Plant Life with and without Oxygen: A Metabolomics Approach
Abstract
:1. Introduction
2. Specificity of Plant Metabolomic Profiles during Oxygen Lack
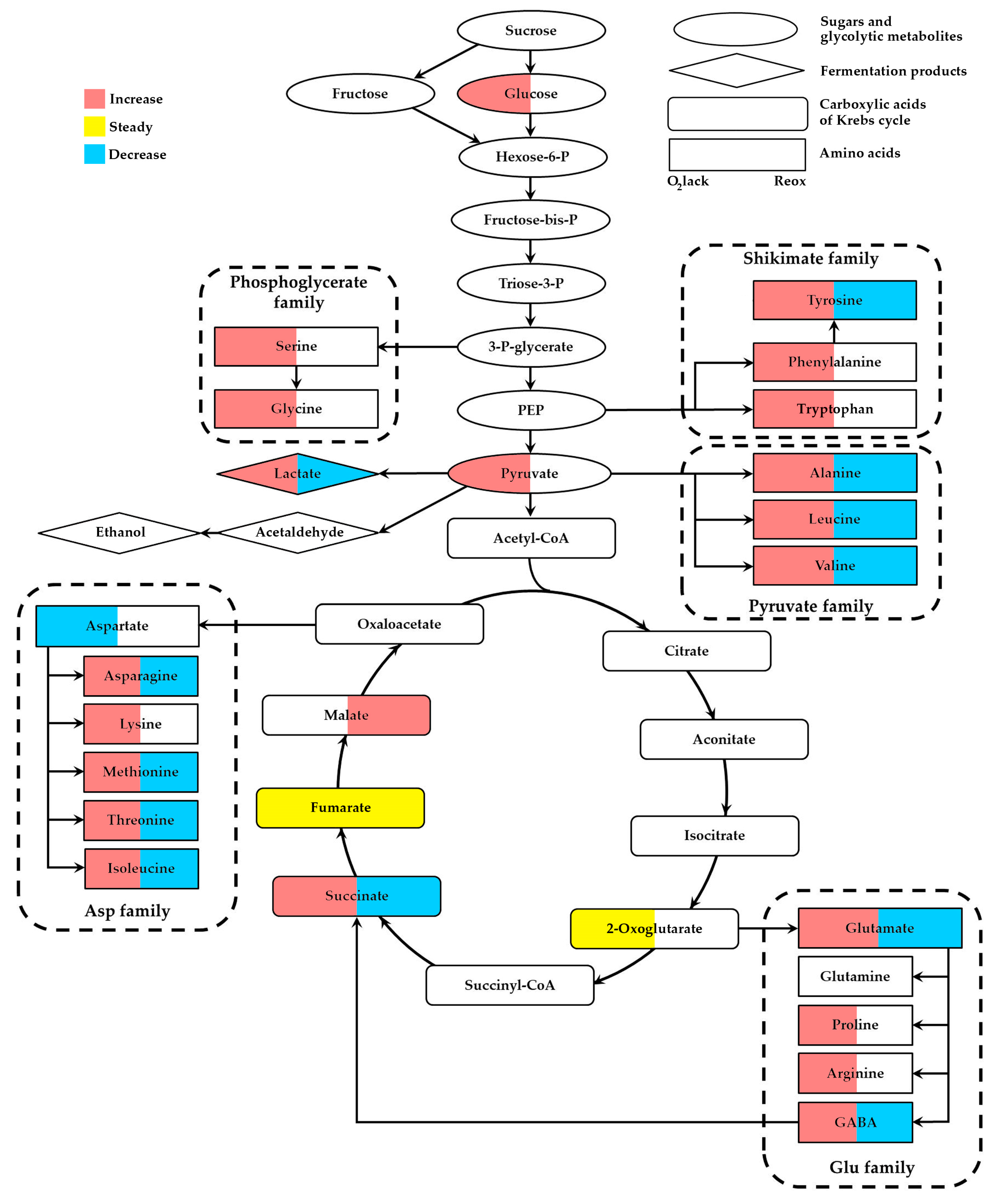
2.1. The Level of Glucose, Fructose and Other Carbohydrates
2.2. Intermediates of Glycolysis
2.3. Metabolites of Fermentation
2.4. Metabolites of the Krebs Cycle
2.5. Amino Acid Metabolism
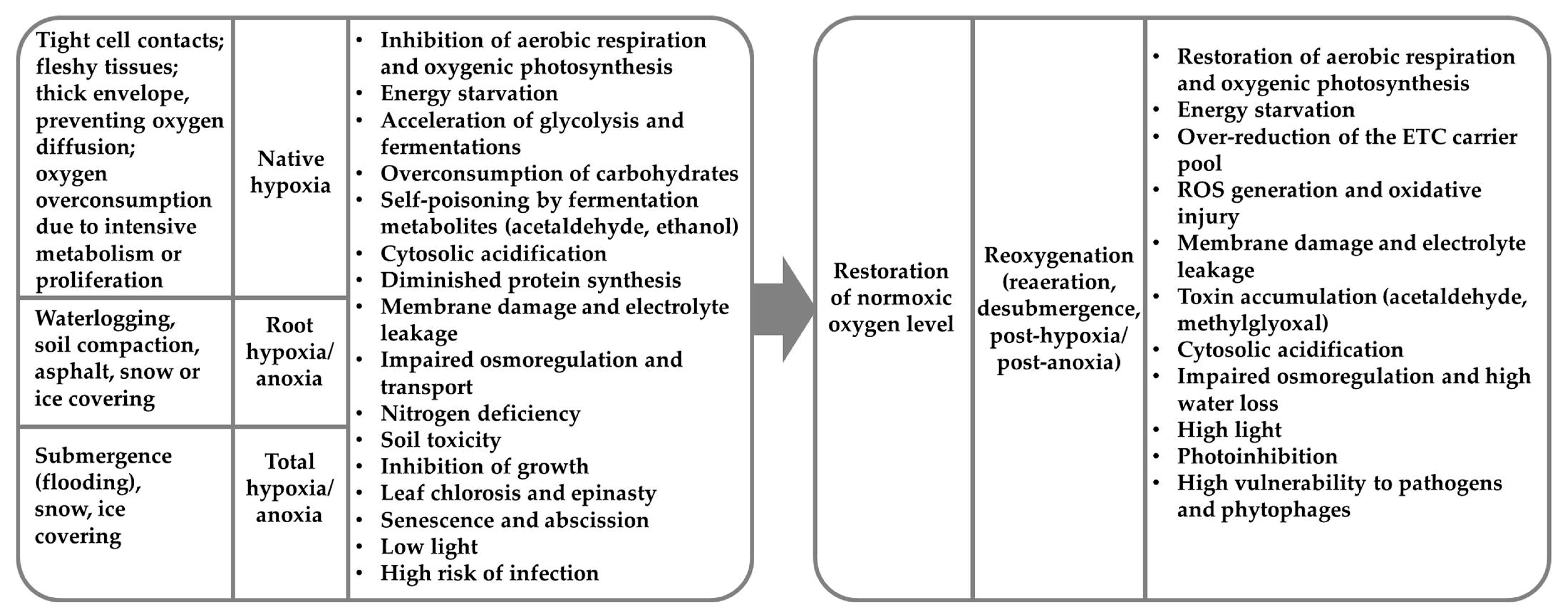
3. Native Hypoxia and Metabolomics
4. Plant Metabolomic Profiles during Reoxygenation
5. Oxidative Stress Metabolomics
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Chirkova, T.; Yemelyanov, V. The study of plant adaptation to oxygen deficiency in Saint Petersburg University. Biol. Commun. 2018, 63, 17–31. [Google Scholar] [CrossRef]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and waterlogging stress in plants: A review highlighting research opportunities and understudied aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.S.; Dolferus, R.; Ellis, M.; Rahman, M.; Wu, Y.; Hoeren, F.U.; Grover, A.; Ismond, K.P.; Good, A.G.; Peacock, W.J. Molecular strategies for improving waterlogging tolerance in plants. J. Exp. Bot. 2000, 51, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Vartapetian, B.B.; Jackson, M.B. Plant adaptations to anaerobic stress. Ann. Bot. 1997, 79, 3–20. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Tamang, B.G.; Fukao, T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180. [Google Scholar] [CrossRef]
- Shikov, A.E.; Chirkova, T.V.; Yemelyanov, V.V. Post-anoxia in plants: Reasons, consequences, and possible mechanisms. Russ. J. Plant Physiol. 2020, 67, 45–59. [Google Scholar] [CrossRef]
- Loreti, E.; Perata, P. The many facets of hypoxia in plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef]
- Weits, D.A.; van Dongen, J.; Francesco, L. Molecular oxygen as a signaling component in plant development. New Phytol. 2021, 229, 24–35. [Google Scholar] [CrossRef]
- Yeung, E.; Bailey-Serres, J.; Sasidharan, R. After the deluge: Plant revival post-flooding. Trends Plant Sci. 2019, 24, 443–454. [Google Scholar] [CrossRef]
- Leon, J.; Castillo, M.C.; Gayubas, B. The hypoxia–reoxygenation stress in plants. J. Exp. Bot. 2021, 72, 5841–5856. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Shishova, M.F. The role of phytohormones in the control of plant adaptation to oxygen depletion. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N.A., Nazar, R., Iqbal, N., Anjum, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 229–248. [Google Scholar] [CrossRef]
- Pedersen, O.; Rich, S.M.; Colmer, T.D. Surviving floods: Leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. Plant J. 2009, 58, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Mommer, L.; Visser, E.J.W. Underwater photosynthesis in flooded terrestrial plants: A matter of leaf plasticity. Ann. Bot. 2005, 96, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Colmer, T.D.; Sand-Jensen, K. Underwater photosynthesis of submerged plants―Recent advances and methods. Front. Plant Sci. 2013, 4, 140. [Google Scholar] [CrossRef]
- Winkel, A.; Colmer, T.D.; Ismail, A.M.; Pedersen, O. Internal aeration of paddy field rice (Oryza sativa) during complete submergence—importance of light and floodwater O2. New Phytol. 2013, 197, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Kende, H.; van der Knaap, E.; Cho, H.-T. Deepwater rice: A model plant to study stem elongation. Plant Physiol. 1998, 118, 1105–1110. [Google Scholar] [CrossRef]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A.C.J. The role of ethylene in metabolic acclimations to low oxygen. New Phytol. 2021, 229, 64–70. [Google Scholar] [CrossRef]
