Mice Lacking PLAP-1/Asporin Show Alteration of Periodontal Ligament Structures and Acceleration of Bone Loss in Periodontitis
Abstract
:1. Introduction
2. Results
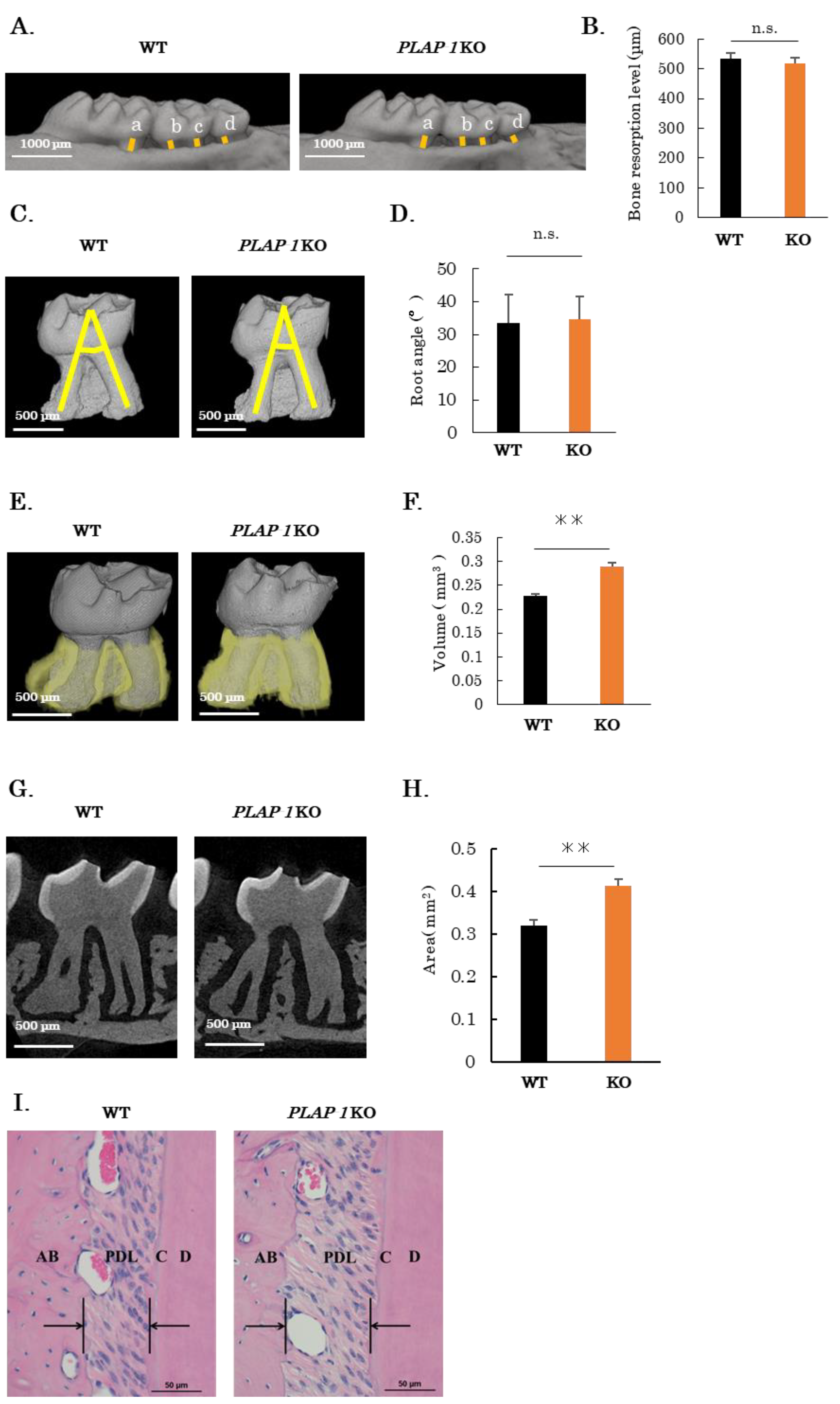
2.1. Increased PDL Space in PLAP-1 KO Mice
2.2. Altered Collagen Bundles and Extracellular Matrix Expression in the PDL of PLAP-1 KO Mice
2.3. Altered Structure and Mechanical Properties of Collagen Fibrils of PDL in PLAP-1 KO Mice
2.4. Increased Alveolar Bone Resorption in PLAP-1 KO Mice in Ligature-Induced Periodontitis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Quantification of Alveolar Bone Loss
4.3. Quantification of Root Angle and PDL Volume
4.4. Histological Analysis and Immunohistochemistry
4.5. Gene Expression Analysis
4.6. Transmission Electron Microscopic (TEM) Analysis
4.7. Periodontal Traction Model
4.8. Ligature-Induced Periodontitis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamada, S.; Murakami, S.; Matoba, R.; Ozawa, Y.; Yokokoji, T.; Nakahira, Y.; Ikezawa, K.; Takayama, S.-I.; Matsubara, K.; Okada, H.; et al. Expression profile of active genes in human periodontal ligament and isolation of PLAP-1, a novel SLRP family gene. Gene 2001, 275, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.P.; Takanosu, M.; Boyd, T.C.; Mayne, P.M.; Eberspaecher, H.; Zhou, W.; de Crombrugghe, B.; Hook, M.; Mayne, R. Expression pattern and gene characterization of asporin. a newly discovered member of the leucine-rich repeat protein family. J. Biol. Chem. 2001, 276, 12212–12221. [Google Scholar] [CrossRef]
- Yamada, S.; Tomoeda, M.; Ozawa, Y.; Yoneda, S.; Terashima, Y.; Ikezawa, K.; Ikegawa, S.; Saito, M.; Toyosawa, S.; Murakami, S. PLAP-1/asporin, a novel negative regulator of periodontal ligament mineralization. J. Biol. Chem. 2007, 282, 23070–23080. [Google Scholar] [CrossRef]
- Kizawa, H.; Kou, I.; Iida, A.; Sudo, A.; Miyamoto, Y.; Fukuda, A.; Mabuchi, A.; Kotani, A.; Kawakami, A.; Yamamoto, S.; et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat. Genet. 2005, 37, 138–144. [Google Scholar] [CrossRef]
- Beertsen, W.; McCulloch, C.A.; Sodek, J. The periodontal ligament: A unique, multifunctional connective tissue. Periodontol. 2000 1997, 13, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Berkovitz, B.K. Periodontal ligament: Structural and clinical correlates. Dent. Update 2004, 31, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kalamajski, S.; Oldberg, A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010, 29, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, S.; Goldoni, S.; Calder, B.W.; Simpson, H.C.; Owens, R.T.; McQuillan, D.J.; Young, M.F.; Iozzo, R.V.; Birk, D.E. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J. Biol. Chem. 2009, 284, 8888–8897. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.; Heinegård, D.; Oldberg, A. Decorin-binding sites for collagen type I are mainly located in leucine-rich repeats 4-5. J. Biol. Chem. 1995, 270, 20712–20716. [Google Scholar] [CrossRef]
- Keene, D.R.; San Antonio, J.D.; Mayne, R.; McQuillan, D.J.; Sarris, G.; Santoro, S.A.; Iozzo, R.V. Decorin binds near the C terminus of type I collagen. J. Biol. Chem. 2000, 275, 21801–21804. [Google Scholar] [CrossRef]
- Kalamajski, S.; Aspberg, A.; Lindblom, K.; Heinegard, D.; Oldberg, A. Asporin competes with decorin for collagen binding, binds calcium and promotes osteoblast collagen mineralization. Biochem. J. 2009, 423, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Passanezi, E.; Sant’Ana, A.C.P. Role of occlusion in periodontal disease. Periodontol. 2000 2019, 79, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A. Dental plaque-induced gingival diseases. Ann. Periodontol. 1999, 4, 7–19. [Google Scholar] [CrossRef]
- Yamaba, S.; Yamada, S.; Kajikawa, T.; Awata, T.; Sakashita, H.; Tsushima, K.; Fujihara, C.; Yanagita, M.; Murakami, S. PLAP-1/Asporin Regulates TLR2- and TLR4-induced Inflammatory Responses. J. Dent. Res. 2015, 94, 1706–1714. [Google Scholar] [CrossRef]
- Awata, T.; Yamada, S.; Tsushima, K.; Sakashita, H.; Yamaba, S.; Kajikawa, T.; Yamashita, M.; Takedachi, M.; Yanagita, M.; Kitamura, M.; et al. PLAP-1/Asporin Positively Regulates FGF-2 Activity. J. Dent. Res. 2015, 94, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Trombetta-eSilva, J.; Rosset, E.A.; Hepfer, R.G.; Wright, G.J.; Baicu, C.; Yao, H.; Bradshaw, A.D. Decreased Mechanical Strength and Collagen Content in SPARC-Null Periodontal Ligament Is Reversed by Inhibition of Transglutaminase Activity. J. Bone Miner. Res. 2015, 30, 1914–1924. [Google Scholar] [CrossRef]
- Miletich, I.; Sharpe, P.T. Normal and abnormal dental development. Hum. Mol. Genet. 2003, 12 (Suppl. 1), R69–R73. [Google Scholar] [CrossRef]
- Parry, D.A. The molecular and fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys. Chem. 1988, 29, 195–209. [Google Scholar] [CrossRef]
- Corsi, A.; Xu, T.; Chen, X.D.; Boyde, A.; Liang, J.; Mankani, M.; Sommer, B.; Iozzo, R.V.; Eichstetter, I.; Robey, P.G.; et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J. Bone Miner. Res. 2002, 17, 1180–1189. [Google Scholar] [CrossRef]
