Combining Genetic and Transcriptomic Approaches to Identify Transporter-Coding Genes as Likely Responsible for a Repeatable Salt Tolerance QTL in Citrus
Abstract
:1. Introduction
2. Results
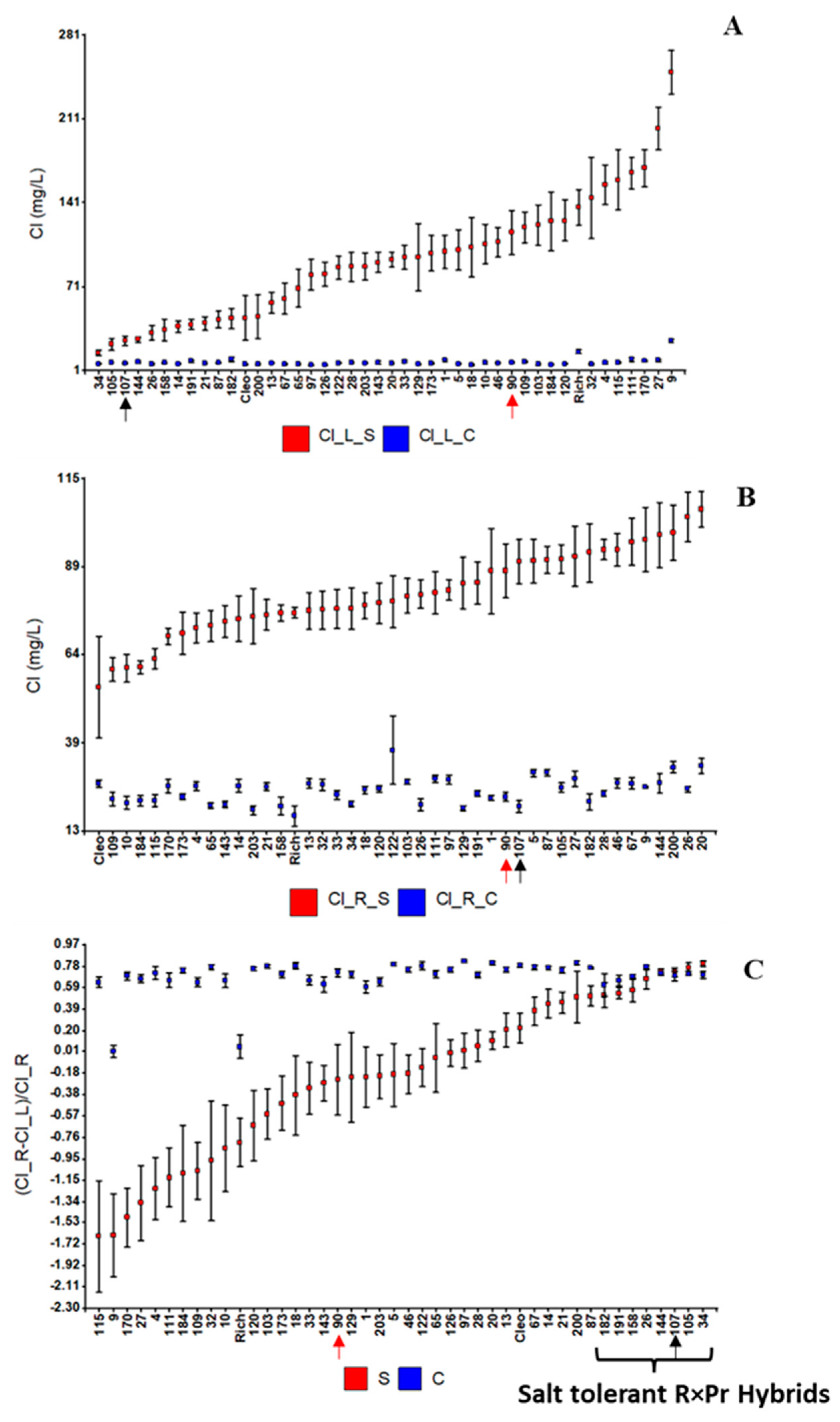
2.1. Salt Tolerance in the GP Experiment
2.2. Salt Tolerance in the Non-Grafted Population (NG Experiment)
2.3. Genomic and Transcriptomic Analysis of Genes in the Salt Tolerance QTL LCl-6
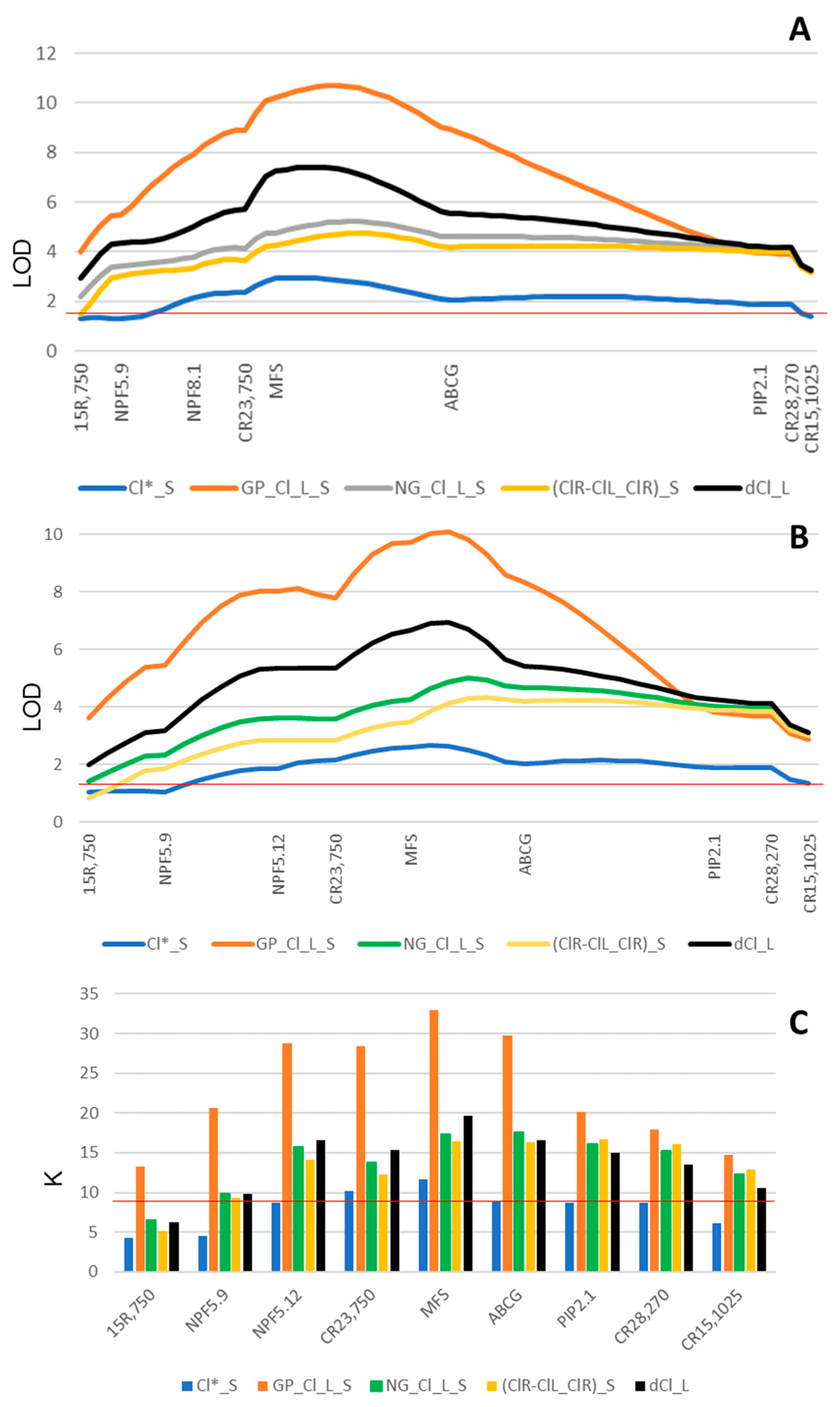
2.4. Incorporating LCl-6 Candidate Genes into the Linkage Maps to Improve the Mapping Resolution of QTL Analysis
3. Discussion
3.1. The Salt Tolerance QTL LCl-6 Has Been Consistently Detected across Three Experiments
3.2. Salt Tolerance Mechanism(s) behind LCL-6
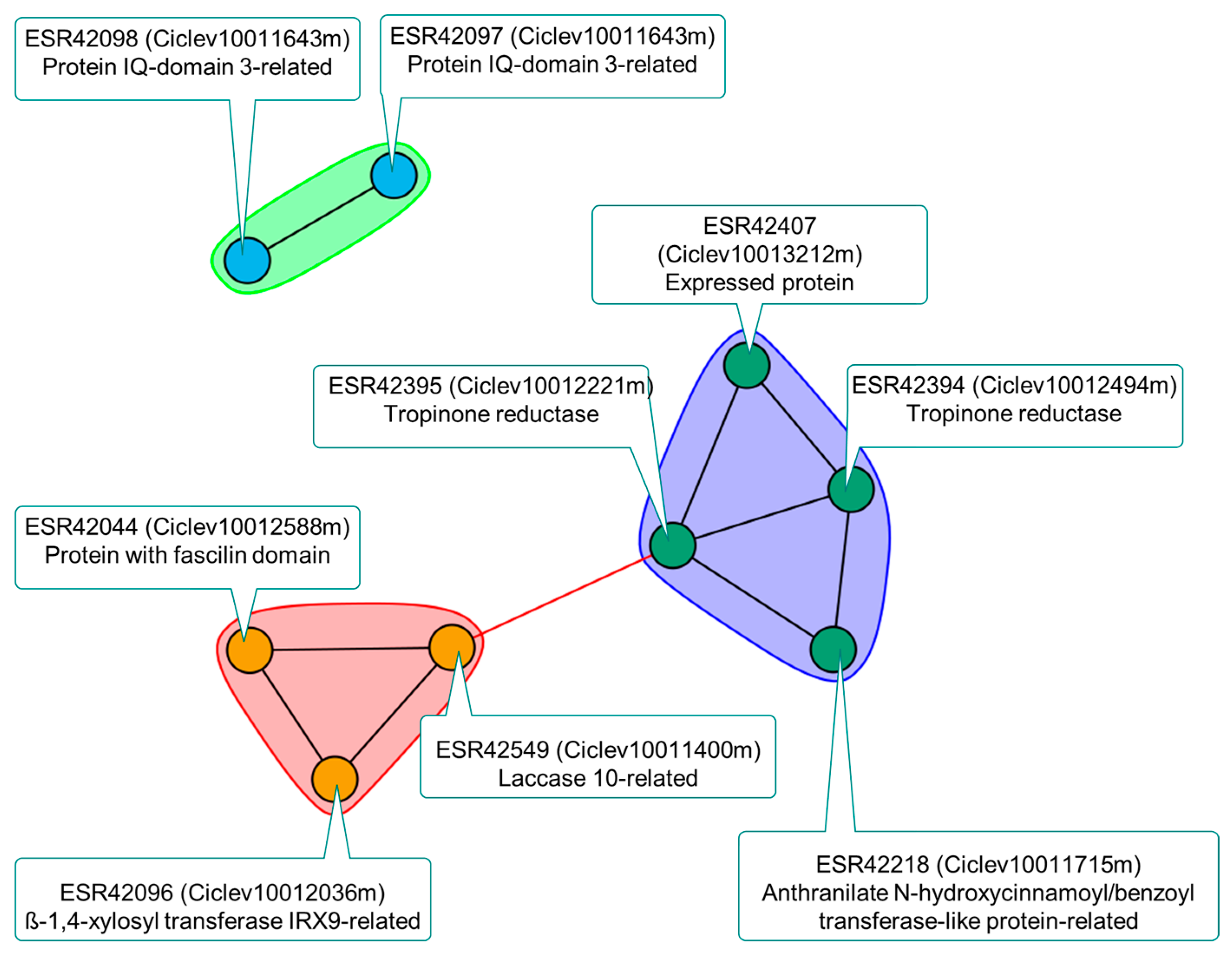
3.3. Salt Tolerance Candidate Genes Underlying LCl-6 in the R×Pr Population
4. Materials and Methods
4.1. Plant Materials
4.2. Trait Evaluation
4.3. Statistical Analysis
4.4. Genetic Analyses
4.4.1. Linkage Map and QTL Analyses
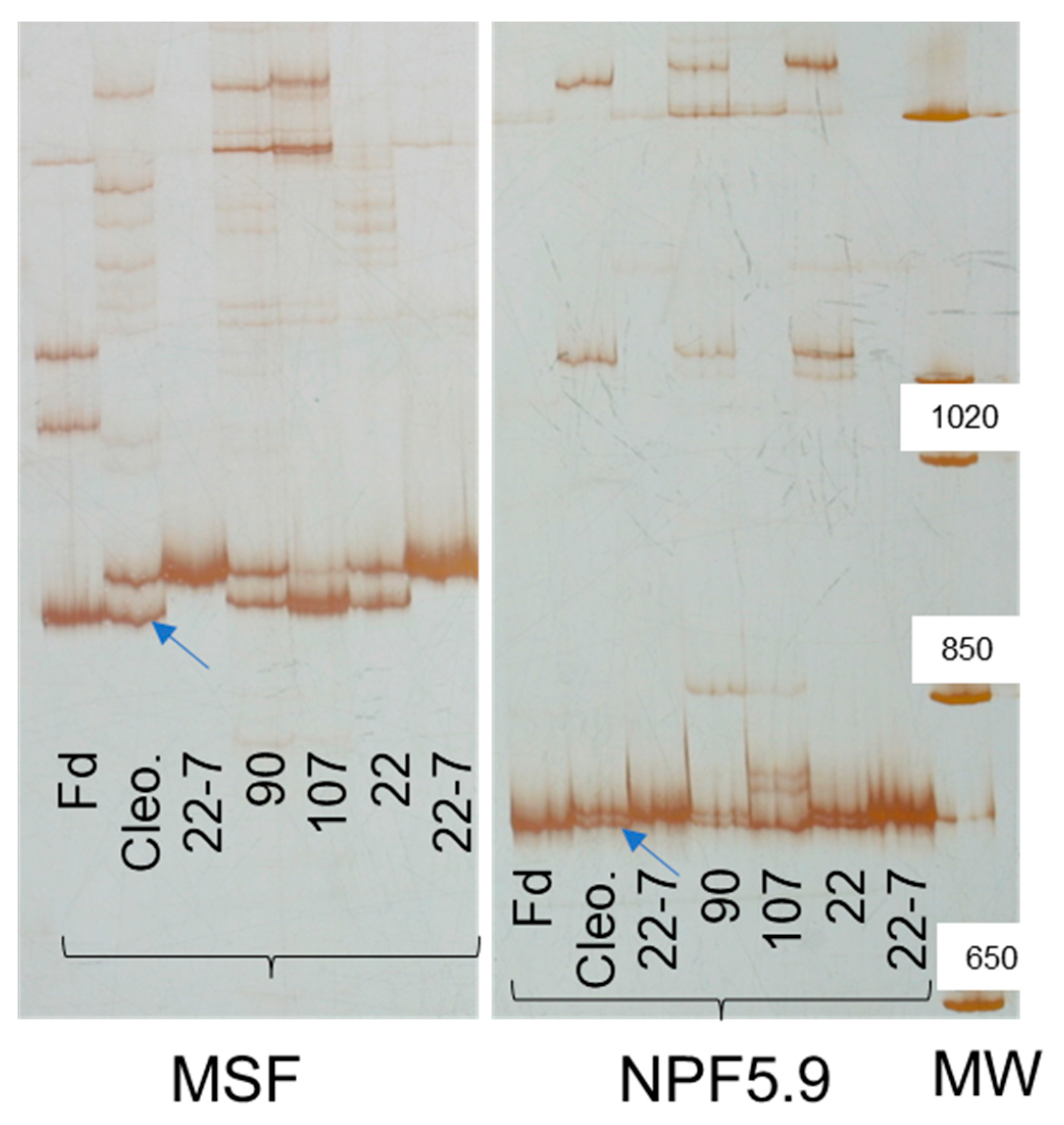
4.4.2. Candidate Genes and Linkage Analysis
4.5. DNA Sequencing of Selected Materials
4.6. RNA-Sequencing Analysis of the Root of Selected Materials
4.6.1. RNA Isolation of Selected Materials
4.6.2. Library Preparation and Sequencing
4.6.3. Read Pre-Processing and Counting
4.6.4. Differential Expression, Correlation, and Clustering Analyses
4.6.5. Functional Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Extent of Salt-Affected Soils. In Portal de Suelos de la FAO; FAO: Rome, Italy, 2023. [Google Scholar]
- Munns, R.; Gilliham, M. Salinity Tolerance of Crops—What Is the Cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Maas, E.V. Salinity and Citriculture. Tree Physiol. 1993, 12, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Bingham, F.T.; Mahler, R.J.; Parra, J.; Stolzy, L.H. Long-Term Effects of Irrigation-Salinity Management on a Valencia Orange Orchard. Soil Sci. 1974, 117, 369. [Google Scholar] [CrossRef]
- Cerda, A.; Nieves, M.; Guillen, M.G. Salt Tolerance of Lemon Trees as Affected by Rootstock. Irrig. Sci. 1990, 11, 245–249. [Google Scholar] [CrossRef]
- Sulistyowati, L. Effect of Soil Salinity and Water Content on Stem Rot Caused by Phytophthora Citrophthora and Accumulation of Phytoalexin in Citrus Rootstocks. Phytopathology 1992, 82, 771. [Google Scholar] [CrossRef]
- Dunn, D.C.; Duncan, L.W.; Romeo, J.T. Changes in Arginine, Pal Activity, and Nematode Behavior in Salinity-Stressed Citrus in Honour of Professor G.H. Neil Towers 75th Birthday. Phytochemistry 1998, 49, 413–417. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple Abiotic Stresses Occurring with Salinity Stress in Citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- Castle, W.S. A Career Perspective on Citrus Rootstocks, Their Development, and Commercialization. HortScience 2010, 45, 11–15. [Google Scholar] [CrossRef]
- Raga, V.; Bernet, G.P.; Carbonell, E.A.; Asins, M.J. Segregation and Linkage Analyses in Two Complex Populations Derived from the Citrus Rootstock Cleopatra Mandarin. Inheritance of Seed Reproductive Traits. Tree Genet. Genomes 2012, 8, 1061–1071. [Google Scholar] [CrossRef]
- Ream, C.L.; Furr, J.R. Salt Tolerance of Some Citrus Species, Relatives, and Hybrids Tested as Rootstocks1. J. Am. Soc. Hortic. Sci. 1976, 101, 265–267. [Google Scholar]
- Storey, R.; Walker, R.R. Citrus and Salinity. Sci. Hortic. 1998, 78, 39–81. [Google Scholar] [CrossRef]
- Levy, Y.; Lifshitz, J.; De Malach, Y.; David, Y. The Response of Several Citrus Genotypes to High-Salinity Irrigation Water. HortScience 1999, 34, 878–881. [Google Scholar] [CrossRef]
- García, M.R.; Bernet, G.P.; Puchades, J.; Gómez, I.; Carbonell, E.A.; Asins, M.J. Reliable and Easy Screening Technique for Salt Tolerance of Citrus Rootstocks under Controlled Environments. Aust. J. Agric. Res. 2002, 53, 653. [Google Scholar] [CrossRef]
- Sykes, S.R. Chloride and Sodium Excluding Capacities of Citrus Rootstock Germplasm Introduced to Australia from the People’s Republic of China. Sci. Hortic. 2011, 128, 443–449. [Google Scholar] [CrossRef]