- van Veen, H.; Akman, M.; Jamar, D.C.L.; Vreugdenhil, D.; Kooiker, M.; van Tienderen, P.; Voesenek, L.A.C.J.; Schranz, M.E.; Sasidharan, R. Group VII Ethylene Response Factor diversification and regulation in four species from flood-prone environments. Plant Cell Environ. 2014, 37, 2421–2432. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.C.J.; van Dongen, J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378. [Google Scholar] [CrossRef]
- Tweeddale, H.; Notley-McRobb, L.; Ferenci, T. Effect of slow growth on metabolism of Escherichia coli, as revealed by global metabolite pool (“Metabolome”) analysis. J. Bacteriol. 1998, 180, 5109–5116. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fu, X. Reprogramming of plant central metabolism in response to abiotic stresses: A metabolomics view. Int. J. Mol. Sci. 2022, 23, 5716. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.T.; Frohlich, A.; Ramirez-Aguilar, S.J.; Schauer, N.; Fernie, A.R.; Erban, A.; Kopka, J.; Clark, J.; Langer, A.; Geigenberger, P. Transcript and metabolite profiling of the adaptive response to mild decreases in oxygen concentration in the roots of arabidopsis plants. Ann. Bot. 2009, 103, 269–280. [Google Scholar] [CrossRef]
- Rocha, M.; Licausi, F.; Araujo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicas. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef]
- Barding, G.A.; Fukao, T.; Beni, S.; Bailey-Serres, J.; Larive, C.K. Differential metabolic regulation governed by the rice SUB1A gene during submergence stress and identification of alanylglycine by 1H NMR spectroscopy. J. Proteome Res. 2012, 11, 320–330. [Google Scholar] [CrossRef]
- Fukushima, A.; Kuroha, T.; Nagai, K.; Hattori, Y.; Kobayashi, M.; Nishizawa, T.; Kojima, M.; Utsumi, Y.; Oikawa, A.; Seki, M.; et al. Metabolite and phytohormone profiling illustrates metabolic reprogramming as an escape strategy of deepwater rice during partially submerged stress. Metabolites 2020, 10, 68. [Google Scholar] [CrossRef]
- António, C.; Päpke, C.; Rocha, M.; Diab, H.; Limami, A.M.; Obata, T.; Fernie, A.R.; van Dongen, J.T. Regulation of primary metabolism in response to low oxygen availability as revealed by carbon and nitrogen isotope redistribution. Plant Physiol. 2016, 170, 43–56. [Google Scholar] [CrossRef]
- Coutinho, I.D.; Henning, L.M.M.; Döpp, S.A.; Nepomuceno, A.; Moraes, A.C.; Marcolino-Gomes, J.; Richter, C.; Schwalbe, H.; Colnago, L.A. Flooded soybean metabolomics analysis reveals important primary and secondary metabolites involved in the hypoxia stress response and tolerance. Environ. Exp. Bot. 2018, 153, 176–187. [Google Scholar] [CrossRef]
- Herzog, M.; Fukao, T.; Winkel, A.; Konnerup, D.; Lamichhane, S.; Alpuerto, J.B.; Hasler-Sheetal, H.; Pedersen, O. Physiology, gene expression, and metabolome of two wheat cultivars with contrasting submergence tolerance. Plant Cell Environ. 2018, 41, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shingaki-Wells, R.N.; Petereit, J.; Alexova, R.; Harvey Millar, A. Temperature-dependent metabolic adaptation of Triticum aestivum seedlings to anoxia. Sci. Rep. 2018, 8, 6151. [Google Scholar] [CrossRef] [PubMed]
- Hasler-Sheetal, H.; Fragner, L.; Holmer, M.; Weckwerth, W. Diurnal effects of anoxia on the metabolome of the seagrass Zostera marina. Metabolomics 2015, 11, 1208–1218. [Google Scholar] [CrossRef]
