Src Tyrosine Kinase Inhibitory and Antioxidant Activity of Black Chokeberry and Bilberry Fruit Extracts Rich in Chlorogenic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Content of Polyphenols in Black Chokeberry and Bilberry Fruit Extracts
2.2. Content of Phenolic Acids and Flavonoids in Black Chokeberry and Bilberry Fruit Extracts
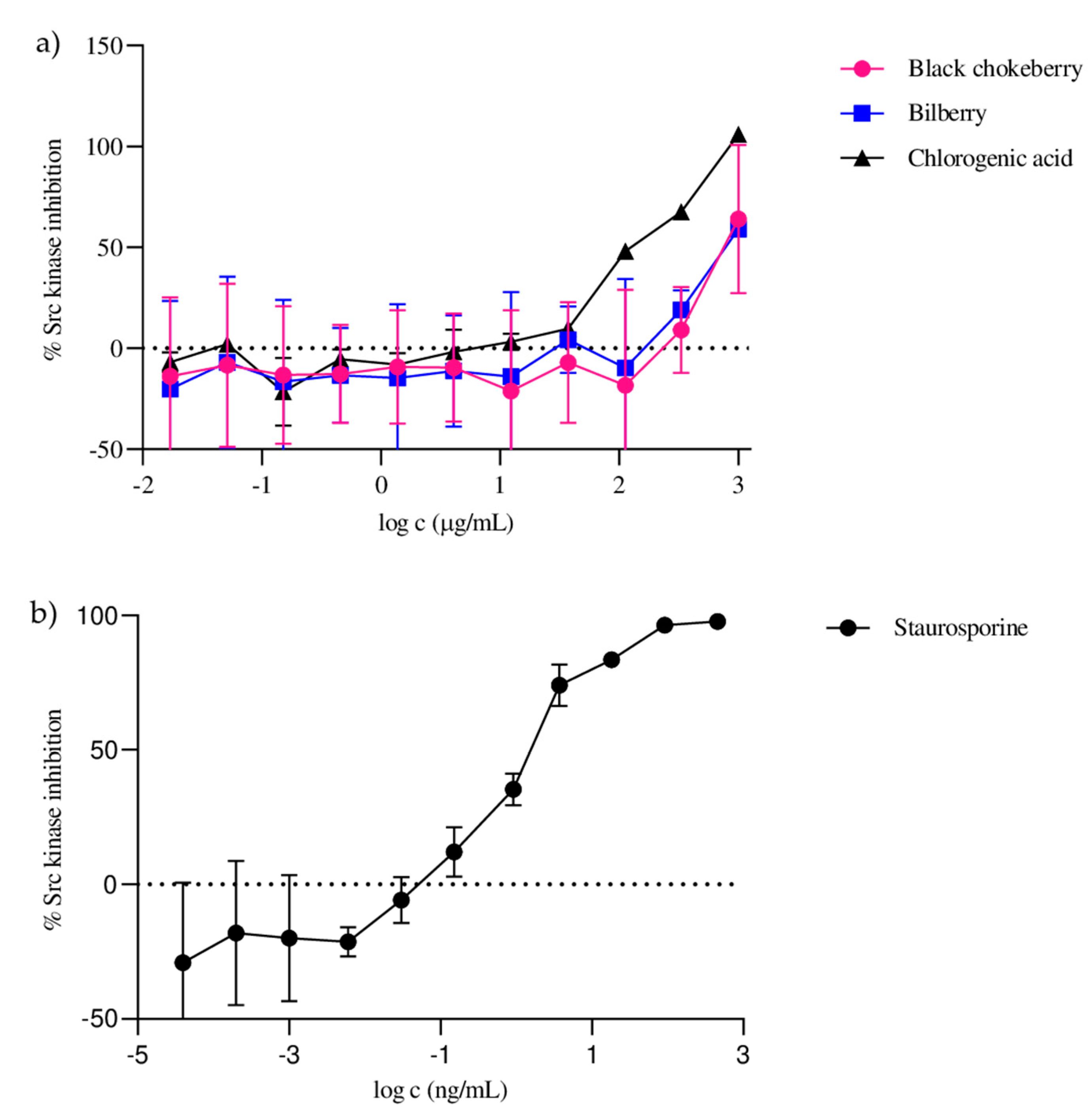
2.3. Src Tyrosine Kinase Inhibitory Activity of Black Chokeberry and Bilberry Fruit Extracts
2.4. Antioxidant Activity of Black Chokeberry and Bilberry Fruit Extracts
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Extract Preparation
3.3. TLC Analysis
3.4. Total Polyphenols Determination
3.5. Determination of Hydroxycinnamic Acid Derivatives
3.6. Total Anthocyanins Determination
3.7. Total Flavonoids Determination
3.8. Determination of Phenolic Acids and Flavonoids by High-Performance Liquid Chromatography
3.9. Src Tyrosine Kinase Inhibition Assay
3.10. DPPH Radical-Scavenging Assay
3.11. NO Radical-Scavenging Assay
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Grupcheva, C.; Galunska, B. Comparative Phytochemical Analysis of Aronia melanocarpa L. Fruit Juices on Bulgarian Market. Plants 2022, 11, 1655. [Google Scholar] [CrossRef]
- Banach, M.; Wiloch, M.; Zawada, K.; Cyplik, W.; Kujawski, W. Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts. Molecules 2020, 25, 4055. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-W.; Kim, J.-E.; Nam, Y.-E.; Kim, W.-S.; Lee, I.; Yim, S.-V.; Kwon, O. Eight-Week Supplementation of Aronia Berry Extract Promoted the Glutathione Defence System against Acute Aerobic Exercise-Induced Oxidative Load Immediately and 30 Min Post-Exercise in Healthy Adults: A Double-Blind, Randomised Controlled Trial. J. Hum. Nutr. Diet. 2023, 36, 1589–1599. [Google Scholar] [CrossRef]
- Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Parfenov, A.; Sharonova, N.; Nikitin, E.; Zobov, V. Radical Scavenging Actions and Immunomodulatory Activity of Aronia melanocarpa Propylene Glycol Extracts. Plants 2021, 10, 2458. [Google Scholar] [CrossRef]
