Unveiling the Immune Microenvironment’s Role in Breast Cancer: A Glimpse into Promising Frontiers
Abstract
:1. Introduction
2. Decoding the Multifaceted Roles of Tumor-Infiltrating Lymphocytes (TILs) in BC
2.1. TILs Subgroups and Frequency
2.2. Distribution, Density, and Functional Characteristics within the Tumor
2.3. Investigating the Prognostic Value of TILs in Breast Cancer
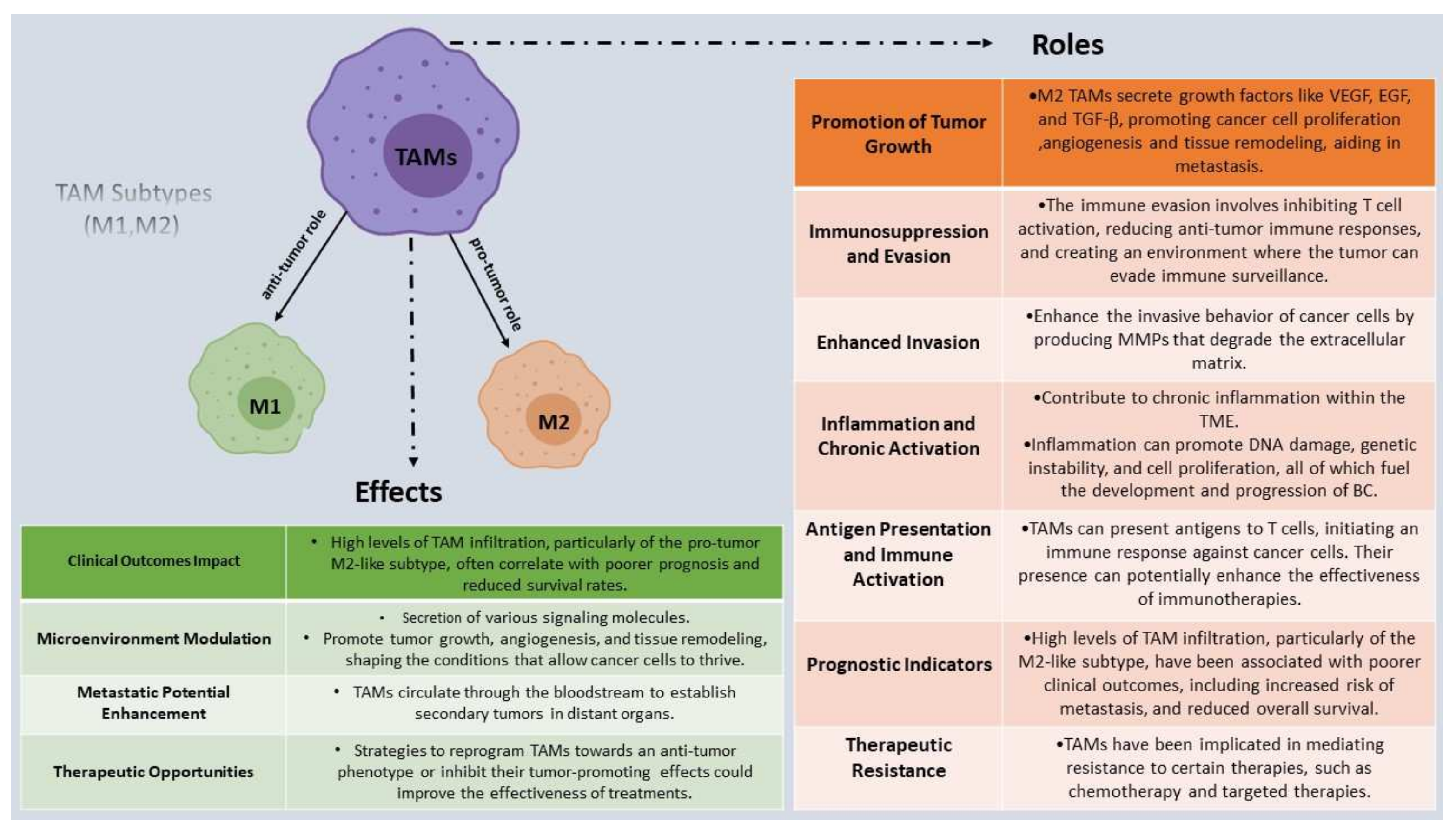
3. The Multifaceted Roles of Tumor-Associated Macrophages (TAMs) in Breast Cancer
3.1. The Presence of TAMs in the Breast Cancer Microenvironment
3.2. TAMs Subtypes and Their Effect on Tumor Progression, Invasion, and Metastasis
3.3. Therapeutic Strategies Targeting TAMs in Breast Cancer
4. Cytokine Expression in the Breast Cancer Microenvironment
5. Immune Evasion in Breast Cancer
6. The Role of Immune Checkpoints in Breast Cancer
7. Other Immunotherapy Approaches for Breast Cancer Patients
7.1. CAR-T Cell Therapy
7.2. Therapeutic Cancer Vaccines
7.3. Immune Modulators
7.4. Combination of Therapies
7.5. Novel Therapies
8. Biomarkers and Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. 2019, 20, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Ghorayeb, T.; Saliba, F.; Allam, S.; Bou Zerdan, M.; Yaghi, M.; Bilani, N.; Jaafar, R.; Nahleh, Z. Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers 2022, 14, 1253. [Google Scholar] [CrossRef]
- Ab Mumin, N.; Ramli Hamid, M.T.; Wong, J.H.D.; Rahmat, K.; Ng, K.H. Magnetic Resonance Imaging Phenotypes of Breast Cancer Molecular Subtypes: A Systematic Review. Acad. Radiol. 2022, 29, S89–S106. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Shaath, H.; Elango, R.; Alajez, N.M. Molecular Classification of Breast Cancer Utilizing Long Non-Coding RNA (lncRNA) Transcriptomes Identifies Novel Diagnostic lncRNA Panel for Triple-Negative Breast Cancer. Cancers 2021, 13, 5350. [Google Scholar] [CrossRef] [PubMed]
- Valenza, C.; Taurelli Salimbeni, B.; Santoro, C.; Trapani, D.; Antonarelli, G.; Curigliano, G. Tumor Infiltrating Lymphocytes across Breast Cancer Subtypes: Current Issues for Biomarker Assessment. Cancers 2023, 15, 767. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Miah, S.; Bagu, E.; Goel, R.; Ogunbolude, Y.; Dai, C.; Ward, A.; Vizeacoumar, F.S.; Davies, G.; Vizeacoumar, F.J.; Anderson, D.; et al. Estrogen receptor signaling regulates the expression of the breast tumor kinase in breast cancer cells. BMC Cancer 2019, 19, 78. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, QLD, Australia, 2022; ISBN 978-0-645-33203-2. [Google Scholar]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An update on the pathological classification of breast cancer. Histopathology 2023, 82, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Bao, H.; Meng, Y.-H.; Zhu, J.-L.; Chu, X.-D.; Chu, X.-L.; Pan, J.-H. Tumour budding is a novel marker in breast cancer: The clinical application and future prospects. Ann. Med. 2022, 54, 1303–1312. [Google Scholar] [CrossRef]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Garattini, E.; Bolis, M.; Di Marino, D.; Maraccani, L.; Morelli, E.; Grolla, A.A.; Fagiani, F.; Corsini, E.; Travelli, C.; et al. OXER1 and RACK1-associated pathway: A promising drug target for breast cancer progression. Oncogenesis 2020, 9, 105. [Google Scholar] [CrossRef]
- Wali, V.B.; Patwardhan, G.A.; Pelekanou, V.; Karn, T.; Cao, J.; Ocana, A.; Yan, Q.; Nelson, B.; Hatzis, C.; Pusztai, L. Identification and Validation of a Novel Biologics Target in Triple Negative Breast Cancer. Sci. Rep. 2019, 9, 14934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, H.; Luo, Y.; Tuo, B.; Liu, X.; Li, T. Effect of metabolism on the immune microenvironment of breast cancer. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2023, 1878, 188861. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Ye, Q. Metabolism and immunity in breast cancer. Front. Med. 2021, 15, 178–207. [Google Scholar] [CrossRef]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, J.; Hu, Z.; Cai, Y.; Xu, Y.; Xu, K. Exploration of the immune microenvironment of breast cancer in large population cohorts. Front. Endocrinol. 2022, 13, 955630. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes. Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Shapiro, D.J. The immune system and inflammation in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Amens, J.N.; Bahçecioglu, G.; Zorlutuna, P. Immune System Effects on Breast Cancer. Cell. Mol. Bioeng. 2021, 14, 279–292. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.-K.; Du, W.-X.; Li, R.-G.; Yang, J.; Li, J.; Li, F.; Tan, H.-B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Peña-Romero, A.C.; Orenes-Piñero, E. Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers. Cancers 2022, 14, 1681. [Google Scholar] [CrossRef]
- Maffuid, K.; Cao, Y. Decoding the Complexity of Immune–Cancer Cell Interactions: Empowering the Future of Cancer Immunotherapy. Cancers 2023, 15, 4188. [Google Scholar] [CrossRef] [PubMed]
- Wöckel, A.; Albert, U.-S.; Janni, W.; Scharl, A.; Kreienberg, R.; Stüber, T. The Screening, Diagnosis, Treatment, and Follow-Up of Breast Cancer. Dtsch. Arztebl. Int. 2018, 115, 316–323. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Badr, N.M.; Berditchevski, F.; Shaaban, A.M. The Immune Microenvironment in Breast Carcinoma: Predictive and Prognostic Role in the Neoadjuvant Setting. Pathobiology 2019, 87, 61–74. [Google Scholar] [CrossRef]
- Howard, F.M.; Villamar, D.; He, G.; Pearson, A.T.; Nanda, R. The emerging role of immune checkpoint inhibitors for the treatment of breast cancer. Expert Opin. Investig. Drugs 2022, 31, 531–548. [Google Scholar] [CrossRef]
- Tower, H.; Ruppert, M.; Britt, K. The Immune Microenvironment of Breast Cancer Progression. Cancers 2019, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Ali, A.; Dutta, S.; Banday, S.; Malonia, S.K. Emerging Trends in Immunotherapy for Cancer. Diseases 2022, 10, 60. [Google Scholar] [CrossRef]
- Grimmett, E.; Al-Share, B.; Alkassab, M.B.; Zhou, R.W.; Desai, A.; Rahim, M.M.A.; Woldie, I. Cancer vaccines: Past, present and future; a review article. Discov. Oncol. 2022, 13, 31. [Google Scholar] [CrossRef]
