Targeting Signalling Pathways in Chronic Wound Healing
Abstract
1. Introduction
1.1. The Physiology of Wound Healing
1.2. Pathophysiology of Wound Healing
2. The PI3K/AKT Pathway
2.1. The PI3K/AKT Signalling Pathway in Wound Healing
2.2. Natural Extracts Promoting the PI3K/AKT Pathway
2.3. Stem-Cell-Based Treatment Promoting the PI3K/AKT Pathway
3. The Canonical Wnt/β-Catenin Pathway
3.1. Overview of the Wnt/β-Catenin Pathway
3.2. Targeting Wnt/β-Catenin Signalling to Enhance Chronic Wound Healing
4. TGF-β Signalling
4.1. Overview of the TGF-β Signalling Pathway
4.2. Targeting TGF-β Signalling to Enhance Chronic Wound Healing
5. Nrf2 Signalling Pathway
5.1. Overview of the Nrf2 Signalling Pathway
5.2. Targeting Nrf2 Signalling to Enhance Chronic Wound Healing
6. Notch Signalling Pathway
6.1. Overview of Notch Signalling Pathway
6.2. Role of Notch Signalling during Normal Wound Healing
6.3. Dysregulated Notch Signalling in Diabetic Wounds and Hypertrophic Scars
6.4. Targeting Notch Signalling to Enhance Healing of Chronic Wounds
6.4.1. Inhibition of Notch Signalling to Promote Diabetic Wound Healing
6.4.2. The Activation of Notch Signalling to Enhance the Healing of Pressure Ulcers and Chronic Limb Ischaemia Ulcers
6.4.3. Novel Regulators of Notch Signalling as Potential Targets to Enhance Chronic Wound Healing
7. HIF-1 Signalling Pathway
7.1. Overview of HIF-1 Signalling Pathway
7.2. Role of HIF-1 Signalling during Wound Healing
7.3. Targeting HIF-1 Signalling to Promote Healing in Chronic Wounds
7.4. Targeting Novel Regulators of HIF-1 Signalling
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Rep. Reg. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 56–582. [Google Scholar] [CrossRef]
- Vaidyanathan, L. Growth Factors in Wound Healing—A Review. Biomed. Pharmacol. J. 2021, 14, 1469–1480. [Google Scholar] [CrossRef]
- Verma, K.D.; Lewis, F.; Mejia, M.; Chalasani, M.; Marcus, K.A. Food and Drug Administration perspective: Advancing product development for non-healing chronic wounds. Wound Repair Regen. 2022, 30, 299–302. [Google Scholar] [CrossRef]
- Goggins, B.J.; Minahan, K.; Outteridge, N.; Knight, D.; Horvat, J.; Keely, S. Hypoxia Inducible Factor (HIF)-1 accelerates epithelial wound healing through integrin regulation. FASEB J. 2017, 31, 465.11. [Google Scholar] [CrossRef]
- Chigurupati, S.; Arumugam, T.V.; Son, T.G.; Lathia, J.D.; Jameel, S.; Mughal, M.R.; Tang, S.; Jo, D.; Camandola, S.; Giunta, M.; et al. Involvement of Notch Signaling in Wound Healing. PLoS ONE 2007, 2, e1167. [Google Scholar] [CrossRef]
- Choi, S.; Yoon, M.; Choi, K. Approaches for Regenerative Healing of Cutaneous Wound with an Emphasis on Strategies Activating the Wnt/β-Catenin Pathway. Adv. Wound Care 2021, 11, 7–86. [Google Scholar] [CrossRef]
- Escuin-Ordinas, H.; Li, S.; Xie, M.W.; Sun, L.; Hugo, W.; Huang, R.R.; Jiao, J.; de-Faria, F.M.; Realegeno, S.; Krystofinski, P.; et al. Cutaneous wound healing through paradoxical MAPK activation by BRAF inhibitors. Nat. Commun. 2016, 7, 12348. [Google Scholar] [CrossRef]
- Wu, X.; Sun, Q.; He, S.; Wu, Y.; Du, S.; Gong, L.; Yu, J.; Guo, H. Ropivacaine inhibits wound healing by suppressing the proliferation and migration of keratinocytes via the PI3K/AKT/mTOR Pathway. BMC Anesthesiol. 2022, 22, 106. [Google Scholar] [CrossRef]
- Basu, P.; Martins-Green, M. Signaling Pathways Associated with Chronic Wound Progression: A Systems Biology Approach. Antioxidants 2022, 11, 1506. [Google Scholar] [CrossRef]
- Geng, K.; Ma, X.; Jiang, Z.; Gu, J.; Huang, W.; Wang, W.; Xu, Y.; Xu, Y. WDR74 facilitates TGF-β/Smad pathway activation to promote M2 macrophage polarization and diabetic foot ulcer wound healing in mice. Cell Biol. Toxicol. 2023, 39, 1577–1591. [Google Scholar] [CrossRef]
- Feng, J.; Dong, C.; Long, Y.; Mai, L.; Ren, M.; Li, L.; Zhou, T.; Yang, Z.; Ma, J.; Yan, L.; et al. Elevated Kallikrein-binding protein in diabetes impairs wound healing through inducing macrophage M1 polarization. Cell Commun. Signal. 2019, 17, 60. [Google Scholar] [CrossRef]
- Wei, P.; Zhong, C.; Yang, X.; Shu, F.; Xiao, S.; Gong, T.; Luo, P.; Li, L.; Chen, Z.; Zheng, Y.; et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burn. Trauma 2020, 8, tkaa020. [Google Scholar] [CrossRef]