- Gill, N.K.; Rios, D.; Osorio-Camacena, E.; Mojica, B.E.; Kaur, B.; Soderstrom, M.A.; Gonzalez, M.; Plaat, B.; Poblete, C.; Kaur, N.; et al. Anticancer Effects of Extracts from Three Different Chokeberry Species. Nutr. Cancer 2021, 73, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Kozuka, M.; Konda, D.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Improvement of Blood Glucose Levels and Obesity in Mice given Aronia Juice by Inhibition of Dipeptidyl Peptidase IV and α-Glucosidase. J. Nutr. Biochem. 2016, 31, 106–112. [Google Scholar] [CrossRef]
- Qin, B.; Anderson, R.A. An Extract of Chokeberry Attenuates Weight Gain and Modulates Insulin, Adipogenic and Inflammatory Signaling Pathways in Epididymal Adipose Tissue of Rats Fed a Fructose-Rich Diet. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhao, X.; Liu, S.; Liu, Y.; Wang, D. Aronia melanocarpa Prevents Alcohol-Induced Chronic Liver Injury via Regulation of Nrf2 Signaling in C57BL/6 Mice. Oxid. Med. Cell Longev. 2020, 2020, e4054520. [Google Scholar] [CrossRef]
- Lee, H.Y.; Weon, J.B.; Jung, Y.S.; Kim, N.Y.; Kim, M.K.; Ma, C.J. Cognitive-Enhancing Effect of Aronia melanocarpa Extract against Memory Impairment Induced by Scopolamine in Mice. Evid. Based Complement. Alternat. Med. 2016, 2016, e6145926. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Herbal Medicinal Products Committee HMPC. European Union Herbal Monograph on Vaccinium myrtillus L., Fructus Siccus. EMA/HMPC/678995/2013. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-vaccinium-myrtillus-l-fructus-siccus_en.pdf (accessed on 7 July 2023).
- European Medicines Agency. Herbal Medicinal Products Committee HMPC. European Union Herbal Monograph on Vaccinium myrtillus L., Fructus Recens. EMA/HMPC/375808/2014. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-vaccinium-myrtillus-l-fructus-recens_en.pdf (accessed on 7 July 2023).
