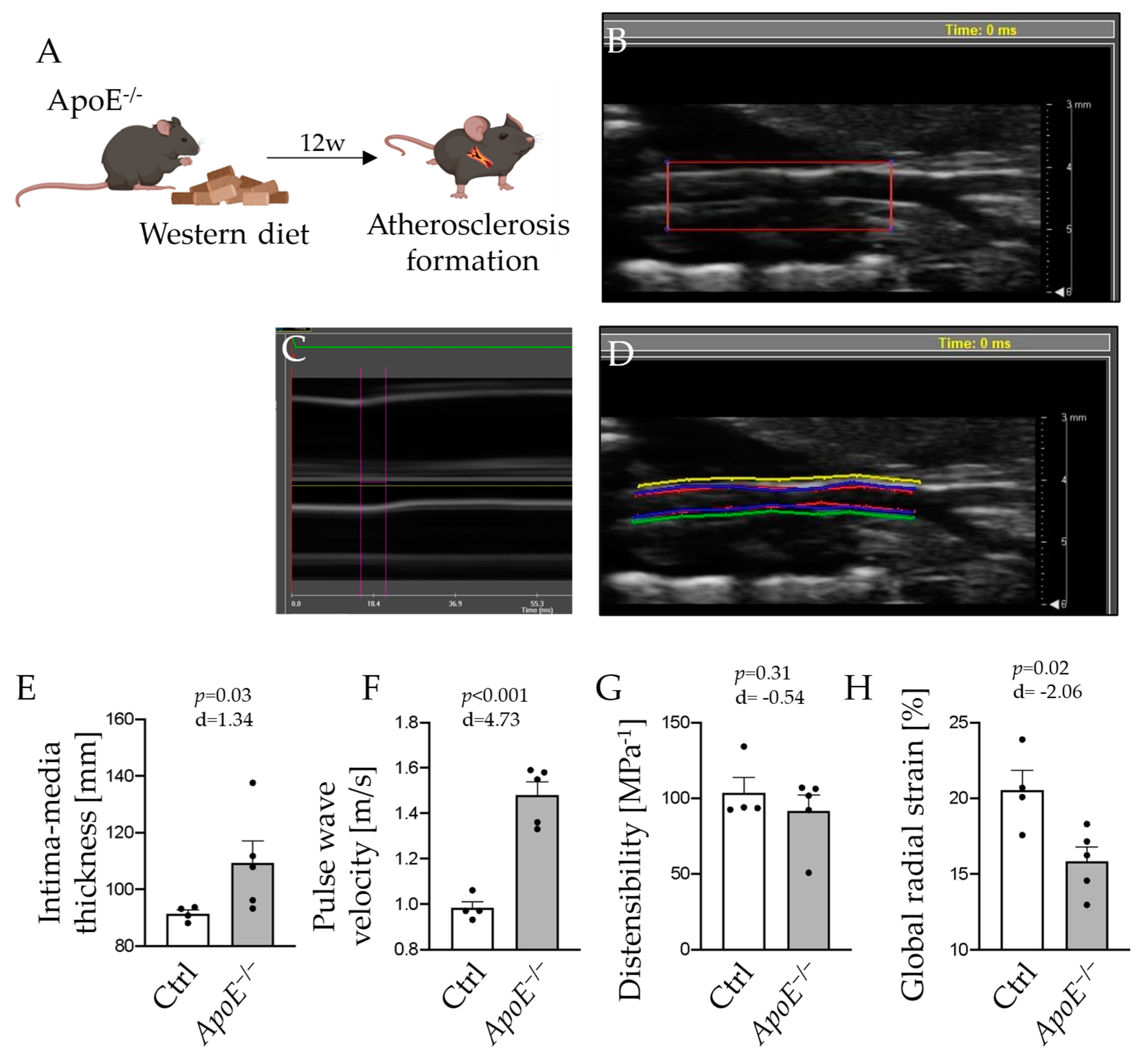
2.2. Morphologic and Functional Alterations of the Carotid Artery in Atherosclerosis Manifest in Vascular Ultrasound Measurements
2.3. Aortic High Resolution Ultrasound Analysis Is Consistent with Histological Postmortem Evaluation of Vascular Disease
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. Implantation of Osmotic Minipumps
4.3. Ultrasound Analysis
4.4. Tissue Preparation
4.5. Picrosirius Red Staining
4.6. Collagen Quantification
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nienaber, C.A.; Clough, R.E.; Sakalihasan, N.; Suzuki, T.; Gibbs, R.; Mussa, F.; Jenkins, M.P.; Thompson, M.M.; Evangelista, A.; Yeh, J.S.; et al. Aortic dissection. Nat. Rev. Dis. Primers 2016, 2, 16053. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Palasubramaniam, J.; Wang, X.; Peter, K. Myocardial Infarction-From Atherosclerosis to Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e176–e185. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, I.; Little, P.J.; Liu, Z.; Xu, Y.; Kamato, D.; Berk, B.C.; Weng, J.; Xu, S. Mouse models of atherosclerosis in translational research. Trends Pharmacol. Sci. 2022, 43, 920–939. [Google Scholar] [CrossRef] [PubMed]
- Lysgaard Poulsen, J.; Stubbe, J.; Lindholt, J.S. Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Hoshide, S.; Kario, K.; Tomitani, N.; Kabutoya, T.; Chia, Y.C.; Park, S.; Shin, J.; Turana, Y.; Tay, J.C.; Buranakitjaroen, P.; et al. Highlights of the 2019 Japanese Society of Hypertension Guidelines and perspectives on the management of Asian hypertensive patients. J. Clin. Hypertens. 2020, 22, 369–377. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Sanches, I.C.; Jorge, L.; Llesuy, S.F.; Irigoyen, M.C.; De Angelis, K. Ovarian status modulates cardiovascular autonomic control and oxidative stress in target organs. Biol. Sex. Differ. 2020, 11, 15. [Google Scholar] [CrossRef]
- Nezu, T.; Hosomi, N.; Aoki, S.; Matsumoto, M. Carotid Intima-Media Thickness for Atherosclerosis. J. Atheroscler. Thromb. 2016, 23, 18–31. [Google Scholar] [CrossRef]
- Berritto, D.; Iacobellis, F.; Rossi, C.; Reginelli, A.; Cappabianca, S.; Grassi, R. Ultra high-frequency ultrasound: New capabilities for nail anatomy exploration. J. Dermatol. 2017, 44, 43–46. [Google Scholar] [CrossRef]
- Nezu, T.; Hosomi, N. Usefulness of Carotid Ultrasonography for Risk Stratification of Cerebral and Cardiovascular Disease. J. Atheroscler. Thromb. 2020, 27, 1023–1035. [Google Scholar] [CrossRef]
- Rekhter, M.D. Collagen synthesis in atherosclerosis: Too much and not enough. Cardiovasc. Res. 1999, 41, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Iino, H.; Okano, T.; Daimon, M.; Sasaki, K.; Chigira, M.; Nakao, T.; Mizuno, Y.; Yamazaki, T.; Kurano, M.; Yatomi, Y.; et al. Usefulness of Carotid Arterial Strain Values for Evaluating the Arteriosclerosis. J. Atheroscler. Thromb. 