- Xiao, W.; Tang, H.; Wu, M.; Liao, Y.; Li, K.; Li, L.; Xu, X. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2017, 37, BSR20170658. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Peng, Y.; Zhao, Y.; Qin, Y.; Zhang, Y.; Xiao, Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J. Nanobiotechnol. 2021, 19, 202. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, H.; Shi, X.; Zhao, J.; Chen, Y.; Wu, F.; Yang, J.; Li, X. Asiaticoside nitric oxide gel accelerates diabetic cutaneous ulcers healing by activating Wnt/β-catenin signaling pathway. Int. Immunopharmacol. 2020, 79, 106109. [Google Scholar] [CrossRef]
- Saleh, M.A.; Shabaan, A.A.; May, M.; Ali, Y.M. Topical application of indigo-plant leaves extract enhances healing of skin lesion in an excision wound model in rats. J. Appl. Biomed. 2022, 20, 124–129. [Google Scholar] [CrossRef]
- Nasrullah, M.Z. Caffeic Acid Phenethyl Ester Loaded PEG-PLGA Nanoparticles Enhance Wound Healing in Diabetic Rats. Antioxidants 2022, 12, 60. [Google Scholar] [CrossRef]
- Fan, J.; Liu, H.; Wang, J.; Zeng, J.; Tan, Y.; Wang, Y.; Yu, X.; Li, W.; Wang, P.; Yang, Z.; et al. Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J. Cell. Mol. Med. 2021, 25, 652–665. [Google Scholar] [CrossRef]
- Shaabani, E.; Sharifiaghdam, M.; Lammens, J.; De Keersmaecker, H.; Vervaet, C.; De Beer, T.; Motevaseli, E.; Ghahremani, M.H.; Mansouri, P.; De Smedt, S.; et al. Increasing Angiogenesis Factors in Hypoxic Diabetic Wound Conditions by siRNA Delivery: Additive Effect of LbL-Gold Nanocarriers and Desloratadine-Induced Lysosomal Escape. Int. J. Mol. Sci. 2021, 22, 9216. [Google Scholar] [CrossRef]
- Qiu, S.; Jia, Y.; Sun, Y.; Han, P.; Xu, J.; Wen, G.; Chai, Y. Von Hippel-Lindau (VHL) Protein Antagonist VH298 Improves Wound Healing in Streptozotocin-Induced Hyperglycaemic Rats by Activating Hypoxia-Inducible Factor- (HIF-) 1 Signalling. J. Diabetes Res. 2019, 2019, 1897174. [Google Scholar] [CrossRef]
- Bouvard, C.; Galy-Fauroux, I.; Grelac, F.; Carpentier, W.; Lokajczyk, A.; Gandrille, S.; Colliec-Jouault, S.; Fischer, A.; Helley, D. Low-Molecular-Weight Fucoidan Induces Endothelial Cell Migration via the PI3K/AKT Pathway and Modulates the Transcription of Genes Involved in Angiogenesis. Mar. Drugs 2015, 13, 7446–7462. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Ma, J.; Sun, X.; Li, F.; Lv, J. Naringin enhances endothelial progenitor cell (EPC) proliferation and tube formation capacity through the CXCL12/CXCR4/PI3K/Akt signaling pathway. Chem. Biol. Interact. 2018, 286, 45–51. [Google Scholar] [CrossRef]
- Wu, M.; Huang, J.; Shi, J.; Shi, L.; Zeng, Q.; Wang, H. Ruyi Jinhuang Powder accelerated diabetic ulcer wound healing by regulating Wnt/β-catenin signaling pathway of fibroblasts In Vivo and In Vitro. J. Ethnopharmacol. 2022, 293, 115321. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, H.; Tang, T.; Liu, L.; Pan, B.; Chen, J.; Cheng, D.; Cai, X.; Sun, Y.; Zhu, F.; et al. Tetrahedral framework nucleic acids promote diabetic wound healing via the Wnt signalling pathway. Cell Prolif. 2022, 55, e13316. [Google Scholar] [CrossRef]
- Ebrahim, N.; Dessouky, A.A.; Mostafa, O.; Hassouna, A.; Yousef, M.M.; Seleem, Y.; Gebaly, E.A.E.A.M.E.; Allam, M.M.; Farid, A.S.; Saffaf, B.A.; et al. Adipose mesenchymal stem cells combined with platelet-rich plasma accelerate diabetic wound healing by modulating the Notch pathway. Stem Cell Res. Ther. 2021, 12, 392. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, W.; Gao, Q.; Su, M.; Yang, Y.; Liu, Z.; Zhu, B. Arnebin-1 promotes angiogenesis by inducing eNOS, VEGF and HIF-1α expression through the PI3K-dependent pathway. Int. J. Mol. Med. 2015, 36, 685–697. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, X.; Cheng, L.; Dai, J.; Wang, C.; Han, P.; Chai, Y. Wound healing improvement with PHD-2 silenced fibroblasts in diabetic mice. PLoS ONE 2013, 8, e84548. [Google Scholar] [CrossRef][Green Version]
- Dallas, A.; Trotsyuk, A.; Ilves, H.; Bonham, C.A.; Rodrigues, M.; Engel, K.; Barrera, J.A.; Kosaric, N.; Stern-Buchbinder, Z.A.; White, A.; et al. Acceleration of Diabetic Wound Healing with PHD2- and miR-210-Targeting Oligonucleotides. Tissue Eng. Part A 2019, 25, 44–54. [Google Scholar] [CrossRef]
- Chen, H.; Jia, P.; Kang, H.; Zhang, H.; Liu, Y.; Yang, P.; Yan, Y.; Zuo, G.; Guo, L.; Jiang, M.; et al. Upregulating Hif-1α by Hydrogel Nanofibrous Scaffolds for Rapidly Recruiting Angiogenesis Relative Cells in Diabetic Wound. Adv. Healthc. Mater. 2016, 5, 907–918. [Google Scholar] [CrossRef]
- Li, G.; Ko, C.; Li, D.; Yang, C.; Wang, W.; Yang, G.; Di Primo, C.; Wong, V.K.W.; Xiang, Y.; Lin, L.; et al. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat. Commun. 2021, 12, 3363. [Google Scholar] [CrossRef]
