Interaction of Methylene Blue with Severe Acute Respiratory Syndrome Coronavirus 2 Envelope Revealed by Molecular Modeling
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemdan, S.S. The shift in the behavior of methylene blue toward the sensitivity of medium: Solvatochromism, solvent parameters, regression analysis and investigation of cosolvent on the acidity constants. J. Fluoresc. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Crossley, K.B. Methylene blue—A therapeutic dye for all seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and molecular actions of methylene blue in the nervous system. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Nedu, M.-E.; Tertis, M.; Cristea, C.; Georgescu, A.V. Comparative study regarding the properties of methylene blue and proflavine and their optimal concentrations for in vitro and in vivo applications. Diagnostics 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Hanekamp, T.; Stayton, M.M. Methylene blue: An alternative, multi-purpose stain for detection, analysis and isolation of nucleic acids. Biopolym. Cell. 1997, 13, 250–253. [Google Scholar] [CrossRef]
- Schmidt, T.F.; Caseli, L.; Oliveira, O.N., Jr.; Itri, R. Binding of methylene blue onto Langmuir monolayers representing cell membranes may explain its efficiency as photosensitizer in photodynamic therapy. Langmuir 2015, 31, 4205–4212. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Sapra, A. Gram Staining; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ghiringhelli, P.D.; Romanowski, V. Quick methylene blue staining for visualizing virus plaques in titration experiments. Biotechniques 1994, 17, 464–465. [Google Scholar] [PubMed]
- Yaroslavsky, A.N.; Feng, X.; Muzikansky, A.; Hamblin, M.R. Fluorescence polarization of methylene blue as a quantitative marker of breast cancer at the cellular level. Sci. Rep. 2019, 9, 940. [Google Scholar] [CrossRef]
- Wan, F.-Y.; Zhang, G.-J. Enhancement of lysosomal proton permeability induced by photooxidation of membrane thiol groups. Arch. Biochem. Biophys. 2002, 402, 268–274. [Google Scholar] [CrossRef]
- Klosowski, E.M.; de Souza, B.T.L.; Mito, M.S.; Constantin, R.P.; Mantovanelli, G.C.; Mewes, J.M.; Bizzera, P.F.V.; da Costa Menezes, P.V.M.; Gilglioni, E.H.; Utsunomiya, K.S.; et al. The photodynamic and direct actions of methylene blue on mitochondrial energy metabolism: A balance of the useful and harmful effects of this photosensitizer. Free Radic. Biol. Med. 2020, 153, 34–53. [Google Scholar] [CrossRef]