- Balal, R.M.; Khan, M.M.; Shahid, M.A.; Mattson, N.S.; Abbas, T.; Ashfaq, M.; Garcia-Sanchez, F.; Ghazanfer, U.; Gimeno, V.; Iqbal, Z. Comparative Studies on the Physiobiochemical, Enzymatic, and Ionic Modifications in Salt-Tolerant and Salt-Sensitive Citrus Rootstocks under NaCl Stress. J. Amer. Soc. Hort. Sci. 2012, 137, 86–95. [Google Scholar] [CrossRef]
- Raga, V.; Intrigliolo, D.S.; Bernet, G.P.; Carbonell, E.A.; Asins, M.J. Genetic Analysis of Salt Tolerance in a Progeny Derived from the Citrus Rootstocks Cleopatra Mandarin and Trifoliate Orange. Tree Genet. Genomes 2016, 12, 34. [Google Scholar] [CrossRef]
- Cooper, W.; Olson, E.; Maxwell, N.; Otey, G. Review of Studies on Adaptability of Citrus Varieties as Rootstocks for Grapefruit in Texas. J. Rio Grande Vall. Hort. Soc. 1956, 10, 6–19. [Google Scholar]
- Romero-Aranda, R.; Moya, J.L.; Tadeo, F.R.; Legaz, F.; Primo-Millo, E.; Talon, M. Physiological and Anatomical Disturbances Induced by Chloride Salts in Sensitive and Tolerant Citrus: Beneficial and Detrimental Effects of Cations. Plant Cell Environ. 1998, 21, 1243–1253. [Google Scholar] [CrossRef]
- Brumós, J.; Colmenero-Flores, J.M.; Conesa, A.; Izquierdo, P.; Sánchez, G.; Iglesias, D.J.; López-Climent, M.F.; Gómez-Cadenas, A.; Talón, M. Membrane Transporters and Carbon Metabolism Implicated in Chloride Homeostasis Differentiate Salt Stress Responses in Tolerant and Sensitive Citrus Rootstocks. Funct. Integr. Genomics. 2009, 9, 293–309. [Google Scholar] [CrossRef]
- Brumós, J.; Talón, M.; Bouhlal, R.; Colmenero-Flores, J.M. Cl− Homeostasis in Includer and Excluder Citrus Rootstocks: Transport Mechanisms and Identification of Candidate Genes. Plant Cell Environ. 2010, 33, 2012–2027. [Google Scholar] [CrossRef]
- De-Paz, J.M.; Visconti, F.; Tudela, L.; Quinones, A.; Intrigliolo, D.S.; Jordá, M.; Bonet, L. La fitotoxicidad por cloruro en el cultivo del caqui: Descripcion del problema. Agrícola Vergel Frutic. Hortic. Floric. 2016, 35, 91–96. [Google Scholar]
- Li, B.; Qiu, J.; Jayakannan, M.; Xu, B.; Li, Y.; Mayo, G.M.; Tester, M.; Gilliham, M.; Roy, S.J. AtNPF2.5 Modulates Chloride (Cl−) Efflux from Roots of Arabidopsis thaliana. Front. Plant Sci. 2017, 7, 2013. [Google Scholar] [CrossRef] [PubMed]
- Celis, N.; Suarez, D.L.; Wu, L.; Li, R.; Arpaia, M.L.; Mauk, P. Salt Tolerance and Growth of 13 Avocado Rootstocks Related Best to Chloride Uptake. HortScience 2018, 53, 1737–1745. [Google Scholar] [CrossRef]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the Move. Trends Plant Sci. 2017, 22, 236–248. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Díaz-Rueda, P.; Rivero-Núñez, C.M.; Brumós, J.; Rubio-Casal, A.E.; de Cires, A.; Colmenero-Flores, J.M.; Rosales, M.A. Chloride Nutrition Improves Drought Resistance by Enhancing Water Deficit Avoidance and Tolerance Mechanisms. J. Exp. Bot. 2021, 72, 5246–5261. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Brumós, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride Regulates Leaf Cell Size and Water Relations in Tobacco Plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef]
- Xie, R.; Pan, X.; Zhang, J.; Ma, Y.; He, S.; Zheng, Y.; Ma, Y. Effect of Salt-Stress on Gene Expression in Citrus Roots Revealed by RNA-Seq. Funct. Integr. Genom. 2018, 18, 155–173. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Xu, W.; Liu, J.-H.; Li, C. Genome-Wide Identification of NRT Gene Family and Expression Analysis of Nitrate Transporters in Response to Salt Stress in Poncirus Trifoliata. Genes 2022, 13, 1115. [Google Scholar] [CrossRef]
- Tozlu, I.; Guy, C.L.; Moore, G.A. QTL Analysis of Na+ and Cl− Accumulation Related Traits in an Intergeneric BC1 Progeny of Citrus and Poncirus under Saline and Nonsaline Environments. Genome 1999, 42, 692–705. [Google Scholar] [CrossRef]
- Guo, B.; Sleper, D.A.; Lu, P.; Shannon, J.G.; Nguyen, H.T.; Arelli, P.R. QTLs Associated with Resistance to Soybean Cyst Nematode in Soybean: Meta-Analysis of QTL Locations. Crop Sci. 2006, 46, 595–602. [Google Scholar] [CrossRef]
- Moya, J.L.; Tadeo, F.R.; Gómez-Cadenas, A.; Primo-Millo, E.; Talón, M. Transmissible Salt Tolerance Traits Identified through Reciprocal Grafts between Sensitive Carrizo and Tolerant Cleopatra Citrus Genotypes. J. Plant Physiol. 2002, 159, 991–998. [Google Scholar] [CrossRef]