- Zhang, E.; Gao, B.; Shi, H.; Huang, L.; Yang, L.; Wu, X.; Wang, Z. 20(S)-Protopanaxadiol enhances angiogenesis via HIF-1α-mediated VEGF secretion by activating p70S6 kinase and benefits wound healing in genetically diabetic mice. Exp. Mol. Med. 2017, 49, e387. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Jia, Y.; Xu, J.; Chai, Y. Roxadustat promotes angiogenesis through HIF-1α/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats. Wound Repair Regen. 2019, 27, 324–334. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Assar, D.H.; Abdelnaby, A.; Asa, S.A.; Abdelhiee, E.Y.; Ibrahim, S.S.; Abdel-Daim, M.M.; Almeer, R.; Atiba, A. Healing potential of Spirulina platensis for skin wounds by modulating bFGF, VEGF, TGF-ß1 and α-SMA genes expression targeting angiogenesis and scar tissue formation in the rat model. Biomed. Pharmacother. 2021, 137, 111349. [Google Scholar] [CrossRef]
- Chang, Z.; Kishimoto, Y.; Hasan, A.; Welham, N.V. TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis. Models Mech. 2014, 7, 83–91. [Google Scholar] [CrossRef]
- Lopez-de la Mora, D.A.; Sanchez-Roque, C.; Montoya-Buelna, M.; Sanchez-Enriquez, S.; Lucano-Landeros, S.; Macias-Barragan, J.; Armendariz-Borunda, J. Role and New Insights of Pirfenidone in Fibrotic Diseases. Int. J. Med. Sci. 2015, 12, 840–847. [Google Scholar]
- Chumpolphant, S.; Suwatronnakorn, M.; Issaravanich, S.; Tencomnao, T.; Prasansuklab, A. Polyherbal formulation exerts wound healing, anti-inflammatory, angiogenic and antimicrobial properties: Potential role in the treatment of diabetic foot ulcers. Saudi J. Biol. Sci. 2022, 29, 103330. [Google Scholar] [CrossRef]
- Li, W.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: Role of TGF-β/Smads and Ang-1/Tie2 signaling pathways. EXCLI J. 2018, 17, 399–419. [Google Scholar]
- Tan, W.S.; Arulselvan, P.; Ng, S.; Mat Taib, C.N.; Sarian, M.N.; Fakurazi, S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Complement. Altern. Med. 2019, 19, 20. [Google Scholar] [CrossRef]
- Yang, X.; Fan, W.; Huang, R.; Liu, G. β-acetoxyisovaleryl alkannin (AAN-II) from Alkanna tinctoria promotes the healing of pressure-induced venous ulcers in a rabbit model through the activation of TGF-β/Smad3 signaling. Cell. Mol. Biol. Lett. 2021, 26, 35. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Yue, J.; Gou, X.; Wu, X. Epidermal Stem Cells in Skin Wound Healing. Adv. Wound Care 2017, 6, 297–307. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Chakroborty, D.; Goswami, S.; Basu, S.; Sarkar, C. Catecholamines in the regulation of angiogenesis in cutaneous wound healing. FASEB J. 2020, 34, 14093–14102. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Russo, B.; Brembilla, N.C.; Chizzolini, C. Interplay between Keratinocytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders. Front. Immunol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Jere, S.W.; Houreld, N.N.; Abrahamse, H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019, 50, 52–59. [Google Scholar] [CrossRef]
- Hou, B.; Cai, W.; Chen, T.; Zhang, Z.; Gong, H.; Yang, W.; Qiu, L. Vaccarin hastens wound healing by promoting angiogenesis via activation of MAPK/ERK and PI3K/AKT signaling pathways in vivo. Acta Cir. Bras. 2020, 34, e201901202. [Google Scholar] [CrossRef]
- Yu, T.; Gao, M.; Yang, P.; Liu, D.; Wang, D.; Song, F.; Zhang, X.; Liu, Y. Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing. J. Cell. Physiol. 2019, 234, 4217–4231. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, C.; Zhao, R.; Wang, L.; Li, S.; Liu, M.; Zhang, M.; Jiang, S.; Tian, Z.; Wang, M.; et al. Effects of PI3K/Akt Pathway in Wound Healing Process of Mice Skin. Fa Yi Xue Za Zhi 2016, 32, 7–12. [Google Scholar]
- Ruttanapattanakul, J.; Wikan, N.; Okonogi, S.; Na Takuathung, M.; Buacheen, P.; Pitchakarn, P.; Potikanond, S.; Nimlamool, W. Boesenbergia rotunda extract accelerates human keratinocyte proliferation through activating ERK1/2 and PI3K/Akt kinases. Biomed. Pharmacother. 2021, 133, 111002. [Google Scholar] [CrossRef]
- Huang, L.; Cai, H.; Zhang, M.; Liao, R.; Huang, X.; Hu, F. Ginsenoside Rg1 promoted the wound healing in diabetic foot ulcers via miR-489–3p/Sirt1 axis. J. Pharmacol. Sci. 2021, 147, 271–283. [Google Scholar] [CrossRef]
- Ayavoo, T.; Murugesan, K.; Gnanasekaran, A. Roles and mechanisms of stem cell in wound healing. Stem Cell Investig. 2021, 8, 4. [Google Scholar] [CrossRef]
- Xiu, C.; Zheng, H.; Jiang, M.; Li, J.; Zhou, Y.; Mu, L.; Liu, W. MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN. Int. J. Stem Cells 2022, 15, 359–371. [Google Scholar] [CrossRef]
