Spectral Physics of Stable Cu(III) Produced by Oxidative Addition of an Alkyl Halide
Abstract
1. Introduction
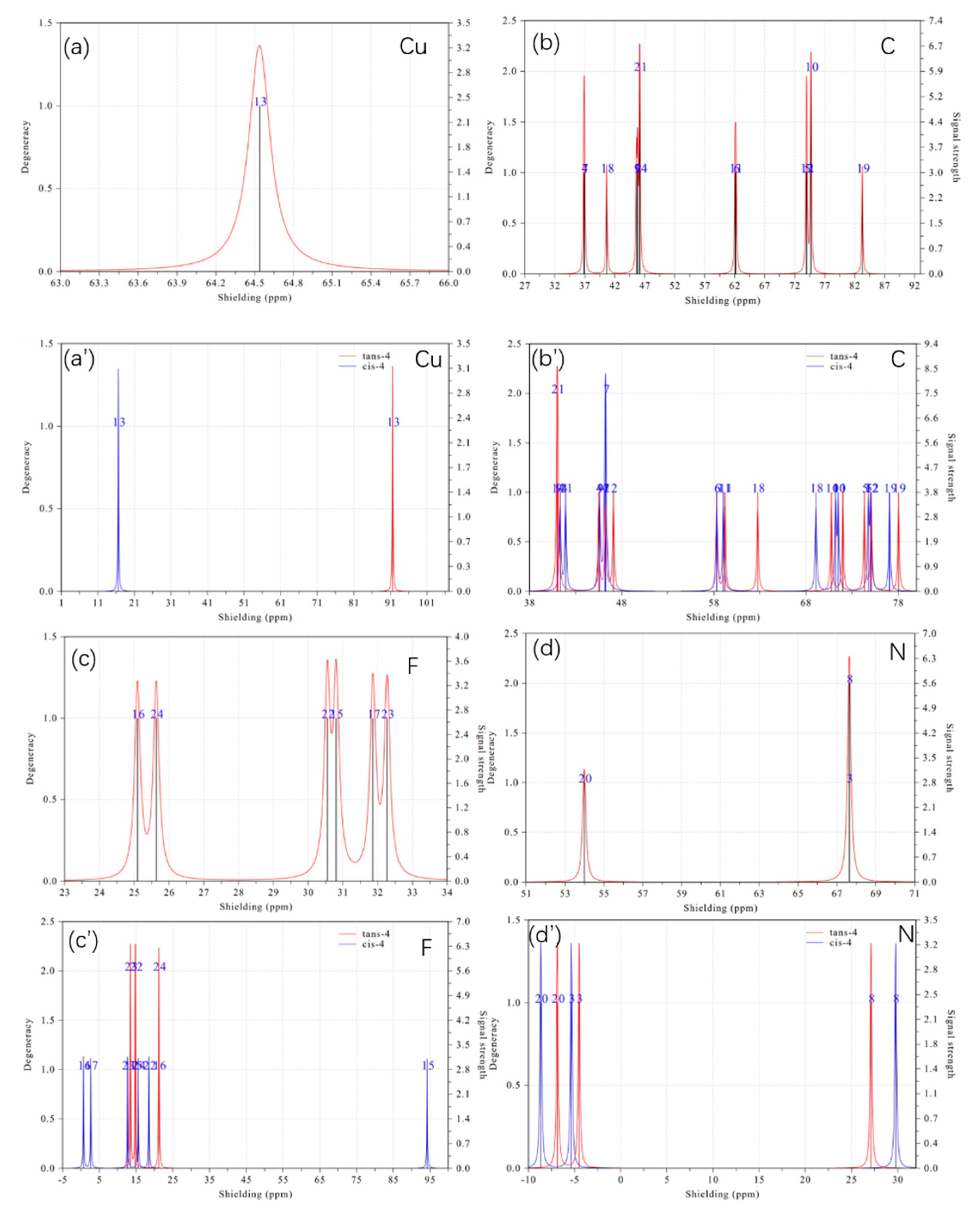
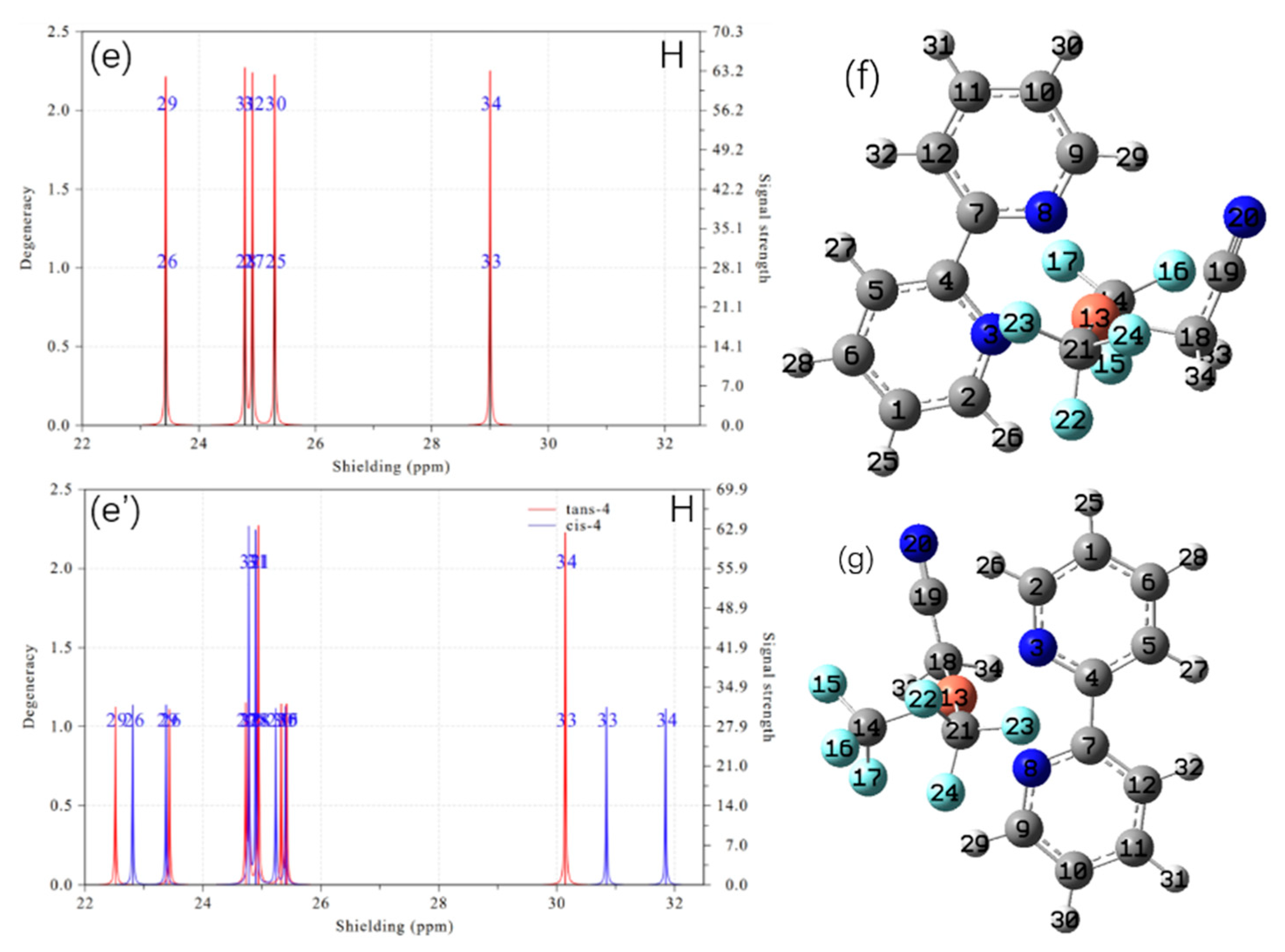
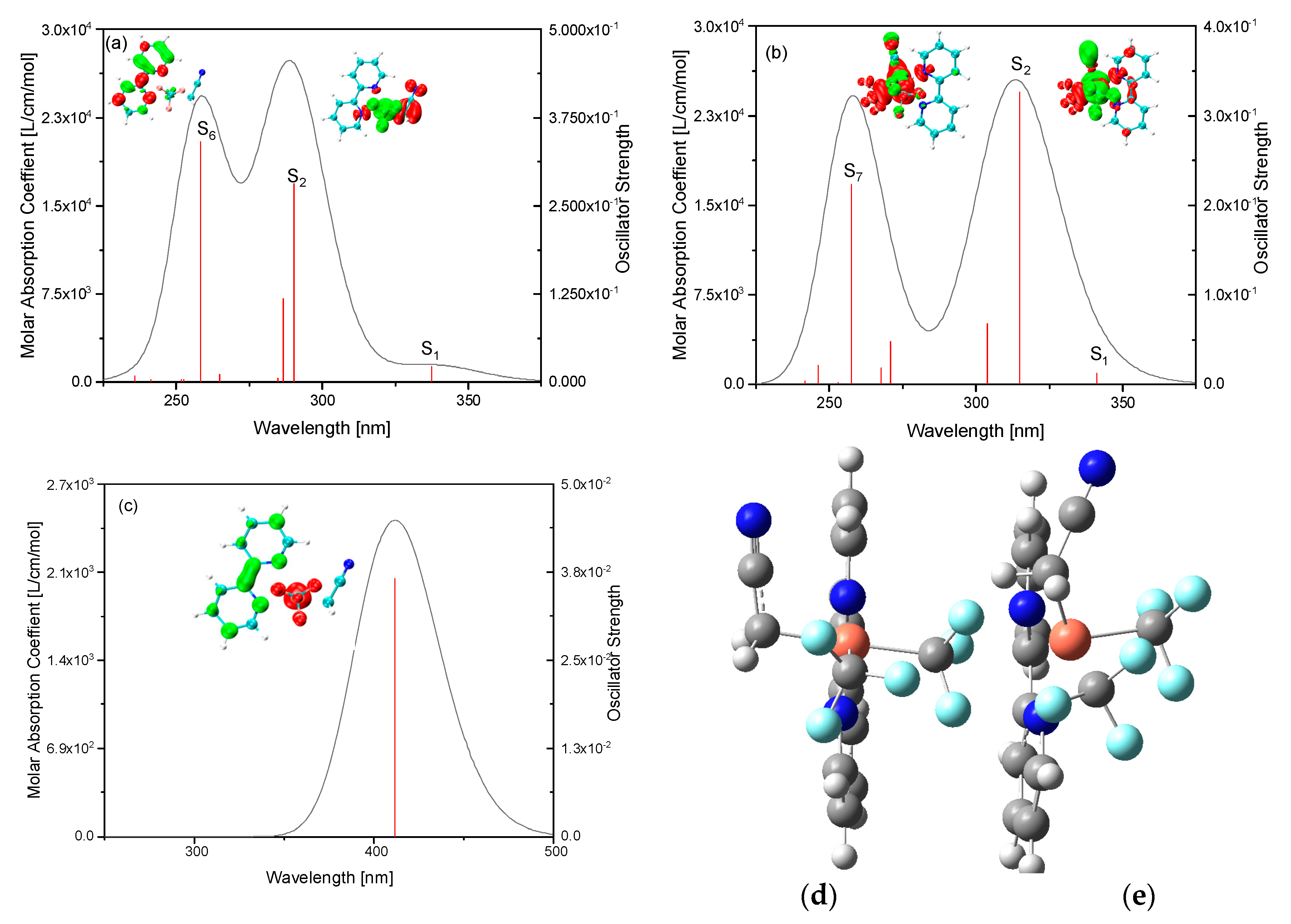
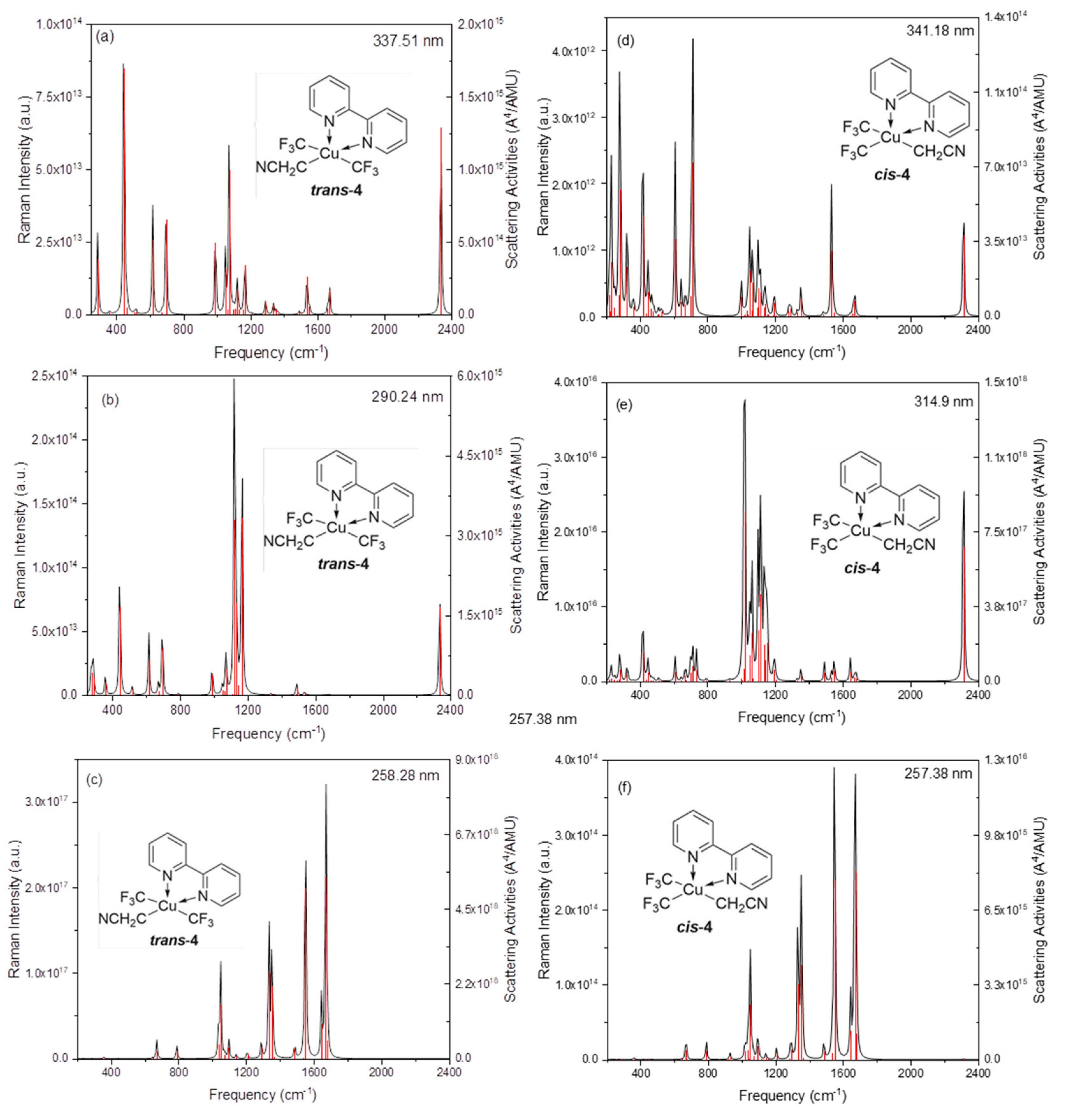
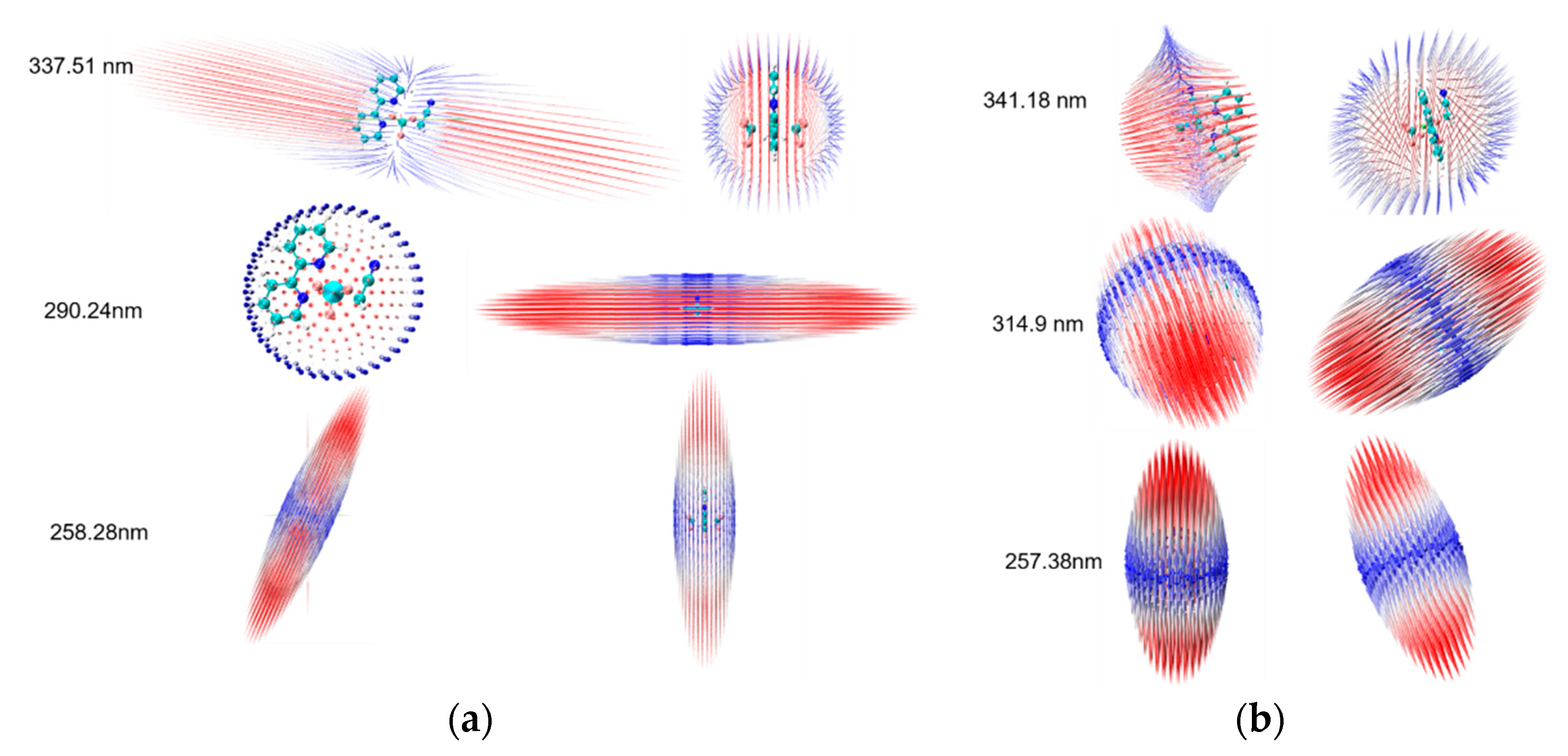
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Shen, Q. Well-defined organometallic Copper(III) complexes: Preparation, characterization and reactivity. Coord. Chem. Rev. 2021, 439, 213923. [Google Scholar] [CrossRef]
- Hickman, A.J.; Sanford, M.S. High-valent organometallic copper and palladium in catalysis. Nature 2012, 484, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hruszkewycz, D.; McCann, S.; Stahl, S.S. Cu-Catalyzed Aerobic Oxidation: Overview and New Developments; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Cho, S.H.; Kim, J.Y.; Kwak, J.; Chang, S. Recent advances in the transition metal-catalyzed twofold oxidative C-H bond activation strategy for C-C and C-N bond formation. Chem. Soc. Rev. 2011, 40, 5068–5083. [Google Scholar] [CrossRef]
- Que, L., Jr.; Tolman, W.B. Bis(μ-oxo)dimetal “Diamond” Cores in Copper and Iron Complexes Relevant to Biocatalysis. Angew. Chem. Int. Ed. 2002, 41, 1114–1137. [Google Scholar]
- Trammell, R.; Rajabimoghadam, K.; Garcia-Bosch, I. Copper-Promoted Functionalization of Organic Molecules: From Biologically Relevant Cu/O2 Model System to Organometallic Transformations. Chem. Rev. 2019, 119, 2954–3031. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, E.; van Klink, G.P.M.; van Koten, G.; de Vries, J.G. The mechanism of the modified Ullmann reaction. Dalton Trans. 2010, 39, 10338–10351. [Google Scholar] [CrossRef] [PubMed]
- Casitas, A.; Ribas, X. The role of organometallic copper (III) complexes in homogeneous catalysis. Chem. Sci. 2013, 4, 2301–2318. [Google Scholar] [CrossRef]
- Li, S.-J.; Lan, Y. Is Cu(III) a necessary intermediate in Cu-mediated coupling reactions? A mechanistic point of view. Chem. Commun. 2020, 56, 6609–6619. [Google Scholar] [CrossRef]