- Roeder, B.A.; Kokini, K.; Sturgis, J.E.; Robinson, J.P.; Voytik-Harbin, S.L. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J. Biomech. Eng. 2002, 124, 214–222. [Google Scholar] [CrossRef]
- Reed, C.C.; Iozzo, R.V. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2002, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Z.; Liu, S.Y.; Xu, S.Y.; Ni, G.X. Asporin and osteoarthritis. Osteoarthr. Cartil. 2015, 23, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Kizawa, H.; Saitoh, M.; Kou, I.; Miyazono, K.; Ikegawa, S. Mechanisms for asporin function and regulation in articular cartilage. J. Biol. Chem. 2007, 282, 32185–32192. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V. The family of the small leucine-rich proteoglycans: Key regulators of matrix assembly and cellular growth. Crit. Rev. Biochem. Mol. Biol. 1997, 32, 141–174. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.W.; Termine, J.D.; Dejter, S.W.; Whitson, S.W.; Yanagishita, M.; Kimura, J.H.; Hascall, V.C.; Kleinman, H.K.; Hassell, J.R.; Nilsson, B. Proteoglycans of developing bone. J. Biol. Chem. 1983, 258, 6588–6594. [Google Scholar] [CrossRef]
- Tenório, D.M.; Santos, M.F.; Zorn, T.M. Distribution of biglycan and decorin in rat dental tissue. Braz. J. Med. Biol. Res. 2003, 36, 1061–1065. [Google Scholar] [CrossRef]
- Häkkinen, L.; Westermarck, J.; Kähäri, V.M.; Larjava, H. Human granulation-tissue fibroblasts show enhanced proteoglycan gene expression and altered response to TGF-beta 1. J. Dent. Res. 1996, 75, 1767–1778. [Google Scholar] [CrossRef]
- Matheson, S.; Larjava, H.; Häkkinen, L. Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues. J. Periodontal Res. 2005, 40, 312–324. [Google Scholar] [CrossRef]
- Häkkinen, L.; Strassburger, S.; Kähäri, V.M.; Scott, P.G.; Eichstetter, I.; Lozzo, R.V.; Larjava, H. A role for decorin in the structural organization of periodontal ligament. Lab. Investig. 2000, 80, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Chiu, R.; Li, W.; Herber, R.P.; Marshall, S.J.; Young, M.; Ho, S.P. Effects of biglycan on physico-chemical properties of ligament-mineralized tissue attachment sites. Arch. Oral. Biol. 2012, 57, 177–187. [Google Scholar] [CrossRef]
- Ameye, L.; Young, M.F. Mice deficient in small leucine-rich proteoglycans: Novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 2002, 12, 107R–116R. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ozaki, N.; Tsushima, K.; Yamaba, S.; Fujihara, C.; Awata, T.; Sakashita, H.; Kajikawa, T.; Kitagaki, J.; Yamashita, M.; et al. Transcriptome Reveals Cathepsin K in Periodontal Ligament Differentiation. J. Dent. Res. 2016, 95, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Maccarana, M.; Svensson, R.B.; Knutsson, A.; Giannopoulos, A.; Pelkonen, M.; Weis, M.; Eyre, D.; Warman, M.; Kalamajski, S. Asporin-deficient mice have tougher skin and altered skin glycosaminoglycan content and structure. PLoS ONE 2017, 12, e0184028. [Google Scholar] [CrossRef]