- Peng, Z.; Bredeson, J.V.; Wu, G.A.; Shu, S.; Rawat, N.; Du, D.; Parajuli, S.; Yu, Q.; You, Q.; Rokhsar, D.S.; et al. A Chromosome-scale Reference Genome of Trifoliate Orange (Poncirus trifoliata) Provides Insights into Disease Resistance, Cold Tolerance and Genome Evolution in Citrus. Plant J. 2020, 104, 1215–1232. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Shao, J.; Zhou, C.; Hartung, J.S. Transcriptome Analysis of Sweet Orange Trees Infected with ‘Candidatus Liberibacter Asiaticus’ and Two Strains of Citrus Tristeza Virus. BMC Genom. 2016, 17, 349. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Iacono, F. Effect of Salinity and Ramsey Rootstock on Ion Concentrations and Carbon Dioxide Assimilation in Leaves of Drip-Irrigated, Field-Grown Grapevines (Vitis Vinifera L. Cv. Sultana). Aust. J. Grape Wine Res. 1997, 3, 66–74. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Dai, S.; Wang, R.; Li, N.; Shen, X.; Zhou, X.; Lu, C.; Zheng, X.; Hu, Z.; et al. NaCl-Induced Alternations of Cellular and Tissue Ion Fluxes in Roots of Salt-Resistant and Salt-Sensitive Poplar Species. Plant Physiol. 2009, 149, 1141–1153. [Google Scholar] [CrossRef]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl- Transport Contributing to Salt Tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Melgar, J.C.; García-Sánchez, F. Salinity Tolerance and Leaf Water Use Efficiency in Citrus. J. Am. Soc. Hort. Sci. 2010, 135, 33–39. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant. Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Smith, M.L.; Boman, B.J. Tree Growth, Mineral Nutrition and Nutrient Leaching Losses from Soil of Salinized Citrus. Agric. Ecosyst. Environ. 1993, 45, 319–334. [Google Scholar] [CrossRef]
- Tozlu, I.; Moore, G.A.; Guy, C.L. Regulation of Growth and Differential Tissue Dry Mass Accumulation by Citrus Grandis, Poncirus Trifoliata and Their F1 under Salinized and Non-Salinized Environments. Funct. Plant Biol. 2000, 27, 27. [Google Scholar] [CrossRef]
- Adams, S.N.; Ac-Pangan, W.O.; Rossi, L. Effects of Soil Salinity on Citrus Rootstock ‘US-942’ Physiology and Anatomy. HortScience 2019, 54, 787–792. [Google Scholar] [CrossRef]
- Allelign Ashagre, H.; Zaltzman, D.; Idan-Molakandov, A.; Romano, H.; Tzfadia, O.; Harpaz-Saad, S. FASCICLIN-LIKE 18 Is a New Player Regulating Root Elongation in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 645286. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Ancillo, G.; Legaz, F.; Primo-Millo, E.; Forner-Giner, M.A. Influence of Salinity on Pip Gene Expression in Citrus Roots and Its Relationship with Root Hydraulic Conductance, Transpiration and Chloride Exclusion from Leaves. Environ. Exp. Bot. 2012, 78, 163–166. [Google Scholar] [CrossRef]
- De Paula Santos Martins, C.; Pedrosa, A.M.; Du, D.; Gonçalves, L.P.; Yu, Q.; Gmitter, F.G.; Costa, M.G.C. Genome-Wide Characterization and Expression Analysis of Major Intrinsic Proteins during Abiotic and Biotic Stresses in Sweet Orange (Citrus Sinensis L. Osb.). PLoS ONE 2015, 10, e0138786. [Google Scholar] [CrossRef]
- Qiu, J.; McGaughey, S.A.; Groszmann, M.; Tyerman, S.D.; Byrt, C.S. Phosphorylation Influences Water and Ion Channel Function of AtPIP2.1. Plant Cell Environ. 2020, 43, 2428–2442. [Google Scholar] [CrossRef]
- He, Y.-N.; Peng, J.-S.; Cai, Y.; Liu, D.-F.; Guan, Y.; Yi, H.-Y.; Gong, J.-M. Tonoplast-Localized Nitrate Uptake Transporters Involved in Vacuolar Nitrate Efflux and Reallocation in Arabidopsis. Sci. Rep. 2017, 7, 6417. [Google Scholar] [CrossRef]
- Yang, X.; Han, W.; Qi, J.; Li, Y.; Chen, X.; Zhang, Y.; Wu, J.; Li, G.; Gao, J.; Dong, X. Identification of NPF Family Genes in Brassica Rapa Reveal Their Potential Functions in Pollen Development and Response to Low Nitrate Stress. Int. J. Mol. Sci. 2023, 24, 754. [Google Scholar] [CrossRef]
- Xiang, J.; Zhou, X.; Zhang, X.; Liu, A.; Xiang, Y.; Yan, M.; Peng, Y.; Chen, X. The Arabidopsis AtUNC-93 Acts as a Positive Regulator of Abiotic Stress Tolerance and Plant Growth via Modulation of ABA Signaling and K+ Homeostasis. Front. Plant Sci. 2018, 9, 718. [Google Scholar] [CrossRef]