2019, 26, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Derwich, W.; Wittek, A.; Pfister, K.; Nelson, K.; Bereiter-Hahn, J.; Fritzen, C.P.; Blase, C.; Schmitz-Rixen, T. High Resolution Strain Analysis Comparing Aorta and Abdominal Aortic Aneurysm with Real Time Three Dimensional Speckle Tracking Ultrasound. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sun, Z.; Hill, M.A.; Hans, C.P. Measurement of Pulse Propagation Velocity, Distensibility and Strain in an Abdominal Aortic Aneurysm Mouse Model. J. Vis. Exp. 2020, 156, e60515. [Google Scholar]
- Nandlall, S.D.; Goldklang, M.P.; Kalashian, A.; Dangra, N.A.; D’Armiento, J.M.; Konofagou, E.E. Monitoring and staging abdominal aortic aneurysm disease with pulse wave imaging. Ultrasound Med. Biol. 2014, 40, 2404–2414. [Google Scholar] [CrossRef]
- Wilson, K.A.; Lindholt, J.S.; Hoskins, P.R.; Heickendorff, L.; Vammen, S.; Bradbury, A.W. The relationship between abdominal aortic aneurysm distensibility and serum markers of elastin and collagen metabolism. Eur. J. Vasc. Endovasc. Surg. 2001, 21, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.A.; Lee, A.J.; Lee, A.J.; Hoskins, P.R.; Fowkes, F.G.; Ruckley, C.V.; Bradbury, A.W. The relationship between aortic wall distensibility and rupture of infrarenal abdominal aortic aneurysm. J. Vasc. Surg. 2003, 37, 112–117. [Google Scholar] [CrossRef]
- Tsuruda, T.; Yamashita, A.; Otsu, M.; Koide, M.; Nakamichi, Y.; Sekita-Hatakeyama, Y.; Hatakeyama, K.; Funamoto, T.; Chosa, E.; Asada, Y.; et al. Angiotensin II Induces Aortic Rupture and Dissection in Osteoprotegerin-Deficient Mice. J. Am. Heart Assoc. 2022, 11, e025336. [Google Scholar] [CrossRef]
- Polzer, S.; Gasser, T.C.; Vlachovský, R.; Kubíček, L.; Lambert, L.; Man, V.; Novák, K.; Slažanský, M.; Burša, J.; Staffa, R. Biomechanical indices are more sensitive than diameter in predicting rupture of asymptomatic abdominal aortic aneurysms. J. Vasc. Surg. 2020, 71, 617–626.e6. [Google Scholar] [CrossRef]
- Romary, D.J.; Berman, A.G.; Goergen, C.J. High-frequency murine ultrasound provides enhanced metrics of BAPN-induced AAA growth. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H981–H990. [Google Scholar] [CrossRef]
- Trachet, B.; Fraga-Silva, R.A.; Piersigilli, A.; Tedgui, A.; Sordet-Dessimoz, J.; Astolfo, A.; Van der Donckt, C.; Modregger, P.; Stampanoni, M.F.; Segers, P.; et al. Dissecting abdominal aortic aneurysm in Ang II-infused mice: Suprarenal branch ruptures and apparent luminal dilatation. Cardiovasc. Res. 2015, 105, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Hu, M.; Shen, M.; Kassiri, Z. Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Chernogubova, E.; Jin, H.; Meurer, F.; Eckstein, H.H.; Kim, M.; Maegdefessel, L. Four Surgical Modifications to the Classic Elastase Perfusion Aneurysm Model Enable Haemodynamic Alterations and Extended Elastase Perfusion. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Moulakakis, K.G.; Papadakis, I.; Ikonomidis, I.; Alepaki, M.; Lekakis, J.; Liapis, C.D. Changes in aortic pulse wave velocity of patients undergoing endovascular repair of abdominal aortic aneurysms. J. Endovasc. Ther. 2012, 19, 661–666. [Google Scholar] [CrossRef]