- Deng, C.; Wynshaw-Boris, A.; Zhou, F.; Kuo, A.; Leder, P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 1996, 84, 911–921. [Google Scholar] [CrossRef]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef] [PubMed]





| GenBank Acc. | Gene | Sequence |
|---|---|---|
| NM_025711 | PLAP-1 | 5’-ATGATGACGATAACGATGATGACGA-3’ 5’-TGTTGTTTGGAACCGATGTCAGA-3’ |
| NM_007742 | Col1a1 | 5’-CAGGGTATTGCTGGACAACGTG-3’ 5’-GGACCTTGTTTGCCAGGTTCA-3’ |
| NM_009930 | Col3a1 | 5’-CAGGCCAGTGGCAATGTAAAGA-3’ 5’-CTCATTGCCTTGCGTGTTTGATA-3’ |
| NM_007542 | Bgn | 5’-GATGATTGAGAATGGGAGCCTGA-3’ 5’-TCCGAAGCCCATAGGACAGAAG-3’ |
| NM_001190451 | Den | 5’-CTGGGCTGGCACAGCATAAGTA-3’ 5’-CGGACAGGGTTGCCGTAAAG-3’ |
| NM_013556 | Hprt | 5’-TTGTTGTTGGATATGCCCTTGACTA-3’ 5’-AGGCAGATGGCCACAGGACTA-3’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, M.; Yamada, S.; Sasaki, J.; Suzuki, S.; Kajikawa, T.; Iwayama, T.; Fujihara, C.; Imazato, S.; Murakami, S. Mice Lacking PLAP-1/Asporin Show Alteration of Periodontal Ligament Structures and Acceleration of Bone Loss in Periodontitis. Int. J. Mol. Sci. 2023, 24, 15989. https://doi.org/10.3390/ijms242115989
Kinoshita M, Yamada S, Sasaki J, Suzuki S, Kajikawa T, Iwayama T, Fujihara C, Imazato S, Murakami S. Mice Lacking PLAP-1/Asporin Show Alteration of Periodontal Ligament Structures and Acceleration of Bone Loss in Periodontitis. International Journal of Molecular Sciences. 2023; 24(21):15989. https://doi.org/10.3390/ijms242115989
Chicago/Turabian StyleKinoshita, Masaki, Satoru Yamada, Junichi Sasaki, Shigeki Suzuki, Tetsuhiro Kajikawa, Tomoaki Iwayama, Chiharu Fujihara, Satoshi Imazato, and Shinya Murakami. 2023. "Mice Lacking PLAP-1/Asporin Show Alteration of Periodontal Ligament Structures and Acceleration of Bone Loss in Periodontitis" International Journal of Molecular Sciences 24, no. 21: 15989. https://doi.org/10.3390/ijms242115989
APA StyleKinoshita, M., Yamada, S., Sasaki, J., Suzuki, S., Kajikawa, T., Iwayama, T., Fujihara, C., Imazato, S., & Murakami, S. (2023). Mice Lacking PLAP-1/Asporin Show Alteration of Periodontal Ligament Structures and Acceleration of Bone Loss in Periodontitis. International Journal of Molecular Sciences, 24(21), 15989. https://doi.org/10.3390/ijms242115989