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A Comparison of Fruit Quality Parameters of Wild Bilberry (Vaccinium myrtillus L.) Growing at Different Locations. J. Sci. Food Agric. 2015, 95, 776–785. [Google Scholar] [CrossRef]
- Ciulca, S.; Roma, G.; Alexa, E.; Radulov, I.; Cocan, I.; Madosa, E.; Ciulca, A. Variation of Polyphenol Content and Antioxidant Activity in Some Bilberry (Vaccinium myrtillus L.) Populations from Romania. Agronomy 2021, 11, 2557. [Google Scholar] [CrossRef]
- Dare, A.P.; Günther, C.S.; Grey, A.C.; Guo, G.; Demarais, N.J.; Cordiner, S.; McGhie, T.K.; Boldingh, H.; Hunt, M.; Deng, C.; et al. Resolving the developmental distribution patterns of polyphenols and related primary metabolites in bilberry (Vaccinium myrtillus) fruit. Food Chem. 2022, 374, 131703. [Google Scholar] [CrossRef]
- Vaneková, Z.; Rollinger, J.M. Bilberries: Curative and Miraculous—A Review on Bioactive Constituents and Clinical Research. Front. Pharmacol. 2022, 13, 909914. [Google Scholar] [CrossRef]
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic Profiles and Antioxidant and Antiradical Activity of Italian Berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. Subsp. Gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa Fruits as a Rich Dietary Source of Chlorogenic Acids and Anthocyanins: 1H-NMR, HPLC-DAD, and Chemometric Studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Restivo, I.; Basilicata, M.G.; Giardina, I.C.; Massaro, A.; Pepe, G.; Salviati, E.; Pecoraro, C.; Carbone, D.; Cascioferro, S.; Parrino, B.; et al. A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation. Antioxidants 2023, 12, 1621. [Google Scholar] [CrossRef] [PubMed]
- Stote, K.S.; Burns, G.; Mears, K.; Sweeney, M.; Blanton, C. The Effect of Berry Consumption on Oxidative Stress Biomarkers: A Systematic Review of Randomized Controlled Trials in Humans. Antioxidants 2023, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Burton, R.A.; Wu, H.; Lipchik, A.M.; Craddock, B.P.; Mo, H.; Parker, L.L.; Miller, W.T.; Post, C.B. Substrate Binding to Src: A New Perspective on Tyrosine Kinase Substrate Recognition from NMR and Molecular Dynamics. Protein Sci. 2020, 29, 350–359. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The Crucial Role of Protein Phosphorylation in Cell Signaling and Its Use as Targeted Therapy. Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Rivera-Torres, J.; San José, E. Src Tyrosine Kinase Inhibitors: New Perspectives on Their Immune, Antiviral, and Senotherapeutic Potential. Front. Pharmacol. 2019, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Clementi, L.; Sabetta, S.; Mattei, V.; Botta, L.; Angelucci, A. Src Family Kinases as Therapeutic Targets in Advanced Solid Tumors: What We Have Learned So Far. Cancers 2020, 12, 1448. [Google Scholar] [CrossRef] [PubMed]