- Cole, A.P.; Root, D.E.; Mukherjee, P.; Solomon, E.I.; Stack, T.D.P. A Trinuclear Intermediate in the Copper-Mediated Reduction of O2: Four Electrons from Three Copper. Science 1996, 273, 1848–1850. [Google Scholar] [CrossRef]
- Sinha, W.; Sommer, M.G.; Deibel, N.; Ehret, F.; Bauer, M.; Sarkar, B.; Kar, S. Experimental and Theoretical Investigations of the Existence of CuII, CuIII, and CuIV in Copper Corrolato Complexes. Angew. Chem. Int. Ed. 2015, 54, 13769–13774. [Google Scholar] [CrossRef]
- Srnec, M.; Navratil, R.; Andris, E.; Jasik, J.; Rouithova, J. Experimentally calibrated analysis of the electronic structure of CuO+: Implications for reactivity. Angew. Chem. Int. Ed. 2018, 52, 17053–17054. [Google Scholar] [CrossRef] [PubMed]
- Lemon, C.M.; Huynh, M.; Maher, A.G.; Anderson, B.L.; Bloch, E.D.; Powers, D.C.; Nocera, D.G. Electronic structure of copper corroles. Angew. Chem. Int. Ed. 2016, 55, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, A.D.; Gagnon, N.L.; Dhar, D.; Yee, G.M.; Tolman, W.B. Determination of the Cu(III)-OH Bond Distance by Resonance Raman Spectroscopy Using a Normalized Version of Badger’s Rule. J. Am. Chem. Soc. 2017, 139, 4477–4485. [Google Scholar] [CrossRef] [PubMed]
- Neisen, B.D.; Gagnon, N.L.; Dhar, D.; Spaeth, A.D.; Tolman, W.B. Formally Copper(III)-Alkylperoxo Complexes as Models of Possible Intermediates in Monooxygenase Enzymes. J. Am. Chem. Soc. 2017, 139, 10220–10223. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Yee, G.M.; Markle, T.F.; Mayer, J.M.; Tolman, W.B. Reactivity of the copper (III)-hydroxide unit with phenols. Chem. Sci. 2017, 8, 1075–1085. [Google Scholar] [CrossRef]
- Bailey, W.D.; Dhar, D.; Cramblitt, A.C.; Tolman, W.B. Mechanistic dichotomy in proton-coupled electron-transfer reactions of phenols with a copper superoxide complex. Am. Chem. Soc. 2019, 141, 5470–5480. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Elwell, C.E.; Bouchey, C.J.; Zerk, T.J.; Tolman, W.B.; Cramer, C. Mechanisms for hydrogen-atom abstraction by mononuclear copper (III) cores: Hydrogen-atom transfer or concerted proton-coupled electron transfer? J. Am. Chem. Soc. 2019, 141, 17236–17244. [Google Scholar] [CrossRef]