- Parveen, M.; Miyagi, A.; Kawai-Yamada, M.; Rashid, M.H.; Asaeda, T. Metabolic and biochemical responses of Potamogeton anguillanus Koidz. (Potamogetonaceae) to low oxygen conditions. J. Plant Physiol. 2019, 232, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Puzanskiy, R.K.; Smirnov, P.D.; Vanisov, S.A.; Dubrovskiy, M.D.; Shavarda, A.L.; Shishova, M.F.; Yemelyanov, V.V. Metabolite profiling of leaves of three Epilobium species. Ecol. Genet. 2022, 20, 279–293. [Google Scholar] [CrossRef]
- Jethva, J.; Schmidt, R.R.; Sauter, M.; Selinski, J. Try or die: Dynamics of plant respiration and how to survive low oxygen conditions. Plants 2022, 11, 205. [Google Scholar] [CrossRef]
- Mustroph, A.; Barding, G.A., Jr.; Kaiser, K.A.; Larive, C.K.; Bailey-Serres, J. Characterization of distinct root and shoot responses to low-oxygen stress in Arabidopsis with a focus on primary C- and N-metabolism. Plant Cell Environ. 2014, 37, 2366–2380. [Google Scholar] [CrossRef]
- Paul, M.V.; Iyer, S.; Amerhauser, C.; Lehmann, M.; van Dongen, J.T.; Geigenberger, P. Oxygen sensing via the ethylene response transcription factor RAP2.12 affects plant metabolism and performance under both normoxia and hypoxia. Plant Physiol. 2016, 172, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-J.; Lin, C.-Y.; Ting, C.-Y.; Shih, M.-C. Ethylene-regulated glutamate dehydrogenase fine-tunes metabolism during anoxia-reoxygenation. Plant Physiol. 2016, 172, 1548–1562. [Google Scholar] [CrossRef]
- Savchenko, T.; Rolletschek, H.; Heinzel, N.; Tikhonov, K.; Dehesh, K. Waterlogging tolerance rendered by oxylipin-mediated metabolic reprogramming in Arabidopsis. J. Exp. Bot. 2019, 70, 2919–2932. [Google Scholar] [CrossRef]
- Shingaki-Wells, R.N.; Huang, S.; Taylor, N.L.; Carroll, A.J.; Zhou, W.; Harvey Millar, A. Differential molecular responses of rice and wheat coleoptiles to anoxia reveal novel metabolic adaptations in amino acid metabolism for tissue tolerance. Plant Physiol. 2011, 156, 1706–1724. [Google Scholar] [CrossRef]
- Locke, A.M.; Barding, G.A., Jr.; Sathnur, S.; Larive, C.K.; Bailey-Serres, J. Rice SUB1A constrains remodelling of the transcriptome and metabolome during submergence to facilitate postsubmergence recovery. Plant Cell Environ. 2018, 41, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Y.; Gui, R.; Wang, Z.; Li, Z.; Han, Y.; Guo, X.; Sun, J. Comparative multi-omics analysis of hypoxic germination tolerance in weedy rice embryos and coleoptiles. Genomics 2021, 113, 3337–3348. [Google Scholar] [CrossRef]
- Behr, J.H.; Bouchereau, A.; Berardocco, S.; Seal, C.E.; Flowers, T.J.; Zorb, C. Metabolic and physiological adjustment of Suaeda maritima to combined salinity and hypoxia. Ann. Bot. 2017, 119, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.H.; Bednarz, H.; Godde, V.; Niehaus, K.; Zorb, C. Metabolic responses of sugar beet to the combined effect of root hypoxia and NaCl-salinity. J. Plant Physiol. 2021, 267, 153545. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Hauberg, J.; Howell, K.A.; Carroll, A.; Rennenberg, H.; Harvey Millar, A.; Whelan, J. Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiol. 2009, 149, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Duan, S.-G.; Xia, Y.; Li, J.-T.; Liu, L.-X.; Tang, M.; Tang, J.; Sun, W.; Yi, Y. Transcriptomic, physiological, and metabolomic response of an alpine plant, Rhododendron delavayi, to waterlogging stress and post-waterlogging recovery. Int. J. Mol. Sci. 2023, 24, 10509. [Google Scholar] [CrossRef]