- Zhukhovitsky, V.; Shevlyagina, N.; Zubasheva, M.; Russu, L.; Gushchin, V.; Meerovich, G.; Strakhovskaya, M. Infectivity and morphology of bovine coronavirus inactivated in vitro by cationic photosensitizers. Viruses 2022, 14, 1053. [Google Scholar] [CrossRef] [PubMed]
- Gendrot, M.; Andreani, J.; Duflot, I.; Boxberger, M.; Le Bideau, M.; Mosnier, J.; Jardot, P.; Fonta, I.; Rolland, C.; Bogreau, H.; et al. Methylene blue inhibits replication of SARS-CoV-2 in vitro. Int. J. Antimicrob. Agents 2020, 56, 106202. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Nagbanshi, M.; Goldau, N.; Mendes Jorge, M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Mueller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Medaglia, C.; Cerny, A.; Cerny, T.; Zwygart, A.C.A.; Cerny, E.; Tapparel, C. Methylene Blue has a potent antiviral activity against SARS-CoV-2 and H1N1 influenza virus in the absence of UV-activation in vitro. Sci. Rep. 2021, 11, 14295. [Google Scholar] [CrossRef] [PubMed]
- Fryk, J.J.; Marks, D.C.; Hobson-Peters, J.; Prow, N.A.; Watterson, D.; Hall, R.A.; Young, P.R.; Reichenberg, S.; Sumian, C.; Faddy, H.M. Dengue and chikungunya viruses in plasma are effectively inactivated after treatment with methylene blue and visible light. Transfusion 2016, 56, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Müller, T.H.; Seltsam, A. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion 2018, 58, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin, M.R. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212. [Google Scholar] [CrossRef] [PubMed]
- Muehler, D.; Brandl, E.; Hiller, K.-A.; Cieplik, F.; Maisch, T. Membrane damage as mechanism of photodynamic inactivation using Methylene blue and TMPyP in Escherichia coli and Staphylococcus aureus. Photochem. Photobiol. Sci. 2022, 21, 209–220. [Google Scholar] [CrossRef]
- Soares, J.C.M.; Luiz, M.T.; Junior, J.A.O.; Besegato, J.F.; de Melo, P.B.G.; de Souza Rastelli, A.N.; Chorilli, M. Antimicrobial photodynamic therapy mediated by methylene blue-loaded polymeric micelles against Streptococcus mutans and Candida albicans biofilms. Photodiagnosis Photodyn. Ther. 2023, 41, 103285. [Google Scholar] [CrossRef]
- Lutkus, L.V.; Rickenbach, S.S.; McCormick, T.M. Singlet oxygen quantum yields determined by oxygen consumption. J. Photochem. Photobiol. A Chem. 2019, 378, 131–135. [Google Scholar] [CrossRef]