- Van Der Steen, N.; Giovannetti, E.; Carbone, D.; Leonetti, A.; Rolfo, C.D.; Peters, G.J. Resistance to epidermal growth factor receptor inhibition in non-small cell lung cancer. Cancer Drug Resist. 2018, 1, 230–249. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Brian, B.F.; Freedman, T.S. The Src-family Kinase Lyn in Immunoreceptor Signaling. Endocrinology 2021, 162, bqab152. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, D.; Zhang, L.; Wei, W. Src Family Protein Kinase Controls the Fate of B Cells in Autoimmune Diseases. Inflammation 2021, 44, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Szilveszter, K.P.; Németh, T.; Mócsai, A. Tyrosine Kinases in Autoimmune and Inflammatory Skin Diseases. Front. Immunol. 2019, 10, 1862. [Google Scholar] [CrossRef]
- Ayala-Aguilera, C.C.; Valero, T.; Lorente-Macías, A.; Baillache, D.J.; Croke, S.; Unciti-Broceta, A. Small Molecule Kinase Inhibitor Drugs (1995–2021): Medical Indication, Pharmacology, and Synthesis. J. Med. Chem. 2022, 65, 1047–1131. [Google Scholar] [CrossRef]
- Pecoraro, C.; Carbone, D.; Cascioferro, S.M.; Parrino, B.; Diana, P. Multi or Single-Kinase Inhibitors to Counteract Drug Resistance in Cancer: What is New? Curr. Med. Chem. 2023, 30, 776–782. [Google Scholar] [CrossRef]
- Nishal, S.; Jhawat, V.; Gupta, S.; Phaugat, P. Utilization of kinase inhibitors as novel therapeutic drug targets: A review. Oncol. Res. 2022, 30, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Vignolini, P.; Ieri, F.; Heimler, D. Polyphenols and Volatile Compounds in Commercial Chokeberry (Aronia melanocarpa) Products. Nat. Prod. Commun. 2016, 11, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Tolić, M.T.; Landeka Jurčević, I.; Panjkota Krbavčić, I.; Marković, K.; Vahčić, N. Phenolic Content, Antioxidant Capacity and Quality of Chokeberry (Aronia melanocarpa) Products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, M.A.; Perova, I.B.; Eller, K.I.; Akimov, M.Y.; Sukhanova, A.M.; Rodionova, G.M.; Ramenskaya, G.V. Investigation of Polyphenolic Compounds in Different Varieties of Black Chokeberry Aronia melanocarpa. Molecules 2023, 28, 4101. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 288. [Google Scholar] [CrossRef]
- Georgescu, C.; Frum, A.; Virchea, L.-I.; Sumacheva, A.; Shamtsyan, M.; Gligor, F.-G.; Olah, N.K.; Mathe, E.; Mironescu, M. Geographicf Variability of Berry Phytochemicals with Antioxidant and Antimicrobial Properties. Molecules 2022, 27, 4986. [Google Scholar] [CrossRef] [PubMed]
- Brasanac-Vukanovic, S.; Mutic, J.; Stankovic, D.M.; Arsic, I.; Blagojevic, N.; Vukasinovic-Pesic, V.; Tadic, V.M. Wild Bilberry (Vaccinium myrtillus L., Ericaceae) from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef] [PubMed]
- Veljković, M.; Pavlović, D.R.; Stojiljković, N.; Ilić, S.; Jovanović, I.; Poklar Ulrih, N.; Rakić, V.; Veličković, L.; Sokolović, D. Bilberry: Chemical Profiling, In Vitro and In Vivo Antioxidant Activity and Nephroprotective Effect against Gentamicin Toxicity in Rats. Phytother. Res. 2017, 31, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, M. Black Chokeberry (Aronia melanocarpa) Polyphenols Reveal Different Antioxidant, Antimicrobial and Neutrophil-Modulating Activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules 2022, 27, 4375. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. White versus blue: Does the wild ‘albino’ bilberry (Vaccinium myrtillus L.) differ in fruit quality compared to the blue one? Food Chem. 2016, 211, 876–882. [Google Scholar] [CrossRef]