- Sanclemente, M.-A.; Ma, F.; Liu, P.; Della Porta, A.; Singh, J.; Wu, S.; Colquhoun, T.; Johnson, T.; Guan, J.-C.; Koch, K.E. Sugar modulation of anaerobic-response networks in maize root tips. Plant Physiol. 2021, 185, 295–317. [Google Scholar] [CrossRef]
- Lothier, J.; Diab, H.; Cukier, C.; Limami, A.M.; Tcherkez, G. Metabolic responses to waterlogging differ between roots and shoots and reflect phloem transport alteration in Medicago truncatula. Plants 2020, 9, 1373. [Google Scholar] [CrossRef]
- Abadie, C.; Blanchet, S.; Carroll, A.; Tcherkez, G. Metabolomics analysis of postphotosynthetic effects of gaseous O2 on primary metabolism in illuminated leaves. Funct. Plant Biol. 2017, 44, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Andrzejczak, O.A.; Havelund, J.F.; Wang, W.-Q.; Kovalchuk, S.; Hagensen, C.E.; Hasler-Sheetal, H.; Jensen, O.N.; Rogowska-Wrzesinska, A.; Møller, I.M.; Hebelstrup, K.H. The hypoxic proteome and metabolome of barley (Hordeum vulgare L.) with and without phytoglobin priming. Int. J. Mol. Sci. 2020, 21, 1546. [Google Scholar] [CrossRef] [PubMed]
- Alpuerto, J.B.; Hussain, R.M.F.; Fukao, T. The key regulator of submergence tolerance, SUB1A, promotes photosynthetic and metabolic recovery from submergence damage in rice leaves. Plant Cell Environ. 2016, 39, 672–684. [Google Scholar] [CrossRef]
- Considine, M.J.; Diaz-Vivancos, P.; Kerchev, P.; Signorelli, S.; Agudelo-Romero, P.; Gibbs, D.J.; Foyer, C.H. Learning to breathe: Developmental phase transitions in oxygen status. Trends Plant Sci. 2017, 22, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.A.; Rubio, M.; Dolle, M.E.; Campisi, J.; Vijg, J. Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell 2003, 2, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Lombardi, L.; Iacopino, S.; Pencik, A.; Novak, O.; Perata, P.; Giuntoli, B.; Licausi, F. Endogenous hypoxia in lateral root primordia controls root architecture by antagonizing auxin signaling in Arabidopsis. Mol. Plant 2019, 12, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Weits, D.A.; Kunkowska, A.B.; Kamps, N.C.W.; Portz, K.M.S.; Packbier, N.K.; Nemec Venza, Z.; Gaillochet, C.; Lohmann, J.U.; Pedersen, O.; van Dongen, J.T.; et al. An apical hypoxic niche sets the pace of shoot meristem activity. Nature 2019, 569, 714–717. [Google Scholar] [CrossRef]
- Meitha, K.; Agudelo-Romero, P.; Signorelli, S.; Gibbs, D.J.; Considine, J.A.; Foyer, C.H.; Considine, M.J. Developmental control of hypoxia during bud burst in grapevine. Plant Cell Environ. 2018, 41, 1154–1170. [Google Scholar] [CrossRef]
- Kerpen, L.; Niccolini, L.; Licausi, F.; van Dongen, J.T.; Weits, D.A. Hypoxic conditions in crown galls induce plant anaerobic responses that support tumor proliferation. Front. Plant Sci. 2019, 10, 56. [Google Scholar] [CrossRef]
- Valeri, M.C.; Novi, G.; Weits, D.A.; Mensuali, A.; Perata, P.; Loreti, E. Botrytis cinerea induces local hypoxia in Arabidopsis leaves. New Phytol. 2021, 229, 173–185. [Google Scholar] [CrossRef]
- Gong, X.; Zhao, Y.; Cai, S.; Fu, S.; Yang, C.; Zhang, S.; Zhang, X. Single cell analysis with probe ESI-mass spectrometry: Detection of metabolites at cellular and subcellular levels. Anal. Chem. 2014, 86, 3809–3816. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wang, M. Design, optimization and application of small molecule biosensor in metabolic engineering. Front. Microbiol. 2017, 8, 2012. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Fang, X.; Tran, S.; Zhai, N.; Yang, Z.; Guo, F.; Chen, L.; Yu, J.; Ison, M.S.; et al. Transcriptional landscapes of de novo root regeneration from detached Arabidopsis leaves revealed by time-lapse and single-cell RNA sequencing analyses. Plant Commun. 2022, 3, 100306. [Google Scholar] [CrossRef]