- Urabe, G.; Hoshina, K.; Shimanuki, T.; Nishimori, Y.; Miyata, T.; Deguchi, J. Structural analysis of adventitial collagen to feature aging and aneurysm formation in human aorta. J. Vasc. Surg. 2016, 63, 1341–1350. [Google Scholar] [CrossRef]
- Gueldner, P.H.; Marini, A.X.; Li, B.; Darvish, C.J.; Chung, T.K.; Weinbaum, J.S.; Curci, J.A.; Vorp, D.A. Mechanical and matrix effects of short and long-duration exposure to beta-aminopropionitrile in elastase-induced model abdominal aortic aneurysm in mice. JVS Vasc. Sci. 2023, 4, 100098. [Google Scholar] [CrossRef]
- Plump, A.S.; Smith, J.D.; Hayek, T.; Aalto-Setälä, K.; Walsh, A.; Verstuyft, J.G.; Rubin, E.M.; Breslow, J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992, 71, 343–353. [Google Scholar] [CrossRef]
- Segers, P.; Rietzschel, E.R.; Chirinos, J.A. How to Measure Arterial Stiffness in Humans. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1034–1043. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Wagenhäuser, M.U.; Mehrkens, D.; Adam, M.; Tsao, P.S.; Ramasubramanian, A.K. An Automated Algorithm to Quantify Collagen Distribution in Aortic Wall. J. Histochem. Cytochem. 2019, 67, 267–274. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hof, A.; Guthoff, H.; Ahdab, M.; Landerer, M.; Schäkel, J.; Niehues, J.; Schorscher, M.; Zimmermann, O.; Winkels, H.; von Stein, P.; et al. Vascular Ultrasound for In Vivo Assessment of Arterial Pathologies in a Murine Model of Atherosclerosis and Aortic Aneurysm. Int. J. Mol. Sci. 2023, 24, 15261. https://doi.org/10.3390/ijms242015261
Hof A, Guthoff H, Ahdab M, Landerer M, Schäkel J, Niehues J, Schorscher M, Zimmermann O, Winkels H, von Stein P, et al. Vascular Ultrasound for In Vivo Assessment of Arterial Pathologies in a Murine Model of Atherosclerosis and Aortic Aneurysm. International Journal of Molecular Sciences. 2023; 24(20):15261. https://doi.org/10.3390/ijms242015261
Chicago/Turabian StyleHof, Alexander, Henning Guthoff, Maysam Ahdab, Max Landerer, Jasper Schäkel, Jana Niehues, Maximilian Schorscher, Oscar Zimmermann, Holger Winkels, Philipp von Stein, and et al. 2023. "Vascular Ultrasound for In Vivo Assessment of Arterial Pathologies in a Murine Model of Atherosclerosis and Aortic Aneurysm" International Journal of Molecular Sciences 24, no. 20: 15261. https://doi.org/10.3390/ijms242015261
APA StyleHof, A., Guthoff, H., Ahdab, M., Landerer, M., Schäkel, J., Niehues, J., Schorscher, M., Zimmermann, O., Winkels, H., von Stein, P., Geißen, S., Baldus, S., Adam, M., Mollenhauer, M., & Mehrkens, D. (2023). Vascular Ultrasound for In Vivo Assessment of Arterial Pathologies in a Murine Model of Atherosclerosis and Aortic Aneurysm. International Journal of Molecular Sciences, 24(20), 15261. https://doi.org/10.3390/ijms242015261