- Wu, T.; MacMillan, S.N.; Rajabimoghadam, K.; Siegler, M.A.; Lancaster, K.M.; Garcia-Bosch, I. Structure, Spectroscopy, and Reactivity of a Mononuclear Copper Hydroxide Complex in Three Molecular Oxidation States. J. Am. Chem. Soc. 2020, 142, 12265–12276. [Google Scholar] [CrossRef]
- Bower, J.K.; Cypcar, A.D.; Henriquez, B.; Stieber, S.C.E.; Zhang, S. C (sp3)–H fluorination with a copper (II)/(III) redox couple. J. Am. Chem. Soc. 2020, 142, 8514–8521. [Google Scholar] [CrossRef]
- Hu, H.; Snyder, J.P. Organocuprate conjugate addition: The square-planar “CuIII” intermediate. Am. Chem. Soc. 2007, 129, 7210–7211. [Google Scholar] [CrossRef]
- Bertz, S.H.; Cope, S.; Murphy, M.; Ogle, C.A.; Taylor, B.J. Rapid injection NMR in mechanistic organocopper chemistry. Preparation of the elusive copper (III) intermediate. J. Am. Chem. Soc. 2007, 129, 7208–7209. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, T.; Henze, W.; Gschwind, R.M. NMR-detection of Cu(III) intermediates in substitution reactions of alkyl halides with Gilman cuprates. Am. Chem. Soc. 2007, 129, 11362–11363. [Google Scholar] [CrossRef] [PubMed]
- Bertz, S.H.; Cope, S.; Dorton, D.; Murphy, M.; Ogle, C.A. Organocuprate cross-coupling: The central role of the copper (III) intermediate and the importance of the copper (I) precursor. Angew. Chem. Int. Ed. 2007, 46, 7082–7085. [Google Scholar] [CrossRef]
- Bartholomew, E.R.; Bertz, S.H.; Cope, S.; Murphy, M.; Ogle, C.A. Preparation of σ-and π-Allylcopper (III) Intermediates in SN2 and SN2′ Reactions of Organocuprate (I) Reagents with Allylic Substrates. J. Am. Chem. Soc. 2008, 130, 11244–11245. [Google Scholar] [CrossRef] [PubMed]