- Borisjuk, L.; Rolletschek, H. The oxygen status of the developing seed. New Phytol. 2009, 182, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Gorzolka, K.; Kölling, J.; Nattkemper, T.W.; Niehaus, K. Spatio-temporal metabolite profiling of the barley germination process by MALDI MS imaging. PLoS ONE 2016, 11, e0150208. [Google Scholar] [CrossRef]
- Kazmi, R.H.; Willems, L.A.J.; Joosen, R.V.L.; Khan, N.; Ligterink, W.; Hilhorst, H.W.M. Metabolomic analysis of tomato seed germination. Metabolomics 2017, 13, 145. [Google Scholar] [CrossRef]
- Feenstra, A.D.; Alexander, L.E.; Song, Z.H.; Korte, A.R.; Yandeau-Nelson, M.D.; Nikolau, B.J.; Lee, Y.J. Spatial mapping and profiling of metabolite distributions during germination. Plant Physiol. 2017, 174, 2532–2548. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lyv, Y.; Zheng, W.; Yang, C.; Li, Y.; Wang, X.; Chen, R.; Wang, C.; Luo, J.; Qu, L. Comparative metabolomics reveals two metabolic modules affecting seed germination in rice (Oryza sativa). Metabolites 2021, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Jayawardhane, J.; Wijesinghe, M.K.P.S.; Bykova, N.V.; Igamberdiev, A.U. Metabolic changes in seed embryos of hypoxia-tolerant rice and hypoxia-sensitive barley at the onset of germination. Plants 2021, 10, 2456. [Google Scholar] [CrossRef]
- Narsai, R.; Howell, K.A.; Carroll, A.; Ivanova, A.; Millar, A.H.; Whelan, J. Defining core metabolic and transcriptomic responses to oxygen availability in rice embryos and young seedlings. Plant Physiol. 2009, 151, 306–322. [Google Scholar] [CrossRef]
- Cukrov, D. Progress toward understanding the molecular basis of fruit response to hypoxia. Plants 2018, 7, 78. [Google Scholar] [CrossRef]
- Biais, B.; Beauvoit, B.; Allwood, J.W.; Deborde, C.; Maucourt, M.; Goodacre, R.; Rolin, D.; Moing, A. Metabolic acclimation to hypoxia revealed by metabolite gradients in melon fruit. J. Plant Physiol. 2010, 167, 242–245. [Google Scholar] [CrossRef]
- Moing, A.; Aharoni, A.; Biais, B.; Rogachev, I.; Meir, S.; Brodsky, L.; Allwood, J.W.; Erban, A.; Dunn, W.B.; Kay, L.; et al. Extensive metabolic cross-talk in melon fruit revealed by spatial and developmental combinatorial metabolomics. New Phytol. 2011, 190, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Beauvoit, B.P.; Biais, B.; Chabane, M.; Allwood, J.W.; Deborde, C.; Maucourt, M.; Goodacre, R.; Cabasson, C.; Moing, A.; et al. Central metabolism is tuned to the availability of oxygen in developing melon fruit. Front. Plant Sci. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Yu, O.; Tang, J.; Gu, X.; Wan, X.; Fang, C. Metabolic profiling of strawberry (Fragaria×ananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2011, 62, 1103–1118. [Google Scholar] [CrossRef] [PubMed]
- Haugeneder, A.; Trinkl, J.; Härtl, K.; Hoffmann, T.; Allwood, J.W.; Schwab, W. Answering biological questions by analysis of the strawberry metabolome. Metabolomics 2018, 14, 145. [Google Scholar] [CrossRef]
- Kuroki, S.; Oshita, S.; Sotome, I.; Kawagoe, Y.; Seo, Y. Visualization of 3-D network of gas-filled intercellular spaces in cucumber fruit after harvest. Postharvest Biol. Technol. 2004, 33, 255–262. [Google Scholar] [CrossRef]