- Papalexi, E.; Satija, R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol. 2018, 18, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308.e36. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-X.; Xu, J.-D.; Liu, X.-L.; Xu, J.-W.; Wang, W.-J.; Li, Q.-Q.; Chen, Q.; Xu, Z.-D.; Liu, X.-P. RACK1: A superior independent predictor for poor clinical outcome in breast cancer. Int. J. Cancer 2010, 127, 1172–1179. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Long, A.; Chiappini, C.; Travelli, C.; Govoni, S.; Racchi, M. Ribosomes as a nexus between translation and cancer progression: Focus on ribosomal Receptor for Activated C Kinase 1 (RACK1) in breast cancer. Br. J. Pharmacol. 2022, 179, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Masi, M.; Racchi, M.; Corsini, E. Endocrine-Disrupting Chemicals’ (EDCs) Effects on Tumour Microenvironment and Cancer Progression: Emerging Contribution of RACK1. Int. J. Mol. Sci. 2020, 21, 9229. [Google Scholar] [CrossRef]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. npj Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; de Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumor-Infiltrating Lymphocytes and Associations with Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated with Lapatinib and Trastuzumab. JAMA Oncol. 2015, 1, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.; Tanaka, A.; Sakaguchi, S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell 2023, 41, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Catacchio, I.; Silvestris, N.; Scarpi, E.; Schirosi, L.; Scattone, A.; Mangia, A. Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients. Transl. Oncol. 2019, 12, 585–595. [Google Scholar] [CrossRef]
- Li, R.; Cao, L. The role of tumor-infiltrating lymphocytes in triple-negative breast cancer and the research progress of adoptive cell therapy. Front. Immunol. 2023, 14, 1194020. [Google Scholar] [CrossRef] [PubMed]
- McRitchie, B.R.; Akkaya, B. Exhaust the exhausters: Targeting regulatory T cells in the tumor microenvironment. Front. Immunol. 2022, 13, 940052. [Google Scholar] [CrossRef] [PubMed]
- Triki, H.; Charfi, S.; Bouzidi, L.; Ben Kridis, W.; Daoud, J.; Chaabane, K.; Sellami-Boudawara, T.; Rebai, A.; Cherif, B. CD155 expression in human breast cancer: Clinical significance and relevance to natural killer cell infiltration. Life Sci. 2019, 231, 116543. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Q.; Wang, Z.; Jiang, J.; Yu, S.-C.; Ping, Y.-F.; Yang, J.; Xu, S.-L.; Ye, X.-Z.; Xu, C.; et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014, 74, 5746–5757. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.M.; Sajjadi, E.; Venetis, K.; Frascarelli, C.; Cursano, G.; Guerini-Rocco, E.; Fusco, N.; Ivanova, M. Immune Biomarkers in Triple-Negative Breast Cancer: Improving the Predictivity of Current Testing Methods. J. Pers. Med. 2023, 13, 1176. [Google Scholar] [CrossRef]
- Pan, X.; Lu, Y.; Lan, R.; Liu, Z.; Qin, Z.; Wang, H.; Liu, Z. Mitosis detection techniques in H&E stained breast cancer pathological images: A comprehensive review. Comput. Electr. Eng. 2021, 91, 107038. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, S. Tertiary lymphoid structures are critical for cancer prognosis and therapeutic response. Front. Immunol. 2023, 13, 1063711. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D. Biomarkers for breast cancer immunotherapy: PD-L1, TILs, and beyond. Expert Opin. Investig. Drugs 2022, 31, 549–555. [Google Scholar] [CrossRef]
- König, L.; Mairinger, F.D.; Hoffmann, O.; Bittner, A.-K.; Schmid, K.W.; Kimmig, R.; Kasimir-Bauer, S.; Bankfalvi, A. Dissimilar patterns of tumor-infiltrating immune cells at the invasive tumor front and tumor center are associated with response to neoadjuvant chemotherapy in primary breast cancer. BMC Cancer 2019, 19, 120. [Google Scholar] [CrossRef]
- Quintana, Á.; Arenas, E.J.; Bernadó, C.; Navarro, J.F.; González, J.; Esteve-Codina, A.; Moliné, T.; Marti, M.; Curigliano, G.; Schmid, P.; et al. Evaluation of triple negative breast cancer with heterogeneous immune infiltration. Front. Immunol. 2023, 14, 1149747. [Google Scholar] [CrossRef] [PubMed]
- Ochi, T.; Bianchini, G.; Ando, M.; Nozaki, F.; Kobayashi, D.; Criscitiello, C.; Curigliano, G.; Iwamoto, T.; Niikura, N.; Takei, H.; et al. Predictive and prognostic value of stromal tumour-infiltrating lymphocytes before and after neoadjuvant therapy in triple negative and HER2-positive breast cancer. Eur. J. Cancer 2019, 118, 41–48. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing tumor infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv. Anat. Pathol. 2017, 24, 235. [Google Scholar] [CrossRef]
- Garaud, S.; Buisseret, L.; Solinas, C.; Gu-Trantien, C.; de Wind, A.; Van den Eynden, G.; Naveaux, C.; Lodewyckx, J.-N.; Boisson, A.; Duvillier, H.; et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight 2019, 4, e129641. [Google Scholar] [CrossRef]
- Ziogas, D.C.; Theocharopoulos, C.; Lialios, P.-P.; Foteinou, D.; Koumprentziotis, I.-A.; Xynos, G.; Gogas, H. Beyond CTLA-4 and PD-1 Inhibition: Novel Immune Checkpoint Molecules for Melanoma Treatment. Cancers 2023, 15, 2718. [Google Scholar] [CrossRef]
- Balta, E.; Wabnitz, G.H.; Samstag, Y. Hijacked Immune Cells in the Tumor Microenvironment: Molecular Mechanisms of Immunosuppression and Cues to Improve T Cell-Based Immunotherapy of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5736. [Google Scholar] [CrossRef]
- Xia, Z.-A.; Lu, C.; Pan, C.; Li, J.; Li, J.; Mao, Y.; Sun, L.; He, J. The expression profiles of signature genes from CD103+LAG3+ tumour-infiltrating lymphocyte subsets predict breast cancer survival. BMC Med. 2023, 21, 268. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Liu, M.; Jiang, J. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: A meta-analysis. BMC Cancer 2020, 20, 1150. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zhang, P.; Xue, S.; Chen, Y.; Sun, L.; Yang, R. Predictive and prognostic values of tumor infiltrating lymphocytes in breast cancers treated with neoadjuvant chemotherapy: A meta-analysis. Breast 2022, 66, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M. Panelists of the St Gallen Consensus Conference Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, C.; Bendahl, P.-O.; Ekholm, M.; Fernö, M.; Forsare, C.; Krüger, U.; Nordenskjöld, B.; Stål, O.; Rydén, L. Tumour-infiltrating lymphocytes as a prognostic and tamoxifen predictive marker in premenopausal breast cancer: Data from a randomised trial with long-term follow-up. Breast Cancer Res. 2020, 22, 140. [Google Scholar] [CrossRef]
- Yilmaz, C.; Cavdar, D.K. Biomarker Discordances and Alterations Observed in Breast Cancer Treated with Neoadjuvant Chemotherapy: Causes, Frequencies, and Clinical Significances. Curr. Oncol. 2022, 29, 9695–9710. [Google Scholar] [CrossRef]
- De Angelis, C.; Nagi, C.; Hoyt, C.C.; Liu, L.; Roman, K.; Wang, C.; Zheng, Y.; Veeraraghavan, J.; Sethunath, V.; Nuciforo, P.; et al. Evaluation of the predictive role of tumor immune infiltrate in HER2-positive breast cancer patients treated with neoadjuvant anti-HER2 therapy without chemotherapy. Clin. Cancer Res. 2020, 26, 738–745. [Google Scholar] [CrossRef]
- Sukumar, J.; Gast, K.; Quiroga, D.; Lustberg, M.; Williams, N. Triple-negative breast cancer: Promising prognostic biomarkers currently in development. Expert Rev. Anticancer Ther. 2021, 21, 135–148. [Google Scholar] [CrossRef]