- Wainwright, M. Methylene blue derivatives—Suitable photoantimicrobials for blood product disinfection? Int. J. Antimicrob. Agents 2000, 16, 381–394. [Google Scholar] [CrossRef]
- Hideki, A.; Wagner, S.J. Analysis of viral DNA, protein and envelope damage after methylene blue, phthalocyanine derivative or merocyanine 540 photosensitization. Photochem. Photobiol. 1995, 61, 402–409. [Google Scholar] [CrossRef]
- Edward, S.J.J.; Tabatabaie, T.; Maidt, L.; Smith, R.H.; Nguyen, X.; Pye, Q.; Floydet, R.A. Potential mechanisms of photodynamic inactivation of virus by methylene blue I. RNA–protein crosslinks and other oxidative lesions in Qβ Bacteriophage. Photochem. Photobiol. 1998, 67, 350–357. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.; Cunha, Â.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [PubMed]
- Seghatchian, J.; Struff, W.G.; Reichenberg, S. Main properties of the THERAFLEX MB-plasma system for pathogen reduction. Transfus. Med. Hemother. 2011, 38, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Fathy, G.; Asaad, M.K.; Rasheed, H.M. Daylight photodynamic therapy with methylene blue in plane warts: A randomized double-blind placebo-controlled study. Photodermatol. Photoimmun. Photomed. 2017, 33, 185–192. [Google Scholar] [CrossRef]
- Ramalho, K.M.; Cunha, S.R.; Gonçalves, F.; Escudeiro, G.S.; Steiner-Oliveira, C.; Horliana, A.C.R.T.; de Paula Eduardo, C. Photodynamic therapy and Acyclovir in the treatment of recurrent herpes labialis: A controlled randomized clinical trial. Photodiag. Photodyn. Ther. 2021, 33, 102093. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Hamadah, O. Association of photodynamic therapy and photobiomodulation as a promising treatment of herpes labialis: A systematic review. Photobiomodul. Photomed. Laser Surg. 2022, 40, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Antiviral photodynamic therapy: Inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagnosis Photodyn. Ther. 2021, 33, 102112. [Google Scholar] [CrossRef]
- Gendrot, M.; Jardot, P.; Delandre, O.; Boxberger, M.; Andreani, J.; Duflot, I.; Le Bideau, M.; Mosnier, J.; Fonta, I.; Hutter, S.; et al. In vitro evaluation of the antiviral activity of methylene blue alone or in combination against SARS-CoV-2. J. Clin. Med. 2021, 10, 3007. [Google Scholar] [CrossRef]