- Shi, Y.; He, M. Differential Gene Expression Identified by RNA-Seq and QPCR in Two Sizes of Pearl Oyster (Pinctada fucata). Gene 2014, 538, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Everaert, C.; Luypaert, M.; Maag, J.L.V.; Cheng, Q.X.; Dinger, M.E.; Hellemans, J.; Mestdagh, P. Benchmarking of RNA-Sequencing Analysis Workflows Using Whole-Transcriptome RT-QPCR Expression Data. Sci. Rep. 2017, 7, 1559. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T. Do Results Obtained with RNA-Sequencing Require Independent Verification? Biofilm 2021, 3, 100043. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Gu, T.-Y.; Qi, Z.-A.; Yan, J.; Fang, Z.-J.; Lu, Y.-T.; Li, H.; Gong, J.-M. Two NPF Transporters Mediate Iron Long-Distance Transport and Homeostasis in Arabidopsis. Plant Commun. 2021, 2, 100244. [Google Scholar] [CrossRef]
- Ruiz, C.; Paz Breto, M.; Asíns, M.J. A Quick Methodology to Identify Sexual Seedlings in Citrus Breeding Programs Using SSR Markers. Euphytica 2000, 112, 89–94. [Google Scholar] [CrossRef]
- Asins, M.J.; Raga, M.V.; Roca, D.; Carbonell, E.A. QTL and Candidate Gene Analyses of Rootstock-Mediated Mandarinfruit Yield and Quality Traits under Contrasting Iron Availabilities. Tree Genet. Genomes 2020, 16, 79. [Google Scholar] [CrossRef]
- Gilliam, J.W. Rapid Measurement of Chlorine in Plant Materials. Soil Sci. Soc. Am. J. 1971, 35, 512–513. [Google Scholar] [CrossRef]
- Laitinen, R.A.E.; Nikoloski, Z. Genetic Basis of Plasticity in Plants. J. Exp. Bot. 2019, 70, 739–745. [Google Scholar] [CrossRef]
- Des Marais, D.L.; Hernandez, K.M.; Juenger, T.E. Genotype-by-Environment Interaction and Plasticity: Exploring Genomic Responses of Plants to the Abiotic Environment. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 5–29. [Google Scholar] [CrossRef]
- Villalta, I.; Bernet, G.P.; Carbonell, E.A.; Asins, M.J. Comparative QTL Analysis of Salinity Tolerance in Terms of Fruit Yield Using Two Solanum Populations of F7 Lines. Theor. Appl. Genet. 2007, 114, 1001–1017. [Google Scholar] [CrossRef]
- Van Ooijen, J.W. MapQTL® 6, Software for the Mapping of Quantitative Trait Loci in Experimental Populations of Diploid Species; Kyazma BV: Wageningen, The Netherlands, 2009. [Google Scholar]
- Grattapaglia, D.; Sederoff, R. Genetic Linkage Maps of Eucalyptus grandis and Eucalyptus urophylla Using a Pseudo-Testcross: Mapping Strategy and RAPD Markers. Genetics 1994, 137, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Van Ooijen, J.W. JoinMap® 4, Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Kyazma BV: Wageningen, The Netherlands, 2006. [Google Scholar]
- Ben Yahmed, J.; Costantino, G.; Amiel, P.; Talon, M.; Ollitrault, P.; Morillon, R.; Luro, F. Diversity in the Trifoliate Orange Taxon Reveals Two Main Genetic Groups Marked by Specific Morphological Traits and Water Deficit Tolerance Properties. J. Agric. Sci. 2016, 154, 495–514. [Google Scholar] [CrossRef]
- Herrero, R.; Asíns, M.J.; Pina, J.A.; Carbonell, E.A.; Navarro, L. Genetic Diversity in the Orange Subfamily Aurantioideae. II. Genetic Relationships among Genera and Species. Theoret. Appl. Genet. 1996, 93, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the Origin and Evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. AgriGO v2.0: A GO Analysis Toolkit for the Agricultural Community, 2017 Update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve Years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Patterson, M.; Marschall, T.; Pisanti, N.; van Iersel, L.; Stougie, L.; Klau, G.W.; Schönhuth, A. WhatsHap: Weighted Haplotype Assembly for Future-Generation Sequencing Reads. J. Comput. Biol. 2015, 22, 498–509. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A Multiple Sequence Alignment Method with Reduced Time and Space Complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Romero-Aranda, M.R.; Espinosa, J.; González-Fernández, P.; Jaime-Fernández, E.; Traverso, J.Á.; Asins, M.J.; Belver, A. Role of Na+ Transporters HKT1;1 and HKT1;2 in Tomato Salt Tolerance. I. Function Loss of Cheesmaniae Alleles in Roots and Aerial Parts. Plant Physiol. Biochem. 2021, 168, 282–293. [Google Scholar] [CrossRef]