- Carre, A.; Hu, M.; James, A.; Kawai, K.; Galvez, M.; Longaker, M.; Lorenz, H. β-Catenin–Dependent Wnt Signaling: A Pathway in Acute Cutaneous Wounding. Plast. Reconstr. Surg. 2018, 141, 669–678. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Lim, X.; Tan, S.H.; Koh, W.L.C.; Chau, R.M.W.; Yan, K.S.; Kuo, C.J.; van Amerongen, R.; Klein, A.M.; Nusse, R. Interfollicular Epidermal Stem Cells Self-Renew via Autocrine Wnt Signaling. Sci. (Am. Assoc. Adv. Sci.) 2013, 342, 1226–1230. [Google Scholar] [CrossRef]
- Lu, S.; Liu, H.; Lu, L.; Wan, H.; Lin, Z.; Qian, K.; Yao, X.; Chen, Q.; Liu, W.; Yan, J.; et al. WISP1 overexpression promotes proliferation and migration of human vascular smooth muscle cells via AKT signaling pathway. Eur. J. Pharmacol. 2016, 788, 90–97. [Google Scholar] [CrossRef]
- Qi, W.; Yang, C.; Dai, Z.; Che, D.; Feng, J.; Mao, Y.; Cheng, R.; Wang, Z.; He, X.; Zhou, T.; et al. High Levels of Pigment Epithelium–Derived Factor in Diabetes Impair Wound Healing through Suppression of Wnt Signaling. Diabetes 2015, 64, 1407–1419. [Google Scholar] [CrossRef]
- Guo, X.; Schaudinn, C.; Blume-Peytavi, U.; Vogt, A.; Rancan, F. Effects of Adipose-Derived Stem Cells and Their Conditioned Medium in a Human Ex Vivo Wound Model. Cells 2022, 11, 1198. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, T.; Lin, Y. Functionalizing Framework Nucleic-Acid-Based Nanostructures for Biomedical Application. Adv. Mater. 2022, 34, e2107820. [Google Scholar] [CrossRef]
- Ekin, K.Ş.; Bahtiyar, H.; Yeşim, K.K.; Erkan, Y. Could radial extracorporeal shock wave therapy have an effect on wound healing in clinical practice by creating genotoxic damage? An in vitro study in mouse fibroblasts. Jt. Dis. Relat. Surg. 2021, 32, 658–667. [Google Scholar]
- Aschermann, I.; Noor, S.; Venturelli, S.; Sinnberg, T.; Mnich, C.D.; Busch, C. Extracorporal Shock Waves Activate Migration, Proliferation and Inflammatory Pathways in Fibroblasts and Keratinocytes, and Improve Wound Healing in an Open-Label, Single-Arm Study in Patients with Therapy-Refractory Chronic Leg Ulcers. Cell. Physiol. Biochem. 2017, 41, 890–906. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, B.; Hong, L.; Shi, K.; Wu, W.; Yu, J. Extracorporeal shock wave therapy with low-energy flux density inhibits hypertrophic scar formation in an animal model. Int. J. Mol. Med. 2018, 41, 1931–1938. [Google Scholar] [CrossRef]
- Chen, R.; Lin, Y.; Liu, K.; Wang, C.; Ramachandran, S.; Wang, C.; Kuo, Y. The Acceleration of Diabetic Wound Healing by Low-Intensity Extracorporeal Shockwave Involves in the GSK-3β Pathway. Biomedicines 2020, 9, 21. [Google Scholar] [CrossRef]
- Finnson, K.W.; McLean, S.; Di Guglielmo, G.M.; Philip, A. Dynamics of Transforming Growth Factor Beta Signaling in Wound Healing and Scarring. Adv. Wound Care 2013, 2, 195–214. [Google Scholar] [CrossRef]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer—A Betrayal Within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef]
- Moulin, V.; Lawny, F.; Barritault, D.; Caruelle, J.P. Platelet releasate treatment improves skin healing in diabetic rats through endogenous growth factor secretion. Cell. Mol. Biol. 1998, 44, 961–971. [Google Scholar]
- Park, J.; Jeong, S.; Song, E.; Song, J.; Kim, H.; Kim, S. Acceleration of the healing process of full-thickness wounds using hydrophilic chitosan–silica hybrid sponge in a porcine model. J. Biomater. Appl. 2018, 32, 1011–1023. [Google Scholar] [CrossRef]
- Xiaojie, W.; Banda, J.; Qi, H.; Chang, A.K.; Bwalya, C.; Chao, L.; Li, X. Scarless wound healing: Current insights from the perspectives of TGF-β, KGF-1, and KGF-2. Cytokine Growth Factor Rev. 2022, 66, 26–37. [Google Scholar] [CrossRef]
- Yuan, R.; Dai, X.; Li, Y.; Li, C.; Liu, L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol. Med. Rep. 2021, 24, 758. [Google Scholar] [CrossRef]
- Wu, L.; Siddiqui, A.; Morris, D.E.; Cox, D.A.; Roth, S.I.; Mustoe, T.A. Transforming growth factor beta 3 (TGF beta 3) accelerates wound healing without alteration of scar prominence. Histologic and competitive reverse-transcription-polymerase chain reaction studies. Arch. Surg. 1997, 132, 753–760. [Google Scholar] [CrossRef]
- Xu, Q.; Miao, Y.; Ren, J.; Sun, Y.; Li, C.; Cai, X.; Wang, Z. Silencing of Nesprin-2 inhibits the differentiation of myofibroblasts from fibroblasts induced by mechanical stretch. Int. Wound J. 2022, 19, 978–986. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Zheng, D.; Ho, C.; Wen, D.; Sun, J.; Huang, L.; Liu, Y.; Li, Q.; Zhang, Y. HDAC5-mediated Smad7 silencing through MEF2A is critical for fibroblast activation and hypertrophic scar formation. Int. J. Biol. Sci. 2022, 18, 5724–5739. [Google Scholar] [CrossRef]