- Ravi, V.; Saxena, S.; Panda, P.S. Basic virology of SARS-CoV 2. Indian J. Med. Microbiol. 2022, 40, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.; Kholina, E.; Khruschev, S.; Kovalenko, I.; Rubin, A.; Strakhovskaya, M. What binds cationic photosensitizers better: Brownian dynamics reveals key interaction sites on spike proteins of SARS-CoV, MERS-CoV, and SARS-CoV-2. Viruses 2021, 13, 1615. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.; Kholina, E.; Khruschev, S.; Kovalenko, I.; Rubin, A.; Strakhovskaya, M. Electrostatic map of the SARS-CoV-2 virion specifies binding sites of the antiviral cationic photosensitizer. Int. J. Mol. Sci. 2022, 23, 7304. [Google Scholar] [CrossRef] [PubMed]
- Bojadzic, D.; Alcazar, O.; Buchwald, P. Methylene blue inhibits the SARS-CoV-2 spike–ACE2 protein-protein interaction—A mechanism that can contribute to its antiviral activity against COVID-19. Front. Pharmacol. 2021, 11, 2255. [Google Scholar] [CrossRef] [PubMed]
- Saud, Z.; Tyrrell, V.J.; Zaragkoulias, A.; Protty, M.B.; Statkute, E.; Rubina, A.; Bentley, K.; White, D.A.; Rodrigues, P.D.S.; Murphy, R.C.; et al. The SARS-CoV2 envelope differs from host cells, exposes procoagulant lipids, and is disrupted in vivo by oral rinses. J. Lipid Res. 2022, 63, 100208. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, F.K. The proteins of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2 or n-COV19), the cause of COVID-19. Protein J. 2020, 39, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Neuman, B.W.; Kiss, G.; Kunding, A.H.; Bhella, D.; Baksh, M.F.; Connelly, S.; Droese, B.; Klaus, J.P.; Makino, S.; Sawicki, S.G.; et al. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011, 174, 11–22. [Google Scholar] [CrossRef]
- Yao, H.; Song, Y.; Chen, Y.; Wu, N.; Xu, J.; Sun, C.; Zhang, J.; Weng, T.; Zhang, Z.; Wu, Z.; et al. Molecular architecture of the SARS-CoV-2 virus. Cell 2020, 183, 730–738. [Google Scholar] [CrossRef]
- Santos-Mendoza, T. The Envelope (E) Protein of SARS-CoV-2 as a Pharmacological Target. Viruses 2023, 15, 1000. [Google Scholar] [CrossRef]
- Artese, A.; Svicher, V.; Costa, G.; Salpini, R.; Di Maio, V.C.; Alkhatib, M.; Ambrosio, F.A.; Santoro, M.M.; Assaraf, Y.G.; Alcaro, S.; et al. Current status of antivirals and druggable targets of SARS-CoV-2 and other human pathogenic coronaviruses. Drug Resist. Updat. 2020, 53, 100721. [Google Scholar] [CrossRef]
- Villala, J. SARS-CoV-2 Protein S Fusion Peptide Is Capable of Wrapping Negatively-Charged Phospholipids. Membranes 2023, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Verdiá-Báguena, C.; Nieto-Torres, J.L.; Alcaraz, A.; DeDiego, M.L.; Enjuanes, L.; Aguilella, V.M. Analysis of SARS-CoV E protein ion channel activity by tuning the protein and lipid charge. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2026–2031. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Anti-infective dyes in the time of COVID. Dyes Pigment. 2021, 196, 109813. [Google Scholar] [CrossRef] [PubMed]