- Spathas, N.; Goussia, A.C.; Koliou, G.-A.; Gogas, H.; Zagouri, F.; Batistatou, A.; Charchanti, A.V.; Papoudou-Bai, A.; Bobos, M.; Chrisafi, S.; et al. Association between CD8+ Tumor Infiltrating Lymphocytes and the Clinical Outcome of Patients with Operable Breast Cancer Treated with Adjuvant Dose-Dense Chemotherapy—A 10 Year Follow-Up Report of a Hellenic Cooperative Oncology Group Observational Study. Cancers 2022, 14, 5635. [Google Scholar] [CrossRef]
- Zhu, Y.; Tzoras, E.; Matikas, A.; Bergh, J.; Valachis, A.; Zerdes, I.; Foukakis, T. Expression patterns and prognostic implications of tumor-infiltrating lymphocytes dynamics in early breast cancer patients receiving neoadjuvant therapy: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 999843. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhao, F.; Huo, X.; Ren, D.; Du, F.; Zheng, F.; Zhao, J. Meta-Analysis of HER2-Enriched Subtype Predicting the Pathological Complete Response within HER2-Positive Breast Cancer in Patients Who Received Neoadjuvant Treatment. Front. Oncol. 2021, 11, 632357. [Google Scholar] [CrossRef]
- Goldberg, J.; Pastorello, R.G.; Vallius, T.; Davis, J.; Cui, Y.X.; Agudo, J.; Waks, A.G.; Keenan, T.; McAllister, S.S.; Tolaney, S.M.; et al. The Immunology of Hormone Receptor Positive Breast Cancer. Front. Immunol. 2021, 12, 674192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, J. Breast cancer immunology and immunotherapy: Targeting the programmed cell death protein-1/programmed cell death protein ligand-1. Chin. Med. J. 2020, 133, 853. [Google Scholar] [CrossRef]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Hirakawa, H.; Takahashi, Y.; Nakagawa, S.; Watanabe, G.; Tada, H.; Suzuki, A.; Ohuchi, N.; et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: A retrospective multicenter study. Breast Cancer Res. 2015, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Foulkes, W.D.; Leung, S.; Gao, D.; Lau, S.; Kos, Z.; Nielsen, T.O. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. 2014, 16, 432. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef]
- Haque, A.S.M.R.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef]
- Ma, C.; He, D.; Tian, P.; Wang, Y.; He, Y.; Wu, Q.; Jia, Z.; Zhang, X.; Zhang, P.; Ying, H.; et al. miR-182 targeting reprograms tumor-associated macrophages and limits breast cancer progression. Proc. Natl. Acad. Sci. USA 2022, 119, e2114006119. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Yousefi, M.; Nosrati, R.; Salmaninejad, A.; Dehghani, S.; Shahryari, A.; Saberi, A. Organ-specific metastasis of breast cancer: Molecular and cellular mechanisms underlying lung metastasis. Cell. Oncol. 2018, 41, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis and Metastasis—Current Status. Curr. Med. Chem. 2021, 28, 8203–8236. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A.; Sopik, V. Is invasion a necessary step for metastases in breast cancer? Breast Cancer Res. Treat. 2018, 169, 9–23. [Google Scholar] [CrossRef]
- Santoni, M.; Romagnoli, E.; Saladino, T.; Foghini, L.; Guarino, S.; Capponi, M.; Giannini, M.; Cognigni, P.D.; Ferrara, G.; Battelli, N. Triple negative breast cancer: Key role of Tumor-Associated Macrophages in regulating the activity of anti-PD-1/PD-L1 agents. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Muscarella, R.A.; Jones, D. The Multifaceted Effects of Breast Cancer on Tumor-Draining Lymph Nodes. Am. J. Pathol. 2021, 191, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Sage, J. Investigating Tumor Heterogeneity in Mouse Models. Annu. Rev. Cancer Biol. 2020, 4, 99–119. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.; Zu, X. Tumor-associated macrophages: An important player in breast cancer progression. Thorac. Cancer 2022, 13, 269–276. [Google Scholar] [CrossRef]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Yoen, H.; Kim, S.-Y.; Lee, D.-W.; Lee, H.-B.; Cho, N. Prediction of Tumor Progression During Neoadjuvant Chemotherapy and Survival Outcome in Patients with Triple-Negative Breast Cancer. Korean J. Radiol. 2023, 24, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Qu, Q.; Chen, X.; Huang, O.; Wu, J.; Shen, K. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0152500. [Google Scholar] [CrossRef]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Huang, Y.-K.; Busuttil, R.A.; Boussioutas, A. The Role of Innate Immune Cells in Tumor Invasion and Metastasis. Cancers 2021, 13, 5885. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Jin, Y.; Xu, X.; Shao, J.; Jin, C. Antitumor therapy for breast cancer: Focus on tumor-associated macrophages and nanosized drug delivery systems. Cancer Med. 2023, 12, 11049–11072. [Google Scholar] [CrossRef] [PubMed]
- Deligne, C.; Midwood, K.S. Macrophages and Extracellular Matrix in Breast Cancer: Partners in Crime or Protective Allies? Front. Oncol. 2021, 11, 620773. [Google Scholar] [CrossRef]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci. 2022, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhao, T.; Luo, R.; Qiu, R.; Li, Z. Tumor-Associated Macrophages: Key Players in Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 772615. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Roles of CCL2-CCR2 Axis in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 8530. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Ren, X.; Yu, H.; Zhan, Y. Targeting the CCL2/CCR2 Axis in Cancer Immunotherapy: One Stone, Three Birds? Front. Immunol. 2021, 12, 771210. [Google Scholar] [CrossRef]
- Hao, X.; Gao, X.; Yin, S.; Jiang, Z. Prospect of neoadjuvant/adjuvant immunotherapy in early-stage triple-negative breast cancer. Transl. Breast Cancer Res. 2023, 4, 6. [Google Scholar] [CrossRef]
- Sawa-Wejksza, K.; Kandefer-Szerszeń, M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch. Immunol. Ther. Exp. 2018, 66, 97–111. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Sabatino, R.; Antonelli, A.; Battistelli, S.; Schwendener, R.; Magnani, M.; Rossi, L. Macrophage Depletion by Free Bisphosphonates and Zoledronate-Loaded Red Blood Cells. PLoS ONE 2014, 9, e101260. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.-J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Imam, B.; Aziz, K.; Khan, M.; Zubair, T.; Iqbal, A. Role of Bisphosphonates in Postmenopausal Women with Osteoporosis to Prevent Future Fractures: A Literature Review. Cureus 2019, 11, e5328. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, B.; Lohrisch, C.; Sheade, J.; Fleming, G.F. Personalizing Adjuvant Endocrine Therapy for Early-Stage Hormone Receptor–Positive Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Amir, E.; Paterson, A.; Zhu, X.; Clemons, M. Are adjuvant bisphosphonates now standard of care of women with early stage breast cancer? A debate from the Canadian Bone and the Oncologist New Updates meeting. J. Bone Oncol. 2015, 4, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Lei, K.-F.; Han, F. Tumor microenvironment: Recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3855–3864. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Bai, X.; Shu, Y.; Ahmad, O.; Shen, P. Targeting tumor-associated macrophages as an antitumor strategy. Biochem. Pharmacol. 2021, 183, 114354. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Wuescher, L.M.; Worth, R.; Yildirim-Ayan, E. Mechano-Immunomodulation: Mechanoresponsive Changes in Macrophage Activity and Polarization. Ann. Biomed. Eng. 2019, 47, 2213–2231. [Google Scholar] [CrossRef]