- Bertz, S.H.; Hardin, R.A.; Murphy, M.D.; Ogle, C.A.; Richter, J.D.; Thomas, A.A. Rapid Injection NMR Reveals η3 ‘π-Allyl’CuIII Intermediates in Addition Reactions of Organocuprate Reagents. J. Am. Chem. Soc. 2012, 134, 9557–9560. [Google Scholar] [CrossRef] [PubMed]
- Maurya, Y.K.; Noda, K.; Yamasumi, K.; Mori, S.; Uchiyama, T.; Kamitani, K.; Hirai, T.; Ninomiya, K.; Nishibori, M.; Hori, Y.; et al. Ground-state copper (III) stabilized by N-confused/N-linked corroles: Synthesis, characterization, and redox reactivity. J. Am. Chem. Soc. 2018, 140, 6883–6892. [Google Scholar] [CrossRef]
- Adinarayana, B.; Thomas, A.P.; Suresh, C.H.; Srinivasan, A. A 6, 11, 16-Triarylbiphenylcorrole with an adj-CCNN Core: Stabilization of an Organocopper (III) Complex. Angew. Chem. Int. Ed. 2015, 54, 10478–10482. [Google Scholar] [CrossRef]
- Ke, X.-S.; Hong, Y.; Tu, P.; He, Q.; Lynch, V.M.; Kim, D.; Sessler, J.L. Hetero Cu(III)–Pd (II) complex of a dibenzo [g, p] chrysene-fused bis-dicarbacorrole with stable organic radical character. J. Am. Chem. Soc. 2017, 139, 15232–15238. [Google Scholar] [CrossRef]
- Ribas, X.; Jackson, D.A.; Donnadieu, B.; Mahía, J.; Parella, T.; Xifra, R.; Hedman, B.; Hodgson, K.O.; Llobet, A.; Stack, T.D.P. Aryl C-H Activation by CuII To Form an Organometallic Aryl–CuIII Species: A Novel Twist on Copper Disproportionation. Angew. Chem. Int. Ed. 2002, 41, 2991–2994. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Wang, T.; Zhang, X.; Long, C.; Wu, Y.-D.; Wang, M.-X. Mechanistic study on cu (II)-catalyzed oxidative cross-coupling reaction between arenes and boronic acids under aerobic conditions. J. Am. Chem. Soc. 2018, 140, 5579–5587. [Google Scholar] [CrossRef]
- Liu, Y.; Resch, S.G.; Klawitter, I.; Cutsai, G.E., III; Demeshko, S.; Dechert, S.; Kühn, F.E.; DeBeer, S.; Meyer, F. An Adaptable N-Heterocyclic Carbene Macrocycle Hosting Copper in Three Oxidation States. Angew. Chem. Int. Ed. 2020, 59, 5696–5705. [Google Scholar] [CrossRef]
- Brothers, P.J.; Roper, W.R. Transition-metal dihalocarbene complexes. Chem. Rev. 1988, 88, 1293–1326. [Google Scholar] [CrossRef]