- Biais, B.; Bénard, C.; Beauvoit, B.; Colombie, S.; Prodhomme, D.; Ménard, G.; Bernillon, S.; Gehl, B.; Gautier, H.; Ballias, P.; et al. Remarkable reproducibility of enzyme activity profiles in tomato fruits grown under contrasting environments provides a roadmap for studies of fruit metabolism. Plant Physiol. 2014, 164, 1204–1221. [Google Scholar] [CrossRef]
- Colombie, S.; Nazaret, C.; Benard, C.; Biais, B.; Mengin, V.; Sole, M.; Fouillen, L.; Dieuaide-Noubhani, M.; Mazat, J.-P.; Beauvoit, B.; et al. Modelling central metabolic fluxes by constraint-based optimization reveals metabolic reprogramming of developing Solanum lycopersicum (tomato) fruit. Plant J. 2015, 81, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.T.; Verboven, P.; Mebatsion, H.K.; Verlinden, B.E.; Vandewalle, S.; Nicolaï, B.M. Microscale mechanisms of gas exchange in fruit tissue. New Phytol. 2009, 182, 163–174. [Google Scholar] [CrossRef]
- Ho, Q.T.; Verboven, P.; Verlinden, B.E.; Herremans, E.; Wevers, M.; Carmeliet, J.; Nicolaï, B.M. A three-dimensional multiscale model for gas exchange in fruit. Plant Physiol. 2011, 155, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yan, J.; Li, W.; Wang, Q.; Wang, C.; Guo, J.; Geng, D.; Guan, Q.; Ma, F. Integrative analyses of widely targeted metabolic profiling and transcriptome data reveals molecular insight into metabolomic variations during apple (Malus domestica) fruit development and ripening. Int. J. Mol. Sci. 2020, 21, 4797. [Google Scholar] [CrossRef] [PubMed]
- Cukrov, D.; Zermiani, M.; Brizzolara, S.; Cestaro, A.; Licausi, F.; Luchinat, C.; Santucci, C.; Tenori, L.; Van Veen, H.; Zuccolo, A.; et al. Extreme hypoxic conditions induce selective molecular responses and metabolic reset in detached apple fruit. Front. Plant Sci. 2016, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Brizzolara, S.; Cukrov, D.; Mercadini, M.; Martinelli, F.; Ruperti, B.; Tonutti, P. Short-term responses of apple fruit to partial reoxygenation during extreme hypoxic storage conditions. J. Agric. Food Chem. 2019, 67, 4754–4763. [Google Scholar] [CrossRef] [PubMed]
- Terzoudis, K.; Hertog, M.L.A.T.M.; Nicolaï, B.M. Dynamic labelling reveals central carbon metabolism responses to stepwise decreasing hypoxia and reoxygenation during postharvest in pear fruit. Postharvest Biol. Technol. 2022, 186, 111816. [Google Scholar] [CrossRef]
- Fu, X.; Xu, Y. Dynamic metabolic changes in arabidopsis seedlings under hypoxia stress and subsequent reoxygenation recovery. Stresses 2023, 3, 86–101. [Google Scholar] [CrossRef]
- Yuan, L.-B.; Chen, M.-X.; Wang, L.-N.; Sasidharan, R.; Voesenek, L.A.C.J.; Xiao, S. Multi-stress resilience in plants recovering from submergence. Plant Biotechnol. J. 2023, 21, 466–481. [Google Scholar] [CrossRef]
- Blokhina, O.B.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Lastochkin, V.V.; Prikaziuk, E.G.; Chirkova, T.V. Activities of catalase and peroxidase in wheat and rice plants under conditions of anoxia and post-anoxic aeration. Russ. J. Plant Physiol. 2022, 69, 117. [Google Scholar] [CrossRef]
- Shikov, A.E.; Lastochkin, V.V.; Chirkova, T.V.; Mukhina, Z.M.; Yemelyanov, V.V. Post-anoxic oxidative injury is more severe than oxidative stress induced by chemical agents in wheat and rice plants. Acta Physiol. Plant. 2022, 44, 90. [Google Scholar] [CrossRef]