- Mun, J.-Y.; Leem, S.-H.; Lee, J.H.; Kim, H.S. Dual Relationship between Stromal Cells and Immune Cells in the Tumor Microenvironment. Front. Immunol. 2022, 13, 864739. [Google Scholar] [CrossRef]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Masih, M.; Agarwal, S.; Kaur, R.; Gautam, P.K. Role of chemokines in breast cancer. Cytokine 2022, 155, 155909. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Z.; Lu, W.; Chen, Z.; Chen, L.; Han, S.; Wu, X.; Cai, T.; Cai, Y. Chemokines and chemokine receptors: A new strategy for breast cancer therapy. Cancer Med. 2020, 9, 3786–3799. [Google Scholar] [CrossRef] [PubMed]
- Portella, L.; Bello, A.M.; Scala, S. CXCL12 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2021, 1302, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liao, X.; Qiu, S.; Xu, H.; Zhang, S.; Wang, S.; Ai, J.; Yang, L. CXCL8 in Tumor Biology and Its Implications for Clinical Translation. Front. Mol. Biosci. 2022, 9, 723846. [Google Scholar] [CrossRef] [PubMed]
- Tzang, B.-S.; Chen, V.C.-H.; Hsieh, C.-C.; Wang, W.-K.; Weng, Y.-P.; Ho, H.-Y.; Hsu, Y.-T.; Hsaio, H.-P.; Weng, J.-C.; Chen, Y.-L. Differential associations of proinflammatory and anti-inflammatory cytokines with depression severity from noncancer status to breast cancer course and subsequent chemotherapy. BMC Cancer 2020, 20, 686. [Google Scholar] [CrossRef] [PubMed]
- Divella, R.; Gadaleta Caldarola, G.; Mazzocca, A. Chronic Inflammation in Obesity and Cancer Cachexia. J. Clin. Med. 2022, 11, 2191. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Nalabolu, M.R.; Palasamudram, K.; Jamil, K. Adiponectin and Leptin Molecular Actions and Clinical Significance in Breast Cancer. Int. J. Hematol. Oncol. Stem Cell Res. 2014, 8, 31–40. [Google Scholar]
- Andò, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef]
- Kim, B.-G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel therapies emerging in oncology to target the TGF-β pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGF-β biology in cancer progression and tumor immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Bang, J.-H.; Nam, A.-R.; Park, J.E.; Jin, M.H.; Bang, Y.-J.; Oh, D.-Y. The prognostic role of soluble TGF-beta and its dynamics in unresectable pancreatic cancer treated with chemotherapy. Cancer Med. 2020, 9, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Gunaydin, G. CAFs Interacting with TAMs in Tumor Microenvironment to Enhance Tumorigenesis and Immune Evasion. Front. Oncol. 2021, 11, 668349. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Elizalde, P.V.; Schillaci, R. Tumor Necrosis Factor α Blockade: An Opportunity to Tackle Breast Cancer. Front. Oncol. 2020, 10, 584. [Google Scholar] [CrossRef]
- Sparano, J.A.; O’Neill, A.; Graham, N.; Northfelt, D.W.; Dang, C.T.; Wolff, A.C.; Sledge, G.W.; Miller, K.D. Inflammatory cytokines and distant recurrence in HER2-negative early breast cancer. npj Breast Cancer 2022, 8, 16. [Google Scholar] [CrossRef]
- Adinew, G.M.; Taka, E.; Mochona, B.; Badisa, R.B.; Mazzio, E.A.; Elhag, R.; Soliman, K.F.A. Therapeutic Potential of Thymoquinone in Triple-Negative Breast Cancer Prevention and Progression through the Modulation of the Tumor Microenvironment. Nutrients 2021, 14, 79. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Zhang, C.; Fei, Y.; Wang, H.; Hu, S.; Liu, C.; Hu, R.; Du, Q. CAFs orchestrates tumor immune microenvironment—A new target in cancer therapy? Front. Pharmacol. 2023, 14, 1113378. [Google Scholar] [CrossRef] [PubMed]
- Czekay, R.-P.; Cheon, D.-J.; Samarakoon, R.; Kutz, S.M.; Higgins, P.J. Cancer-Associated Fibroblasts: Mechanisms of Tumor Progression and Novel Therapeutic Targets. Cancers 2022, 14, 1231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, T.; Yuan, Y.; Zhu, Y. What is new in cancer-associated fibroblast biomarkers? Cell Commun. Signal. 2023, 21, 96. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Sakurai, M.; Yamamoto, Y.; Suzuki, E.; Tsuda, M.; Kataoka, T.R.; Hirata, M.; Nishie, M.; Nojiri, T.; Kumazoe, M.; et al. Alteration of specific cytokine expression patterns in patients with breast cancer. Sci. Rep. 2019, 9, 2924. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Martínez-Pérez, C.; Kay, C.; Meehan, J.; Gray, M.; Dixon, J.M.; Turnbull, A.K. The IL6-like Cytokine Family: Role and Biomarker Potential in Breast Cancer. J. Pers. Med. 2021, 11, 1073. [Google Scholar] [CrossRef]
- Fabre, J.A.S.; Giustiniani, J.; Garbar, C.; Merrouche, Y.; Antonicelli, F.; Bensussan, A. The Interleukin-17 Family of Cytokines in Breast Cancer. Int. J. Mol. Sci. 2018, 19, 3880. [Google Scholar] [CrossRef]
- Moaaz, M.; Lotfy, H.; Motawea, M.A.; Fadali, G. The interplay of interleukin-17A and breast cancer tumor microenvironment as a novel immunotherapeutic approach to increase tumor immunogenicity. Immunobiology 2021, 226, 152068. [Google Scholar] [CrossRef] [PubMed]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-M.; Lam, H.Y.P.; Hsu, H.-J.; Jiang, S.-J. Interleukin-10: A double-edged sword in breast cancer. Tzu Chi Med. J. 2021, 33, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Maddalon, A.; Masi, M.; Iulini, M.; Linciano, P.; Galbiati, V.; Marinovich, M.; Racchi, M.; Buoso, E.; Corsini, E. Effects of endocrine active contaminating pesticides on RACK1 expression and immunological consequences in THP-1 cells. Environ. Toxicol. Pharmacol. 2022, 95, 103971. [Google Scholar] [CrossRef]
- Maddalon, A.; Cari, L.; Iulini, M.; Alhosseini, M.N.; Galbiati, V.; Marinovich, M.; Nocentini, G.; Corsini, E. Impact of endocrine disruptors on peripheral blood mononuclear cells in vitro: Role of gender. Arch. Toxicol. 2023, 97, 3129–3150. [Google Scholar] [CrossRef]
- Buoso, E.; Kenda, M.; Masi, M.; Linciano, P.; Galbiati, V.; Racchi, M.; Dolenc, M.S.; Corsini, E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021, 12, 743991. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Bates, J.P.; Derakhshandeh, R.; Jones, L.; Webb, T.J. Mechanisms of immune evasion in breast cancer. BMC Cancer 2018, 18, 556. [Google Scholar] [CrossRef] [PubMed]