- Romine, A.M.; Nebra, N.; Konovalov, A.I.; Martin, E.; Benet-Buchholz, J.; Grushin, V.V. Easy access to the copper (III) anion [Cu (CF3) 4]−; Angew. Chem. Int. Ed. 2015, 54, 2745–2749. [Google Scholar] [CrossRef]
- Shen, H.; Liu, Z.; Zhang, P.; Tan, X.; Zhang, Z.; Li, C. Trifluoromethylation of alkyl radicals in aqueous solution. J. Am. Chem. Soc. 2017, 139, 9843–9846. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Z.; Shen, H.; Zhang, P.; Zhang, Z.; Li, C. Silver-catalyzed decarboxylative trifluoromethylation of aliphatic carboxylic acids. J. Am. Chem. Soc. 2017, 139, 12430–12433. [Google Scholar] [CrossRef]
- Guo, S.; AbuSalim, D.I.; Cook, S.P. Aqueous benzylic C–H trifluoromethylation for late-stage functionalization. J. Am. Chem. Soc. 2018, 140, 12378–12382. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Lee, G.S.; Park, B.; Kim, D.; Hong, S.H. Direct C (sp3)−H trifluoromethylation of unactivated alkanes enabled by multifunctional trifluoromethyl copper complexes. Angew. Chem. Int. Ed. 2021, 60, 5467–5474. [Google Scholar] [CrossRef]
- Guo, S.; AbuSalim, D.I.; Cook, S.P. 1, 2-(Bis) trifluoromethylation of Alkynes: A One-Step Reaction to Install an Underutilized Functional Group. Angew. Chem. Int. Ed. 2019, 58, 11704–11708. [Google Scholar] [CrossRef]
- Le, C.; Chen, T.Q.; Liang, T.; Zhang, P.; MacMillan, D.W.C. A radical approach to the copper oxidative addition problem: Trifluoromethylation of bromoarenes. Science 2018, 360, 1010–1014. [Google Scholar] [CrossRef]
- Kornfilt, D.J.P.; MacMillan, D.W.C. Copper-catalyzed trifluoromethylation of alkyl bromides. J. Am. Chem. Soc. 2019, 141, 6853–6858. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, H.; Liu, S.; Leng, X.; Lan, Y.; Shen, Q. A Key Intermediate in Copper-Mediated Arene Trifluoromethylation,[nBu4N][Cu (Ar)(CF3) 3]: Synthesis, Characterization, and C (sp2)−CF3 Reductive Elimination. Angew. Chem. Int. Ed. 2019, 58, 8510–8514. [Google Scholar] [CrossRef] [PubMed]