- Strakhovskaya, M.G.; Meerovich, G.A.; Kuskov, A.N.; Gonchukov, S.A.; Loschenov, V.B. Photoinactivation of coronaviruses: Going along the optical spectrum. Laser. Phys. Lett. 2020, 17, 93001. [Google Scholar] [CrossRef]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Müller, T.H.; Seltsam, A. Inactivation of three emerging viruses–severe acute respiratory syndrome coronavirus, Crimean–Congo haemorrhagic fever virus and Nipah virus—In platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang. 2020, 115, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Marrink, S.J.; Risselada, H.J.; Yefimov, S.; Tieleman, D.P.; De Vries, A.H. The MARTINI force field: Coarse grained model for biomolecular simulations. J. Phys. Chem. B 2007, 111, 7812–7824. [Google Scholar] [CrossRef] [PubMed]
- Pezeshkian, W.; Grünewald, F.; Narykov, O.; Lu, S.; Arkhipova, V.; Solodovnikov, A.; Wassenaar, T.A.; Marrink, S.J.; Korkin, D. Molecular architecture and dynamics of SARS-CoV-2 envelope by integrative modeling. Structure 2023, 31, 492–503.e7. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An automated force field topology builder (ATB) and repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Ileri Ercan, N.; Stroeve, P.; Tringe, J.W.; Faller, R. Molecular dynamics modeling of methylene blue-DOPC lipid bilayer interactions. Langmuir 2018, 34, 4314–4323. [Google Scholar] [CrossRef]
- Empereur-Mot, C.; Pesce, L.; Doni, G.; Bochicchio, D.; Capelli, R.; Perego, C.; Pavan, G.M. Swarm-CG: Automatic parametrization of bonded terms in MARTINI-based coarse-grained models of simple to complex molecules via fuzzy self-tuning particle swarm optimization. ACS Omega 2020, 5, 32823–32843. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; Van Gunsteren, W.F. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.A.; Kovalenko, I.B.; Khruschev, S.S.; Ustinin, D.M.; Antal, T.K.; Riznichenko, G.Y.; Rubin, A.B. Comparative analysis of plastocyanin–cytochrome f complex formation in higher plants, green algae and cyanobacteria. Physiol. Plant. 2019, 166, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, I.B.; Knyazeva, O.S.; Antal, T.K.; Ponomarev, V.Y.; Riznichenko, G.Y.; Rubin, A.B. Multiparticle Brownian dynamics simulation of experimental kinetics of cytochrome bf oxidation and photosystem I reduction by plastocyanin. Phiol. Plant. 2017, 161, 88–96. [Google Scholar] [CrossRef]