- Pruneri, G.; Gray, K.P.; Vingiani, A.; Viale, G.; Curigliano, G.; Criscitiello, C.; Láng, I.; Ruhstaller, T.; Gianni, L.; Goldhirsch, A.; et al. Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res. Treat. 2016, 158, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.; Seliger, B. The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care 2018, 13, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Dushyanthen, S.; Beavis, P.A.; Salgado, R.; Denkert, C.; Savas, P.; Combs, S.; Rimm, D.L.; Giltnane, J.M.; Estrada, M.V.; et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin. Cancer Res. 2016, 22, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.; Ferrari, P.; Rossi, G.; Carpi, A. Tumour growth and immune evasion as targets for a new strategy in advanced cancer. Endocr.-Relat. Cancer 2018, 25, R577–R604. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Song, Y.; Wang, Z.; Wang, Y.; Luo, F.; Xu, Y.; Zhao, Y.; Wu, Z.; Xu, Y. Mechanism of immune evasion in breast cancer. Onco Targets Ther. 2017, 10, 1561–1573. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, C.; Liu, T.; Dai, X.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front. Immunol. 2020, 11, 1371. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Antonangeli, F.; Natalini, A.; Garassino, M.C.; Sica, A.; Santoni, A.; Di Rosa, F. Regulation of PD-L1 Expression by NF-κB in Cancer. Front. Immunol. 2020, 11, 584626. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Basudan, A.M. The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin. Pr. 2022, 13, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Beckermann, K.E.; Johnson, D.B.; Das, S. Combining anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and -programmed cell death protein 1 (PD-1) agents for cancer immunotherapy. Expert Opin. Biol. Ther. 2021, 21, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadegan, M.; Bavandpour, P.; Nowroozi, M.R.; Amini, E.; Kourosh-Arami, M.; Momeni, S.A.; Bokaie, S.; Sharifi, L. The Potential of T Cell Immunoglobulin and Mucin-Domain Containing-3 (Tim-3) in Designing Novel Immunotherapy for Bladder Cancer. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 2131–2146. [Google Scholar] [CrossRef]
- Kern, R.; Panis, C. CTLA-4 Expression and Its Clinical Significance in Breast Cancer. Arch. Immunol. Ther. Exp. 2021, 69, 16. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrari, P.; Carpi, A. Immune Checkpoint Inhibitors and Other Immune Therapies in Breast Cancer: A New Paradigm for Prolonged Adjuvant Immunotherapy. Biomedicines 2022, 10, 2511. [Google Scholar] [CrossRef]
- Savoia, P.; Astrua, C.; Fava, P. Ipilimumab (Anti-Ctla-4 Mab) in the treatment of metastatic melanoma: Effectiveness and toxicity management. Hum. Vaccin. Immunother. 2016, 12, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Gil Del Alcazar, C.R.; Huh, S.J.; Ekram, M.B.; Trinh, A.; Liu, L.L.; Beca, F.; Zi, X.; Kwak, M.; Bergholtz, H.; Su, Y.; et al. Immune Escape in Breast Cancer During In Situ to Invasive Carcinoma Transition. Cancer Discov. 2017, 7, 1098–1115. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Toor, S.M.; Khalaf, S.; Elkord, E. Breast Cancer Cells and PD-1/PD-L1 Blockade Upregulate the Expression of PD-1, CTLA-4, TIM-3 and LAG-3 Immune Checkpoints in CD4+ T Cells. Vaccines 2019, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Dermani, F.K.; Samadi, P.; Rahmani, G.; Kohlan, A.K.; Najafi, R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J. Cell. Physiol. 2019, 234, 1313–1325. [Google Scholar] [CrossRef]
- Thomas, R.; Al-Khadairi, G.; Decock, J. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer Treatment: Promising Future Prospects. Front. Oncol. 2021, 10, 600573. [Google Scholar] [CrossRef]
- Tonse, R.; Rubens, M.; Appel, H.; Tom, M.C.; Hall, M.D.; Odia, Y.; McDermott, M.W.; Ahluwalia, M.S.; Mehta, M.P.; Kotecha, R. Systematic review and meta-analysis of PD-L1 expression discordance between primary tumor and lung cancer brain metastasis. Neuro-Oncol. Adv. 2021, 3, vdab166. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, R.; Yang, A.-G.; Zheng, G. Diversity of immune checkpoints in cancer immunotherapy. Front. Immunol. 2023, 14, 1121285. [Google Scholar] [CrossRef]
- Sobhani, N.; Tardiel-Cyril, D.R.; Davtyan, A.; Generali, D.; Roudi, R.; Li, Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers 2021, 13, 1440. [Google Scholar] [CrossRef]
- Ai, L.; Chen, J.; Yan, H.; He, Q.; Luo, P.; Xu, Z.; Yang, X. Research Status and Outlook of PD-1/PD-L1 Inhibitors for Cancer Therapy. Drug Des. Devel. Ther. 2020, 14, 3625–3649. [Google Scholar] [CrossRef]
- Szlezinger, K.; Pogoda, K.; Jagiełło-Gruszfeld, A.; Kłosowska, D.; Górski, A.; Borysowski, J. Eligibility criteria in clinical trials in breast cancer: A cohort study. BMC Med. 2023, 21, 240. [Google Scholar] [CrossRef]
- Wein, L.; Luen, S.J.; Savas, P.; Salgado, R.; Loi, S. Checkpoint blockade in the treatment of breast cancer: Current status and future directions. Br. J. Cancer 2018, 119, 4–11. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Palomeras, S.; Ruiz-Martínez, S.; Puig, T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Molecules 2018, 23, 2193. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Liu, J.-W.; Lu, C.; Wei, J.-F. CAR-T Cell Therapy for Breast Cancer: From Basic Research to Clinical Application. Int. J. Biol. Sci. 2022, 18, 2609–2626. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-J.; Ma, D.; Liu, Y.-Y.; Xiao, Y.; Gong, Y.; Jiang, Y.-Z.; Shao, Z.-M.; Hu, X.; Di, G.-H. Bulk and single-cell transcriptome profiling reveal the metabolic heterogeneity in human breast cancers. Mol. Ther. 2021, 29, 2350–2365. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ye, F.; Kong, Y.; Hu, X.; Deng, X.; Xie, J.; Song, C.; Ou, X.; Wu, S.; Wu, L.; et al. The Single-Cell Landscape of Intratumoral Heterogeneity and The Immunosuppressive Microenvironment in Liver and Brain Metastases of Breast Cancer. Adv. Sci. 2023, 10, e2203699. [Google Scholar] [CrossRef]
- Wang, X.; Xie, T.; Luo, J.; Zhou, Z.; Yu, X.; Guo, X. Radiomics predicts the prognosis of patients with locally advanced breast cancer by reflecting the heterogeneity of tumor cells and the tumor microenvironment. Breast Cancer Res. 2022, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, S.; Zou, Y.; Tang, Y.; Tian, W.; Wong, C.-W.; Wu, S.; Ou, X.; Zhao, W.; Cai, M.; et al. Turning up a new pattern: Identification of cancer-associated fibroblast-related clusters in TNBC. Front. Immunol. 2022, 13, 1022147. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef]
- Huober, J.; Barrios, C.H.; Niikura, N.; Jarząb, M.; Chang, Y.-C.; Huggins-Puhalla, S.L.; Pedrini, J.; Zhukova, L.; Graupner, V.; Eiger, D.; et al. Atezolizumab with Neoadjuvant Anti-Human Epidermal Growth Factor Receptor 2 Therapy and Chemotherapy in Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer: Primary Results of the Randomized Phase III IMpassion050 Trial. J. Clin. Oncol. 2022, 40, 2946–2956. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; O’Neill, A.; Gradishar, W.; Hobday, T.J.; Goldstein, L.J.; Mayer, I.A.; Bloom, S.; Brufsky, A.M.; Tevaarwerk, A.J.; Sparano, J.A.; et al. Double-Blind Phase III Trial of Adjuvant Chemotherapy with and without Bevacizumab in Patients with Lymph Node-Positive and High-Risk Lymph Node-Negative Breast Cancer (E5103). J. Clin. Oncol. 2018, 36, 2621–2629. [Google Scholar] [CrossRef]