- Paeth, M.; Tyndall, S.B.; Chen, L.-Y.; Hong, J.-C.; Carson, W.P.; Liu, X.; Sun, X.; Liu, J.; Yang, K.; Hale, E.M.; et al. Csp3–Csp3 bond-forming reductive elimination from well-defined copper (III) complexes. J. Am. Chem. Soc. 2019, 141, 3153–3159. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Liu, S.; Lu, Z.; Lu, C.; Leng, X.; Lan, Y.; Shen, Q. C (sp3)-CF3 Reductive elimination from a five-coordinate neutral copper (III) complex. J. Am. Chem. Soc. 2020, 142, 9785–9791. [Google Scholar] [CrossRef]
- Wing, R.M. Carborane analogs of. pi.-allyls. The crystal and molecular structure of triphenylmethylphosphonium bis (3)-1, 2-dicarboryl) cuprate (III). J. Am. Chem. Soc. 1968, 90, 4828–4834. [Google Scholar] [CrossRef]
- Varadarajan, A.; Johnson, S.E.; Gomez, F.A.; Chakrabarti, S.; Knobler, C.B.; Hawthorne, M.F. Synthesis and structural characterization of pyrazole-bridged metalla-bis (dicarbollide) derivatives of cobalt, nickel, copper, and iron: Models for venus flytrap cluster reagents. J. Am. Chem. Soc 1992, 114, 9003–9011. [Google Scholar] [CrossRef]
- García-López, J.; Yañez-Rodríguez, V.; Roces, L.; García-Granda, S.; Martínez, A.; Guevara-García, A.; Castro, G.R.; Jiménez-Villacorta, F.; Iglesias, M.J.; Ortiz, F.L. Synthesis and characterization of a coupled binuclear CuI/CuIII complex. J. Am. Chem. Soc. 2010, 132, 10665–10667. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, M.; Yu, H.-T.; Zhang, W.-X.; Xi, Z. Organocopper (III) spiro complexes: Synthesis, structural characterization, and redox transformation. J. Am. Chem. Soc. 2017, 139, 13688–13691. [Google Scholar] [CrossRef] [PubMed]
- Santo, R.; Miyamoto, R.; Tanaka, R.; Nishioka, T.; Sato, K.; Toyota, K.; Obata, M.; Yano, S.; Kinoshita, I.; Ichimura, A.; et al. Diamagnetic–Paramagnetic Conversion of Tris (2-pyridylthio) methylcopper (III) through a Structural Change from Trigonal Bipyramidal to Octahedral. Angew. Chem. Int. Ed. 2006, 45, 7611–7614. [Google Scholar] [CrossRef]
- Snyder, J.P. Elusiveness of CuIII complexation. preference for trifluoromethyl oxidation in the formation of [CuI (CF3)4]−salts. Angew. Chem. Int. Ed. Engl. 1995, 34, 80–81. [Google Scholar] [CrossRef]