- Liao, Z.; Chen, M.; Guo, L.; Gong, Y.; Tang, F.; Sun, X.; Tang, K. Rapid Isolation of High-Quality Total RNA from Taxus and Ginkgo. Prep. Biochem. Biotechnol. 2004, 34, 209–214. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Bullones, A.; Lima-Cabello, E.; Fernández-Pozo, N.; Castro, A.J.; Alché, J.d.D.; Claros, M.G. New Insights of Pollen Tube Growth in Olea Europaea L. Subsp. Europaea by Means of Bioinformatic Transcriptome Profiling. Plants 2023, 12, 2894. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ge, X.; Peng, F.; Li, W.; Li, J.J. Exaggerated False Positives by Popular Differential Expression Methods When Analyzing Human Population Samples. Genome Biol. 2022, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Smyth, G.K. Testing Significance Relative to a Fold-Change Threshold Is a TREAT. Bioinformatics 2009, 25, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Altman, N.; Krzywinski, M. P Values and the Search for Significance. Nat. Methods 2017, 14, 3–4. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists Rise up against Statistical Significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef]
- Emamjomeh, A.; Saboori Robat, E.; Zahiri, J.; Solouki, M.; Khosravi, P. Gene Co-Expression Network Reconstruction: A Review on Computational Methods for Inferring Functional Information from Plant-Based Expression Data. Plant Biotechnol. Rep. 2017, 11, 71–86. [Google Scholar] [CrossRef]
- Johnson, K.A.; Krishnan, A. Robust Normalization and Transformation Techniques for Constructing Gene Coexpression Networks from RNA-Seq Data. Genome Biol. 2022, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Sia, J.; Zhang, W.; Jonckheere, E.; Cook, D.; Bogdan, P. Inferring Functional Communities from Partially Observed Biological Networks Exploiting Geometric Topology and Side Information. Sci. Rep. 2022, 12, 10883. [Google Scholar] [CrossRef] [PubMed]
- Usadel, B.; Obayashi, T.; Mutwil, M.; Giorgi, F.M.; Bassel, G.W.; Tanimoto, M.; Chow, A.; Steinhauser, D.; Persson, S.; Provart, N.J. Co-Expression Tools for Plant Biology: Opportunities for Hypothesis Generation and Caveats. Plant Cell Environ. 2009, 32, 1633–1651. [Google Scholar] [CrossRef]
- Kleinberg, J.M. Authoritative Sources in a Hyperlinked Environment. J. ACM 1999, 46, 604–632. [Google Scholar] [CrossRef]
- Buchfink, B.; Reuter, K.; Drost, H.-G. Sensitive Protein Alignments at Tree-of-Life Scale Using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
| Trait | Group | Position | Marker | LOD | ac | ad | bc | bd | PEV |
|---|---|---|---|---|---|---|---|---|---|
| Ca_L_S | 4c (R) | 0.00 | 15R,750 | 3.02 | 25,445.20 | 36,035.50 | 23,826.60 | 26,975.70 | 21.60 |
| Cl_L_S | 3b | 15.08 | C2iC1i,470 | 2.80 | 62.10 | 55.40 | 81.08 | 45.74 | 20.20 |
| Cl_L_S | 3b (Pr) | 32.51 | TAA27,235 | 3.81 | 60.53 | 51.58 | 83.46 | 54.24 | 26.50 |
| Cl_L_S | 4c (R) | 9.96 | CR23,750 | 9.08 | 38.82 | 47.32 | 90.37 | 76.14 | 52.00 |
| Cl_L_S | 3a | 34.57 | CR31,100 | 2.42 | 80.54 | 64.20 | 40.57 | 85.82 | 17.80 |
| Fe_L_C | 3b | 17.23 | 5F4R,600 | 2.62 | 77.48 | 69.74 | 65.80 | 79.66 | 19.10 |
| Fe_L_S | 4c (R) | 6.00 | 15R,750-CR23,750 | 2.60 | 71.56 | 74.68 | 54.50 | 63.11 | 19.00 |
| FW1_S | 12 (R) | 45.86 | CMS20,170-6F5R,1200 | 2.88 | 87.90 | 91.06 | 66.38 | 65.28 | 22.10 |
| K_L_S | 4c (R) | 18.96 | CR23,750-CR28,270 | 2.56 | 10,614.20 | 10,767.70 | 16,442.40 | 14,337.00 | 18.70 |
| NFp_C | 10+5b (R) | 175.19 | CMS46,190 | 2.88 | 10.51 | 11.75 | 21.28 | 12.14 | 20.80 |
| NFp_C | 4c (R) | 9.96 | CR23,750 | 2.48 | 17.07 | 17.20 | 9.39 | 12.26 | 18.10 |
| NFp_S | 3b (Pr) | 6.98 | CR71,310 | 4.25 | 10.52 | 11.93 | 6.54 | 18.84 | 29.10 |
| NFp_S | 4c (R) | 19.96 | CR23,750-CR28,270 | 5.18 | 15.72 | 13.46 | 5.11 | 8.09 | 34.20 |
| SSC1_C | 4c (R) | 14.96 | CR23,750-CR28,270 | 2.39 | 8.89 | 9.08 | 8.23 | 8.63 | 18.70 |
| SSC1_S | 12 (R) | 25.84 | CHI_M598-6F5R,1200 | 2.95 | 8.57 | 8.76 | 9.66 | 9.24 | 22.60 |
| SSC1_S | 4c (R) | 18.96 | CR23,750-CR28,270 | 7.10 | 9.77 | 9.63 | 8.06 | 9.05 | 46.00 |
| SSC2_C | 4b (R) | 90.52 | 520AR,350-Py65C,506 | 3.52 | 8.46 | 8.68 | 9.36 | 7.96 | 26.80 |
| SSC2_S | 4c (R) | 27.78 | CR28,270-CR15,1025 | 3.05 | 9.26 | 9.55 | 8.53 | 8.67 | 23.60 |