- Baselga, J.; Costa, F.; Gomez, H.; Hudis, C.A.; Rapoport, B.; Roche, H.; Schwartzberg, L.S.; Petrenciuc, O.; Shan, M.; Gradishar, W.J. A phase 3 tRial comparing capecitabinE in combination with SorafenIb or pLacebo for treatment of locally advanced or metastatIc HER2-Negative breast CancEr (the RESILIENCE study): Study protocol for a randomized controlled trial. Trials 2013, 14, 228. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Zamagni, C.; Gómez, P.; Bermejo, B.; Nagai, S.E.; Melichar, B.; Chan, A.; Mángel, L.; Bergh, J.; Costa, F.; et al. RESILIENCE: Phase III Randomized, Double-Blind Trial Comparing Sorafenib with Capecitabine Versus Placebo with Capecitabine in Locally Advanced or Metastatic HER2-Negative Breast Cancer. Clin. Breast Cancer 2017, 17, 585–594.e4. [Google Scholar] [CrossRef]
- Pernas, S.; Guerriero, J.L.; Naumenko, S.; Goel, S.; Regan, M.M.; Hu, J.; Harrison, B.T.; Lynce, F.; Lin, N.U.; Partridge, A.; et al. Early on-treatment transcriptional profiling as a tool for improving pathological response prediction in HER2-positive inflammatory breast cancer. Ther. Adv. Med. Oncol. 2022, 14, 17588359221113268. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.A.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef]
- Fasching, P.A.; Loibl, S.; Hu, C.; Hart, S.N.; Shimelis, H.; Moore, R.; Schem, C.; Tesch, H.; Untch, M.; Hilfrich, J.; et al. BRCA1/2 Mutations and Bevacizumab in the Neoadjuvant Treatment of Breast Cancer: Response and Prognosis Results in Patients with Triple-Negative Breast Cancer from the GeparQuinto Study. J. Clin. Oncol. 2018, 36, 2281–2287. [Google Scholar] [CrossRef]
- Untch, M.; von Minckwitz, G.; Gerber, B.; Schem, C.; Rezai, M.; Fasching, P.A.; Tesch, H.; Eggemann, H.; Hanusch, C.; Huober, J.; et al. Survival Analysis after Neoadjuvant Chemotherapy with Trastuzumab or Lapatinib in Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer in the GeparQuinto (G5) Study (GBG 44). J. Clin. Oncol. 2018, 36, 1308–1316. [Google Scholar] [CrossRef]
- Ruíz-Borrego, M.; Guerrero-Zotano, A.; Bermejo, B.; Ramos, M.; Cruz, J.; Baena-Cañada, J.M.; Cirauqui, B.; Rodríguez-Lescure, Á.; Alba, E.; Martínez-Jáñez, N.; et al. Phase III evaluating the addition of fulvestrant (F) to anastrozole (A) as adjuvant therapy in postmenopausal women with hormone receptor-positive HER2-negative (HR+/HER2−) early breast cancer (EBC): Results from the GEICAM/2006-10 study. Breast Cancer Res. Treat. 2019, 177, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. npj Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.-T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Ghosh, S.; Jha, S.; Hazra, S.; Srivastava, N.; Chakraborty, U.; Roy, A.G. Recent advancement in breast cancer treatment using CAR T cell therapy:- A review. Adv. Cancer Biol.-Metastasis 2023, 7, 100090. [Google Scholar] [CrossRef]
- Nasiri, F.; Kazemi, M.; Mirarefin, S.M.J.; Mahboubi Kancha, M.; Ahmadi Najafabadi, M.; Salem, F.; Dashti Shokoohi, S.; Evazi Bakhshi, S.; Safarzadeh Kozani, P.; Safarzadeh Kozani, P. CAR-T cell therapy in triple-negative breast cancer: Hunting the invisible devil. Front. Immunol. 2022, 13, 1018786. [Google Scholar] [CrossRef]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Hassan, R.; Thomas, A.; Alewine, C.; Le, D.T.; Jaffee, E.M.; Pastan, I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J. Clin. Oncol. 2016, 34, 4171–4179. [Google Scholar] [CrossRef]
- Hamilton, D.H.; Roselli, M.; Ferroni, P.; Costarelli, L.; Cavaliere, F.; Taffuri, M.; Palena, C.; Guadagni, F. Brachyury, a vaccine target, is overexpressed in triple-negative breast cancer. Endocr. Relat. Cancer 2016, 23, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Moradi-Kalbolandi, S.; Hosseinzadeh, A.; Kazemi, M.H.; Khorramdelazad, H.; Safari, E.; Farahmand, L. Breast cancer immunotherapy: Current and novel approaches. Int. Immunopharmacol. 2021, 98, 107886. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Zhou, W.; Weng, J.; Feng, H.; Liang, P.; Li, Y.; Shi, F. Application of HER2 peptide vaccines in patients with breast cancer: A systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 489. [Google Scholar] [CrossRef] [PubMed]
- Cejalvo, J.M.; Falato, C.; Villanueva, L.; Tolosa, P.; González, X.; Pascal, M.; Canes, J.; Gavilá, J.; Manso, L.; Pascual, T.; et al. Oncolytic viruses: A new immunotherapeutic approach for breast cancer treatment? Cancer Treat. Rev. 2022, 106, 102392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Baldin, A.V.; Isayev, O.; Werner, J.; Zamyatnin, A.A.; Bazhin, A.V. Cancer Vaccines: Antigen Selection Strategy. Vaccines 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-León, M.L.; Jiménez-Cortegana, C.; Silva Romeiro, S.; Garnacho, C.; de la Cruz-Merino, L.; García-Domínguez, D.J.; Hontecillas-Prieto, L.; Sánchez-Margalet, V. Defining the Emergence of New Immunotherapy Approaches in Breast Cancer: Role of Myeloid-Derived Suppressor Cells. Int. J. Mol. Sci. 2023, 24, 5208. [Google Scholar] [CrossRef]
- Moragon, S.; Hernando, C.; Martinez-Martinez, M.T.; Tapia, M.; Ortega-Morillo, B.; Lluch, A.; Bermejo, B.; Cejalvo, J.M. Immunological Landscape of HER-2 Positive Breast Cancer. Cancers 2022, 14, 3167. [Google Scholar] [CrossRef]
- Emens, L.A.; Molinero, L.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Diéras, V.; Iwata, H.; Barrios, C.H.; Nechaeva, M.; Nguyen-Duc, A.; et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-Negative Breast Cancer: Biomarker Evaluation of the IMpassion130 Study. J. Natl. Cancer Inst. 2021, 113, 1005–1016. [Google Scholar] [CrossRef]
- Taylor-Papadimitriou, J.; Burchell, J.M.; Graham, R.; Beatson, R. Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 2018, 46, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Yazdian-Robati, R.; Behravan, J. HER2-Positive Breast Cancer Immunotherapy: A Focus on Vaccine Development. Arch. Immunol. Ther. Exp. 2020, 68, 2. [Google Scholar] [CrossRef]
- Khadela, A.; Soni, S.; Megha, K.; Shah, A.C.; Pandya, A.J.; Kothari, N.; Shah, I.; Avinash, C.B. Contracting triple-negative breast cancer with immunotherapeutic armamentarium: Recent advances and clinical prospects. Med. Oncol. 2023, 40, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-R.; Wu, X.-L.; Sun, Y.-L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Signal Transduct. Target. Ther. 2022, 7, 331. [Google Scholar] [CrossRef]