- Walroth, R.C.; Lukens, J.T.; MacMillan, S.N.; Finkelstein, K.D.; Lancaster, K.M. Spectroscopic Evidence for a 3d10 Ground State Electronic Configuration and Ligand Field Inversion in [Cu (CF3)4] 1–. J. Am. Chem. Soc. 2016, 138, 1922–1931. [Google Scholar] [CrossRef]
- Dimucci, I.M.; Lukens, J.T.; Chatterjee, S.; Carsch, K.M.; Titus, C.J.; Lee, S.J.; Nordlund, D.; Betley, T.A.; MacMillan, S.N.; Lancaster, K.M. The myth of d8 copper (III). J. Am. Chem. Soc. 2019, 141, 18508–18520. [Google Scholar] [CrossRef]
- Hoffmann, R.; Alvarez, S.; Mealli, C.; Falceto, A.; Cahill, T.J., III; Zeng, T.; Manca, G. From widely accepted concepts in coordination chemistry to inverted ligand fields. Chem. Rev. 2016, 116, 8173–8192. [Google Scholar] [CrossRef] [PubMed]
- van Koten, G. Organocopper Compounds: From Elusive to Isolable Species, from Early Supramolecular Chemistry with RCuI Building Blocks to Mononuclear R2–nCuII and R3–mCuIII Compounds. A Personal View. Organometallics 2012, 31, 7634–7764. [Google Scholar] [CrossRef][Green Version]
- Melník, M.; Kabešová, M. Copper (III) coordination compounds: Classification and analysis of crystallographic and structural data. Coord. Chem. 2000, 50, 323–338. [Google Scholar] [CrossRef]
- Bartholomew, E.R.; Bertz, S.H.; Cope, S.; Dorton, D.C.; Murphy, M.; Ogle, C.A. Neutral organocopper (III) complexes. Chem. Commun. 2008, 10, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Y.; Wu, J.; Xue, X.; Hartwig, J.F.; Shen, Q. Oxidative addition of an alkyl halide to form a stable Cu(III) product. Science 2023, 381, 1072–1079. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.P.; Petersson, G.A.; Nakatsuji, H.R.; et al. Gaussian 16, Revision A. 03; Gaussian. Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- García, J.C.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.; Beratan, D.N.; Yang, W.J. NCIPLOT: A program for plotting noncovalent interaction regions. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Gross, E.K.U.; Kohn, W. Local density-functional theory of frequency-dependent linear response. Phys. Rev. Lett. 1985, 55, 2850–2852. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Tuer, A.; Krouglov, S.; Cisek, R.; Tokarz, D.; Barzda, V.J. Three-dimensional visualization of the first hyperpolarizability tensor. Comput. Chem. 2011, 32, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, T.; Yuan, A.; Wang, X.; Chen, Q.; Yan, X. Remarkable Size Effect on Photophysical and Nonlinear Optical Properties of All-Carboatomic Rings, Cyclo [18] carbon and Its Analogues. Chem. Asian J. 2021, 16, 2267–2271. [Google Scholar] [CrossRef]
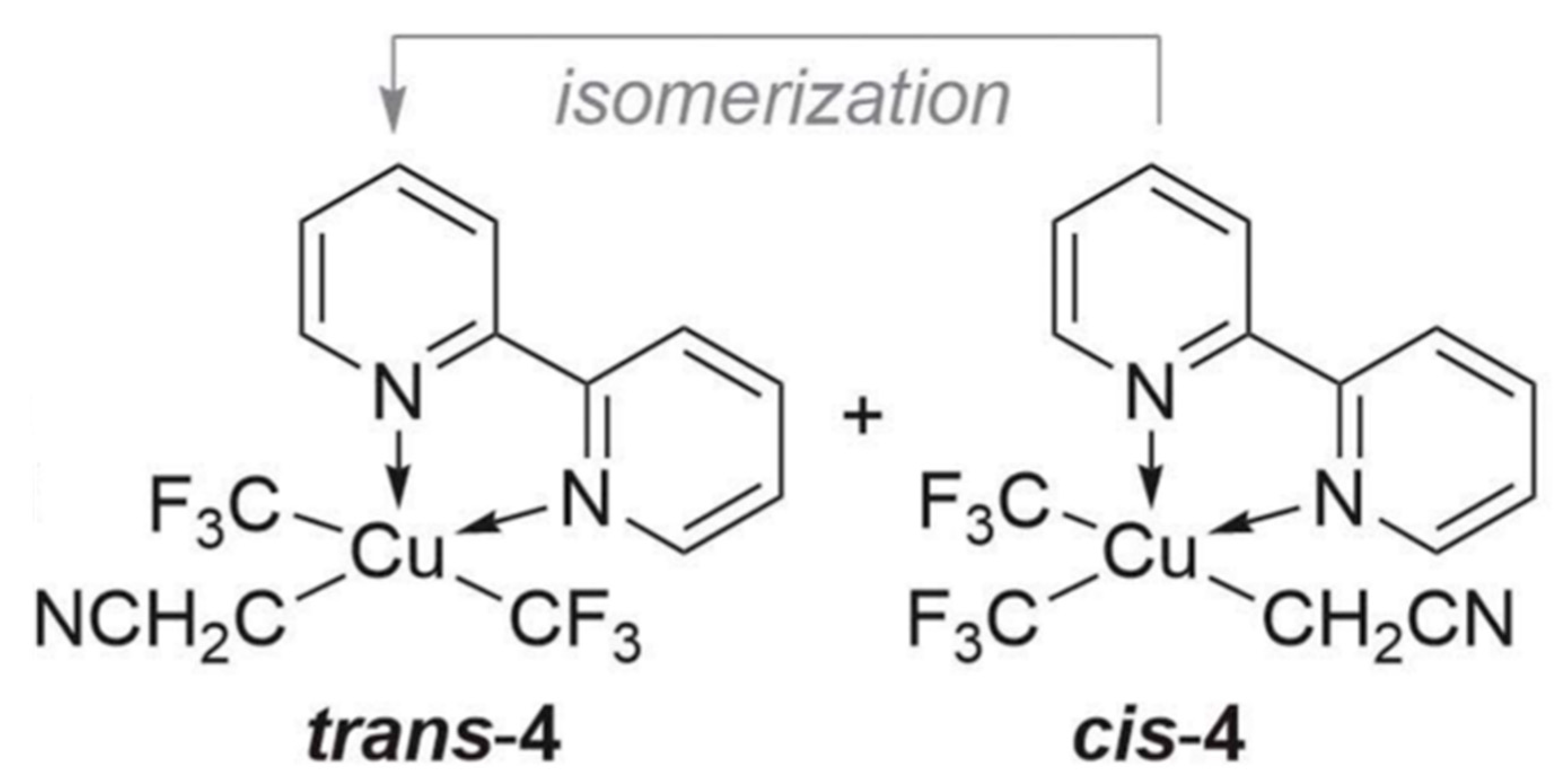

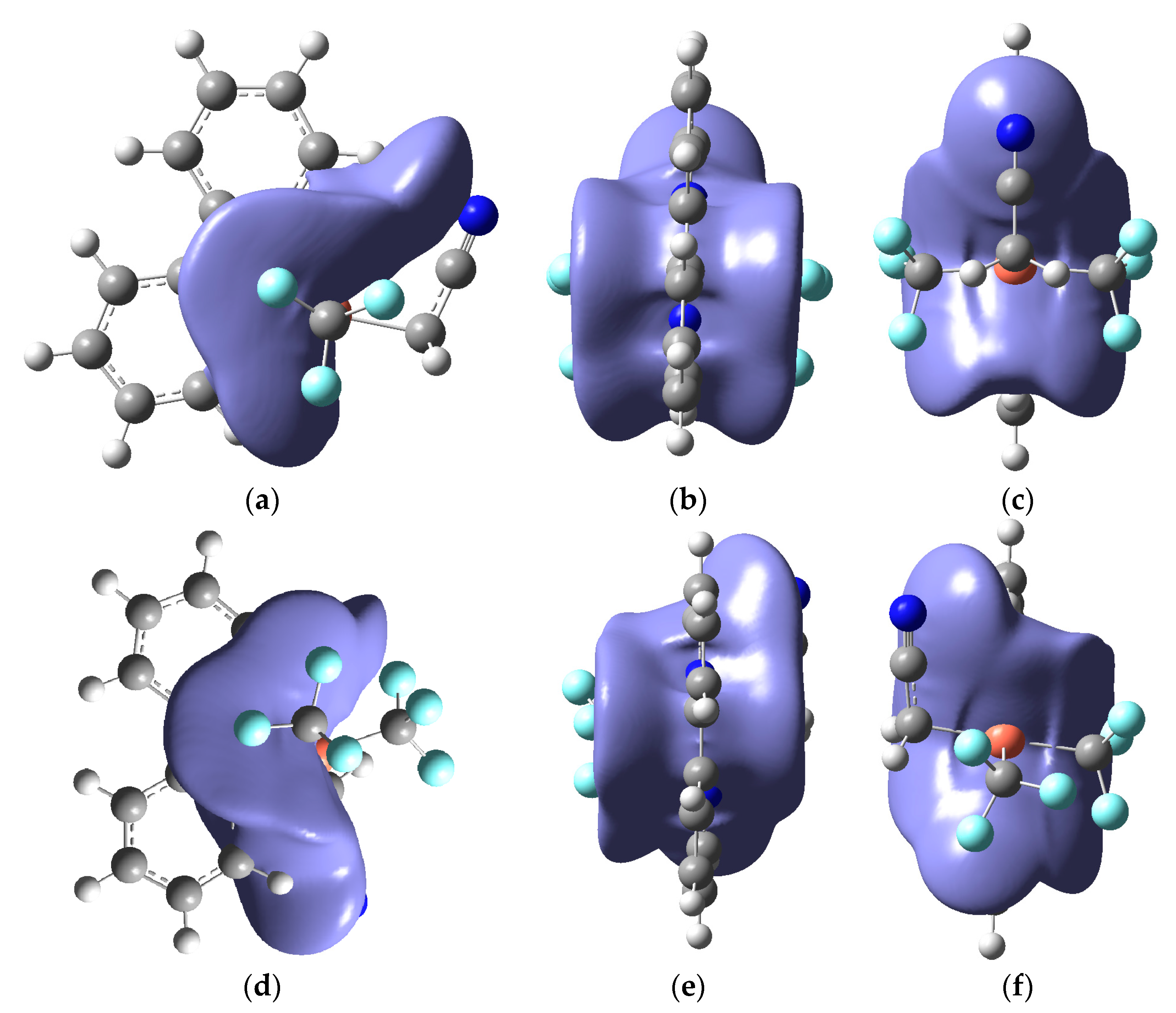
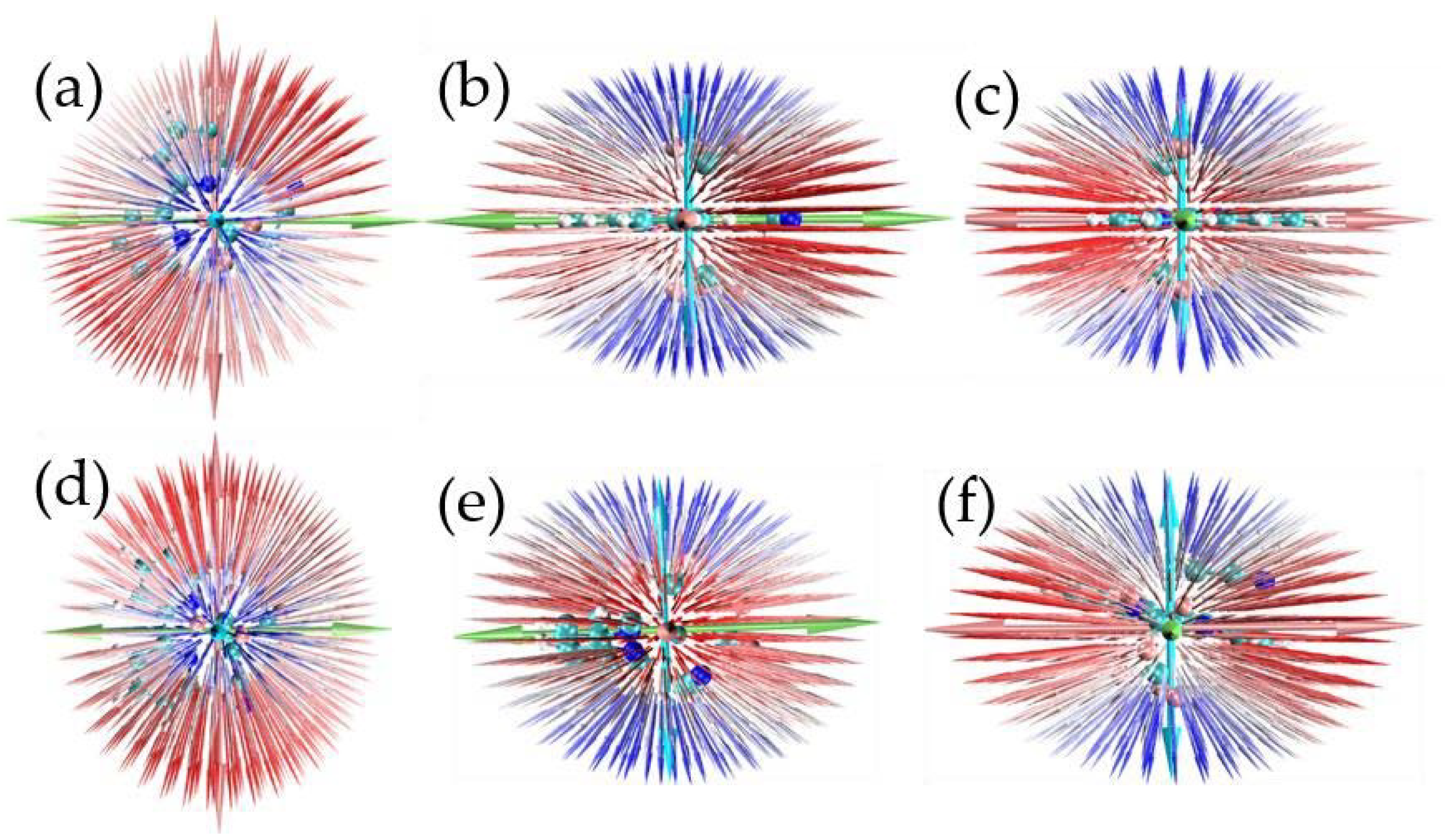
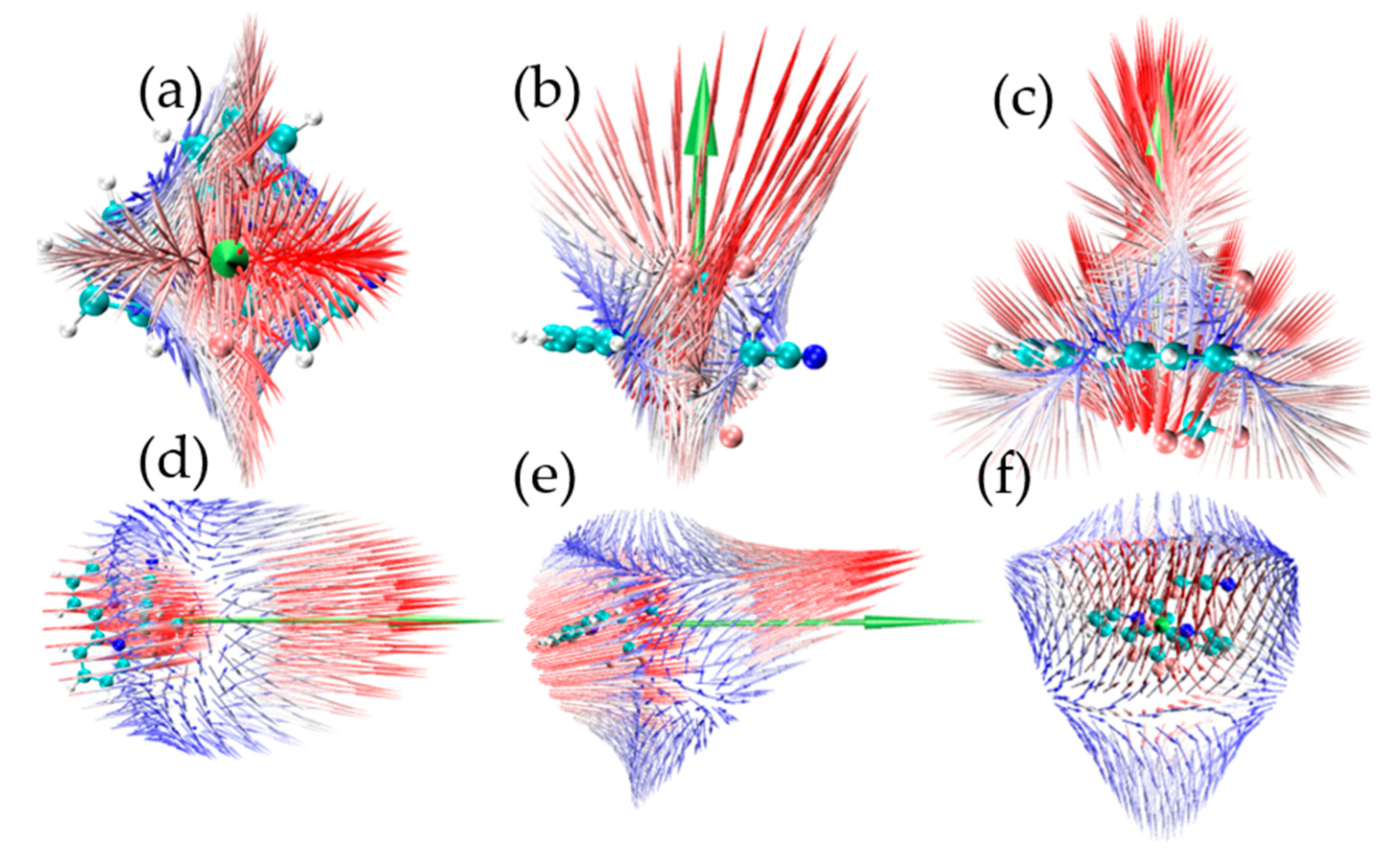

| (x) | (y) | (z) | ||||
|---|---|---|---|---|---|---|
| trans-4 | −9.0 | 0.7 | 0.0 | 237 | 231 | 128 |
| cis-4 | 10.2 | −2.7 | −1.4 | 215 | 253 | 139 |
| trans-4 | 340 | −26 | 0 | 102 | −215 | 133 | 0 | −48 | 0 |
| cis-4 | −610 | −15 | 51 | −67 | 110 | −41 | −70 | 9 | 184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, E.; Sun, M. Spectral Physics of Stable Cu(III) Produced by Oxidative Addition of an Alkyl Halide. Int. J. Mol. Sci. 2023, 24, 15694. https://doi.org/10.3390/ijms242115694
Cao E, Sun M. Spectral Physics of Stable Cu(III) Produced by Oxidative Addition of an Alkyl Halide. International Journal of Molecular Sciences. 2023; 24(21):15694. https://doi.org/10.3390/ijms242115694
Chicago/Turabian StyleCao, En, and Mengtao Sun. 2023. "Spectral Physics of Stable Cu(III) Produced by Oxidative Addition of an Alkyl Halide" International Journal of Molecular Sciences 24, no. 21: 15694. https://doi.org/10.3390/ijms242115694
APA StyleCao, E., & Sun, M. (2023). Spectral Physics of Stable Cu(III) Produced by Oxidative Addition of an Alkyl Halide. International Journal of Molecular Sciences, 24(21), 15694. https://doi.org/10.3390/ijms242115694