- Simatou, A.; Simatos, S.; Goulielmaki, M.; Spandidos, D.A.; Baliou, S.; Zoumpourlis, V. Historical Retrospective of the SRC Oncogene and New Perspectives (Review). Mol. Clin. Oncol. 2020, 13, 21. [Google Scholar] [CrossRef]
- Pelaz, S.G.; Tabernero, A. Src: Coordinating Metabolism in Cancer. Oncogene 2022, 41, 4917–4928. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, J.; Cheng, Q. Proto-Oncogene Tyrosine-Protein Kinase SRC (Src) Inhibition in Microglia Relieves Neuroinflammation in Neuropathic Pain Mouse Models. Bioengineered 2021, 12, 11390–11398. [Google Scholar] [CrossRef]
- Byeon, S.E.; Yi, Y.-S.; Oh, J.; Yoo, B.C.; Hong, S.; Cho, J.Y. The Role of Src Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm. 2012, 2012, 512926. [Google Scholar] [CrossRef]
- Baier, A.; Szyszka, R. Compounds from Natural Sources as Protein Kinase Inhibitors. Biomolecules 2020, 10, 1546. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-Rich Extract from Aronia meloncarpa E. Induces a Cell Cycle Block in Colon Cancer but not Normal Colonic Cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Bermùdez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espín, J.C.; Tomás-Barberan, F.A.; García-Conesa, M.T. Upregulation of Tumor Suppressor Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 in Human Colon Cancer Caco-2 cells Following Repetitive Exposure to Dietary Levels of a Polyphenol-Rich Chokeberry Juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Deng, H.-J.; Yun, B.-S.; Lee, D.-S. Triterpene Acid ( 3-O-p-Coumaroyltormentic Acid) Isolated from Aronia Extracts Inhibits Breast Cancer Stem Cell Formation through Downregulation of c-Myc Protein. Int. J. Mol. Sci. 2018, 19, 2528. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yu, W.; Hao, R.; Fan, J.; Gao, J. Anthocyanins from Aronia melanocarpa Induce Apoptosis in Caco-2 Cells through Wnt/β-Catenin Signaling Pathway. Chem. Biodivers. 2020, 17, e2000654. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Del Bubba, M.; Di Serio, C.; Renai, L.; Scordo, C.V.A.; Checchini, L.; Ungar, A.; Tarantini, F.; Bartoletti, R. Vaccinium myrtillus L. Extract and its Native Polyphenol-Recombined Mixture Have Anti-Proliferative and Pro-Apoptotic Effects on Human Prostate Cancer Cell Lines. Phytother. Res. 2021, 35, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Mauramo, M.; Onali, T.; Wahbi, W.; Vasara, J.; Lampinen, A.; Mauramo, E.; Kivimäki, A.; Martens, S.; Häggman, H.; Sutinen, M.; et al. Bilberry (Vaccinium myrtillus L.) Powder Has Anticarcinogenic Effects on Oral Carcinoma In Vitro and In Vivo. Antioxidants 2021, 10, 1319. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry Anthocyanidins Synergistically Suppress Growth and Invasive Potential of Human Non-Small-Cell Lung Cancer Cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Alhosin, M.; León-González, A.J.; Dandache, I.; Lelay, A.; Rashid, S.K.; Kevers, C.; Pincemail, J.; Fornecker, L.-M.; Mauvieux, L.; Herbrecht, R.; et al. Bilberry Extract (Antho 50) Selectively Induces Redox-Sensitive Caspase 3-related Apoptosis in Chronic Lymphocytic Leukemia Cells by Targeting the Bcl-2/Bad Pathway. Sci. Rep. 2015, 11, 5:8996. [Google Scholar] [CrossRef]
- Nguyen, V.; Tang, J.; Oroudjev, E.; Lee, C.J.; Marasigan, C.; Wilson, L.; Ayoub, G. Cytotoxic Effects of Bilberry Extract on MCF7-GFP-Tubulin Breast Cancer Cells. J. Med. Food 2010, 13, 278–285. [Google Scholar] [CrossRef]
- Onali, T.; Kivimäki, A.; Mauramo, M.; Salo, T.; Korpela, R. Anticancer Effects of Lingonberry and Bilberry on Digestive Tract Cancers. Antioxidants 2021, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic Inflammation: Key Player and Biomarker-Set to Predict and Prevent Cancer Development and Progression Based on Individualized Patient Profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.T.; Pham, H.; Pandol, S.J.; Ptasznik, A. Src as the Link between Inflammation and Cancer. Front. Physiol. 2014, 4, 416. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.; Carle, R.; Muñoz, E. Chokeberry (Aronia melanocarpa (Michx.) Elliot) Concentrate Inhibits NF-κB and Synergizes with Selenium to Inhibit the Release of Pro-Inflammatory Mediators in Macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, H.J. Anti-Inflammatory Activity of Bilberry (Vaccinium myrtillus L.). Curr. Issues Mol. Biol. 2022, 44, 4570–4583. [Google Scholar] [CrossRef]
- Xue, H.; Wei, M.; Ji, L. Chlorogenic Acids: A Pharmacological Systematic Review on Their Hepatoprotective Effects. Phytomedicine 2023, 118, 154961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, F.; Chen, J.; Zhang, S.; Wang, H. Chlorogenic Acid Inhibits Human Glioma U373 Cell Progression via Regulating the SRC/MAPKs Signal Pathway: Based on Network Pharmacology Analysis. Drug Des. Devel Ther. 2021, 15, 1369–1383. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.; Biswas, T.; Roy, K.C.; Mandal, S.; Mandal, C.; Pal, B.C.; Bhattacharya, S.; Rakshit, S.; Bhattacharya, D.K.; Chaudhuri, U.; et al. Chlorogenic Acid Inhibits Bcr-Abl Tyrosine Kinase and Triggers p38 Mitogen-Activated Protein Kinase-Dependent Apoptosis in Chronic Myelogenous Leukemic Cells. Blood 2004, 104, 2514–2522. [Google Scholar] [CrossRef]
- Socodato, R.; Portugal, C.C.; Canedo, T.; Domith, I.; Oliveira, N.A.; Paes-de-Carvalho, R.; Relvas, J.B.; Cossenza, M. c-Src Deactivation by the Polyphenol 3-O-Caffeoylquinic Acid Abrogates Reactive Oxygen Species-Mediated Glutamate Release from Microglia and Neuronal Excitotoxicity. Free Radic. Biol. Med. 2015, 79, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Li, S.; Kang, X.; Deng, J.; Yang, H.; Chen, F.; Jiang, J.; Zhang, J.; Li, W. 1 Phase I Study of Chlorogenic Acid Injection for Recurrent High-Grade Glioma with Long-Term Follow-up. Cancer Biol. Med. 2023, 20, 465–476. [Google Scholar] [CrossRef]