- Riccardi, C.; Ronchetti, S.; Nocentini, G. Glucocorticoid-induced TNFR-related gene (GITR) as a therapeutic target for immunotherapy. Expert Opin. Ther. Targets 2018, 22, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Ronchetti, S.; Ricci, E.; Petrillo, M.G.; Cari, L.; Migliorati, G.; Nocentini, G.; Riccardi, C. Glucocorticoid-induced tumour necrosis factor receptor-related protein: A key marker of functional regulatory T cells. J. Immunol. Res. 2015, 2015, 171520. [Google Scholar] [CrossRef]
- Cari, L.; Nocentini, G.; Migliorati, G.; Riccardi, C. Potential effect of tumor-specific Treg-targeted antibodies in the treatment of human cancers: A bioinformatics analysis. Oncoimmunology 2018, 7, e1387705. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Bell, A.; Ladomersky, E.; Lauing, K.L.; Bollu, L.; Sosman, J.A.; Zhang, B.; Wu, J.D.; Miller, S.D.; Meeks, J.J.; et al. Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and Targeting Strategies. Front. Immunol. 2020, 11, 1185. [Google Scholar] [CrossRef] [PubMed]
- Baxevanis, C.N.; Fortis, S.P.; Perez, S.A. The balance between breast cancer and the immune system: Challenges for prognosis and clinical benefit from immunotherapies. Semin. Cancer Biol. 2021, 72, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Iori, F.; Bruni, A.; Cozzi, S.; Ciammella, P.; Di Pressa, F.; Boldrini, L.; Greco, C.; Nardone, V.; Salvestrini, V.; Desideri, I.; et al. Can Radiotherapy Empower the Host Immune System to Counterattack Neoplastic Cells? A Systematic Review on Tumor Microenvironment Radiomodulation. Curr. Oncol. 2022, 29, 4612–4624. [Google Scholar] [CrossRef]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef]
- Radosa, J.C.; Stotz, L.; Müller, C.; Kaya, A.C.; Solomayer, E.-F.; Radosa, M.P. Clinical Data on Immunotherapy in Breast Cancer. Breast Care 2020, 15, 450–469. [Google Scholar] [CrossRef]
- Ren, Y.; Song, J.; Li, X.; Luo, N. Rationale and Clinical Research Progress on PD-1/PD-L1-Based Immunotherapy for Metastatic Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 8878. [Google Scholar] [CrossRef]
- Han, H.S.; Vikas, P.; Costa, R.L.B.; Jahan, N.; Taye, A.; Stringer-Reasor, E.M. Early-Stage Triple-Negative Breast Cancer Journey: Beginning, End, and Everything in Between. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390464. [Google Scholar] [CrossRef] [PubMed]
- Astor, L. EFS Findings for Neoadjuvant Pembrolizumab/Chemotherapy Suggest a Potential New Standard of Care in Early TNBC, Leading to FDA Approval. Target. Ther. Oncol. 2021, 10, 74. [Google Scholar]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Hein, A.; Kolberg, H.-C.; Häberle, L.; Uhrig, S.; Rübner, M.; Belleville, E.; Hack, C.C.; Fehm, T.N.; Janni, W.; et al. Pembrolizumab in combination with nab-paclitaxel for the treatment of patients with early-stage triple-negative breast cancer—A single-arm phase II trial (NeoImmunoboost, AGO-B-041). Eur. J. Cancer 2023, 184, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Tolaney, S.M. Clinical Development of New Antibody-Drug Conjugates in Breast Cancer: To Infinity and Beyond. BioDrugs 2021, 35, 159–174. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Olivier, T.; Prasad, V. Sacituzumab govitecan in metastatic triple negative breast cancer (TNBC): Four design features in the ASCENT trial potentially favored the experimental arm. Transl. Oncol. 2022, 15, 101248. [Google Scholar] [CrossRef]
- Nambiar, D.K.; Mishra, D.; Singh, R.P. Targeting DNA repair for cancer treatment: Lessons from PARP inhibitor trials. Oncol. Res. 2023, 31, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.; Garber, J.E. PARP inhibition in breast cancer: Progress made and future hopes. npj Breast Cancer 2022, 8, 47. [Google Scholar] [CrossRef]
- Robson, M.E.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Tung, N.; Armstrong, A.; Dymond, M.; et al. OlympiAD extended follow-up for overall survival and safety: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Eur. J. Cancer 2023, 184, 39–47. [Google Scholar] [CrossRef]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.-H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Gonçalves, A.; Rugo, H.S.; Lee, K.-H.; Fehrenbacher, L.; Mina, L.A.; Diab, S.; Blum, J.L.; Chakrabarti, J.; Elmeliegy, M.; et al. Talazoparib in Patients with a Germline BRCA-Mutated Advanced Breast Cancer: Detailed Safety Analyses from the Phase III EMBRACA Trial. Oncologist 2020, 25, e439–e450. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz-Merino, L.; Gion, M.; Cruz, J.; Alonso-Romero, J.L.; Quiroga, V.; Moreno, F.; Andrés, R.; Santisteban, M.; Ramos, M.; Holgado, E.; et al. Pembrolizumab in combination with gemcitabine for patients with HER2-negative advanced breast cancer: GEICAM/2015-04 (PANGEA-Breast) study. BMC Cancer 2022, 22, 1258. [Google Scholar] [CrossRef] [PubMed]
- Dees, S.; Ganesan, R.; Singh, S.; Grewal, I.S. Bispecific Antibodies for Triple Negative Breast Cancer. Trends Cancer 2021, 7, 162–173. [Google Scholar] [CrossRef]
- Bedard, P.; Im, S.-A.; Elimova, E.; Rha, S.; Goodwin, R.; Ferrario, C.; Lee, K.-W.; Hanna, D.; Meric-Bernstam, F.; Mayordomo, J.; et al. Abstract P2-13-07: Zanidatamab (ZW25), a HER2-targeted bispecific antibody, in combination with chemotherapy (chemo) for HER2-positive breast cancer (BC): Results from a phase 1 study. Cancer Res. 2022, 82, P2-13-07. [Google Scholar] [CrossRef]
- Hosseini, M.; Seyedpour, S.; Khodaei, B.; Loghman, A.-H.; Seyedpour, N.; Yazdi, M.-H.; Rezaei, N. Cancer Vaccines for Triple-Negative Breast Cancer: A Systematic Review. Vaccines 2023, 11, 146. [Google Scholar] [CrossRef]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Loi, S. Immunotherapy Approaches for Breast Cancer Patients in 2023. Cold Spring Harb. Perspect. Med. 2023, 13, a041332. [Google Scholar] [CrossRef]
- Stevens, M.J.; West, S.; Gard, G.; Renaud, C.; Nevell, D.; Roderick, S.; Le, A. Utility of adjuvant whole abdominal radiation therapy in ovarian clear cell cancer (OCCC): A pragmatic cohort study of women with classic immuno-phenotypic signature. Radiat. Oncol. 2021, 16, 29. [Google Scholar] [CrossRef]
- Zhong, S.; Jia, Z.; Zhang, H.; Gong, Z.; Feng, J.; Xu, H. Identification and validation of tumor microenvironment-related prognostic biomarkers in breast cancer. Transl. Cancer Res. 2021, 10, 4355–4364. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zou, X.; Zheng, S.; Tang, H.; Zhang, L.; Liu, P.; Xie, X. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920940928. [Google Scholar] [CrossRef]
- Petitprez, F.; Meylan, M.; de Reyniès, A.; Sautès-Fridman, C.; Fridman, W.H. The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies. Front. Immunol. 2020, 11, 784. [Google Scholar] [CrossRef]
- Christodoulou, M.-I.; Zaravinos, A. Single-Cell Analysis in Immuno-Oncology. Int. J. Mol. Sci. 2023, 24, 8422. [Google Scholar] [CrossRef] [PubMed]