| TFW_C | 4b (Pr) | 30.43 | CR72,260 | 3.26 | 782.95 | 1342.33 | 1056.52 | 1392.10 | 23.20 |
| TFW_C | 3b (Pr) | 17.23 | 5F4R,600 | 2.85 | 1181.72 | 1420.13 | 911.87 | 1655.12 | 20.60 |
| TFW_C | 4c (R) | 9.96 | CR23,750 | 4.08 | 1481.83 | 1429.62 | 719.21 | 1098.06 | 28.10 |
| TFW_S | 3b (Pr) | 8.90 | C8iC1rt,650 | 3.42 | 769.45 | 922.67 | 512.03 | 1182.29 | 24.10 |
| TFW_S | 4c (R) | 14.96 | CR23,750-CR28,270 | 5.61 | 1115.86 | 983.97 | 387.39 | 589.08 | 36.50 |
| Exp/E | Trait | Cleopatra | Trifoliate | 90 (SS) | 107 (ST) | Pop |
|---|---|---|---|---|---|---|
| A1 | 1.3 ± 0.0 | 1.8 ± 0.2 | 1.1 ± 0.0 | 1.1 ± 0.1 | 1.3 ± 0.0 | |
| A2 | 0.8 ± 0.0 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.0 | |
| Ca_L | 28,639.1 ± 5688.0 | 30,049.4 ± 4049.4 | 17,460.5 ± 3035.6 | 33,967.9 ± 9743.0 | 28,109.6 ± 619.6 | |
| Cl_L | 10.2 ± 1.3 | 20.7 ± 3.5 | 17.5 ± 7.5 | 12.8 ± 2.3 | 13.3 ± 0.4 | |
| Fe_L | 55.8 ± 2.4 | 103.5 ± 6.0 | 82.7 ± 50.2 | 62.7 ± 8.0 | 72.6 ± 1.7 | |
| FW1 | 97.8 ± 21.9 | 53.5 ± 15.9 | 77.3 ± 18.0 | 122.8 ± 50.1 | 89.7 ± 3.3 | |
| GP/C | FW2 | 63.9 ± 0.0 | 71.1 ± 16.3 | 79.5 ± 24.6 | 158.1 ± 70.3 | 105.6 ± 3.7 |
| K_L | 17,095.0 ± 2611.4 | 12,537.4 ± 1676.2 | 14,715.9 ± 5314.0 | 14,645.5 ± 2101.2 | 16,843.6 ± 378.1 | |
| Na_L | 1111.6 ± 439.0 | 635.6 ± 106.5 | 498.0 ± 193.8 | 641.8 ± 152.09 | 665.3 ± 34.2 | |
| NFp | 6.7 ± 3.3 | 11.7 ± 2.3 | 27.5 ± 8.5 | 10.0 ± 7.0 | 13.7 ± 0.9 | |
| SSC1 | 8.5 ± 0.3 | 9.7 ± 0.1 | 8.2 ± 1.1 | 8.1 ± 0.2 | 8.7 ± 0.1 | |
| SSC2 | 7.2 ± 0.0 | 9.8 ± 0.8 | 8.6 ± 0.9 | 7.5 ± 0.0 | 8.7 ± 0.1 | |
| TChl | 12.8 ± 0.3 | 14.6 ± 0.6 | 15.6 ± 0.9 | 15.2 ± 1.7 | 14.5 ± 0.1 | |
| TFW | 545.8 ± 172.5 | 672.6 ± 75.5 | 1894.6 ± 252.6 | 1006.3 ± 432.2 | 1166.9 ± 64.1 | |
| A1 | 1.4 ± 0.3 | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.1 | 1.5 ± 0.1 | |
| A2 | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.6 ± 0.0 | 0.9 ± 0.1 | 1.0 ± 0.0 | |
| Ca_L | 23,834.1 ± 2561.0 | 17,674.6 ± 339.9 | 29,286.7 ± 7462.5 | 19,806.7 ± 2339.4 | 28,302.8 ± 996.4 | |
| Cl_L | 74.0 ± 7.1 | 110.0 ± 21.6 | 72.3 ± 2.3 | 31.3 ± 1.4 | 66.0 ± 3.5 | |
| Fe_L | 52.6 ± 2.3 | 48.7 ± 2.8 | 86.6 ± 12.2 | 83.7 ± 27.1 | 65.6 ± 2.0 | |
| FW1 | 76.0 ± 14.4 | 91.5 ± 21.1 | 134.4 ± 58.6 | 83.6 ± 9.1 | 75.5 ± 3.2 | |
| GP/S | FW2 | 112.3 ± 27.9 | 59.1 ± 3.1 | 88.3 ± 0.0 | 91.2 ± 12.7 | 82.5 ± 3.4 |
| K_L | 13,709.9 ± 2305.3 | 21,367.5 ± 1700.9 | 16,603.8 ± 4939.4 | 13,042.6 ± 1501.2 | 13,213.8 ± 601.1 | |
| Na_L | 8020.5 ± 348.5 | 4091.0 ± 179.3 | 2541.9 ± 268.4 | 3162.0 ± 348.4 | 3910.2 ± 181.9 | |
| NFp | 5.7 ± 2.2 | 5.7 ± 2.7 | 7.0 ± 6.0 | 15.0 ± 5.0 | 10.1 ± 0.8 | |
| SSC1 | 9.6 ± 0.2 | 8.3 ± 0.3 | 7.9 ± 0.6 | 9.4 ± 0.4 | 9.1 ± 0.1 | |
| SSC2 | 9.1 ± 0.4 | 7.7 ± 0.4 | 7.8 ± 0.0 | 8.5 ± 0.4 | 8.9 ± 0.1 | |
| TChl | 11.4 ± 2.1 | 10.5 ± 2.9 | 15.3 ± 0.1 | 15.5 ± 0.7 | 13.8 ± 0.3 | |
| TFW | 434.6 ± 54.5 | 407.6 ± 133.1 | 670.0 ± 477.0 | 1352.8 ± 413.3 | 729.0 ± 51.7 | |
| C_L | 39.4 ± 0.9 | 41.8 ± 0.7 | 36.2 ± 1.5 | 36.1 ± 0.5 | 39.0 ± 0.2 | |
| C_R | 44.4 ± 0.0 | 42.1 ± 0.7 | 43.4 ± 0.7 | 43.3 ± 0.2 | 43.1 ± 0.2 | |
| Ca_L | 23,932.3 ± 2685.1 | 20,284.0 ± 1305.0 | 26,355.3 ± 1455.1 | 20,183.0 ± 216.0 | 22,202.5 ± 461.4 | |
| Ca_R | 8955.0 ± 265.1 | 9642.0 ± 893.2 | 9293.5 ± 197.5 | 8797.0 ± 927.0 | 10,153.9 ± 290.9 | |
| Cl_L | 5.8 ± 0.4 | 16.3 ± 1.9 | 7.0 ± 0.7 | 6.6 ± 0.5 | 7.4 ± 0.5 | |
| Cl_R | 26.8 ± 1.0 | 17.7 ± 2.9 | 23.1 ± 1.3 | 20.2 ± 1.6 | 25.1 ± 0.6 | |
| ClR-ClL/ClR | 0.8 ± 0.0 | 0.1 ± 0.1 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | |
| NG/C | Fe_L | 153.3 ± 6.5 | 166.3 ± 10.5 | 861.7 ± 636.3 | 152.3 ± 30.7 | 205.6 ± 21.3 |
| Fe_R | 900.3 ± 129.8 | 2225.3 ± 562.0 | 928.0 ± 113.0 | 985.5 ± 458.5 | 1321.2 ± 163.7 | |
| FRDW | 4.0 ± 0.5 | 6.7 ± 2.1 | 4.3 ± 1.3 | 3.5 ± 0.3 | 6.3 ± 0.3 | |
| K_L | 41,733.3 ± 1295.0 | 31,028.0 ± 1691.3 | 48,567.3 ± 3066.9 | 57,188.3 ± 1655.2 | 48,037.6 ± 1198.6 | |
| K_R | 18,175.7 ± 1315.7 | 26,780.7 ± 1087.7 | 31,479.5 ± 3552.5 | 18,780.0 ± 2566.0 | 22,892.4 ± 539.7 | |
| N_L | 3.2 ± 0.2 | 2.7 ± 0.3 | 3.9 ± 0.9 | 4.4 ± 0.6 | 3.3 ± 0.1 | |
| N_R | 1.8 ± 0.0 | 1.9 ± 0.1 | 2.3 ± 0.3 | 1.9 ± 0.1 | 2.2 ± 0.0 | |