- Rahimi, K.; Hosseinpour, L.; Balaneji, S.M.; Goli, R.; Faraji, N.; Babamiri, B. Large wound surgery of diabetic foot ulcer with Split-thickness skin graft (STSG), and maggot debridement therapy (MDT): A case report. Int. J. Surg. Case Rep. 2023, 104, 107947. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Gao, C.; Sun, X.; Wang, L.; Hu, Z.; Li, G.; Wang, J.; Wang, A. Maggot treatment promotes healing of diabetic foot ulcer wounds possibly by upregulating Treg levels. Diabetes Res. Clin. Pract. 2022, 184, 109187. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, F.; Li, B.; Huang, C.; Xu, C.; Lin, K.; Lin, D. NMR-based metabolomic analysis for the effects of Huiyang Shengji extract on rat diabetic skin ulcers. J. Ethnopharmacol. 2020, 261, 112978. [Google Scholar] [CrossRef]
- Lin, Y.; He, X.; Xie, X.; Liu, Q.; Chen, J.; Li, P. Huiyang Shengji Extract Improve Chronic Nonhealing Cutaneous through the TGF-β1/Smad3 Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 8881565. [Google Scholar] [CrossRef]
- Gasca-Lozano, L.E.; Lucano-Landeros, S.; Ruiz-Mercado, H.; Salazar-Montes, A.; Sandoval-Rodríguez, A.; Garcia-Bañuelos, J.; Santos-Garcia, A.; Davila-Rodriguez, J.R.; Navarro-Partida, J.; Bojórquez-Sepúlveda, H.; et al. Pirfenidone Accelerates Wound Healing in Chronic Diabetic Foot Ulcers: A Randomized, Double-Blind Controlled Trial. J. Diabetes Res. 2017, 2017, 3159798. [Google Scholar] [CrossRef]
- Lavery, L.A.; Killeen, A.L.; Farrar, D.; Akgul, Y.; Crisologo, P.A.; Malone, M.; Davis, K.E. The effect of continuous diffusion of oxygen treatment on cytokines, perfusion, bacterial load, and healing in patients with diabetic foot ulcers. Int. Wound J. 2020, 17, 1986–1995. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Stress-Activated Cap’n’collar Transcription Factors in Aging and Human Disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef]
- Hiebert, P.; Werner, S. Targeting NRF2 to promote epithelial repair. Biochem. Soc. Trans. 2023, 51, 101. [Google Scholar] [CrossRef]
- He, T.; Bai, X.; Jing, J.; Liu, Y.; Wang, H.; Zhang, W.; Li, X.; Li, Y.; Wang, L.; Xie, S.; et al. Notch signal deficiency alleviates hypertrophic scar formation after wound healing through the inhibition of inflammation. Arch. Biochem. Biophys. 2020, 682, 108286. [Google Scholar] [CrossRef]
- Long, M.; Rojo de la Vega, M.; Wen, Q.; Bharara, M.; Jiang, T.; Zhang, R.; Zhou, S.; Wong, P.K.; Wondrak, G.T.; Zheng, H.; et al. An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes 2016, 65, 780–793. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Kim, J.H.; Yang, B.; Tedesco, A.; Lebig, E.G.D.; Ruegger, P.M.; Xu, K.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress and Skin Microbiome are Critical for Initiation and Development of Chronic Wounds in Diabetic Mice. Sci. Rep. 2019, 9, 19318. [Google Scholar] [CrossRef]
- Watanabe, S.; Togashi, S.; Takahashi, N.; Fukui, T. L-tryptophan as an antioxidant in human placenta extract. J. Nutr. Sci. Vitaminol. 2002, 48, 36–39. [Google Scholar] [CrossRef]
- Huang, L.; Chin, L.; Kimura, K.; Nakahata, Y. Human Placental Extract Delays In Vitro Cellular Senescence through the Activation of NRF2-Mediated Antioxidant Pathway. Antioxidants 2022, 11, 1545. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Ribeiro, B.C.d.S.; Faria, R.V.d.C.; Nogueira, J.d.S.; Santos Valença, S.; Chen, L.; Romana-Souza, B. Olive oil promotes the survival and migration of dermal fibroblasts through Nrf2 pathway activation. Lipids 2022, 58, 59–68. [Google Scholar] [CrossRef]
- He, T.; Sun, P.; Liu, B.; Wan, S.; Fang, P.; Chen, J.; Huang, G.; Min, W. Puffball spores improve wound healing in a diabetic rat model. Front. Endocrinol. 2022, 13, 942549. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Badr, G.; Badr, B.M.; Allam, A.; Ghamdi, A.A.; Al-Wadaan, M.A.; Al-Waili, N.S. Bee venom improves diabetic wound healing by protecting functional macrophages from apoptosis and enhancing Nrf2, Ang-1 and Tie-2 signaling. Mol. Immunol. 2018, 103, 322–335. [Google Scholar] [CrossRef]
- Lu, X.; Liu, M.; Dong, H.; Miao, J.; Stagos, D.; Liu, M. Dietary prenylated flavonoid xanthohumol alleviates oxidative damage and accelerates diabetic wound healing via Nrf2 activation. Food Chem. Toxicol. 2022, 160, 112813. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Zhao, Z.; Chen, J.; Li, C.; Zhao, G. Paeoniflorin accelerates foot wound healing in diabetic rats though activating the Nrf2 pathway. Acta Histochem. 2020, 122, 151649. [Google Scholar] [CrossRef]