- Vafaizadeh, V.; Barekati, Z. Immuno-Oncology Biomarkers for Personalized Immunotherapy in Breast Cancer. Front. Cell Dev. Biol. 2020, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Ramamoorthi, G.; Jia, Y.; Faughn, J.; Wiener, D.; Awshah, S.; Kodumudi, K.; Czerniecki, B.J. Chapter Six—Immunotherapy in breast cancer: Current status and future directions. In Advances in Cancer Research; Wang, X.-Y., Fisher, P.B., Eds.; Immunotherapy of Cancer; Academic Press: Cambridge, MA, USA, 2019; Volume 143, pp. 295–349. [Google Scholar]
- Zhang, W.; Xu, K.; Li, Z.; Wang, L.; Chen, H. Tumor immune microenvironment components and the other markers can predict the efficacy of neoadjuvant chemotherapy for breast cancer. Clin. Transl. Oncol. 2023, 25, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Hammerl, D.; Smid, M.; Timmermans, A.M.; Sleijfer, S.; Martens, J.W.M.; Debets, R. Breast cancer genomics and immuno-oncological markers to guide immune therapies. Semin. Cancer Biol. 2018, 52, 178–188. [Google Scholar] [CrossRef] [PubMed]


| Section | Main Findings/Concepts |
|---|---|
| Introduction | Introduction to the importance of understanding the immune microenvironment in breast cancer. |
| Decoding the multifaceted roles of tumor-infiltrating lymphocytes (TILs) in breast cancer | TILs subgroups—distribution, density, and functional characteristics within the tumor—investigating the prognostic value of TILs in breast cancer. |
| The multifaceted roles of tumor-associated macrophages (TAMs) in Breast Cancer | The presence of TAMs in the breast cancer microenvironment—TAMs subtypes and their effect on tumor progression, invasion, and metastasis—therapeutic strategies targeting TAMs in breast cancer |
| Cytokine expression in breast cancer microenvironment | Discussion on the role of cytokines in the breast cancer microenvironment. |
| Immune evasion in breast cancer | Exploration of mechanisms used by breast cancer to evade the immune system. |
| The role of immune checkpoints in breast cancer | Discussion on the significance of immune checkpoints in breast cancer. |
| Other immunotherapy approaches for breast cancer patients | CAR-T cell therapy—therapeutic cancer vaccines—immune modulators—combination of therapies—novel therapies |
| Biomarkers and future directions | Exploration of potential biomarkers and future research directions in breast cancer immunotherapy. |
| Cell Population | Function | Therapeutic Targeting |
|---|---|---|
| TILs | Immune response against tumors | Immunotherapies |
| Stromal TILs (sTILs) | Organizing TME, cytokine production | Immune modulators |
| CD8+ T Cells | Cytotoxic activity | Checkpoint inhibitors |
| CD8+ TRM Cells | Tissue-resident memory T cells | Localized therapies |
| NK Cells | Anti-tumor cytotoxicity | NK cell-targeted therapies |
| CD4+ Th1/2/17 Cells | Helper T cell functions | Immunomodulators |
| CD4+ Tregs | Immunosuppression | Treg inhibitors |
| CD4+ Tfh T Cells | Follicular helper functions | Immune response modulators |
| Tumor-Infiltrating B Cells | Antibody production | B cell-targeted therapies |
| TAMs | Tumor growth, angiogenesis | TAM-targeted therapies |
| Number of Clinical Trial [References] | Targeted Therapy | Study Phase | Patient Cohort | Key Findings and Outcomes |
|---|---|---|---|---|
| NCT03726879 [194] | Combining standard of care (pertuzumab-trastuzumab [PH], chemotherapy) with cancer immunotherapy | Phase 3 | HER2+ BC | Did not increase pCR rates versus placebo in the ITT or PD-L1-positive populations. |
| NCT00433511 [195] | Effect of bevacizumab | Phase 3 | HER2- BC | Incorporation of bevacizumab into sequential anthracycline- and taxane-containing adjuvant therapy does not improve IDFS or overall survival. |
| NCT05910710 [N/A] | Neoadjuvant pembrolizumab | Phase 3 | TNBC | N/A |
| NCT01234337 [196] | CapecitabinE in combination with SorafenIb or placebo | Phase 3 | Advanced HER2- BC | Definitive PFS data for the combination of sorafenib and capecitabine in advanced HER2- BC and better characterize the benefit-to-risk profile. |
| NCT01234337 [197] | Capecitabine with sorafenib or placebo | Phase 3 | Advanced HER2- BC | The combination of sorafenib with capecitabine did not improve PFS, OS, or ORR in patients with HER2-negative advanced BC. |
| NCT05912062 [198] | BC treated with neoadjuvant HP and paclitaxel | Phase 3 | Early HER2+ | Not only offered different biological information but importantly served as a better predictor of pCR than baseline transcriptional analysis. |
| NCT00174655 [199] | Lymphocytic infiltration | Phase 3 | node-positive, ER-negative/HER2-negative BC | Increasing lymphocytic infiltration was associated with excellent prognosis. |
| NCT00004125/ NCT00003519 [200] | Stromal lymphocytic infiltration | Phase 3 | TNBC | Stromal lymphocytic infiltration constitutes a robust prognostic factor in TNBCs. |
| NCT00567554 [201] | BRCA1/2 Mutations and Bevacizumab | Phase 3 | TNBC | Bevacizumab may increase the pCR after standard neoadjuvant chemotherapy for patients with TNBC with BRCA1/2 mutations. |
| NCT00567554 [202] | Neoadjuvant Chemotherapy with Trastuzumab or Lapatinib | Phase 3 | HER2+ | Prolonged anti-HER2 treatment—neoadjuvant lapatinib for 6 months, followed by adjuvant trastuzumab for 12 months—significantly improved survival compared with anti-HER2 treatment with trastuzumab alone. |
| NCT00543127 [203] | Fulvestrant (Faslodex) + Anastrozole (Arimidex) vs. Anastrozole | Phase 3 | HER2+/HER2- | Statistically significant increase in DFS by adding adjuvant Fulvestrant to Anastrozole, though no firm conclusions can be drawn because of the limited sample size due to the early stop of the trial. |
| NCT03373708 [N/A] | Chemotherapy and Intensive Endocrine Therapy | Phase 2/3 | Luminal B1 | N/A |
| NCT03580395 [N/A] | Apatinib, paclitaxel, cisplatin | Phase 2/3 | TNBC, HER2+ or Luminal B | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsifaki, A.; Alevizopoulos, N.; Dimopoulou, V.; Armakolas, A. Unveiling the Immune Microenvironment’s Role in Breast Cancer: A Glimpse into Promising Frontiers. Int. J. Mol. Sci. 2023, 24, 15332. https://doi.org/10.3390/ijms242015332
Kotsifaki A, Alevizopoulos N, Dimopoulou V, Armakolas A. Unveiling the Immune Microenvironment’s Role in Breast Cancer: A Glimpse into Promising Frontiers. International Journal of Molecular Sciences. 2023; 24(20):15332. https://doi.org/10.3390/ijms242015332
Chicago/Turabian StyleKotsifaki, Amalia, Nektarios Alevizopoulos, Vassiliki Dimopoulou, and Athanasios Armakolas. 2023. "Unveiling the Immune Microenvironment’s Role in Breast Cancer: A Glimpse into Promising Frontiers" International Journal of Molecular Sciences 24, no. 20: 15332. https://doi.org/10.3390/ijms242015332
APA StyleKotsifaki, A., Alevizopoulos, N., Dimopoulou, V., & Armakolas, A. (2023). Unveiling the Immune Microenvironment’s Role in Breast Cancer: A Glimpse into Promising Frontiers. International Journal of Molecular Sciences, 24(20), 15332. https://doi.org/10.3390/ijms242015332