| Na_L | 1767.0 ± 154.4 | 360.3 ± 139.4 | 946.3 ± 330.3 | 817.3 ± 109.5 | 1073.0 ± 91.5 | |
| Na_R | 1142.0 ± 196.7 | 381.7 ± 86.5 | 960.0 ± 134.0 | 290.5 ± 15.5 | 864.7 ± 56.8 | |
| TRDW | 15.5 ± 0.7 | 17.5 ± 3.6 | 15.6 ± 2.4 | 14.7 ± 0.3 | 20.0 ± 0.6 | |
| C_L | 42.8 ± 0.3 | 37.8 ± 1.2 | 36.7 ± 1.1 | 39.2 ± 0.4 | 39.1 ± 0.2 | |
| C_R | 44.5 ± 0.3 | 43.0 ± 0.3 | 43.6 ± 0.3 | 43.3 ± 0.2 | 43.2 ± 0.1 | |
| Ca_L | 19,948.0 ± 772.0 | 19,171.3 ± 726.3 | 24,257.0 ± 429.1 | 14,560.0 ± 1743.0 | 22,202.5 ± 461.4 | |
| Ca_R | 6161.5 ± 697.5 | 7710.7 ± 146.0 | 7839.5 ± 68.5 | 7969.0 ± 588.0 | 7498.5 ± 122.2 | |
| Cl_L | 44.5 ± 18.5 | 137.0 ± 15.0 | 116.0 ± 18.4 | 25.4 ± 3.8 | 92.0 ± 8.0 | |
| Cl_R | 54.5 ± 14.5 | 76.0 ± 1.5 | 88.0 ± 7.8 | 90.8 ± 6.3 | 82.2 ± 1.8 | |
| ClR-ClL/ClR | 0.2 ± 0.1 | −0.8 ± 0.2 | −0.2 ± 0.3 | 0.7 ± 0.0 | −0.2 ± 0.1 | |
| NG/S | Fe_L | 148.5 ± 12.5 | 1029.7 ± 806.2 | 1154.3 ± 947.0 | 162.3 ± 17.3 | 261.8 ± 27.5 |
| Fe_R | 534.0 ± 89.0 | 539.3 ± 175.4 | 905.5 ± 114.5 | 1031.5 ± 30.5 | 835.7 ± 50.5 | |
| FRDW | 8.8 ± 2.9 | 3.7 ± 1.0 | 10.2 ± 0.1 | 4.7 ± 0.7 | 7.5 ± 0.4 | |
| K_L | 29,310.5 ± 3005.5 | 43,560.7 ± 2852.0 | 44,553.3 ± 1380.2 | 41,241.7 ± 939.9 | 42,725.8 ± 900.6 | |
| K_R | 11,289.5 ± 1351.5 | 27,783.3 ± 1288.0 | 19,544.5 ± 471.5 | 22,475.5 ± 1169.5 | 19,709.1 ± 482.1 | |
| N_L | 2.8 ± 0.4 | 3.0 ± 0.4 | 2.5 ± 0.0 | 3.2 ± 0.2 | 2.9 ± 0.1 | |
| N_R | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.1 | 1.8 ± 0.2 | 1.9 ± 0.0 | |
| Na_L | 10,553.0 ± 377.0 | 8323.3 ± 1149.8 | 6783.3 ± 881.0 | 3609.7 ± 579.2 | 7082.8 ± 474.7 | |
| Na_R | 3252.0 ± 1838.0 | 2518.0 ± 193.4 | 4180.5 ± 474.5 | 3012.0 ± 734.0 | 3490.3 ± 167.1 | |
| TRDW | 20.6 ± 3.1 | 13.5 ± 1.7 | 22.9 ± 0.2 | 16.2 ± 0.7 | 21.0 ± 0.6 |
| Trait | LG | cM | Marker | LOD | ac | ad | bc | bd | PEV |
|---|---|---|---|---|---|---|---|---|---|
| (CaR-CaL/CaR)_S | 4c (R) | 9.96 | CR23,750 | 3.31 | −1.48 | −1.44 | −2.30 | −2.25 | 30.40 |
| (ClR-ClL/ClR)_S | 4c (R) | 20.96 | CR23-CR28 | 4.21 | 0.18 | 0.55 | −0.49 | −0.57 | 37.00 |
| C_L_S | 4c (R) | 19.96 | CR23-CR28 | 2.50 | 39.62 | 40.55 | 38.86 | 38.37 | 24.00 |
| Ca_L_C | 3a | 0.00 | CL2.26,395 | 2.49 | 25,124.90 | 22,355.50 | 20,850.10 | 21,678.50 | 23.90 |
| Ca_L_S | 4c (R) | 10.96 | CR23,750 | 4.50 | 18,996.90 | 18,213.40 | 25,142.40 | 23,567.90 | 39.00 |
| Cl_L_S | 4c (R) | 19.96 | CR23-CR28 | 4.58 | 57.72 | 36.56 | 121.88 | 116.64 | 39.40 |
| Cl_R_S | 7 | 128.19 | CR76,1400 | 3.86 | 77.15 | 79.01 | 79.29 | 94.48 | 34.50 |
| dCa_L | 4c (R) | 7.00 | 15R-CR23 | 2.95 | −0.10 | −0.10 | 0.12 | 0.03 | 27.70 |
| dCl_L | 4c (R) | 11.27 | CR23-CR28 | 5.82 | 6.63 | 4.61 | 13.60 | 15.66 | 47.20 |
| dCl_R | 7 | 128.19 | CR76,1400 | 3.86 | 76.15 | 78.01 | 78.29 | 93.48 | 34.50 |
| Fe_L_S | 2 (Pr) | 239.94 | Mybg2,210 | 3.94 | 527.66 | 161.57 | 267.11 | 137.05 | 35.10 |
| Fe_L_S | 4c | 0.00 | 15R,750 | 2.36 | 223.31 | 189.91 | 486.04 | 215.06 | 22.80 |
| Fe_R_C | 7 | 60.62 | CL1.35-COR15 | 4.58 | 1147.92 | 750.15 | 942.96 | 3678.05 | 39.50 |
| Fe_R_C | 7 | 83.01 | 24R,950 | 3.68 | 1135.87 | 968.40 | 972.75 | 2557.25 | 33.20 |
| Fe_R_S | 3a | 7.00 | CL2.26,395 | 2.53 | 411.11 | 977.91 | 963.37 | 802.94 | 24.20 |
| Fe_R_S | 3b (R) | 6.98 | CR71,310 | 2.78 | 1004.75 | 778.82 | 756.57 | 461.35 | 26.30 |
| FRDW_C | 2 | 153.08 | CR19,370 | 3.47 | 5.70 | 4.12 | 8.26 | 6.63 | 31.70 |
| FRDW_C | 4c (R) | 7.00 | 15R-CR23 | 2.88 | 4.11 | 5.29 | 6.73 | 7.24 | 27.10 |
| K_L_C | 7 (R) | 0.00 | Myc2(HaeIII),480 | 3.67 | 49,804.20 | 56,643.20 | 43,620.80 | 46,139.00 | 33.10 |
| K_L_C | 10+5b | 46.81 | TAA41,160 | 4.31 | 53,039.40 | 41,858.30 | 44,812.40 | 52,128.20 | 37.70 |
| K_L_C | 3a (Pr) | 34.57 | CR31,100 | 2.98 | 38,263.80 | 54,458.60 | 49,637.80 | 47,656.50 | 27.90 |
| K_L_S | 7 | 24.43 | CR41-CR20 | 3.40 | 47,939.30 | 41,816.10 | 38,534.00 | 44,202.00 | 31.10 |
| K_L_S | 4b (R) | 66.24 | CR3,320 | 3.57 | 47,744.90 | 42,798.50 | 40,029.70 | 37,750.10 | 32.40 |
| N_R_C | 10+5b | 35.45 | 5F6R,1550 | 3.57 | 2.36 | 2.12 | 2.08 | 2.06 | 32.40 |
| N_R_S | 2 | 153.08 | CR19,370 | 2.80 | 1.80 | 1.77 | 1.94 | 1.87 | 26.40 |
| Na_R_C | 7 | 28.43 | CR20 | 3.82 | 1218.08 | 655.55 | 720.82 | 954.38 | 34.20 |