- Gowers, R.J.; Linke, M.; Barnoud, J.; Reddy, T.J.E.; Melo, M.N.; Seyler, S.L.; Dotson, D.L.; Domanski, J.; Buchoux, S.; Kenney, I.M.; et al. MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations. In Proceedings of the 15th Python in Science Conference, Austin, TX, USA, 11–17 July 2016; Benthall, S., Rostrup, S., Eds.; SciPy: Austin, TX, USA, 2016; pp. 98–105. [Google Scholar] [CrossRef]
- Schrödinger, L.L.C.; DeLano, W. The PyMOL Molecular Graphics System, Version 2.5. Available online: https://pymol.org/ (accessed on 23 October 2023).
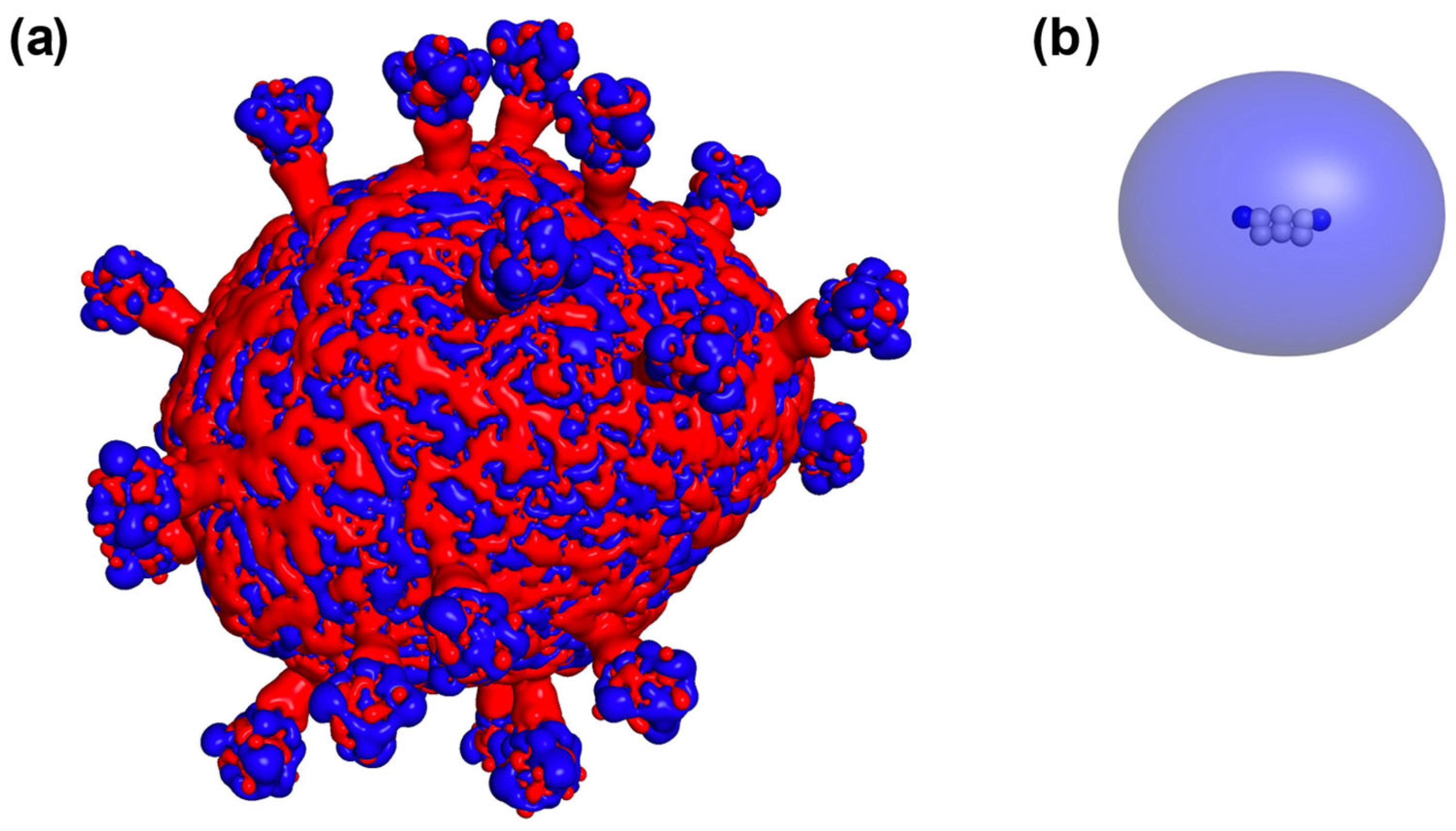

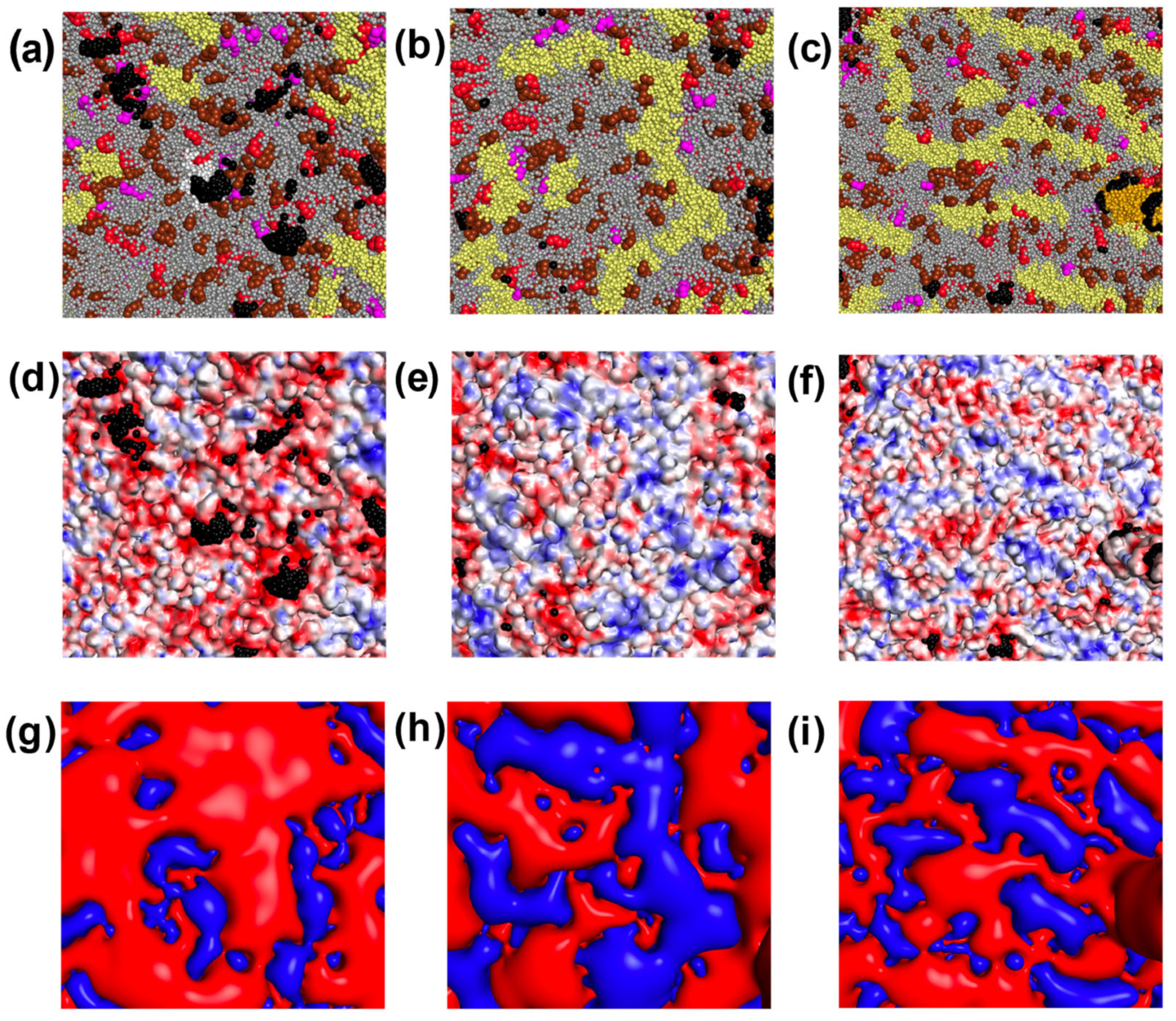
| Components of the Viral Envelope | Fraction, % | ||
|---|---|---|---|
| Proteins | S | 59.5 | |
| M | 3.2 | ||
| E | 2.0 | ||
| Lipids | Negatively charged | POPI | 17.7 |
| CDL2 | 28.2 | ||
| POPS | 4.3 | ||
| Uncharged | POPC | 31.0 | |
| POPE | 15.2 | ||
| CHOL | 0.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalenko, I.; Kholina, E.; Fedorov, V.; Khruschev, S.; Vasyuchenko, E.; Meerovich, G.; Strakhovskaya, M. Interaction of Methylene Blue with Severe Acute Respiratory Syndrome Coronavirus 2 Envelope Revealed by Molecular Modeling. Int. J. Mol. Sci. 2023, 24, 15909. https://doi.org/10.3390/ijms242115909
Kovalenko I, Kholina E, Fedorov V, Khruschev S, Vasyuchenko E, Meerovich G, Strakhovskaya M. Interaction of Methylene Blue with Severe Acute Respiratory Syndrome Coronavirus 2 Envelope Revealed by Molecular Modeling. International Journal of Molecular Sciences. 2023; 24(21):15909. https://doi.org/10.3390/ijms242115909
Chicago/Turabian StyleKovalenko, Ilya, Ekaterina Kholina, Vladimir Fedorov, Sergei Khruschev, Ekaterina Vasyuchenko, Gennady Meerovich, and Marina Strakhovskaya. 2023. "Interaction of Methylene Blue with Severe Acute Respiratory Syndrome Coronavirus 2 Envelope Revealed by Molecular Modeling" International Journal of Molecular Sciences 24, no. 21: 15909. https://doi.org/10.3390/ijms242115909
APA StyleKovalenko, I., Kholina, E., Fedorov, V., Khruschev, S., Vasyuchenko, E., Meerovich, G., & Strakhovskaya, M. (2023). Interaction of Methylene Blue with Severe Acute Respiratory Syndrome Coronavirus 2 Envelope Revealed by Molecular Modeling. International Journal of Molecular Sciences, 24(21), 15909. https://doi.org/10.3390/ijms242115909