- Rankenberg, T.; van Veen, H.; Sedaghatmehr, M.; Liao, C.-Y.; Devaiah, M.B.; Balazadeh, S.; Sasidharan, R. Differential leaf flooding resilience in Arabidopsis thaliana is controlled by age-dependent ORESARA1 activity. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gautam, P.; Lal, B.; Raja, R.; Baig, M.J.; Haldar, D.; Rath, L.; Shahid, M.; Tripathi, R.; Mohanty, S.; Bhattacharyya, P.; et al. Post-flood nitrogen and basal phosphorus management affects survival, metabolic changes and anti-oxidant enzyme activities of submerged rice (Oryza sativa). Funct. Plant Biol. 2014, 41, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Setter, T.L.; Bhekasut, P.; Greenway, H. Desiccation of leaves after de-submergence is one cause for intolerance to complete submergence of the rice cultivar IR 42. Funct. Plant Biol. 2010, 37, 1096–1104. [Google Scholar] [CrossRef]
- van Eck, W.H.J.M.; Lenssen, J.P.M.; Rengelink, R.H.J.; Blom, C.W.P.M.; De Kroon, H. Water temperature instead of acclimation stage and oxygen concentration determines responses to winter floods. Aquat. Bot. 2005, 81, 253–264. [Google Scholar] [CrossRef]
- Panda, D.; Sharma, S.G.; Sarkar, R.K. Chlorophyll fluorescence parameters, CO2 photosynthetic rate and regeneration capacity as a result of complete submergence and subsequent re-emergence in rice (Oryza sativa L.). Aquat. Bot. 2008, 88, 127–133. [Google Scholar] [CrossRef]
- Qin, X.; Li, F.; Chen, X.; Xie, Y. Growth responses and non-structural carbohydrates in three wetland macrophyte species following submergence and de-submergence. Acta Physiol. Plant. 2013, 35, 2069–2074. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, R.; Xiao, W.; Guo, Q.; Wang, Y.; Wang, N.; Wang, Y. Leaf gas exchange, chlorophyll fluorescence, non-structural carbohydrate content and growth responses of Distylium chinense during complete submergence and subaerial re-emergence. Aquat. Bot. 2015, 124, 70–77. [Google Scholar] [CrossRef]
- de Sousa, C.A.F.; Sodek, L. Alanine metabolism and alanine aminotransferase activity in soybean (Glycine max) during hypoxia of the root system and subsequent return to normoxia. Environ. Exp. Bot. 2003, 50, 1–8. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.E.; Chirkova, T.V.; Yemelyanov, V.V. Functions of reactive oxygen species in plant cells under normal conditions and during adaptation. Ecol. Genet. 2021, 19, 343–363. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Bedhomme, M.; Groni, H.; Marchand, C.H.; Puppo, C.; Gontero, B.; Cassier-Chauvat, C.; Decottignies, P.; Lemaire, S.D. Glutathionylation in the photosynthetic model organism Chlamydomonas reinhardtii: A proteomic survey. Mol. Cell. Proteom. 2012, 11, M111.014142. [Google Scholar] [CrossRef]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Fermani, S.; Costa, A.; Lemaire, S.D.; Trost, P. Plant cytoplasmic GAPDH: Redox post-translational modifications and moonlighting properties. Front. Plant Sci. 2013, 4, 450. [Google Scholar] [CrossRef]
- Skryhan, K.; Gurrieri, L.; Sparla, F.; Trost, P.; Blennow, A. Redox regulation of starch metabolism. Front. Plant Sci. 2018, 9, 1344. [Google Scholar] [CrossRef]
- Dumont, S.; Rivoal, J. Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front. Plant Sci. 2019, 10, 166. [Google Scholar] [CrossRef]
- Chaouch, S.; Queval, G.; Vanderauwera, S.; Mhamdi, A.; Vandorpe, M.; Langlois-Meurinne, M.; Van Breusegem, F.; Saindrenan, P.; Noctor, G. Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength-related manner. Plant Physiol. 2010, 153, 1692–1705. [Google Scholar] [CrossRef]
- Chaouch, S.; Queval, G.; Noctor, G. AtRbohF is a crucial modulator of defense associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 2012, 69, 613–627. [Google Scholar] [CrossRef]