- Kindl, M.; Blažeković, B.; Bucar, F.; Vladimir-Knežević, S. Antioxidant and Anticholinesterase Potential of Six Thymus Species. Evid. Based Complement. Alternat Med. 2015, 2015, 403950. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Kolarov, R.; Peić Tukuljac, M.; Kolbas, A.; Kolbas, N.; Barać, G.; Ognjanov, V.; Ljubojević, M.; Prvulović, D. Antioxidant Capacity of Wild-Growing Bilberry, Elderberry, and Strawberry Fruits. Acta Hort. Regiotec. 2021, 24, 119–126. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Chun, E.M.; Mijan, M.A.; Park, S.H.; Moon, S.-K.; Lim, B.O. Anthocyanins Profiling of Bilberry (Vaccinium myrtillus L.) Extract that Elucidates Antioxidant and Anti-Inflammatory Effects. Food Agric. Immunol. 2021, 32, 713–726. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S.; Zgainski, E.M. Drogenanalyse, 2nd ed.; Springer: Berlin, Germany, 2009; pp. 195–197. [Google Scholar]
- Feng, S.; Zhang, C.; Liu, L.; Xu, Z.; Chen, T.; Zhou, L.; Yuan, M.; Li, T.; Ding, C. Comparison of Phenolic Compounds in Olive Leaves by Different Drying and Storage Methods. Separations 2021, 8, 156. [Google Scholar] [CrossRef]
- European Pharmacopoeia Online. Available online: https://pheur.edqm.eu/home (accessed on 2 May 2023).
- Ye, L.; Zhou, S.; Liu, L.; Liu, L.; Waters, D.L.E.; Zhong, K.; Zhou, X.; Ma, X.; Liu, X. Phenolic Compounds and Antioxidant Capacity of Brown Rice in China. Int. J. Food Eng. 2016, 12, 537–546. [Google Scholar] [CrossRef]
- Jelić, D.; Lower-Nedza, A.D.; Brantner, A.H.; Blažeković, B.; Bian, B.; Yang, J.; Brajša, K.; Vladimir-Knežević, S. Baicalin and Baicalein Inhibit Src Tyrosine Kinase and Production of IL-6. J. Chem. 2016, 2016, 2510621. [Google Scholar] [CrossRef]
- Harput, U.S.; Genç, Y.; Khan, N.; Saracoglu, İ. Radical Scavenging Effects of Different Veronica Species. Rec. Nat. Prod. 2011, 5, 100–107. [Google Scholar]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant Potential, Total Phenolic and Total Flavonoid Contents of Rhododendron anthopogonoides and its Protective Effect on Hypoxia-induced Injury in PC12 cells. BMC Complement. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef] [PubMed]
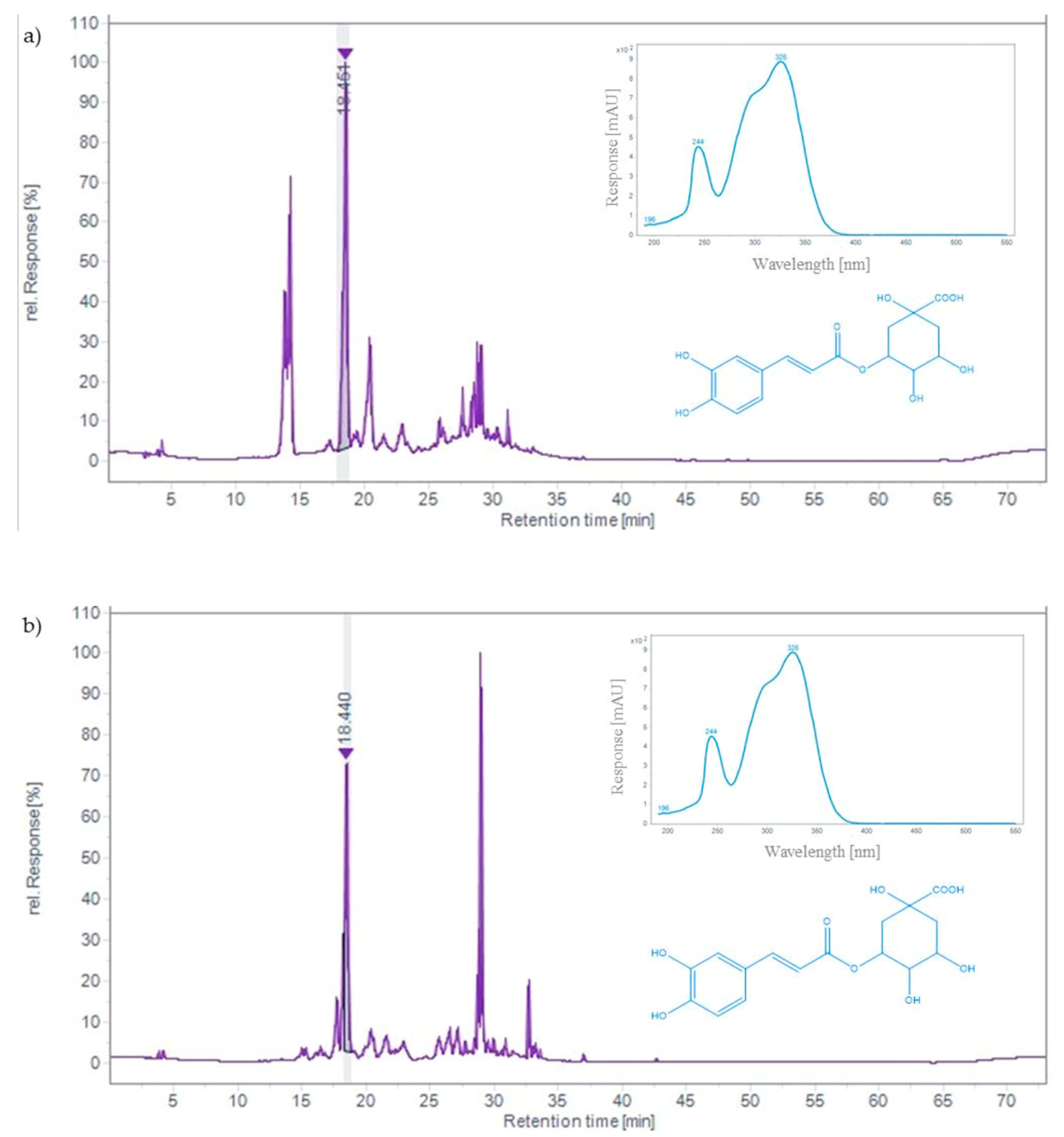
| Sample | Polyphenols | Hydroxycinnamic Acids | Anthocyanins | Flavonoids |
|---|---|---|---|---|
| Black chokeberry | 5.90 ± 0.18 a | 4.09 ± 0.13 a | 0.41 ± 0.02 b | 0.17 ± 0.003 a |
| Bilberry | 4.96 ± 0.09 b | 1.51 ± 0.22 b | 1.06 ± 0.01 a | 0.12 ± 0.002 b |
| Compound | Rt (min) | Black Chokeberry | Bilberry |
|---|---|---|---|
| Neochlorogenic acid | 14.12 | 2.07 ± 0.13 | n.d. |
| Chlorogenic acid | 18.45 | 2.05 ± 0.00 a | 2.54 ± 0.02 b |
| Rutin | 27.58 | 0.34 ± 0.01 | n.d. |
| Hyperoside | 28.42 | 0.50 ± 0.03 a | 0.26 ± 0.01 b |