- Ganesh, G.V.; Ramkumar, K.M. Pterostilbene attenuates hemin-induced dysregulation of macrophage M2 polarization via Nrf2 activation in experimental hyperglycemia. Inflammopharmacology 2023, 31, 2133–2145. [Google Scholar] [CrossRef]
- Vendidandala, N.R.; Yin, T.P.; Nelli, G.; Pasupuleti, V.R.; Nyamathulla, S.; Mokhtar, S.I. Gallocatechin-silver nanoparticle impregnated cotton gauze patches enhance wound healing in diabetic rats by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κB pathways. Life Sci. 2021, 286, 120019. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Jiang, Z.; Luo, P.; Liu, L.; Huang, Y.; Wang, H.; Wang, Y.; Long, L.; Tan, X.; et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat. Commun. 2019, 10, 2538. [Google Scholar] [CrossRef]
- Yang, F.; Shen, C. Sodium Danshensu Cream Promotes the Healing of Pressure Ulcers in Mice through the Nrf2/HO-1 and NF-κB Pathways. Pharmaceuticals 2022, 15, 1548. [Google Scholar] [CrossRef]
- Zhou, Q.; Cai, X.; Huang, Y.; Zhou, Y. Pluronic F127-liposome-encapsulated curcumin activates Nrf2/Keap1 signaling pathway to promote cell migration of HaCaT cells. Mol. Cell Biochem. 2023, 478, 241–247. [Google Scholar] [CrossRef]
- Zhou, X.; Ruan, Q.; Ye, Z.; Chu, Z.; Xi, M.; Li, M.; Hu, W.; Guo, X.; Yao, P.; Xie, W. Resveratrol accelerates wound healing by attenuating oxidative stress-induced impairment of cell proliferation and migration. Burns 2021, 47, 133–139. [Google Scholar] [CrossRef]
- Rabbani, P.S.; Ellison, T.; Waqas, B.; Sultan, D.; Abdou, S.; David, J.A.; Cohen, J.M.; Gomez-Viso, A.; Lam, G.; Kim, C.; et al. Targeted Nrf2 activation therapy with RTA 408 enhances regenerative capacity of diabetic wounds. Diabetes Res. Clin. Pract. 2018, 139, 11–23. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Liu, H.; Zhong, D.; Yin, K.; Li, Y.; Zhu, L.; Xu, C.; Li, M.; Wang, C. Bone marrow stromal cell-derived exosomal circular RNA improves diabetic foot ulcer wound healing by activating the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and inhibiting ferroptosis. Diabet. Med. 2022, 40, e15031. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, S.; Pan, N.; Zhong, G.; Qiu, Z.; Kuang, S.; Lin, Z.; Zhang, Z.; Pan, Y. UCMSCs-derived exosomal circHIPK3 promotes ulcer wound angiogenesis of diabetes mellitus via miR-20b-5p/Nrf2/VEGFA axis. Diabet. Med. 2023, 40, e14968. [Google Scholar] [CrossRef]
- Guo, Z.; Wan, X.; Luo, Y.; Liang, F.; Jiang, S.; Yuan, X.; Mo, Z. The vicious circle of UHRF1 down-regulation and KEAP1/NRF2/HO-1 pathway impairment promotes oxidative stress-induced endothelial cell apoptosis in diabetes. Diabet. Med. 2022, 40, e15026. [Google Scholar] [CrossRef]
- Wang, S.; Shi, M.; Zhou, J.; Wang, W.; Zhang, Y.; Li, Y. Circulating Exosomal miR-181b-5p Promoted Cell Senescence and Inhibited Angiogenesis to Impair Diabetic Foot Ulcer via the Nuclear Factor Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway. Front. Cardiovasc. Med. 2022, 9, 844047. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, J.; Jiang, T.; Yan, C.; Kang, Y.; Zhang, M.; Xiang, K.; Guo, J.; Jiang, G.; Wang, C.; et al. Milk-derived exosomes carrying siRNA-KEAP1 promote diabetic wound healing by improving oxidative stress. Drug Deliv. Transl. Res. 2023, 13, 2286–2296. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Yang, K.; Liu, P.; Wang, J. The signaling pathways of traditional Chinese medicine in promoting diabetic wound healing. J. Ethnopharmacol. 2022, 282, 114662. [Google Scholar] [CrossRef]
- Shen, W.; Huang, J.; Wang, Y. Biological Significance of NOTCH Signaling Strength. Front. Cell Dev. Biol. 2021, 9, 652273. [Google Scholar] [CrossRef]
- Bocci, F.; Onuchic, J.N.; Jolly, M.K. Understanding the Principles of Pattern Formation Driven by Notch Signaling by Integrating Experiments and Theoretical Models. Front. Physiol. 2020, 11, 929. [Google Scholar] [CrossRef]
- Takazawa, Y.; Ogawa, E.; Saito, R.; Uchiyama, R.; Ikawa, S.; Uhara, H.; Okuyama, R. Notch down-regulation in regenerated epidermis contributes to enhanced expression of interleukin-36α and suppression of keratinocyte differentiation during wound healing. J. Dermatol. Sci. 2015, 79, 10–19. [Google Scholar] [CrossRef]
- Qin, S.; Zheng, J.; Xia, Z.; Qian, J.; Deng, C.; Yang, S. CTHRC1 promotes wound repair by increasing M2 macrophages via regulating the TGF-β and notch pathways. Biomed. Pharmacother. 2019, 113, 108594. [Google Scholar] [CrossRef]