| Na_R_S | 7 (R) | 83.01 | 24R,950 | 3.93 | 3046.92 | 3083.47 | 3743.36 | 4692.49 | 35.00 |
| TRDW_C | 2 | 153.08 | CR19,370 | 3.02 | 18.82 | 16.53 | 23.37 | 20.40 | 28.20 |
| TRDW_C | 4c (R) | 23.96 | CR23-CR28 | 3.45 | 14.06 | 20.32 | 22.04 | 20.98 | 31.50 |
| mRNA | Start | DEG in | Description |
|---|---|---|---|
| Ciclev10013718m | 9543190 | PTHR23515:SF3—HIGH AFFINITY NITRATE TRANSPORTER 2.5 | |
| Ciclev10011188m | 10089075 | PTHR10217//PTHR10217:SF494—VOLTAGE AND LIGAND-GATED POTASSIUM CHANNEL | |
| Ciclev10012379m | 10374766 | PTHR19139:SF167—AQUAPORIN PIP2-1-RELATED | |
| Ciclev10012633m | 10396990 | PTHR19139:SF167—AQUAPORIN PIP2-1-RELATED | |
| Ciclev10012375m | 10419232 | 3 | PTHR19139:SF167—AQUAPORIN PIP2-1-RELATED |
| Ciclev10011234m | 12362572 | PTHR19241:SF258—ABC TRANSPORTER G FAMILY MEMBER 17-RELATED | |
| Ciclev10013485m | 12469611 | 3 | PTHR19241:SF258—ABC TRANSPORTER G FAMILY MEMBER 17-RELATED |
| Ciclev10011167m | 12478048 | 3 | PTHR19241:SF258—ABC TRANSPORTER G FAMILY MEMBER 17-RELATED |
| Ciclev10011147m | 12497442 | 3 | PTHR19241:SF258—ABC TRANSPORTER G FAMILY MEMBER 17-RELATED |
| Ciclev10010954m | 12508551 | 3 | PTHR24093:SF289—CALCIUM-TRANSPORTING ATPASE 1 |
| Ciclev10011060m | 13305253 | 3 | PTHR32468:SF10—CATION/H(+) ANTIPORTER 20 |
| Ciclev10011092m | 13371258 | PTHR32468:SF10—CATION/H(+) ANTIPORTER 20 | |
| Ciclev10011096m | 13412581 | PTHR32468:SF10—CATION/H(+) ANTIPORTER 20 | |
| Ciclev10011745m | 13446344 | 1, 2, 3 | PTHR19444—UNC-93 RELATED—Major facilitator superfamily protein |
| Ciclev10013496m | 15046032 | PTHR11654:SF181—PROTEIN NRT1/ PTR FAMILY 2.8 (NPF2.3) | |
| Ciclev10011341m | 15116860 | 1, 3 | PTHR11654:SF79—PROTEIN NRT1/ PTR FAMILY 5.5-RELATED (NPF5.12) |
| Ciclev10011514m | 15540132 | PTHR11662:SF235—ANION TRANSPORTER 3, CHLOROPLASTIC-RELATED | |
| Ciclev10011381m | 15747188 | 1, 3 | PTHR11654//PTHR11654:SF125—OLIGOPEPTIDE TRANSPORTER-RELATED (NPF8.1) |
| Ciclev10013821m | 15754314 | 3 | PTHR11654//PTHR11654:SF125—OLIGOPEPTIDE TRANSPORTER-RELATED (NPF8.2) |
| Ciclev10013337m | 15843855 | 1, 2, 3 | PTHR11654:SF79—PROTEIN NRT1/ PTR FAMILY 5.5-RELATED (NPF5.9) |
| Ciclev10013636m | 15862182 | KOG1237—H+/oligopeptide symporter (NPF5.8) | |
| Ciclev10013488m | 15867971 | PTHR11654:SF79—PROTEIN NRT1/ PTR FAMILY 5.5-RELATED (NPF5.10) | |
| Ciclev10012384m | 15896416 | PTHR19139:SF169—AQUAPORIN PIP1-4-RELATED |
| Comparison | DEGs iDEP | DEGs RSeqFlow Transcriptome | DEG RSeqFlow QTL | ||
|---|---|---|---|---|---|
| Transcriptome | eBayes | Treat | eBayes | Treat | |
| 107_15 vs. 107_0 | 7 | 0 | 0 | 2 | 0 |
| 90_15 vs. 90_0 | 45 | 0 | 0 | 0 | 0 |
| 107_0 vs. 90_0 | 1201 | 1955 | 344 | 62 | 21 |
| 107_15 vs. 90_15 | 670 | 881 | 297 | 16 | 8 |
| (107_15 + 107_0) vs. (90_15 + 90_0) | 1501 | 5505 | 2400 | 130 | 92 |
| (107_15 + 90_15) vs. (107_0 + 90_0) | 42 | 0 | 0 | 14 | 0 |
| (107_15 − 90_15) vs. (107_0 − 90_0) | na | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asins, M.J.; Bullones, A.; Raga, V.; Romero-Aranda, M.R.; Espinosa, J.; Triviño, J.C.; Bernet, G.P.; Traverso, J.A.; Carbonell, E.A.; Claros, M.G.; et al. Combining Genetic and Transcriptomic Approaches to Identify Transporter-Coding Genes as Likely Responsible for a Repeatable Salt Tolerance QTL in Citrus. Int. J. Mol. Sci. 2023, 24, 15759. https://doi.org/10.3390/ijms242115759
Asins MJ, Bullones A, Raga V, Romero-Aranda MR, Espinosa J, Triviño JC, Bernet GP, Traverso JA, Carbonell EA, Claros MG, et al. Combining Genetic and Transcriptomic Approaches to Identify Transporter-Coding Genes as Likely Responsible for a Repeatable Salt Tolerance QTL in Citrus. International Journal of Molecular Sciences. 2023; 24(21):15759. https://doi.org/10.3390/ijms242115759
Chicago/Turabian StyleAsins, Maria J., Amanda Bullones, Veronica Raga, Maria R. Romero-Aranda, Jesus Espinosa, Juan C. Triviño, Guillermo P. Bernet, Jose A. Traverso, Emilio A. Carbonell, M. Gonzalo Claros, and et al. 2023. "Combining Genetic and Transcriptomic Approaches to Identify Transporter-Coding Genes as Likely Responsible for a Repeatable Salt Tolerance QTL in Citrus" International Journal of Molecular Sciences 24, no. 21: 15759. https://doi.org/10.3390/ijms242115759