| Isoquercitrin | 28.71 | 0.67 ± 0.01 a | 1.36 ± 0.01 b |
| Sample | 6.25 µg/mL | 12.5 µg/mL | 25 µg/mL | 50 µg/mL | 100 µg/mL | 200 µg/mL | 400 µg/mL | 800 µg/mL |
|---|---|---|---|---|---|---|---|---|
| Black chokeberry | 8.43 ± 1.93 b | 15.56 ± 1.44 b | 19.44 ± 8.08 b | 32.98 ± 2.78 b | 47.73 ± 2.13 b | 55.12 ± 4.40 b | 75.72 ± 3.51 b | 91.85 ± 3.11 a |
| Bilberry | 6.76 ± 1.14 b | 9.09 ± 1.82 c | 13.80 ± 1.70 b | 20.76 ± 2.90 c | 35.38 ± 2.07 c | 43.11 ± 0.02 c | 57.24 ± 1.53 c | 84.84 ± 0.71 b |
| Chlorogenic acid | 20.87 ± 2.18 a | 36.65 ± 1.36 a | 65.52 ± 1.37 a | 77.66 ± 3.97 a | 91.01 ± 3.98 a | 95.03 ± 0.93 a | 95.24 ± 1.18 a | 95.62 ± 1.53 a |
| Sample | 6.25 µg/mL | 12.5 µg/mL | 25 µg/mL | 50 µg/mL | 100 µg/mL | 200 µg/mL | 400 µg/mL | 800 µg/mL |
|---|---|---|---|---|---|---|---|---|
| Black chokeberry | 15.64 ± 0.23 b | 22.95 ± 5.52 b | 25.36 ± 2.90 b | 32.53 ± 1.45 b | 39.12 ± 0.60 b | 56.82 ± 2.73 b | 80.98 ± 2.60 a | 94.28 ± 1.38 a |
| Bilberry | 2.12 ± 0.26 c | 4.44 ± 3.02 c | 7.39 ± 5.08 c | 10.37 ± 4.03 c | 20.58 ± 1.29 c | 34.14 ± 3.31 c | 55.02 ± 2.83 b | 62.78 ± 0.48 c |
| Chlorogenic acid | 24.94 ± 1.61 a | 37.78 ± 2.99 a | 57.39 ± 0.50 a | 64.88 ± 1.42 a | 68.65 ± 1.01 a | 73.57 ± 1.90 a | 77.44 ± 0.61 a | 78.16 ± 1.72 b |
| Sample | DPPH• Scavenging Activity | NO• Scavenging Activity |
|---|---|---|
| Black chokeberry | 153.3 ± 13.2 b | 161.1 ± 11.4 b |
| Bilberry | 298.8 ± 16.2 a | 352.2 ± 26.1 a |
| Chlorogenic acid | 18.4 ± 0.4 c | 20.4 ± 0.3 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladimir-Knežević, S.; Bival Štefan, M.; Blažeković, B.; Jelić, D.; Petković, T.; Mandić, M.; Šprajc, E.; Lovković, S. Src Tyrosine Kinase Inhibitory and Antioxidant Activity of Black Chokeberry and Bilberry Fruit Extracts Rich in Chlorogenic Acid. Int. J. Mol. Sci. 2023, 24, 15512. https://doi.org/10.3390/ijms242115512
Vladimir-Knežević S, Bival Štefan M, Blažeković B, Jelić D, Petković T, Mandić M, Šprajc E, Lovković S. Src Tyrosine Kinase Inhibitory and Antioxidant Activity of Black Chokeberry and Bilberry Fruit Extracts Rich in Chlorogenic Acid. International Journal of Molecular Sciences. 2023; 24(21):15512. https://doi.org/10.3390/ijms242115512
Chicago/Turabian StyleVladimir-Knežević, Sanda, Maja Bival Štefan, Biljana Blažeković, Dubravko Jelić, Tea Petković, Marta Mandić, Ekaterina Šprajc, and Sandy Lovković. 2023. "Src Tyrosine Kinase Inhibitory and Antioxidant Activity of Black Chokeberry and Bilberry Fruit Extracts Rich in Chlorogenic Acid" International Journal of Molecular Sciences 24, no. 21: 15512. https://doi.org/10.3390/ijms242115512
APA StyleVladimir-Knežević, S., Bival Štefan, M., Blažeković, B., Jelić, D., Petković, T., Mandić, M., Šprajc, E., & Lovković, S. (2023). Src Tyrosine Kinase Inhibitory and Antioxidant Activity of Black Chokeberry and Bilberry Fruit Extracts Rich in Chlorogenic Acid. International Journal of Molecular Sciences, 24(21), 15512. https://doi.org/10.3390/ijms242115512