- Madhuchhanda, R.; King, T.W. Epidermal growth factor regulates NIKS keratinocyte proliferation through Notch signaling. J. Surg. Res. 2013, 185, 6–11. [Google Scholar]
- Shu, B.; Hua Yang, R. Notch1 Signaling Regulates Wound Healing via Changing the Characteristics of Epidermal Stem Cells. J. Stem Cell Res. Ther. 2016, 6, 1000348. [Google Scholar] [CrossRef]
- Wang, P.; Shu, B.; Xu, Y.; Zhu, J.; Liu, J.; Zhou, Z.; Chen, L.; Zhao, J.; Liu, X.; Qi, S.; et al. Basic fibroblast growth factor reduces scar by inhibiting the differentiation of epidermal stem cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell Res. Ther. 2017, 8, 114. [Google Scholar] [CrossRef]
- Patel, J.; Baz, B.; Wong, H.Y.; Lee, J.S.; Khosrotehrani, K. Accelerated Endothelial to Mesenchymal Transition Increased Fibrosis via Deleting Notch Signaling in Wound Vasculature. J. Investig. Dermatol. 2018, 138, 1166–1175. [Google Scholar] [CrossRef]
- Li, B.; Gao, C.; Diao, J.; Wang, D.; Chu, F.; Li, Y.; Wang, G.; Guo, S.; Xia, W. Aberrant Notch signalling contributes to hypertrophic scar formation by modulating the phenotype of keratinocytes. Exp. Dermatol. 2016, 25, 137–142. [Google Scholar] [CrossRef]
- Shao, H.; Li, Y.; Pastar, I.; Xiao, M.; Prokupets, R.; Liu, S.; Yu, K.; Vazquez-Padron, R.I.; Tomic-Canic, M.; Velazquez, O.C.; et al. Notch1 signaling determines the plasticity and function of fibroblasts in diabetic wounds. Life Sci. Alliance 2020, 3, e202000769. [Google Scholar] [CrossRef]
- Narayanan, S.; Sunkari, V.G.; Eliasson, S.; Botusan, I.R.; Grünler, J.; Catrina, A.I.; Radtke, F.; Xu, C.; Zhao, A.; Ekberg, N.R.; et al. Triggering of a Dll4–Notch1 loop impairs wound healing in diabetes. Proc. Natl. Acad. Sci. USA 2019, 116, 6985–6994. [Google Scholar]
- Kimball, A.S.; Joshi, A.D.; Boniakowski, A.E.; Schaller, M.; Chung, J.; Allen, R.; Bermick, J.; William, F.C., IV; Henke, P.K.; Maillard, I.; et al. Notch Regulates Macrophage-Mediated Inflammation in Diabetic Wound Healing. Front. Immunol. 2017, 8, 635. [Google Scholar] [CrossRef]
- Liu, P.; Yang, X.; Han, J.; Zhao, M.; Guo, J.; Si, R.; Zhang, Z.; Wang, A.; Zhang, J. Tazarotene-loaded PLGA nanoparticles potentiate deep tissue pressure injury healing via VEGF-Notch signaling. Mater. Sci. Eng. C 2020, 114, 111027. [Google Scholar] [CrossRef]
- Lv, S.; Cai, H.; Xu, Y.; Dai, J.; Rong, X.; Zheng, L. Thymosin-β 4 induces angiogenesis in critical limb ischemia mice via regulating Notch/NF-κB pathway. Int. J. Mol. Med. 2020, 46, 1347–1358. [Google Scholar] [CrossRef]
- Qiu, T.; Huang, J.; Wang, L.; Zhu, B. Inhibition of miR-200b Promotes Angiogenesis in Endothelial Cells by Activating the Notch Pathway. Cell J. 2022, 24, 779–781. [Google Scholar]
- Dai, Z.; Lu, L.; Yang, Z.; Mao, Y.; Lu, J.; Li, C.; Qi, W.; Chen, Y.; Yao, Y.; Li, L.; et al. Kallikrein-binding protein inhibits LPS-induced TNF-α by upregulating SOCS3 expression. J. Cell. Biochem. 2013, 114, 1020–1028. [Google Scholar] [CrossRef]
- McBride, J.D.; Jenkins, A.J.; Liu, X.; Zhang, B.; Lee, K.; Berry, W.L.; Janknecht, R.; Griffin, C.T.; Aston, C.E.; Lyons, T.J.; et al. Elevated Circulation Levels of an Antiangiogenic SERPIN in Patients with Diabetic Microvascular Complications Impair Wound Healing through Suppression of Wnt Signaling. J. Investig. Dermatol. 2014, 134, 1725–1734. [Google Scholar] [CrossRef]
- Bento, C.F.; Pereira, P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia 2011, 54, 1946–1956. [Google Scholar] [CrossRef]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 39–399. [Google Scholar] [CrossRef]
- DeFrates, K.G.; Franco, D.; Heber-Katz, E.; Messersmith, P.B. Unlocking mammalian regeneration through hypoxia inducible factor one alpha signaling. Biomaterials 2021, 269, 120646. [Google Scholar] [CrossRef]
- McNeill, L.A.; Hewitson, K.S.; Claridge, T.D.; Seibel, J.F.; Horsfall, L.E.; Schofield, C.J. Hypoxia-inducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the β-carbon of asparagine-803. Biochem. J. 2002, 367, 571–575. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef]
- Berchner-Pfannschmidt, U.; Frede, S.; Wotzlaw, C.; Fandrey, J. Imaging of the hypoxia-inducible factor pathway: Insights into oxygen sensing. Eur. Respir. J. 2008, 32, 210–217. [Google Scholar] [CrossRef]