- Han, Y.; Chaouch, S.; Mhamdi, A.; Queval, G.; Zechmann, B.; Noctor, G. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The metabolomics of oxidative stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef]
- Chen, Y.; Hoehenwarter, W. Changes in the phosphoproteome and metabolome link early signaling events to rearrangement of photosynthesis and central metabolism in salinity and oxidative stress response in Arabidopsis. Plant Physiol. 2015, 169, 3021–3033. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Araújo, G.; de Oliveira Paula-Marinho, S.; de Paiva Pinheiro, S.K.; de Castro Miguel, E.; de Sousa Lopes, L.; Marques, E.C.; de Carvalho, H.H.; Gomes-Filho, E. H2O2 priming promotes salt tolerance in maize by protecting chloroplasts ultrastructure and primary metabolites modulation. Plant Sci. 2021, 303, 110774. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.A.; Balogh, E.; Ahres, M.; Szalai, G.; Gondor, O.K.; Darkó, E.; Borbély, P.; Kulman, K.; Mednyánszky, Z.; Simon-Sarkadi, L.; et al. Ascorbate and hydrogen peroxide modify metabolite profile of wheat differently. J. Plant Growth Regul. 2023, 42, 6155–6170. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wang, T.; Li, C. Metabolic response of soybean leaves induced by short-term exposure of ozone. Ecotoxicol. Environ. Saf. 2021, 213, 112033. [Google Scholar] [CrossRef]
- Cho, K.; Shibato, J.; Agrawal, G.K.; Jung, Y.-H.; Kubo, A.; Jwa, N.-S.; Tamogami, S.; Satoh, K.; Kikuchi, S.; Higashi, T.; et al. Integrated transcriptomics, proteomics, and metabolomics analyses to survey ozone responses in the leaves of rice seedling. J. Proteome Res. 2008, 7, 2980–2998. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takahara, K.; Hirabayashi, T.; Matsumura, H.; Fujisawa, S.; Terauchi, R.; Uchimiya, H.; Kawai-Yamada, M. Metabolome analysis of response to oxidative stress in rice suspension cells overexpressing cell death suppressor Bax Inhibitor-1. Plant Cell Physiol. 2010, 51, 9–20. [Google Scholar] [CrossRef]
- Sipari, N.; Lihavainen, J.; Shapiguzov, A.; Kangasjärvi, J.; Keinänen, M. Primary metabolite responses to oxidative stress in early-senescing and paraquat resistant Arabidopsis thaliana rcd1 (Radical-Induced Cell Death1). Front. Plant Sci. 2020, 11, 194. [Google Scholar] [CrossRef]
- Savchenko, T.; Tikhonov, K. Oxidative stress-induced alteration of plant central metabolism. Life 2021, 11, 304. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.-H.; Szczepski, K.; Al-Younis, I.; Lachowicz, J.I.; Jaremko, M. Fluxomics–New metabolomics approaches to monitor metabolic pathways. Front. Pharmacol. 2022, 13, 805782. [Google Scholar] [CrossRef] [PubMed]
- de Falco, B.; Giannino, F.; Carteni, F.; Mazzoleni, S.; Kim, D.-H. Metabolic flux analysis: A comprehensive review on sample preparation, analytical techniques, data analysis, computational modelling, and main application areas. RSC Adv. 2022, 12, 25528–25548. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yemelyanov, V.V.; Puzanskiy, R.K.; Shishova, M.F. Plant Life with and without Oxygen: A Metabolomics Approach. Int. J. Mol. Sci. 2023, 24, 16222. https://doi.org/10.3390/ijms242216222
Yemelyanov VV, Puzanskiy RK, Shishova MF. Plant Life with and without Oxygen: A Metabolomics Approach. International Journal of Molecular Sciences. 2023; 24(22):16222. https://doi.org/10.3390/ijms242216222
Chicago/Turabian StyleYemelyanov, Vladislav V., Roman K. Puzanskiy, and Maria F. Shishova. 2023. "Plant Life with and without Oxygen: A Metabolomics Approach" International Journal of Molecular Sciences 24, no. 22: 16222. https://doi.org/10.3390/ijms242216222