- Xiao, H.; Gu, Z.; Wang, G.; Zhao, T. The Possible Mechanisms Underlying the Impairment of HIF-1α Pathway Signaling in Hyperglycemia and the Beneficial Effects of Certain Therapies. Int. J. Med. Sci. 2013, 10, 1412–1421. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Hutami, I.R.; Izawa, T.; Khurel-Ochir, T.; Sakamaki, T.; Iwasa, A.; Tanaka, E. Macrophage Motility in Wound Healing Is Regulated by HIF-1α via S1P Signaling. Int. J. Mol. Sci. 2021, 22, 8992. [Google Scholar] [CrossRef]
- Peng, W.; He, P.; Liu, L.; Zhu, T.; Zhong, Y.; Xiang, L.; Peng, K.; Yang, J.; Xiang, G. LncRNA GAS5 activates the HIF1A/VEGF pathway by binding to TAF15 to promote wound healing in diabetic foot ulcers. Lab. Investig. 2021, 101, 1071–1083. [Google Scholar] [CrossRef]
- Ko, S.; Nauta, A.; Morrison, S.; Hu, M.; Zimmermann, A.; Chung, M.; Glotzbach, J.; Wong, V.; Walmsley, G.; Peter Lorenz, H.; et al. PHD-2 Suppression in Mesenchymal Stromal Cells Enhances Wound Healing. Plast. Reconstr. Surg. 2018, 141, 55e–67e. [Google Scholar] [CrossRef]
- Wang, C.; Lou, Y.; Tong, M.; Zhang, L.; Zhang, Z.; Feng, Y.; Li, S.; Xu, H.; Mao, C. Asperosaponin VI promotes angiogenesis and accelerates wound healing in rats via up-regulating HIF-1α/VEGF signaling. Acta Pharmacol. Sin. 2018, 39, 393–404. [Google Scholar] [CrossRef]
- Haase, V.H. Hypoxia-inducible factor–prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int. Suppl. 2021, 11, 8–25. [Google Scholar] [CrossRef]
- Guo, J.; Yin, L.; Chen, Y.; Jin, X.; Zhou, X.; Zhu, N.; Liu, X.; Wei, H.; Duan, L. Autologous blood transfusion augments impaired wound healing in diabetic mice by enhancing lncRNA H19 expression via the HIF-1α signaling pathway. Cell Commun. Signal. CCS 2018, 16, 84. [Google Scholar] [CrossRef]
- He, M.; Tu, L.; Shu, R.; Meng, Q.; Du, S.; Xu, Z.; Wang, S. Long Noncoding RNA CASC2 Facilitated Wound Healing through miRNA-155/HIF-1α in Diabetic Foot Ulcers. Contrast Media Mol. Imaging 2022, 2022, 6291497. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Yu, W.; Xiao, Q. High glucose and/or high insulin affects HIF-1 signaling by regulating AIP1 in human umbilical vein endothelial cells. Diabetes Res. Clin. Pract. 2015, 109, 48–56. [Google Scholar] [CrossRef]
- Lin, C.; Lan, Y.; Ou, M.; Ji, L.; Lin, S. Expression of miR-217 and HIF-1α/VEGF pathway in patients with diabetic foot ulcer and its effect on angiogenesis of diabetic foot ulcer rats. J. Endocrinol. Investig. 2019, 42, 1307–1317. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Pan, Z.; Li, Q.; Sun, L.; Li, X.; Gong, M.; Yang, X.; Wang, Y.; Li, H.; et al. GDF11 promotes wound healing in diabetic mice via stimulating HIF-1ɑ-VEGF/SDF-1ɑ-mediated endothelial progenitor cell mobilization and neovascularization. Acta Pharmacol. Sin. 2022, 44, 999–1013. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, Z.; Ho, C.; Wang, J.; Li, Q.; Zhang, Y.; Zhou, J. LRG1 promotes keratinocyte migration and wound repair through regulation of HIF-1α stability. J. Investig. Dermatol. 2020, 140, 455–464. [Google Scholar] [CrossRef]
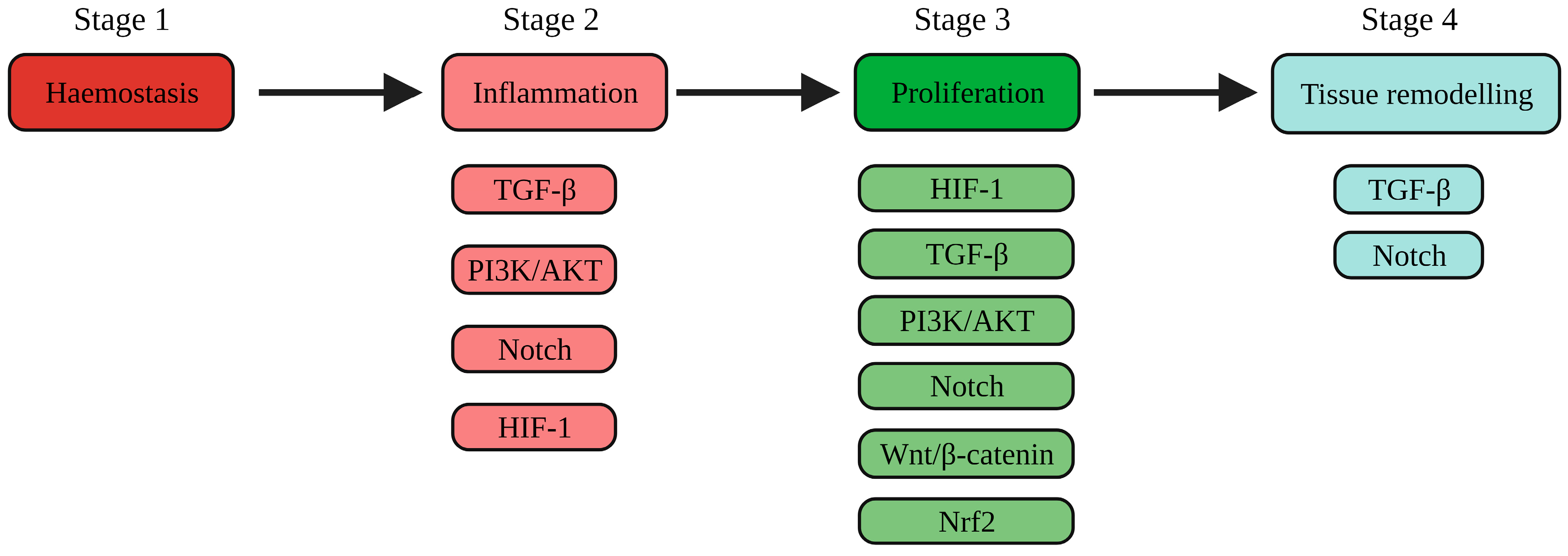
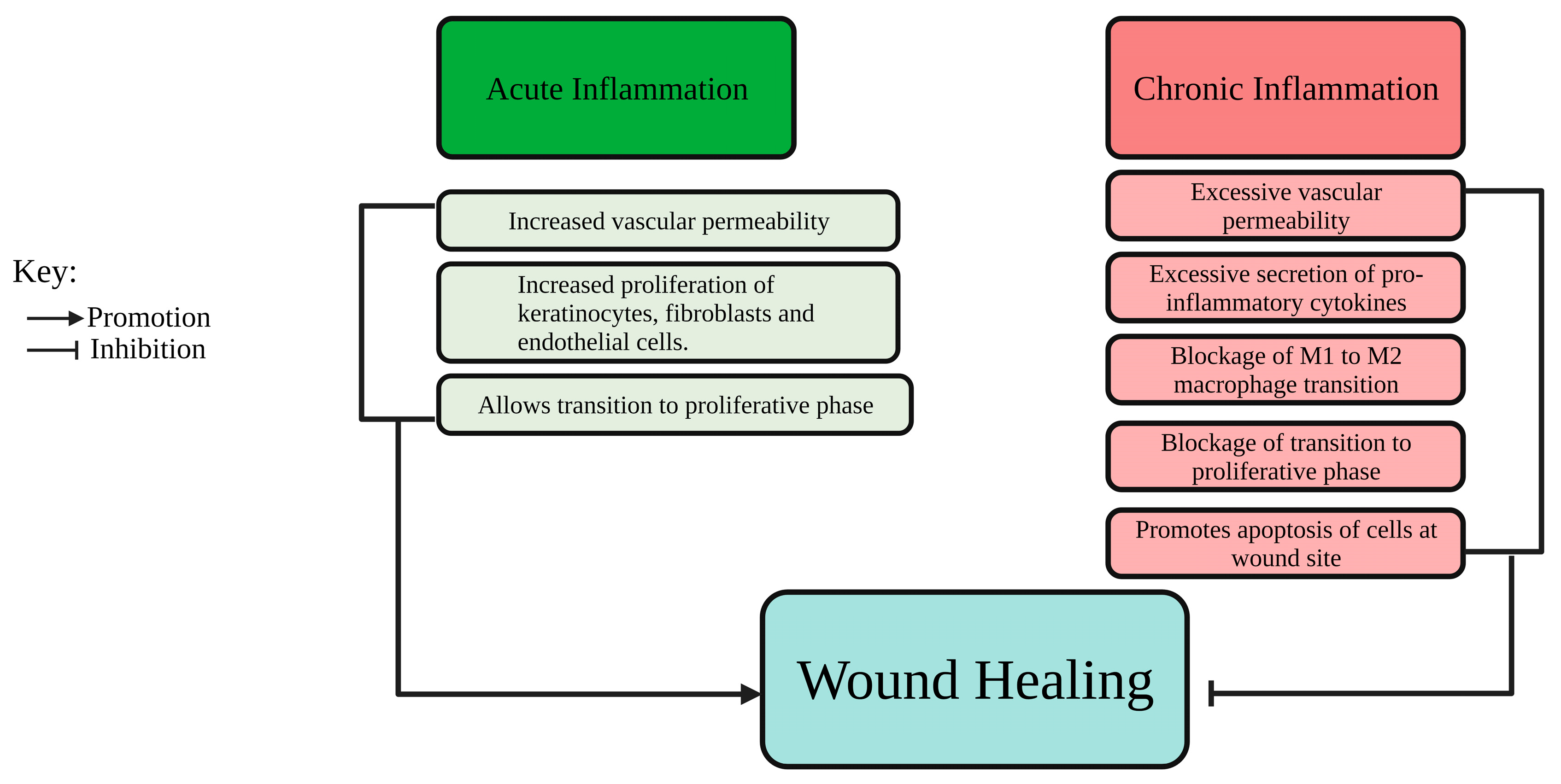
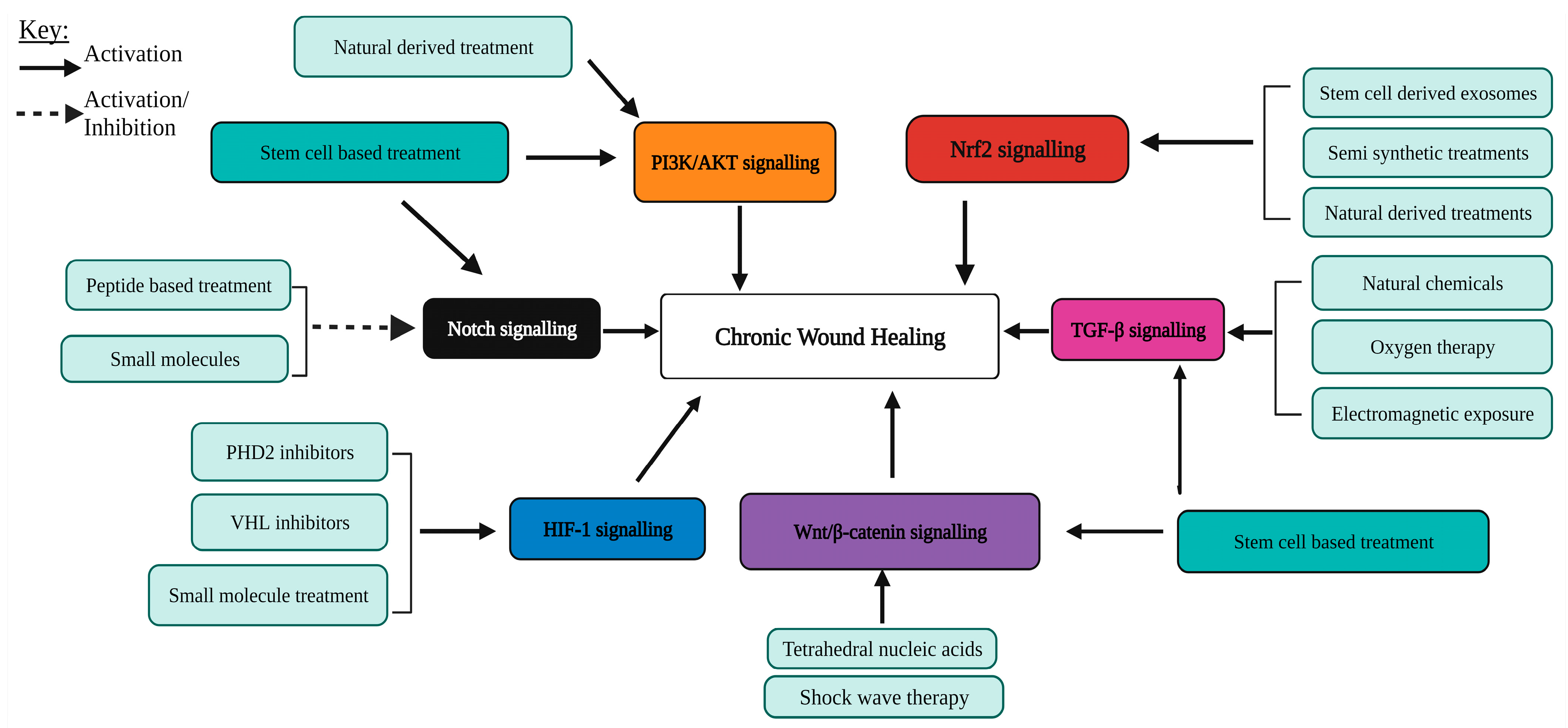
| Processes Required to Enhance Chronic Wound Healing | Activation of Pathways Involved in the Processes of Wound Healing |
|---|---|
| The promotion of M1 to M2 macrophage transition | TGF-β [13] and Notch [14] |
| The promotion of keratinocyte proliferation | PI3K/AKT [15] |
| The promotion of fibroblast proliferation and migration | PI3K/AKT [15,16,17], Wnt/β-catenin [18], TGF-β [19,20], Nrf2 [21] and HIF-1 [22,23] |
| The promotion of angiogenesis | PI3K/AKT [15,24,25], Wnt/β-catenin [26,27], Notch [28] and HIF-1 [23,29,30,31,32,33,34,35] |
| The promotion of remodelling | TGF-β [36,37,38,39,40,41,42] |
| The promotion of keratinocyte migration | TGF-β [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnici, L.; Suleiman, S.; Schembri-Wismayer, P.; Cassar, A. Targeting Signalling Pathways in Chronic Wound Healing. Int. J. Mol. Sci. 2024, 25, 50. https://doi.org/10.3390/ijms25010050
Bonnici L, Suleiman S, Schembri-Wismayer P, Cassar A. Targeting Signalling Pathways in Chronic Wound Healing. International Journal of Molecular Sciences. 2024; 25(1):50. https://doi.org/10.3390/ijms25010050
Chicago/Turabian StyleBonnici, Lian, Sherif Suleiman, Pierre Schembri-Wismayer, and Analisse Cassar. 2024. "Targeting Signalling Pathways in Chronic Wound Healing" International Journal of Molecular Sciences 25, no. 1: 50. https://doi.org/10.3390/ijms25010050
APA StyleBonnici, L., Suleiman, S., Schembri-Wismayer, P., & Cassar, A. (2024). Targeting Signalling Pathways in Chronic Wound Healing. International Journal of Molecular Sciences, 25(1), 50. https://doi.org/10.3390/ijms25010050







